Abstract
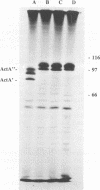
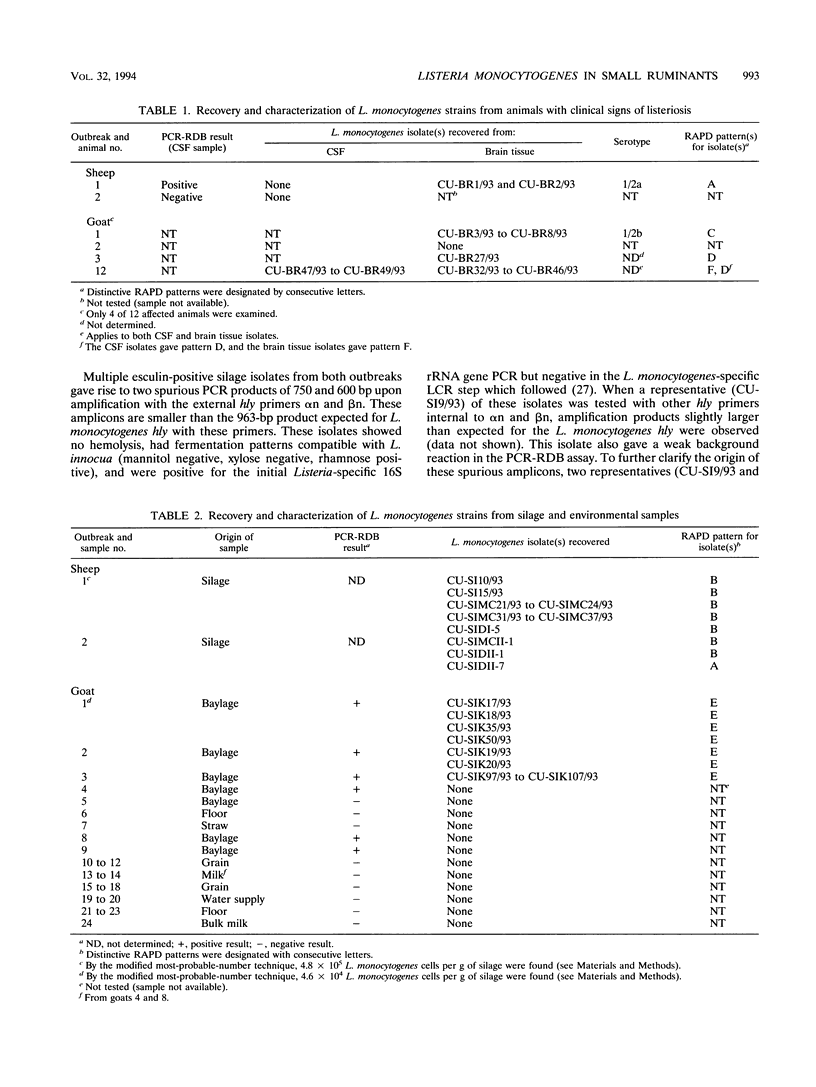
Two outbreaks of epizootic listerial encephalitis, one in sheep and one in goats, were investigated through pathology, microbiology, and DNA amplification-based techniques. Efforts were made to survey the diversity of Listeria monocytogenes strains in the silage consumed by affected animals and to verify the causal relationship between silage and disease outbreak. In both outbreaks, L. monocytogenes was isolated from silage and brain tissue samples. Random amplified polymorphic DNA patterns revealed two distinct L. monocytogenes strains, one of which was identical to the sheep brain isolate, in the silage associated with the outbreak in sheep. Three brain isolates and one silage isolate, all of which had different random amplified polymorphic DNA patterns, were found in the outbreak involving goats. All isolates from both outbreaks were indistinguishable in an in vitro assay for cell-to-cell spread and growth in macrophages. All brain isolates from the goat outbreak had identical intracellular ActA patterns, which were different from the pattern for the silage isolate. While the sheep brain isolate had an ActA pattern different from that of the corresponding silage isolate, the patterns for the brain isolates from the two outbreaks were not identical. This survey demonstrates the diversity of L. monocytogenes in silage and suggests the existence of one or more selective processes by which certain strains are more prone to give rise to disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow R. M., McGorum B. Ovine listerial encephalitis: analysis, hypothesis and synthesis. Vet Rec. 1985 Mar 2;116(9):233–236. doi: 10.1136/vr.116.9.233. [DOI] [PubMed] [Google Scholar]
- Boerlin P., Piffaretti J. C. Typing of human, animal, food, and environmental isolates of Listeria monocytogenes by multilocus enzyme electrophoresis. Appl Environ Microbiol. 1991 Jun;57(6):1624–1629. doi: 10.1128/aem.57.6.1624-1629.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage R. A., Smith G. A., Camilli A., Theriot J. A., Portnoy D. A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsat N., Batt C. A. A combined modified reverse dot-blot and nested PCR assay for the specific non-radioactive detection of Listeria monocytogenes. Mol Cell Probes. 1993 Jun;7(3):199–207. doi: 10.1006/mcpr.1993.1029. [DOI] [PubMed] [Google Scholar]
- Czajka J., Bsat N., Piani M., Russ W., Sultana K., Wiedmann M., Whitaker R., Batt C. A. Differentiation of Listeria monocytogenes and Listeria innocua by 16S rRNA genes and intraspecies discrimination of Listeria monocytogenes strains by random amplified polymorphic DNA polymorphisms. Appl Environ Microbiol. 1993 Jan;59(1):304–308. doi: 10.1128/aem.59.1.304-308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez Rodriguez L., Vazquez Boland J. A., Fernandez Garayzabal J. F., Echalecu Tranchant P., Gomez-Lucia E., Rodriguez Ferri E. F., Suarez Fernandez G. Microplate technique to determine hemolytic activity for routine typing of Listeria strains. J Clin Microbiol. 1986 Jul;24(1):99–103. doi: 10.1128/jcm.24.1.99-103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenlon D. R. Rapid quantitative assessment of the distribution of Listeria in silage implicated in a suspected outbreak of listeriosis in calves. Vet Rec. 1986 Mar 1;118(9):240–242. doi: 10.1136/vr.118.9.240. [DOI] [PubMed] [Google Scholar]
- Fensterbank R., Audurier A., Godu J., Guerrault P., Malo N. Etude des souches de listeria isolées d'animaux malades et de l'ensilage consommé. Ann Rech Vet. 1984;15(1):113–118. [PubMed] [Google Scholar]
- Fernandez-Garayzabal J. F., Blanco M., Vazquez-Boland J. A., Briones V., Garcia J. A., Delgado C., Domingo M., Marco J., Dominguez L. A direct plating method for monitoring the contamination of Listeria monocytogenes in silage. Zentralbl Veterinarmed B. 1992 Sep;39(7):513–518. doi: 10.1111/j.1439-0450.1992.tb01200.x. [DOI] [PubMed] [Google Scholar]
- Gitter M., Stebbings R. S., Morris J. A., Hannam D., Harris C. Relationship between ovine listeriosis and silage feeding. Vet Rec. 1986 Feb 22;118(8):207–208. doi: 10.1136/vr.118.8.207. [DOI] [PubMed] [Google Scholar]
- Jaton K., Sahli R., Bille J. Development of polymerase chain reaction assays for detection of Listeria monocytogenes in clinical cerebrospinal fluid samples. J Clin Microbiol. 1992 Aug;30(8):1931–1936. doi: 10.1128/jcm.30.8.1931-1936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Rocourt J., Hof H., Goebel W. Levels of Listeria monocytogenes hemolysin are not directly proportional to virulence in experimental infections of mice. Infect Immun. 1988 Feb;56(2):534–536. doi: 10.1128/iai.56.2.534-536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low J. C., Chalmers R. M., Donachie W., Freeman R., McLauchlin J., Sisson P. R. Pyrolysis mass spectrometry of Listeria monocytogenes isolates from sheep. Res Vet Sci. 1992 Jul;53(1):64–67. doi: 10.1016/0034-5288(92)90086-h. [DOI] [PubMed] [Google Scholar]
- Niebuhr K., Chakraborty T., Rohde M., Gazlig T., Jansen B., Köllner P., Wehland J. Localization of the ActA polypeptide of Listeria monocytogenes in infected tissue culture cell lines: ActA is not associated with actin "comets". Infect Immun. 1993 Jul;61(7):2793–2802. doi: 10.1128/iai.61.7.2793-2802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen O. F., Beck T., Olsen J. E., Dons L., Rossen L. Listeria monocytogenes isolates can be classified into two major types according to the sequence of the listeriolysin gene. Infect Immun. 1991 Nov;59(11):3945–3951. doi: 10.1128/iai.59.11.3945-3951.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Vázquez-Boland J. A., Dominguez L., Blanco M., Rocourt J., Fernández-Garayzábal J. F., Gutiérrez C. B., Tascón R. I., Rodriguez-Ferri E. F. Epidemiologic investigation of a silage-associated epizootic of ovine listeric encephalitis, using a new Listeria-selective enumeration medium and phage typing. Am J Vet Res. 1992 Mar;53(3):368–371. [PubMed] [Google Scholar]
- Wiedmann M., Barany F., Batt C. A. Detection of Listeria monocytogenes with a nonisotopic polymerase chain reaction-coupled ligase chain reaction assay. Appl Environ Microbiol. 1993 Aug;59(8):2743–2745. doi: 10.1128/aem.59.8.2743-2745.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M., Czajka J., Barany F., Batt C. A. Discrimination of Listeria monocytogenes from other Listeria species by ligase chain reaction. Appl Environ Microbiol. 1992 Nov;58(11):3443–3447. doi: 10.1128/aem.58.11.3443-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]