Abstract
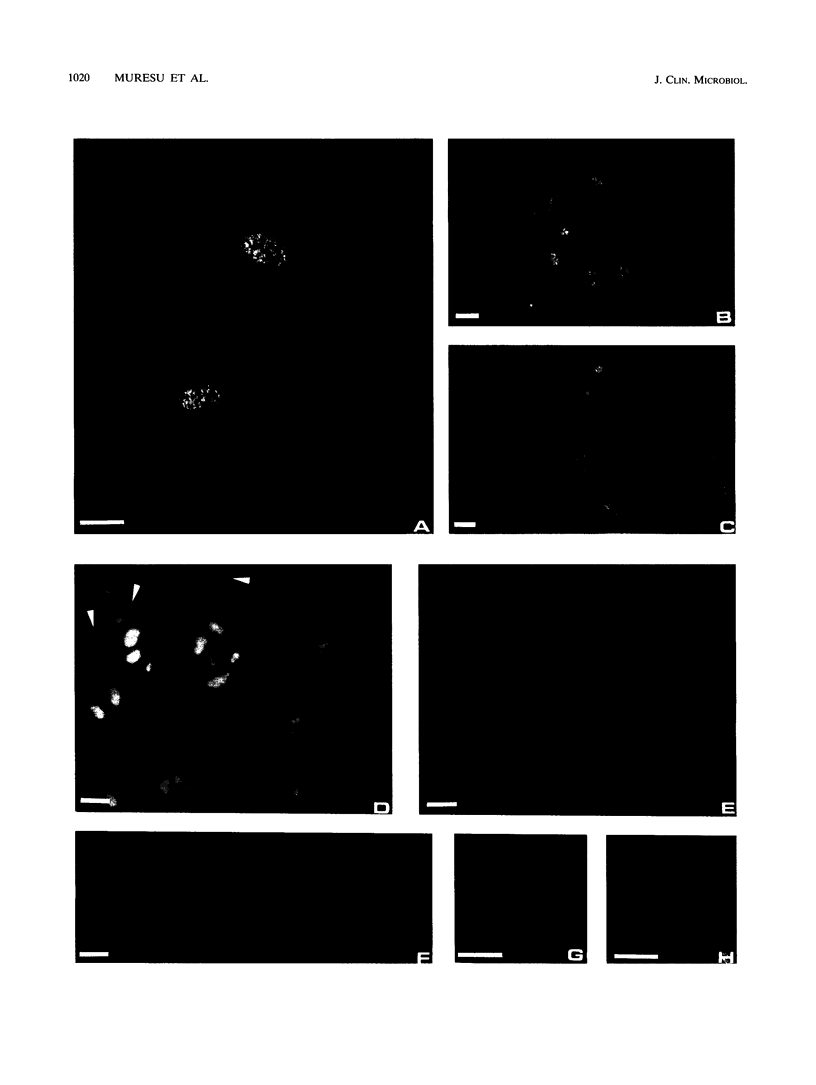
The protozoan flagellate Trichomonas vaginalis is responsible for human trichomoniasis, one of the most widespread sexually transmitted diseases in the world. Several methods are currently used for laboratory diagnosis, including direct microscopic observation, cell culture, immunological techniques, and more recently, DNA probing and gene amplification. This report describes an in situ hybridization technique with specific DNA probes labeled with either biotin, rhodamine, or fluorescein for detection of T. vaginalis with fluorescence microscopy. Repetitive DNA sequences were evident in the nuclei of the protozoa as intensively fluorescent regions, giving a spotted pattern. No cross-reactivity between the probes and the DNAs of mammalian cells, yeasts, or bacteria was noted. This technique is potentially useful for the diagnosis of human trichomoniasis in clinical samples.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Kasmala L. Monoclonal antibody to a major glycoprotein immunogen mediates differential complement-independent lysis of Trichomonas vaginalis. Infect Immun. 1986 Sep;53(3):697–699. doi: 10.1128/iai.53.3.697-699.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L., Smith J., Spence M. Heterogeneity of Trichomonas vaginalis and discrimination among trichomonal isolates and subpopulations with sera of patients and experimentally infected mice. Infect Immun. 1985 Sep;49(3):463–468. doi: 10.1128/iai.49.3.463-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P. J., Nicol C. S. Growth studies of various strains of T. vaginalis and possible improvements in the laboratory diagnosis of trichomoniasis. Br J Vener Dis. 1973 Dec;49(6):536–539. doi: 10.1136/sti.49.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Fleury F. J. Diagnosis of Trichomonas vaginalis infection. JAMA. 1979 Dec 7;242(23):2556–2557. [PubMed] [Google Scholar]
- Fouts A. C., Kraus S. J. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis. 1980 Feb;141(2):137–143. doi: 10.1093/infdis/141.2.137. [DOI] [PubMed] [Google Scholar]
- Jírovec O., Petrů M. Trichomonas vaginalis and trichomoniasis. Adv Parasitol. 1968;6:117–188. doi: 10.1016/s0065-308x(08)60473-x. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Holmes K. K., Spence M. R., Rein M. F., McCormack W. M., Tam M. R. Geographic variation among isolates of Trichomonas vaginalis: demonstration of antigenic heterogeneity by using monoclonal antibodies and the indirect immunofluorescence technique. J Infect Dis. 1985 Nov;152(5):979–984. doi: 10.1093/infdis/152.5.979. [DOI] [PubMed] [Google Scholar]
- Krieger J. N. Urologic aspects of trichomoniasis. Invest Urol. 1981 May;18(8):411–417. [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter P., Boyle A. L., Cremer T., Ward D. C. Analysis of genes and chromosomes by nonisotopic in situ hybridization. Genet Anal Tech Appl. 1991 Feb;8(1):24–35. doi: 10.1016/1050-3862(91)90005-c. [DOI] [PubMed] [Google Scholar]
- Lo Y. M., Mehal W. Z., Fleming K. A. Rapid production of vector-free biotinylated probes using the polymerase chain reaction. Nucleic Acids Res. 1988 Sep 12;16(17):8719–8719. doi: 10.1093/nar/16.17.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip A., Carter-Scott P., Rogers C. An agar culture technique to quantitate Trichomonas vaginalis from women. J Infect Dis. 1987 Feb;155(2):304–308. doi: 10.1093/infdis/155.2.304. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. E., Roberts M. C., Takayama T., Krieger J. N. Development of a polymerase chain reaction-based diagnosis of Trichomonas vaginalis. J Clin Microbiol. 1992 Feb;30(2):465–472. doi: 10.1128/jcm.30.2.465-472.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg R. B., Simon R., Chipperfield E., Catterall R. D. Efficacy of selected diagnostic tests for sexually transmitted diseases. JAMA. 1976 Jan 5;235(1):49–51. [PubMed] [Google Scholar]
- Rubino S., Muresu R., Rappelli P., Fiori P. L., Rizzu P., Erre G., Cappuccinelli P. Molecular probe for identification of Trichomonas vaginalis DNA. J Clin Microbiol. 1991 Apr;29(4):702–706. doi: 10.1128/jcm.29.4.702-706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Spence M. R., Hollander D. H., Smith J., McCaig L., Sewell D., Brockman M. The clinical and laboratory diagnosis of Trichomonas vaginalis infection. Sex Transm Dis. 1980 Oct-Dec;7(4):168–171. doi: 10.1097/00007435-198010000-00004. [DOI] [PubMed] [Google Scholar]
- Tchen P., Fuchs R. P., Sage E., Leng M. Chemically modified nucleic acids as immunodetectable probes in hybridization experiments. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3466–3470. doi: 10.1073/pnas.81.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]