Abstract
High frequency (>1 Hz) repetitive transcranial magnetic stimulation (rTMS) applied to the left prefrontal cortex and low frequency (≤1 Hz) rTMS applied to the right prefrontal cortex have shown antidepressant effects. However, the clinical significance of these effects has often been modest. It was hypothesized that a combination of these two techniques might act synergistically and result in more clinically relevant antidepressant effects. Sixty-two subjects with treatment-resistant major depression (an average of 8 failed medication trials) were randomized to receive combination right low frequency (1 Hz)/left high frequency (10 Hz) rTMS over the dorsolateral prefrontal cortex at 110% of the motor threshold vs sham rTMS. Subjects were treated for 2 weeks (10 weekday sessions) and received 1600 stimulations during each treatment session. Subjects receiving combination treatment were further randomized to receive different orders of treatment: right low frequency first (Slow Right) vs left high frequency first (Fast Left). There were no statistical differences in the active vs sham treatment arms in the primary outcome variable, the Hamilton Depression Rating Scale (HDRS). However compared with subjects in the Sham and Slow Right arms, there was a trend for subjects in the Fast Left arm to show improvement in the HDRS, the Beck Depression Inventory, and the Brief Psychotic Rating Scale with increased number of treatments. The Fast Left arm also showed significant improvement in both blinded clinician and self-ratings of global improvement. These differences were hypothesized to be due to the decreased number of failed medication trials for subjects in Fast Left arm. Neuropsychological performance was not significantly different between the sham and active rTMS arms. Future studies should increase the number of treatment sessions and focus on subjects with moderate treatment resistance.
Keywords: transcranial magnetic stimulation, treatment resistant depression
Introduction
Repetitive transcranial magnetic stimulation (rTMS) provides a minimally invasive technique for electrically stimulating and modifying function in the cerebral cortex. Research has focused on using rTMS as a treatment for major depression. Given its favorable side-effect profile, rTMS offers a potentially valuable treatment option for subjects who do not tolerate or respond to antidepressant medications and/or electroconvulsive therapy. However, while meta-analyses of rTMS studies in depression have shown that rTMS does indeed have statistically significant antidepressant effects, the clinical significance of these effects has not been satisfactorily demonstrated (Holtzheimer et al 2001; Burt et al 2002; Kozel and George 2002; Martin et al 2003). For rTMS to become a clinically meaningful treatment for depression, the technique must be optimized.
The majority of studies demonstrating antidepressant efficacy for rTMS have used high frequency (>1 Hz or “fast”) rTMS over the left dorsolateral prefrontal cortex (DLPFC). Mechanistic support for these parameters is provided by (1) evidence that the left DLPFC is often hypofunctional in depression (Martinot et al 1990; Kennedy et al 1997; Videbech 2000), and (2) evidence that fast rTMS can induce lasting increases in cortical excitability (Pascual-Leone et al 1994). A few studies have also shown antidepressant efficacy for low frequency (≤ 1 Hz or “slow”) rTMS applied to the right DLPFC (Klein et al 1999; Menkes et al 1999; Fitzgerald et al 2003). Slow rTMS has been shown to decrease cortical excitability (Chen et al 1997; Wassermann et al 1998), and data suggest some depressed subjects may have a hyperfunctional right prefrontal cortex (Schaffer et al 1983; Garcia-Toro et al 2001).
Taken together, these data suggest that fast and slow rTMS applied to the left and right DLPFCs, respectively, may act via reciprocal and potentially complementary mechanisms. It is possible that a combination of these approaches could act synergistically to treat depression. While two prior sham-controlled studies have used a combination of rTMS approaches, both suffered from important limitations. Loo et al compared fast rTMS applied to bilateral DLPFCs to sham stimulation and found no benefit for active rTMS (Loo et al 2003). However, fast right-sided rTMS may have antimanic rather than antidepressant effects (Michael and Erfurth 2004), and the use of this approach may have limited the efficacy of fast left-sided rTMS. Hausmann et al (2004) found no benefit for fast left-sided rTMS or combined fast left-sided and slow right-sided rTMS in a sham-controlled study (Hausmann et al 2004). However, all subjects were started on antidepressant medications at the beginning of the study, and all 3 arms showed a significant antidepressant response over time. Given the use of an active comparator (antidepressant medications in the sham arm), this study likely lacked the power to demonstrate a statistical difference between the arms.
In this sham-controlled study, the antidepressant effects of combined left high frequency and right low frequency rTMS were investigated in a treatment-resistant depressed population. It was hypothesized that combination rTMS would be superior to sham stimulation in treating depression, and that these antidepressant effects would be clinically significant. In order to determine if the order of the stimulus (ie, slow left first vs slow right first) made a difference in treatment response, the subjects were randomly assigned to receive either fast left or slow right first during treatment administration.
Methods
Subjects
This study was reviewed and approved by the Emory University Human Investigations Committee. The 62 subjects were between 18 and 70 years old recruited from the community who met the severity of symptoms specified by the American Psychiatric Association criteria for an acute course of electroconvulsive therapy (ECT) (ie, severe major depression and intolerant and/or resistant to antidepressant medication; Weiner 2001) and had treatment resistance to at least 3 antidepressant medications during the present depressive episode. Treatment resistance was defined as no significant improvement in depressive symptoms following a 6-week trial of an antidepressant dosage equivalent to fluoxetine 20 mg. The number of previous medication trials was determined by self-report and psychiatric record review and included the number of failed medication trials in the current depressive episode.
After obtaining informed consent, subjects had a complete neurological and physical examination with a thorough review of systems. Subjects with minor neurological abnormalities (eg, essential tremor, chronic headaches, gait ataxia, prior head injury) underwent neuroimaging unless they had prior neuroimaging at the onset of the neurological dysfunction that showed no gross abnormality. Subjects were also screened with a complete blood count, thyroid function tests, and electrolytes within 3 months of the treatments. Women of childbearing age were screened with a urine pregnancy test.
Subjects were evaluated using a Structured Clinical Interview for DSM-IV (SCID) (First et al 1996), Beck Depression Inventory (BDI) (Beck et al 1961), Brief Psychiatric Rating Scale (BPRS), and the 21-item Hamilton Depression Rating Scale (HDRS) (Hamilton 1967). Entry criteria for the study included meeting SCID criteria for Unipolar Depression (UP) or Bipolar Disorder (BP), depressed phase, and a score on the 17-item HDRS ≥ 20 both at baseline and within 24 hours of the first treatment. Subjects could not have active suicidal ideation (defined as a score > 2 on question nr 9 of the BDI). Subjects were also assessed for handedness and educational level by self-report.
Exclusion criteria included evidence of dementia on neuropsychological testing (see description of tests below), or meeting SCID criteria for Organic Brain Syndrome, Organic Mood Disorder, Substance Dependence within the last 6 months, a diagnosis of a significant central neurological disorders including brain mass, epileptic seizures, stroke, transient ischemic attack within 2 years, cerebral aneurysm, dementia, Parkinson’s disease, Huntington’s chorea, multiple sclerosis, or other major CNS dysfunction. Additional exclusion criteria included pregnancy, the presence of cardiac pacemakers, cochlear implants, or other intracranial implants with the exception of dental fillings, and the presence of psychiatric symptoms of significant severity (eg, refusal of food and medication or the presence of psychosis) that would prevent a 2-week trial of rTMS being tolerated or would require psychiatric hospitalization. Subjects that required continued treatment with antidepressant medications were excluded. Subjects with acute, unstable medical conditions that required stabilization (eg, uncontrolled hypertension) prior to treatment were also excluded. Subjects were also excluded if they had a previous course of TMS.
Neuropsychological measures
The Brief Visuospatial Memory Test-Revised (BVMT-R; Benedict 1997) was used to evaluate visuospatial memory functioning. This measure presents 6 geometric shapes oriented in a 2 by 3 array across 3 study-test trials. The array is examined for 10 seconds and is subsequently removed from the examinee’s view. The examinee is requested to draw the figures as accurately as possible and in the correct location. One point is awarded for correctly placing a figure in its proper location, and one point is awarded for drawing the figure accurately. A total of 12 points are possible on any trial. Age-corrected T-scores (mean of 50, SD of 10) are computed on the basis of raw scores for each trial, for the sum of trials 1 through 3 (immediate memory), and for a 30-minute delayed recall of the figures. There are 6 alternative forms that may be administered to reduce practice effects. Forms 1 and 2 were administered in counterbalanced fashion, such that half of the subjects received Form 1 at Day 0 followed by Form 2 at Day 10 and half of the subjects received Form 2 first, followed by Form 1.
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; [Randolph 1988]) was used to evaluate level of performance across 5 cognitive domains (Immediate Memory, Visuospatial Functioning, Language, Attention, Delayed Memory). It also provides a summary total score. The measure takes approximately 25–35 minutes to administer, and it yields age-corrected standard scores (mean = 100, SD = 10) for each cognitive domain and for the total score. Two parallel forms (Forms A and B) are available that can be used to reduce the effects of repeated practice. As with the BVMT-R, Forms 1 and 2 were administered in counterbalanced fashion, such that half of the subjects received Form A at Day 0 followed by Form B at Day 10 and half of the subjects received Form B first, followed by Form A.
Finally, a Verbal Letter Fluency task (Benton and des Hamsher 1983) was used to evaluate cognitive flexibility. This task required the examinee to generate as many words as possible beginning with a specific letter in 60 seconds; 2 different letters are administered for each of 2 forms. As with the other neuropsychological measures, the 2 forms (CFL and PRW) were administered in counterbalanced fashion. The dependent variable for this task was the total number of words generated across 3 60-second trials (1 60-second trial for each of 3 letters).
Procedures
The device was a Neuronetics High Speed Magnetic Stimulator (Neuronetics Inc, Malvern PA, USA) with a maximum output of 2 Tesla. The magnetic configuration is a figure-8 with an iron core that has an induced field configuration with increased efficiency (Epstein and Davey 2002).
At each treatment, the subject’s motor threshold was determined using the method of limits and all rTMS dosing was administered relative to this value. Motor-evoked potentials from muscles in the hand were elicited with single-pulse magnetic stimulation over the left hemisphere. After determination of the point of maximal stimulation for the contralateral thumb, the method of limits was used to find the motor threshold for that individual (the lowest stimulation intensity capable of inducing 5 motor responses in a series of 10 single magnetic stimuli with the coil centered over the optimal scalp position). Once the optimal area to evoke the MT was determined, the coil was moved 5 cm anterior to treat over the dorsolateral prefrontal cortex.
Subjects were randomized to either sham TMS or a combination of Fast Left (10 Hz) rTMS over the DLPFC followed by Slow Right (1 Hz) DLPFC rTMS or Slow Right followed by Fast Left rTMS in a 1:2:2 ratio, respectively. Sham TMS was administered by tilting the stimulator at a 90-degree angle to the skull so that the electromagnetic pulse is directed away from the cortex and is essentially ineffective. Combination fast and slow rTMS was administered at 110% motor threshold (MT), with 20 5-second stimulations over 10 minutes of 10 Hz over the left DLPFC (total of 1000 stimulations) and 10 minutes of 1 Hz stimulation over the right DLPFC respectively (total of 600 stimulations). Stimulations were administered for 10 days separated by the weekend.
The subject and/or a research assistant blind to the randomization completed all clinical measures at baseline (within 24 hours before the first treatment), and after the 5th and 10th treatment. The measures included the HDRS, BDI, and 7-point Clinical Global Improvement Scale (CGI). The RBANS, BVMT-R, and Verbal Fluency measures were used to monitor for cognitive effects of rTMS. The neuropsychological battery was administered at baseline and after the 10th treatment. The HDRS score was the primary measure of efficacy and remission of symptoms was defined as HDRS ≤ 7 (Ballinger 1999). Treatment response was defined as a 50% or more decline in the HDRS compared with baseline (Frank et al 1991). Subjects who remitted or who showed a partial treatment response after 10 treatments were assessed 2 weeks, 1 month, 2 months, and 3 months after the last treatment to determine if they relapsed (ie, met SCID criteria for a major depression).
Statistical analyses
The primary analysis of the data was performed according to subjects’ original treatment assignment (ie, an intention-to-treat analysis) and all subjects were included in the analyses for as long as they contributed data. Baseline characteristics between treatment arms were compared with the Kruskal-Wallis test for continuous variables and with the χ2 or Fisher’s exact test for proportions. Change in CGI over time for each treatment arm was compared with the sign test.
The data were analyzed both as a comparison of the combined active vs sham treatment arms and a comparison on the 3 arms (Fast Left First, Slow Right Rirst, and Sham). The latter comparison was done to determine the effect of the order of treatment administration on treatment response. Repeated-measures analyses for HDRS, CGI, BDI, and BPRS and percent change from baseline were analyzed with a means model with SAS Proc Mixed (version 8; SAS Institute Inc., Cary, NC, USA), providing separate estimates of the means by time on study and treatment arm. An unstructured variance-covariance form among the repeated measurements was assumed for each outcome and estimates of the standard errors of parameters were used to perform statistical tests and construct 95% confidence intervals. T-tests were used to compare the pair-wise differences between the model-based treatment means (least-squares means) at each time point. The model-based means are unbiased with unbalanced and missing data, if the missing data are noninformative (missing at random). A dropout process is assumed to be missing at random if, conditional on the observed data, the dropout is independent of the unobserved measurements. Statistical tests were 2-sided. A Bonferroni adjustment (p < 0.0167) was used for the 3 pair-wise comparisons performed at each time point.
For each neuropsychological measure, a 3 (arm–placebo, Fast Left First, Slow Right First) by 2 (Time–Day 0 vs Day 10) mixed design analysis of variance (ANOVA) was performed using SPSS (SPSS Inc, Chicago, IL, USA) Version 12.0.2. The General Linear Model procedure was used to evaluate main effects of Arm and Time of Treatment, as well as the interaction between Arm and Time.
Results
Subjects were randomized into 3 arms in a 1:2:2 ratio: Sham stimulation (n = 12), combination active treatment with fast left then slow right (Fast Left; n = 25) or combination treatment with slow right followed by fast left (Slow Right; n = 25). The summary of the demographic and clinical characteristics of the study population is outlined in Table 1.
Table 1.
Demographic and clinical characteristics by randomization arm: the demographic and clinical characteristics of the subjects divided by whether they received 2 weeks of sham transcranial magnetic stimulation (TMS) or combination fast left and slow right TMS starting with either Fast Left treatments or Slow Right treatments. Significant differences were noted in the number of medication trials subjects received prior to TMS
| Variable | Sham (n = 12) | Fast Left (n = 25) | Slow Right (n = 25) | p |
|---|---|---|---|---|
|
Median (P25, P75)a |
||||
| Age (years) | 54.0 (47.0, 64.0) | 49.0 (41.0, 55.0) | 49.0 (39.0, 54.0) | 0.24 |
| Education (years) | 14.0 (12.0, 16.0) | 16.0 (14.0, 17.0) | 16.0 (14.0, 18.0) | 0.21 |
| # previous medication trials | 6.5 (5.0, 11.0) | 7.0 (5.0, 9.0) | 10.0 (8.0, 13.0) | 0.003 |
|
% (n) |
||||
| % male | 58.3 (7) | 28.0 (7) | 64.0 (16) | 0.03 |
| % bipolar | 25.0 (3) | 20.0 (5) | 0.0 (0) | 0.03 |
| % right hand dominant | 66.7 (8) | 96.0 (24) | 76.0 (19) | 0.05 |
| % previous ECT | 50.0 (6) | 28.0 (7) | 54.2 (13) | 0.15 |
| % attempted suicide | 16.7 (2) | 12.0 (3) | 12.5 (3) | 1.00 |
Entries are the median, the 25th, and 75th percentiles (P25, P75) for continuous variables; % (frequency) for categorical variables.
The subjects were treatment resistant. The median number of failed medication trials prior to study entry was 8, and 43% of subjects had failed ECT. The Slow Right arm had the highest number of failed trials of medication (p = 0.006), fewer male (p = 0.03) and bipolar subjects (p = 0.03). The Fast Left arm had all but 1 subject who was right hand dominant (p = 0.05).
Only 3 subjects remitted by the a priori diagnosis of a final HDRS ≤ 7. The 3 subjects were all in the Fast Left arm (12% remitted in the Fast Left arm; 4.8% of all subjects). Including the remitted subjects, 11 of the 62 subjects (18% of all subjects) met criteria for treatment response (ie, 50% decline in the HDRS): 7/25 (28%) Fast Left, 3/25 (12%) Slow Right, 1 Placebo (8%). This was a nonsignificant difference, which showed a trend for improvement with the subjects who received Fast Left treatment first.
Although subjects were allowed to remain on psychotropic medications except for antidepressants, only 6 subjects were on medications and none of these subjects responded to treatment. The daily doses of medication for these 6 subjects were: (1) lorazepam 1 mg and zolpidem 10 mg; (2) lorazepam 1 mg; (3) risperidone 3 mg; (4) zaleplon 5 mg; (5) lorazepam 0.5 mg; (6) lorazepam 0.5 mg.
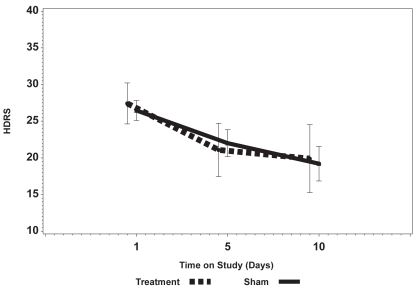
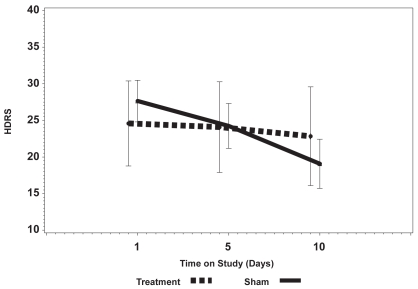
Figures 1a and 1b show the mean change in the HDRS and the BDI over 2 weeks comparing the sham arm with active stimulation (ie, Fast Left and Slow Right combined). There was no significant change in either the total observer-rated HDRS or self-rated BDI scores, although the BDI for the active stimulation arm did show a clear trend toward improving (lower score) with an increased number of treatments and the BDI in the sham arm remained flat. There was also no significant change in the BPRS (not shown).
Figure 1a.
Change in the Hamilton Depression Rating Scale (HDRS) over the 10 days of active vs Sham treatment showing a change in the absolute scores over time without a significant difference between and Sham and active treatment.
Figure 1b.
Change in the Beck Depression Inventory (BDI) over the 10 days of active vs Sham treatment showing a change in the absolute scores over time without a significant difference between Sham and active treatment. There is a trend over time for the BDI to improve in the active group compared with the lack of any change in the Sham group.
Mean HDRS in the 3 study arms were similar at baseline (p = 0.23) and the mean HDRS scores declined in all 3 arms from baseline to day 10. After the 10th treatment, mean HDRS was 19.8, 16.2, and 22.3 for Sham, Fast Left, and Slow Right arms respectively. The mean difference of 6.1 (22.3–16.3) between Slow Right and Fast Left in HDRS at day 10 was statistically significant (p = 0.007).
Mean BDI in the 3 study arms were similar at baseline (p = 0.18). Mean BDI did not change over time for the Sham arm but mean BDI did decline over time in the Fast Left and Slow Right arms. After the 10th treatment, mean BDI was 22.8, 15.3, and 22.8 for Sham, Fast Left, and Slow Right arms, respectively. The mean differences in BDI between Sham and Fast Left at day 10 (mean difference = 7.5, p = 0.07) and Slow Right and Fast Left (mean difference = 7.5, p = 0.02) were not statistically significant based on the Bonferroni adjustment.
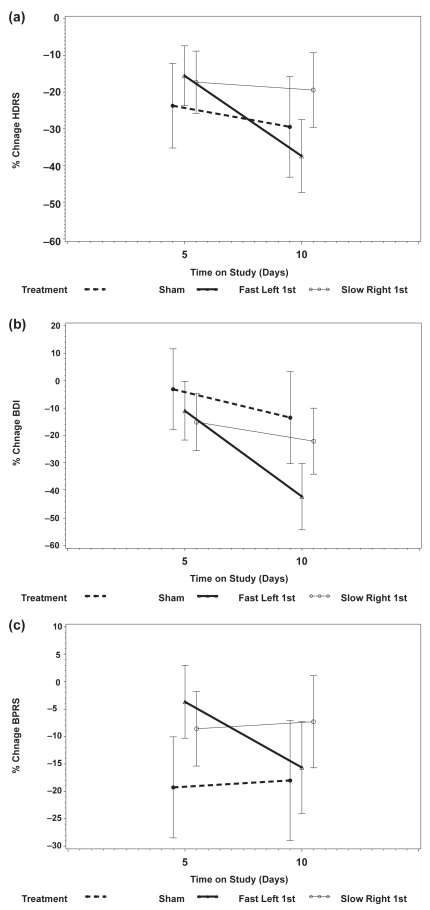
The mean percentage decline from baseline in HDRS was similar for the 3 arms after the 5th treatment (p = 0.51). After the 10th treatment, the percentage decline from baseline in HDRS was 29.4%, 37.4%, and 19.4% for Sham, Fast Left, and Slow Right arms, respectively (p = 0.014 for Fast Left vs Slow Right, Figure 2a).
Figure 2.
The percentage change and 95% confidence intervals in Hamilton Depression Rating Scale (HDRS) (a); Beck Depression Inventory (BDI) (b); and Brief Psychotic Rating Scale (BPRS) (c). All 3 groups showed an improvement over the 2 weeks. The Fast Left group showed continued improvement (ie, continued decrease in the HDRS, BDI, and BPRS) in the second week of the study, whereas the Slow Right and Sham groups appeared to level off or show no improvement.
The BDI in the 3 study arms changed in significantly different ways during treatment (p = 0.003, test for interaction between time on study and treatment arm). The difference in the pattern of change is best seen by comparing the percentage change from baseline to day 10 (Sham arm, mean change = 13.5%; Fast Left arm, mean change = 42.2%; Slow Right, mean change = 22.0%, Figure 2b). The mean percentage decline from baseline to day 10 was significantly different between Sham and Fast Left (p = 0.007) but not statistically different between Sham and Slow Right (p = 0.41) and Fast Left and Slow Right (p = 0.02).
Repeated-measures analyses of BDI were performed separately for several baseline covariates in order to adjust the analyses for covariates. Each baseline covariate was included as a factor in the repeated-measures analyses along with treatment arm, time on study, and the interaction between treatment arm and time on study. BDI was not significantly different based on the number of failed trials of medication (p = 0.10), gender (p = 0.51), bipolar diagnosis (p = 0.82), hand dominance (p = 0.74), or failure of previous ECT (p = 0.45). These analyses were also performed for HDRS, and baseline covariates did not affect the HDRS results.
Mean BPRS in the 3 study arms were similar at baseline (p = 0.11) and after the 5th treatment (p = 0.71) and after the 10th treatment (p = 0.25). Again, however, the pattern of change in Figure 2c shows a decline in the BPRS from day 5 to day 10 in the Fast Left arm and a small increase in the BPRS for the Slow Right and Sham arms.
In summary, all 3 measures of psychopathology tended to level off after the first 5 treatments in the Sham and Slow Right arms, whereas the Fast Left arm appeared to continue to show improvement through the 10th or final treatment. This is best illustrated in Figures 2a and 2b which show the mean percentage of improvement in the HDRS and BDI, respectively. The BDI improved markedly for the Fast Left arm from days 5 to 10 and was relatively stable for Sham and Slow Right arms. This pattern was similar for the HDRS although not quite as marked.
Table 2 summarizes the CGI data. The Fast Left arm showed a significant improvement over time with treatment, whereas the other two arms were relatively stable. There was a shift towards improvement between days 5 and 10 in both the subject (P = 0.006) and clinician (p = 0.003)-rated CGI data for the Fast Left arm.
Table 2.
Patients’ and blinded raters’ assessed improvement on the 7 point Clinical Global Improvement (CGI) scale which ranged from –3 (markedly worse mood) to +3 (markedly improved mood). The percentage of +2 (much improved) and +3 scores are presented; dropouts were counted as 0 or no change
| Day 5 | Day 10 | |
|---|---|---|
| CGI patient | ||
| Sham | 8.3% | 16.6% |
| Fast Left | 16% | 48% |
| Fast Right | 4% | 12% |
| CGI rater | ||
| Sham | 8.3% | 8.3% |
| Fast Left | 16% | 52% |
| Fast Right | 8% | 8% |
A univariate analysis of both the BDI and HDRS scores over time showed that there was no interaction between time and treatment, and therefore there was no significant improvement in depression in the Fast Left or Slow Right arms compared with Sham at day 5 or day 10. However, the CGI data in Table 2 indicate that the Fast Left arm again showed a tendency to improve over time with treatment, whereas the other 2 arms were relatively stable.
A univariate analysis was conducted using the HDRS as the response of interest. The main variables were treatment (Sham, Fast Left, and Slow Right), and time (Day 1, Day 5, and Day 10). Other variables included gender, age, education in years, handedness (left–right), diagnosis (bipolar or unipolar depression), have had ECT before (Yes/No), working at treatment time (Yes/No), the number of suicide attempts, and the number of previous medication trials. Race was not included since all but one subject was Caucasian. Only the number of previous medication trials approached significance (F = 3.94, df = 58, p = 0.052). A mixed-model univariate analysis conducted after adjusting for demographic variables showed that previous medication trials (F = 7.21, df = 56, p = 0.0095) and medication trials x time (F = 5.99, df = 103, p = 0.0035) were significant, whereas medication trials × treatment approached significance (F = 3.94, df = 56, p = 0.0250).
Neuropsychological measures
Means and standard deviations for each of the 3 arms across both time periods are presented in Table 3. There was no significant (p < 0.05) Arm by Time interaction effects or Arm main effects for any of the dependent variables studied. A significant Time main effect was observed for RBANS Immediate Memory (F(1,48) = 4.49, p < 0.04) and for Verbal Letter Fluency (F(1,47) = 7.31, p < 0.01), reflecting a significant improvement at Day 10 relative to Day 0 across all 3 arms. A significant Time main effect was also observed for the RBANS Language Index (F(1,48) = 25.10, p < 0.001), reflecting a significant decline across all three arms at Day 10 relative to Day 0. Inspection of performance on subtests making up this Index revealed significant differences across all three arms on a verbal category member generation task in which participants were asked to generate as many animal names as possible (Form A) or as many fruits and vegetables as possible (Form B). Performance was significantly lower across all 3 arms at the Day 10 assessment point.
Table 3.
Means and standard deviations for neuropsychological measures across group and time
| Group
|
||||||
|---|---|---|---|---|---|---|
| Placebo (n = 11)
|
Fast Left First (n = 20)
|
Slow Right First (n = 20)
|
||||
| Mean | SD | Mean | SD | Mean | SD | |
| BVMT Immediate Day 0 | 31.73 | 11.31 | 37.95 | 11.81 | 32.30 | 10.51 |
| BVMT Immediate Day 10 | 31.45 | 8.25 | 39.95 | 12.33 | 33.85 | 10.63 |
| BVMT Delayed Day 0 | 29.45 | 10.91 | 41.00 | 12.63 | 33.20 | 11.87 |
| BVMT Delayed Day 10 | 34.55 | 12.75 | 38.55 | 12.58 | 33.90 | 11.05 |
| RBANS Immediate Memory Day 0 | 98.73 | 15.20 | 103.20 | 18.54 | 95.90 | 14.24 |
| RBANS Immediate Memory Day 10 | 99.82 | 13.56 | 106.55 | 15.62 | 102.20 | 17.17 |
| RBANS Visuospatial Day 0 | 91.27 | 18.54 | 88.70 | 16.78 | 79.80 | 15.72 |
| RBANS Visuospatial Day 10 | 84.27 | 15.15 | 90.50 | 12.23 | 81.20 | 17.46 |
| RBANS Language Day 0 | 89.00 | 14.00 | 96.55 | 9.23 | 92.20 | 14.51 |
| RBANS Language Day 10 | 81.55 | 9.76 | 88.40 | 11.16 | 85.85 | 11.03 |
| RBANS Attention Day 0 | 88.36 | 21.74 | 95.15 | 18.71 | 97.58a | 14.98 |
| RBANS Attention Day 10 | 91.64 | 21.29 | 100.40 | 17.04 | 97.32a | 13.10 |
| RBANS Delayed Memory Day 0 | 92.00 | 12.20 | 96.40 | 14.46 | 94.37a | 10.93 |
| RBANS Delayed Memory Day 10 | 90.45 | 13.21 | 98.10 | 12.64 | 92.32a | 16.06 |
| RBANS Total Score Day 0 | 89.18 | 17.04 | 94.60 | 15.21 | 89.95a | 9.15 |
| RBANS Total Score Day 10 | 85.91 | 13.82 | 95.55 | 14.38 | 89.95a | 11.38 |
| Verbal Letter Fluency Day 0 | 34.55 | 14.71 | 35.00 | 12.99 | 36.53a | 12.05 |
| Verbal Letter Fluency Day 10 | 36.09 | 14.31 | 40.20 | 15.60 | 38.05a | 12.78 |
n = 19 for Slow Right First group.
Abbreviations: BVMT, Brief Visuospatial Memory Test-Revised; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SD, standard deviation.
The subjects who met criteria for response and remission (n = 11) were assessed at 2 weeks, 1 month, 2 months, and 3 months post TMS. Their treating physician started subjects on medications although some subjects and their doctors chose not to start on medications because of previous intolerance or lack of response. Two subjects refused further follow-up. One of these subjects had a final HDRS of 8 and the other had a final HDRS of 9. The treatment and maintenance data on the remaining 9 subjects are outlined in Table 4. Three subjects met criteria for remission: 1 subject remained well on fluoxetine and olanzapine and 2 other subjects relapsed in 2 weeks, 1 bipolar subject on a combination of lithium and carbamazepine and a second subject with unipolar depression relapsed on no medication. Six subjects met criteria for treatment response: 1 subject remained well through 2 months on no medication and was then lost to follow-up; 1 subject remained well through 3 months on no medication; and 4 subjects relapsed. Of the subjects who relapsed: 1 subject relapsed at 2 weeks on lorazepam and tranylcypromine; 2 subjects relapsed at 1 month, 1 on olanzapine and valproic acid and 1 on no medication; 1 subject relapsed at 3 months on paroxetine.
Table 4.
Time to relapse after response to an acute course of transcranial magnetic stimulation
| Randomization | Final HDRS | Maintenance medication | Time to relapsea |
|---|---|---|---|
| Fast Left | 2 | zaleplon 10 mg, lithium 600 mg, carbamezepine 800 mg, lorazepam 1 mg | 2 weeks |
| Fast Left | 4 | No medication | 2 weeks |
| Fast Left | 7 | fluoxetine 20 mg, quetiapine 400 mg | No relapse |
| Fast Left | 8 | No medication | No relapse |
| Fast Left | 10 | lorazepam 1 mg bid, tranylcypromine 10 mg bid | 2 weeks |
| Sham | 10 | No medication | 1 month |
| Slow Right | 10 | paroxetine 10 mg | 3 months |
| Slow Right | 15 | Olanzapine 10 mg qhs, valproic acid 250 mg bid | 1 month |
| Slow Right | 17 | No medication | 2 months (not seen at 3 months) |
Subjects were started on medication by the treating physician and assessed at 2 weeks, 1, 2, and 3 months to determine if they met criteria for relapse.
Abbreviations: HDRS, Hamilton Depression Rating Score.
Discussion
This study evaluated the acute and long-term efficacy of combined slow right and fast left stimulation of the DLPFC in subjects with treatment-resistant depression. The subjects in this study met criteria for ECT and almost half had failed a trial of ECT prior to rTMS. The average number of failed medication trials was approximately 8.5. Both the Sham and Combination treatment arms showed improvement with time although there was no clear treatment effect for active rTMS. The one clinical variable that did appear to have a significant effect on response was an improved response with a decreased number of previous failed medication trials. This finding is not surprising and may be a measure of treatment resistance. In addition this observation is similar to data from ECT studies (eg, Sackeim et al 1990), which correlates poor response to ECT with previous medication failures. The fact that a previous poor response to ECT was not associated with poor response to rTMS replicates earlier work that showed that subjects may respond differentially to ECT and rTMS (Janicak et al 2002). It is also noteworthy that there were no differences in neuropsychological functioning across the sham and the active arms, suggesting the relative safety of both rTMS procedures over the 10-day treatment period.
The data showed a trend for subjects who received Fast Left treatments first to have a better response as indicated by nonsignificant improvements in the HDRS and significant improvements in the BDI in the second week, together with an improved rating on both the clinician and subject rated global improvement scale. The finding was most likely due to the fact that the subjects in this arm had fewer previous medication trials and were less ill. Others have cited evidence that the level of pharmacological treatment resistance may have a negative correlation with response to TMS (Gershon et al 2003).
However the possibility that administering Slow Right first somehow impeded the efficacy of Fast Left or, alternatively, Fast Left First improved the response to Slow Right cannot be ruled out in this study. Another possibility is that the HDRS, which is weighted toward somatic symptoms of depression (eg, sleep and appetite disturbances), was not sensitive enough to detect the improvement noted on the CGI.
There are several possible reasons why the active treatments did not differentiate from Sham. First, the total number of stimulations for a given treatment setting was relatively low (1600) and the total number of sessions was only 10. Both the number of pulses and the quantity of pulses have been shown to be directly correlated with treatment response (Gershon et al 2003). And although our study is one of the larger randomized controlled trials using rTMS, the sample size may have been too small to detect a difference in the groups (ie, Type II error).
The finding that the subjects in the Fast Left arm were improving in week 2 leaves open the question of whether they would have continued to improve with additional treatment sessions. Overall, the subjects in this study were very treatment resistant, and half had failed ECT, which makes the finding of improvement on the BDI and CGI encouraging. In retrospect, the use of rTMS as an augmenting agent to the antidepressant the subject was taking at the time of initial evaluation may have been more appropriate. Most subjects were having at least a partial response to these medications, although they still met severity criteria for entry into the study.
Our arm is a part of 2 multicenter trials (1 industry sponsored and the other sponsored by the National Institute of Mental Health). Both trials screen subjects for only moderate treatment resistance (1–3 medication failures), increase the number of stimulations per session, and use a more flexible treatment design which allows the treating clinician to treat subjects up to 6 weeks. Data from the present study support the use of this modified rTMS treatment protocol in major depression.
Acknowledgments
The study was funded by an Independent Investigator Award from the National Alliance for Research in Schizophrenia and Depression.
Footnotes
Disclosures
William M McDonald is a consultant to Neuronetics, a company which now owns the patent to the TMS machine used in this study. He and Paul Holtzheimer were investigators in an ongoing multi-center trial funded by Neuronetics and ended their involvement in that trial in January 2005. Charles M Epstein is a consultant for Neuronetics and may receive royalties.
References
- Ballinger JC. Clinical guidelines for establishing remission in subjects with depression and anxiety. J Clin Psychiatry. 1999;60:29–34. [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelsohn M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benedict R. Brief visuospatial memory test-revised professional manual. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- Benton AL, des Hamsher K. Multilingual aphasia examination. Iowa City: AJA Associates, Inc; 1983. [Google Scholar]
- Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta-analysis. Int J Neuropsychopharmacol. 2002;5:73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Davey KR. Iron-core coils for transcranial magnetic stimulation. J Clin Neuorophysiol. 2002;19:376–81. doi: 10.1097/00004691-200208000-00010. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Disorders - Research Version (SCID-I, Version 20) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Fitzgerald PB, Brown TL, Marston NA, et al. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60:1002–8. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse and recurrence. Arch Gen Psychiatry. 1991;48:851–5. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Garcia-Toro M, Montes JM, Talavera JA. Functional cerebral asymmetry in affective disorders: new facts contributed by transcranial magnetic stimulation. J Affect Disord. 2001;66:103–9. doi: 10.1016/s0165-0327(00)00276-7. [DOI] [PubMed] [Google Scholar]
- Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry. 2003;160:835–45. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hausmann A, Kemmler G, Walpoth M, et al. No benefit derived from repetitive transcranial magnetic stimulation in depression: a prospective, single centre, randomised, double blind, sham controlled “add on” trial. J Neurol Neurosurg Psychiatry. 2004;75:320–2. [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, III, Russo J, Avery DH. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull. 2001;35:149–69. [PubMed] [Google Scholar]
- Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry. 2002;51:659–67. doi: 10.1016/s0006-3223(01)01354-3. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Javanmard M, Vaccarino FJ. A review of functional neuroimaging in mood disorders: positron emission tomography and depression. Can J Psychiatry. 1997;42:467–75. doi: 10.1177/070674379704200502. [DOI] [PubMed] [Google Scholar]
- Klein E, Kreinin I, Chistyakov A, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry. 1999;56:315–20. doi: 10.1001/archpsyc.56.4.315. [DOI] [PubMed] [Google Scholar]
- Kozel FA, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract. 2002;8:270–5. doi: 10.1097/00131746-200209000-00003. [DOI] [PubMed] [Google Scholar]
- Loo CK, Mitchell PB, Croker VM, et al. Double-blind controlled investigation of bilateral prefrontal transcranial magnetic stimulation for the treatment of resistant major depression. Psychol Med. 2003;33:33–40. doi: 10.1017/s0033291702006839. [DOI] [PubMed] [Google Scholar]
- Martin JL, Barbanoj MJ, Schlaepfer TE, et al. Repetitive transcranial magnetic stimulation for the treatment of depression: Systematic review and meta-analysis. Br J Psychiatry. 2003;182:480–91. doi: 10.1192/bjp.182.6.480. [DOI] [PubMed] [Google Scholar]
- Martinot JL, Hardy P, Feline A, et al. Left prefrontal glucose hypometabolism in the depressed state: a confirmation. Am J Psychiatry. 1990;147:1313–7. doi: 10.1176/ajp.147.10.1313. [DOI] [PubMed] [Google Scholar]
- Menkes DL, Bodnar P, Ballesteros RA, et al. Right frontal lobe slow frequency repetitive transcranial magnetic stimulation (SF r-TMS) is an effective treatment for depression: a case-control pilot study of safety and efficacy. J Neurol Neurosurg Psychiatry. 1999;67:113–15. doi: 10.1136/jnnp.67.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N, Erfurth A. Treatment of bipolar mania with right prefrontal rapid transcranial magnetic stimulation. J Affect Disord. 2004;78:253–7. doi: 10.1016/S0165-0327(02)00308-7. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, et al. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–58. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Randolph C. Repeatable battery for the assessment of neuropsychological status. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- Sackeim HA, Prudic J, Devanand DP, et al. The impact of medication resistance and continuation pharmacotherapy on relapse following response to electroconvulsive therapy in major depression. J Clin Psychopharmacol. 1990;10:96–104. doi: 10.1097/00004714-199004000-00004. [DOI] [PubMed] [Google Scholar]
- Schaffer CE, Davidson RJ, Saron C. Frontal and parietal electroencephalogram asymmetry in depressed and nondepressed subjects. Biol Psychiatry. 1983;18:753–62. [PubMed] [Google Scholar]
- Videbech P. PET measurements of brain glucose m etabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr Scand. 2000;101:11–20. doi: 10.1034/j.1600-0447.2000.101001011.x. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Wedegaertner FR, Ziemann U, et al. Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neurosci Lett. 1998;250:141–4. doi: 10.1016/s0304-3940(98)00437-6. [DOI] [PubMed] [Google Scholar]
- Weiner R. American Psychiatric Association Task Force on Electroconvulsive Therapy: The practice of electroconvulsive therapy: Recommendations for treatment, training and privileging. Washington, DC: American Psychiatric Association Press; 2001. [Google Scholar]