Abstract
A recent report demonstrated a racial difference in response to furosemide compatible with increased ion reabsorption in the thick ascending limb of the loop of Henle in blacks. Urinary dilution is another function of the loop-diuretic-sensitive Na,K,2Cl cotransporter in the thick ascending limb, and racial differences in urinary diluting capacity have not been reported previously. We assessed diluting segment (cortical thick ascending limb and distal convoluted tubule) function in black and white normotensives in 2 studies utilizing a water loading approach. In both studies, we found that whites excreted a water load more rapidly than blacks. In the first study, the final free water clearance rates (mean±SD) were 7.3±4.7 ml/min in whites (n=17, 7 females, 10 males) and 3.8±3.6 ml/min in blacks (n=14, 9 females, 5 males), <P=0.03. In the second study, final free water clearance rates were 8.3±2.6 ml/min in whites (n=17, 8 females, 9 males) and 6.4±1.8 ml/min in blacks (n=11, 8 females, 3 males), P<0.01. We found no evidence of a racial difference in renal proximal tubular fluid reabsorption as assessed by renal endogenous lithium clearance or in plasma vasopressin level that could explain the difference in free water excretion. We conclude that our observations are most consistent with a lower capacity of ion reabsorption in the renal diluting segment in blacks. Slower excretion of an acute water load may have been an advantage during natural selection of humans living in arid, hot climates.
Keywords: kidney, water, race, renal tubule, ion, vasopressin
Chun, et al, recently demonstrated a racial difference in responses to furosemide compatible with increased activity of the loop-diuretic-sensitive Na,K,2Cl (NKCC2) cotransporter in the thick ascending limb (TAL) of the loop of Henle in blacks compared to whites (1). In addition to helping to concentrate urine, the cortical TAL NKCC2 (in concert with the Na,Cl cotransporter [NCC] in the water-impermeable segments of the distal convoluted tubule [DCT]) generates free water by the net reabsorption of ions from tubular fluid, and a racial difference in NKCC2 activity could therefore affect the ability to dilute urine. Tubular diluting capacity can be characterized by measuring free water generation during water loading (2), and we report here the results of 2 experimental studies of renal responses to water loading in blacks and whites.
Methods
Both studies were approved by the University of Michigan Institutional Review Board, and all subjects gave written informed consent. For both studies we recruited subjects by public advertisement from Ann Arbor, Michigan and the surrounding area. Subjects were healthy normotensive black and white (non-Hispanic) men and women, aged 18-50 years. As per NIH guidelines, race was self-determined. Normotensive status was established during a screening visit as a systolic blood pressure of <140 mmHg and a diastolic blood pressure of <90 mmHg for the average of two seated auscultatory blood pressure measurements performed by an experienced observer. A general health history was obtained and a physical examination performed to exclude other diseases including cancer, recent (within 6 months) stroke or myocardial infarction, diabetes, liver disease, chronic infections, and psychiatric disease of sufficient severity to interfere with a subject's ability to adhere to the protocol. We screened for occult disease with a complete blood count, a thyroid stimulating hormone level, a comprehensive automated biochemical profile and a urinalysis; all values were required to be normal, including a serum creatinine (Cr) of <1.3 mg/dL for women and <1.5 mg/dL for men and a serum potassium (K) >3.5 mmol/L. Pregnancy was excluded by a rapid urine pregnancy test. Drug therapies that could affect renal tubular Na handling (diuretics, non-steroidal anti-inflammatory drugs, caffeine, theophylline) were not permitted.
Protocol #1
Data reported here cover the first 90 minutes (three 30 minute periods) of a longer protocol designed to study the effects of dopaminergic control of renal sodium excretion; those data will be reported elsewhere. Subjects consuming their usual diet came to an outpatient research facility of the University of Michigan Medical Center during the morning. Subjects were asked to drink water prior to coming, as we wanted to ensure that they were well hydrated and could produce adequate urine volumes during the study. Upon arrival they were asked to drink 12 oz (360 mL) of water as rapidly as possible. An intravenous infusion of 5% glucose in water was begun and maintained throughout the study at a rate of 200 mL/hr. Urine samples were collected every 30 minutes, and at 30 and 60 minutes, urine output was replaced orally mL for mL with water. Blood was obtained at baseline and at the end of each hour. Measurements of sodium (Na), K, Cr and osmolality (Osm) were obtained for all urine and serum samples.
Protocol 2
Inclusion and exclusion criteria and recruitment methods were the same as for protocol #1. On Day 1, subjects who had previously agreed to participate in the study came to the outpatient research facility, where the details of the protocol were reviewed, a medical history was obtained and a physical examination was performed. Before leaving the facility, subjects voided and were instructed to begin collecting all urine in 2 plastic jugs; “Day” was the period from leaving the research facility until retiring to sleep, “Night” was the time from retiring through awakening, at which time subjects collected their first morning void, which completed the nighttime collection. The times of retiring and awakening were recorded. Subjects were instructed to collect any voids during the night in the “Night” jug. Ambulatory blood pressure monitoring was performed during Day 1 (Spacelabs Model 90207, Spacelabs, Inc., Redmond, WA).
At 9:00 AM on Day 2, subjects reported in the fasting state to the research facility and returned the urine collections. A catheter for blood sampling was placed in a forearm vein and baseline blood samples obtained. Subjects then voided and began the water loading protocol by drinking 20 mL per kg of body weight of distilled water as rapidly as possible over no longer than 45 minutes. Hourly urine samples were collected for the following 2 hours by voiding. Urine volume was measured for both hourly collections, and the first hour's volume was replaced orally mL for mL with distilled water. In addition to the baseline samples, blood was collected at the end of both urine collections. Serum analyses included Na, lithium (Li), Cr, and Osm. Plasma vasopressin was measured in the specimen obtained at the final collection. Urine Na and Li concentrations and Osm were measured in all urine specimens. Urinary clearances (ClX) were calculated by the standard formula: (Urine X/Serum X) * Urine flow rate, where X can be Cr, Osm or Li. Urinary free water clearance (ClH2O) was calculated as Urine flow rate - ClOsm. A concentration index (C.I.) was calculated as Urine Cr/Serum Cr (3).
Serum and urine Na and K were measured by flame photometry and plasma Osm by freezing point depression. We measured endogenous serum and urine Li concentrations by mass spectrometry using a Finnigan Element inductively coupled-high resolution mass spectrometer in standard Meinhard nebulizer and Scott spray chamber configuration. Both Li-6 and Li-7 isotopes were analyzed in low resolution mode, as well as Li-7 in medium resolution mode to confirm that isotopic interferences were negligible. The nominal instrument detection limit for Li is <0.002uM. This permits substantial dilution of the samples prior to analysis, which helps to minimize matrix effects. External calibrations are used for both urine and serum samples; the methods were validated by comparison with results obtained by standard additions. Serum and urine creatinine assays were performed using a modified Jaffe reaction. Plasma vasopressin was measured by radioimmunoassay.
Statistical comparisons between races were by unpaired t-test. Statistical significance was accepted at the p< 0.05 level. All data are expressed as mean ± SD.
Results
Characteristics of the subjects who participated in the 2 studies are shown in Tables 1 and 2. With minor variations, the composition of the study groups was similar in the 2 studies.
Table 1.
Baseline characteristics of the subjects for Study 1 (mean ± SD)
| Parameter | Black n=14 | White n=17 | p(BvW) |
|---|---|---|---|
| Female sex - no. (%) | 9 (64.3) | 7(41.2) | 0.21 |
| Age (y) | 32.1 ± 10.3 | 29.3 ± 9.3 | 0.43 |
| Blood Pressure (mm Hg)* | |||
| Systolic | 118.1 ± 11.3 | 116.9 ± 11.4 | 0.77 |
| Diastolic | 74.4 ± 7.0 | 65.2 ± 5.9 | 0.01 |
| BMI (kg/m2) | 26.3 ± 6.0 | 29.4 ± 6.5 | 0.19 |
| ClCr (mL/min) | 93.6 ± 11.5 | 90.0 ± 11.8 | 0.67 |
| Serum Cr (mg/dL) | 0.98 ± 0.16 | 0.94 ± 0.14 | 0.45 |
| Serum Na (mmol/L) | 138.6 ± 3.4 | 137.8 ± 1.3 | 0.38 |
| Serum K (mmol/L) | 4.0 ± 0.3 | 4.1 ± 0.2 | 0.35 |
| Serum Osmolality (mOsm/L) | 283.5 ± 3.5 | 285.3 ± 4.7 | 0.23 |
Average of 2 seated auscultatory readings
Table 2.
Baseline characteristics of the subjects for Study 2 (mean ± SD)
| Parameter | Black n=11 | White n=17 | p (B v W) |
|---|---|---|---|
| Female sex - no. (%) | 8 (72.7) | 8 (47.1) | 0.23 |
| Age (y) | 32.5 ± 9.5 | 27.2 ± 5.6 | 0.13 |
| Ambulatory Blood Pressure (mm Hg)* | |||
| Systolic | 115.2 ± 10.6 | 116.3 ± 8.2 | 0.75 |
| Diastolic | 72.0 ± 8.9 | 69.8 ± 5.7 | 0.43 |
| BMI (kg/m2) | 28.5 ± 8.7 | 25.0 ± 5.8 | 0.21 |
| ClCr (mL/min) | 94.7 ± 14.5 | 94.0 ± 14.8 | 0.90 |
| Serum Cr (mg/dL) | 0.94 ± 0.14 | 0.93 ± 0.18 | 0.91 |
| Serum Na (mmol/L) | 139.0 ± 1.9 | 140.5 ± 2.9 | 0.14 |
| Serum K (mmol/L) | 4.2 ± 0.17 | 4.3 ± 0.32 | 0.25 |
Average for 24 hours by oscillometeric automated device
Study 1
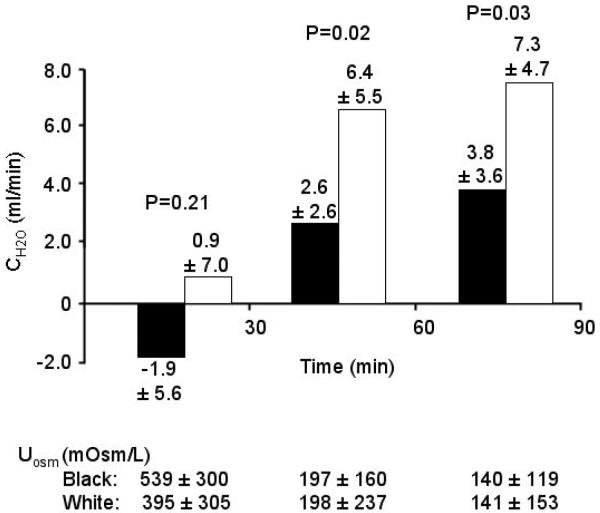
Baseline characteristics, including serum Osm, did not differ between the races (Table 1) except for a higher diastolic blood pressure in blacks. Urine flow rate was higher for all 3 periods in whites (Period 1 (0-30 min): 11.9±7.9 vs. 4.8±3.5 mL/min, p<0.01; Period 2 (30-60 min): 10.3±6.1 vs. 4.8 ±2.7 mL/min, p<0.01; Period 3 (60-90 min): 9.6±4.2 vs. 6.9±3.7 mL/min p<0.05, white vs. black for all comparisons). CH2Owas higher in whites during Periods 2 and 3 (Figure 1). Because flow rate differed during the first 30 minutes and water was replaced orally mL for mL at the 30 and 60 minute time points, the total amount of water consumed during the study differed between the races (1144±395 vs. 763±163 mL, white vs. black, p=0.002). The total urine volume excreted during the 90 minutes following water loading was greater in whites than blacks (994±419 vs. 490±241 mL, white vs. black, p=0.001), as was the average percentage of the water load excreted (81.1±22.5 vs. 60.8±21.4 %, white vs. black, p=0.015). UOsm declined in both races and was similar during all collection periods (Figure 1).
Figure 1. Study 1.
Comparison of CH2O in blacks (filled bars) and whites during water loading. Pairwise comparisons by t-test are significantly different for the second and third periods but not for the first period. UOsm is not different for any period.
Urinary excretion of Na during the 90 minutes of the study was greater, although not significantly so, in whites (39.4±20.1 vs. 20.1±11.7 mmoles, white vs. black, p=0.07); there was no difference during any of the individual periods.
Study 2
There were no significant differences between the white and black groups for urine volume, flow rate, Na or osmolar excretion or C.I. during the day or night (Table 3). Total osmolar excretion (day plus night) was also similar between the groups: 746±274 vs. 707±366 mosmoles, white vs. black, p=0.77). K excretion was lower in blacks during the daytime, but not at night (Table 3).
Table 3.
Day and night urine volume, flow rate and composition for Study 2 (mean ± SD)
| Parameter | Black n=11 | White n=17 | p (BvW) |
|---|---|---|---|
| Day | |||
| Volume (mL) | 934 ± 751 | 1070 ± 403 | 0.59 |
| Flow rate (mL/min) | 1.18 ± 0.95 | 1.38 ± 0.48 | 0.55 |
| Na excretion (mmoles) | 83.4 ± 59.8 | 101.0 ± 57.3 | 0.45 |
| K excretion (mmoles) | 26.1 ± 16.7 | 45.6 ± 20.9 | 0.01 |
| UOsm (mOsm/L) | 583 ± 243 | 477 ± 204 | 0.25 |
| Osmolar excretion (mosmoles) | 460.4 ± 317.3 | 494.2 ± 246.7 | 0.77 |
| Concentration index | 129.0 ± 86.9 | 91.2 ± 37.9 | 0.20 |
| Night | |||
| Volume (mL) | 455 ± 212 | 478 ± 207 | 0.78 |
| Flow rate (mL/min) | 0.94 ± 0.48 | 0.93 ± 0.36 | 0.94 |
| Na excretion (mmoles) | 43.3 ± 32.4 | 55.2 ± 31.7 | 0.35 |
| K excretion (mmoles) | 13.5 ± 12.0 | 13.6 ± 9.6 | 0.99 |
| UOsm (mOsm/L) | 610 ± 218 | 556 ± 226 | 0.54 |
| Osmolar excretion (mosmoles) | 247.0 ± 108.9 | 251.7 ± 107.4 | 0.91 |
| Concentration index | 167.8 ± 102.2 | 131.5 ± 68.7 | 0.32 |
Urinary endogenous CLi did not differ between the races (day: 19.9±8.0 vs. 18.3±10.9 mL/min, white vs. black [n=10], p=0.53; night: 16.0±5.8 vs. 16.7±11.2 mL/min, white vs. black [n=10], p=0.92). Because of sample mishandling, one black subject did not have urinary Li values determined for the day or night urines.
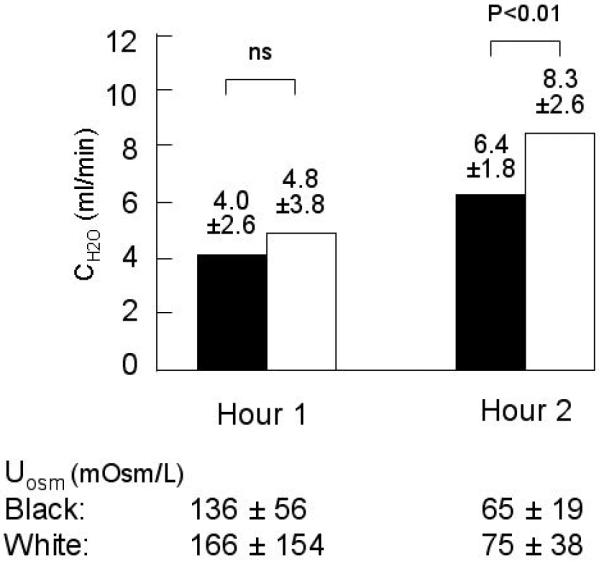
During water loading, the amount of water consumed did not differ between the groups (2091±410 vs. 2156±566 mL, white vs. black, p=0.77). The total urine volume excreted during the 120 minutes of water loading was greater, although not significantly so, in whites compared to blacks (1131±471 vs. 930±256 mL, white vs. black, p=0.15). Urine flow rate did not differ between the groups during the first hour (8.8±4.2 vs. 8.3±2.6 mL/min, white vs. black, p=0.55); flow rate increased more in whites than blacks and was significantly greater during the second hour (11.4±3.1 vs. 8.8±2.8 mL/min, white vs. black, p<0.01). CH2O also increased significantly in both groups from the first to the second hour of water loading: during the second hour, CH2Owas significantly greater in whites (Figure 2), while CLiwas similar (26.9±9.6 vs. 22.3±12.2 mL/min, white vs. black, p=0.32). UOsm during the first and second hourly collections did not differ by race (Figure 2).
Figure 2. Study 2.
Symbols are the same as in Figure 1. Hour 1 CH2O is not different. CH2O increased in both blacks and whites from hour 1 to 2 (both p ≤ 0.001). Hour 2 urine CH2O is significantly higher in whites. UOsmis not different for either period.
Plasma vasopressin was below the lower limit of the assay (<0.5 pg/mL) in all subjects at the end of 2 hours, and serum Na, K, Cr and Osm did not differ between the groups at the end of either hour 1 or 2 (data not shown).
Discussion
The observations in our 2 studies are consistent in showing that whites excrete a water load more rapidly than blacks. The racial difference observed in the first study could have been affected by the differences in water intake resulting from the subjects' ad lib and unmeasured water consumption prior to their presentation to the research facility and by the procedure of replacing urine volume mL for mL at the end of each period. However, in the second study subjects presented in the fasting state, and the amount of water ingested was very similar between the groups; the lower urine flow rate and lesser CH2O in blacks therefore appears to be due to a difference in renal water handling. We acknowledge that since the water loads were delivered orally in that study, it is possible that there is a racial difference in the gastrointestinal absorption of water or its distribution in the body (4), but we have no way of assessing those possibilities and contend that our observations most likely result from a difference in renal water handling.
The recent report from Chun, et al, demonstrating that there is a racial difference in urinary responses to furosemide administration concluded that TAL NKCC2 activity may be higher in blacks (1): our demonstration of lower free water clearance in blacks is more consistent with decreased TAL NKCC2 activity. It is important to stress that quite different functions were assessed in the 2 studies, neither of which provides an unequivocal assessment of TAL function. We used a water loading protocol to assess TAL function, but because free water is generated by solute reabsorption in both the water impermeable cortical segment of the TAL and in the DCT, we cannot distinguish between those segments (2). However, based on the observed effects of furosemide and thiazides on free water clearance in humans (5) and the lack of a significant defect in concentrating ability in human genetic disorders with markedly impaired NCC activity (6), the TAL is thought to be quantitatively much more important than the DCT. Nonetheless, clinical observations of thiazide-induced hyponatremia suggest that particularly in some elderly individuals, DCT function can be a quantitatively important determinant of water balance (7).
In addition to ion reabsorption in the TAL, vasopressin-mediated water reabsorption in the collecting tubule is an important determinant of CH2O. Vigorous water loading is expected to largely eliminate, or at least greatly attenuate, the action of vasopressin, although because the vasopressin assay is not sensitive enough to ensure that suppression of vasopressin is complete, we cannot exclude an effect at the collecting duct. In addition, it is possible that non-vasopressin dependent back-diffusion of water in the collecting duct is greater in blacks than whites. However, the similarity of the minimum urine osmolarities observed in blacks and whites in our study argues against an important contribution of water reabsorption in the collecting duct.
Urinary ClH2O is influenced by differences in tubular flow rate and the delivery of Na and K from the proximal tubule (8): we addressed the possibility of a racial difference in proximal tubular ion reabsorption by measuring urinary ClLi(9). Our observation that ClLi did not differ between the races prior to or during water loading suggests that a difference in the delivery of fluid from the proximal tubule does not account for the racial difference in ClH2O. Since average minimum UOsm is similar in blacks and whites following water loading, and in both groups is far below that of the plasma, the difference in CH2O between the groups reflects the rate at which the diluting segment can generate free water and implies that the determinants of minimum UOsm, including the osmolality of the medullary interstitium and the efficacy of the countercurrent mechanism as well as the permeabilities of the TAL and collecting duct are intact.
We did not observe significant differences in urine volume or UOsm between whites and blacks during the day or night preceding our second water loading study. This is in contrast to the observations of Bankir, Perucca and Weinberger, who observed a difference in urine flow rate and C.I. during the day, although not at night (3), and of Chun, et al, who observed a racial difference in urine volume and osmolality in a 12-hour overnight collection (1). In part the differing observations might be accounted for by differences between the protocols followed by those investigators and ourselves, as they hospitalized subjects and imposed a controlled diet, while our subjects were studied as outpatients, and no dietary or fluid restrictions were imposed. We also note, however, that in our study both UOsm and C.I. did trend in the direction of greater concentration in blacks, particularly during the daytime, and our smaller sample size may have been insufficient to permit detection of a statistically significant difference.
Perspective
We agree with Bankir, Perucca and Weinberger that natural selection could have played a role in shaping racial differences in renal water handling and suggest that in addition to enhanced urine concentrating ability (3), slower excretion of a water load could have conferred a selective advantage during the evolution of humans in the hot, arid climate of East Africa. Much of the interest in adaptations to such environments has focused on sodium homeostasis, but it is likely that obligatory water requirements are much more stringent than those for sodium. For instance, while average sodium excretion varied by some 200-fold between the populations with the lowest and highest excretions in INTERSALT, urine volume varied by only about 3-fold, and even that might overestimate the magnitude of variation in water intake, since there is no way to estimate insensible losses in that study (10). Since water is available only intermittently to hunter-gatherers, slowing excretion of an acute water load could help to optimize water retention and improve reproductive success.. Such a phenotype would be particularly advantageous if coupled with an ability to increase maximum urinary concentrating ability.
Acknowledgments
Sources of funding:: Supported in part by Grant Number M01-RR000042 from the National Center for Research Resources (NCRR) of the National Institutes of Health (NIH) and by the Faculty Group Practice of the University of Michigan. The contents are solely the responsibility of the authors and do not represent the official views of NCRR or NIH.
Footnotes
Disclosures: None
References
- 1.Chun T-Y, Bankir L, Eckert GJ, Bichet DG, Saha C, Zaidi S-A, Wagner MA, Pratt JH. Ethnic differences in renal responses to furosemide. Hypertension. 2008;52:1–8. doi: 10.1161/HYPERTENSIONAHA.108.109801. [DOI] [PubMed] [Google Scholar]
- 2.Levinsky NG, Lieberthal W. Clearance techniques. In: Windhager EE, editor. Handbook of Physiology. A critical, comprehensive presentation of physiological knowledge and concepts. Vol 1. Oxford University Press; New York, USA: 1982. pp. 227–247. Section 8: Renal Physiology. [Google Scholar]
- 3.Bankir L, Perucca J, Weinberger MH. Ethnic differences in urine concentration: possible relationship to blood pressure. Clin J Am Soc Nephrol. 2007;2:304–312. doi: 10.2215/CJN.03401006. [DOI] [PubMed] [Google Scholar]
- 4.Shafiee MA, Charest AF, Cheema-Dhadli S, Glick DN, Napolova O, Roozbeh J, Semenova E, Sharman A, Halperin ML. Defining conditions that lead to the retention of water: the importance of the arterial sodium concentration. Kidney International. 2005;67:613–621. doi: 10.1111/j.1523-1755.2005.67117.x. [DOI] [PubMed] [Google Scholar]
- 5.Seldin DW, Eknoyan G, Suki WN, Rector FC. Localization of diuretic action from the pattern of water and electrolyte excretion. Ann N Y Acad Sci. 1966;139:328–343. doi: 10.1111/j.1749-6632.1966.tb41207.x. [DOI] [PubMed] [Google Scholar]
- 6.Jeck N, Schlingmann KP, Reinalter SC, Komhoff M, Peters M, Waldegger S, Seyberth HW. Salt handling in the distal nephron: lessons learned from inherited human disorders. Am J Physiol Regul Integr Comp Physiol. 2005;288:R782–R785. doi: 10.1152/ajpregu.00600.2004. [DOI] [PubMed] [Google Scholar]
- 7.Clark BA, Shannon RP, Rosa RM, Epstein FH. Increased susceptibility to thiazide-induced hyponatremia in the elderly. J Am Soc Nephrol. 1994;5:1106–1111. doi: 10.1681/ASN.V541106. [DOI] [PubMed] [Google Scholar]
- 8.Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19:1076–1078. doi: 10.1681/ASN.2007091042. [DOI] [PubMed] [Google Scholar]
- 9.Thomsen K. Lithium clearance as a measure of sodium and water delivery from the proximal tubule. Kidney International. 1990;37(Suppl 28):S-10–S16. [PubMed] [Google Scholar]
- 10.The Intersalt Co-operative Research Group J Hum Hypertens. 1989;3:331. Appendix Tables A-21, A-22. [PubMed] [Google Scholar]