Abstract
Objectives
This study analyzed various clinical and histopathologic factors for patients with early stage squamous cell carcinoma (SCC) of the oral tongue to define a high risk group for regional recurrence and finally to find out the indication of elective neck dissection (END).
Methods
Retrospective chart review was performed for 63 patients with T1-T2N0 SCC of the oral tongue who underwent partial glossectomy with/without END. Clinical and histopathologic factors assessed were age, gender, clinical T stage, tumor cell differentiation, depth of invasion, pathologic nodal status, and intrinsic muscle involvement, perineural invasion, lymphovascular emboli and resection margin involvement.
Results
Five year overall survival rate was 97.1% in stage I and 76.2% in stage II, and 5-yr disease free survival rate was 76.7% in stage I and 43.5% in stage II. Rates of occult nodal metastasis in stage I and II were 15.4% and 42.9%, respectively. Overall regional recurrence rate was 15.9%, which consisted of 10.2% in stage I and 35.7% in stage II. The success rate of salvage treatment was 100% in stage I and 40% in stage II. Higher T stage, higher histologic grade, depth of invasion ≥3 mm, presence of intrinsic muscle involvement were significantly related to regional recurrence (P=0.035, P=0.011, P=0.016, P=0.009, respectively). In stage I, the non-END group (n=36) showed 13.9% of regional recurrence rate, while END group (n=13) did not have any regional recurrence (P=0.198). Five year disease free survival rate of END group was significantly higher than non-END group (100% and 68.7%, respectively, P=0.045).
Conclusion
We recommend to perform END in early stage SCC of the oral tongue if the primary tumor has T2 stage, and T1 stage with higher histologic grade, depth of invasion more than 3 mm, or presence of intrinsic muscle involvement.
Keywords: Early stage, Squamous cell carcinoma, Oral tongue, Regional recurrence
INTRODUCTION
It is well known that the presence of neck nodal metastasis is the most important prognostic factor of survival and the regional recurrence is the most frequent cause of treatment failure after surgical removal for patients with squamous cell carcinoma (SCC) of the oral tongue (1). Treatment failures with even fatal outcome can occur in patients who initially have presented very small primary tumor with clinically negative neck (2, 3).
Several studies demonstrated 13-33% and 27-53% of occult nodal metastasis in stage I and II, respectively (2, 4). However, there has been a lot of controversy over the management of occult neck disease of early stage oral tongue cancer for more than 3 decades: elective neck dissection (END) versus observation. Some studies have shown no survival benefit, reduction of recurrence or other advantages when the clinically negative neck is treated with END (1, 5, 6), while others have described reduced regional recurrence or prolonged survival (3, 7-9). Therefore, to choose properly between close observation or END as the management for clinically negative neck in patients with early stage SCC of the oral tongue (T1-2N0M0), it is essential to find the patients who have a great risk for occult nodal metastasis and regional recurrence. Several studies have evaluated the value of certain clinicohistologic factors in predicting the probability of occult nodal metastasis, such as age, gender, T stage, N stage, depth of invasion, histologic grade, perineural and lymphvascular invasion, resection margin and etc (10, 11).
The aim of this study was to analyze various clinical and histopathologic factors for patients with T1-T2N0 SCC of the oral tongue treated primarily by surgery, in an attempt to better define a subgroup at high risk for regional recurrence and finally to find out the indication of END.
MATERIALS AND METHODS
Subjects
We reviewed the medical records of 105 patients of SCC of the oral tongue who had been treated initially at the Department of Otorhinolaryngology-Head and Neck Surgery in Seoul National University Hospital between 1987 and 2006. The subjects of this study were limited to patients with stage I/II (T1-2N0M0) SCC of the oral tongue. N0 was considered when there is no palpable lymph node by physical examination and size of the lymph node is less than 1 cm by computed tomography (CT) or magnetic resonance imaging without any area suggestive of central necrosis or metastasis. M0 was considered when there is no evidence of distant metastasis by liver sonography, bone scan and chest x-ray, or by PET. Staging was based on the AJCC's Manual for Staging of Cancer (2002, Sixth edition).
Thirty-five patients with stage III and IV were excluded. And 7 of 70 patients with early stage tumor who ether developed secondary malignancy, underwent neoadjuvant chemotherapy or be treated by radiotherapy alone were excluded. Ultimately, 63 patients were eligible for this retrospective study. All patients underwent partial glossectomy intraorally with/without unilateral END.
The male to female ratio was 1.25:1 (35 males and 28 females). The mean age of the patients was 56 yr (range, 26-88 yr). The preoperative clinical stage was cT1N0M0 in 49 and cT2N0M0 in 14 patients. The follow-up period ranged from 12 to 191 months, with a median of 59 months.
Clinical and histopathologic factors assessed included age (<40 vs. ≥40 yr), gender, clinical T stage (T1 vs. T2 stage), tumor cell differentiation (well vs. moderate vs. poor), depth of invasion (<3 vs. ≥3 mm), pathologic nodal status, and presence or absence of intrinsic muscle involvement, perineural invasion, lymphovascular emboli and resection margin involvement. Histopathologic findings were reviewed by a single pathologist with hematoxylin- and eosin-stained histologic sections of tumor.
Statistical methods
All statistical analyses were performed using SPSS software for Windows (version 13.0, SPSS, Chicago, IL, USA). Variables were independently tested for their relation to presence of regional recurrence using the χ2 test or Fisher's exact test. Multiple logistic regression was used to identify the multivariate correlates of regional recurrence.
We used the Kaplan-Meier method and the log-rank test to compare the overall survival and disease-free survival between the patients with stage I and II, and between those with END and without it. P-value less than 0.05 was considered indicative of statistical significance.
RESULTS
Regional recurrence and occult nodal metastasis
Of 63 patients, 10 showed regional recurrence during follow-up period, and overall regional recurrence rate was 15.9%. The average time from initial treatment to regional recurrence was 8 months (range 2-43 months). Twenty patients including 13 in stage I and 7 in stage II underwent END, and 5 of them were revealed to have pathologic positive nodes. Rates of occult nodal metastasis in stage I and II were 15.4% (2/13) and 42.9% (3/7), respectively.
Survival for early SCC of the oral tongue in Seoul National University Hospital
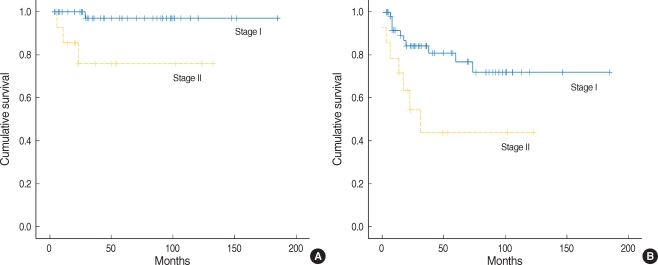
Five year overall survival rate was 97.1% in stage I and 76.2% in stage II. Patients in stage I showed higher 5-yr overall survival rate than in stage II with statistical significance (P=0.006) (Fig. 1A). Five year disease free survival rate of stage I was higher than that of stage II with statistical significance, 76.7% vs. 43.5%, respectively (P=0.012) (Fig. 1B).
Fig. 1.
(A) Five year overall survival rate in stage I and II of squamous cell carcinoma of the oral tongue. (B) Five year disease free survival rate in stage I and II of squamous cell carcinoma of the oral tongue.
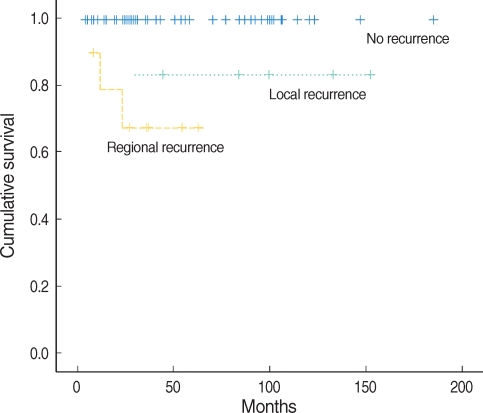
In 46 patients without any type of recurrence, 5-yr survival rate was 100%. However, 5-yr survival rate was 83.3% in 7 patients with local recurrence, and 67.5% in 10 with regional recurrence. Patients with regional recurrence showed lower survival rate than patients with local recurrence with statistical significance (P=0.001) (Fig. 2).
Fig. 2.
Survival according to type of recurrence in squamous cell carcinoma of the oral tongue.
Among 10 patients with regional recurrence after initial surgical treatment, all 5 of stage I were successfully salvaged by modified radical neck dissection with/without postoperative radiotherapy. However, only 2 of 5 patients of stage II were salvaged by modified radical neck dissection with/without postoperative radiotherapy. One patient was alive with disease, and other 2 were dead of disease on the last follow-up in spite of neck dissection, postoperative radiotherapy or adjuvant chemotherapy. Therefore success rate of salvage treatment was 100% in stage I and 40% in stage II.
Factors related to regional recurrence
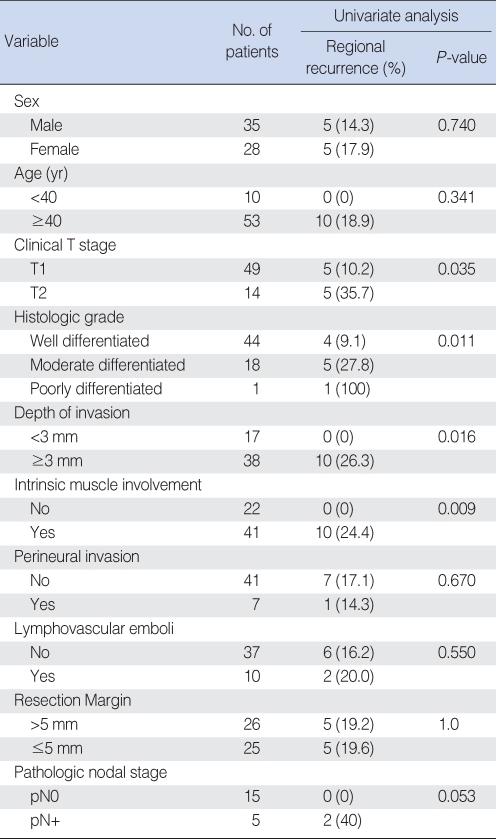
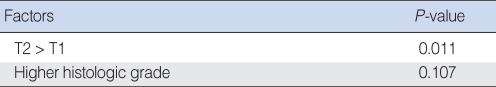
In univariate analysis using the χ2 test or Fisher's exact test, clinical factors including gender and age groups divided at 40 yr were not significantly related to regional recurrence. Histopathological factors including resection margin ≤5 mm, perineural invasion and lymphovascular emboli of the primary tumor were not significantly related to regional recurrence. In cases with pathologically positive node, there was a tendency of higher incidence of regional recurrence than in cases with negative one, 0% vs. 40%, respectively, but it did not reach statistical significance (P=0.053). The incidence of regional recurrence was 10.2% and 35.7% in clinical T1 and T2 stage, respectively. Higher T stage was significantly related to regional recurrence (P=0.035). Patient with higher histologic grade, depth of invasion ≥3 mm and intrinsic muscle involvement from histopathologic findings showed a significantly higher incidence of regional recurrence (P=0.011, P=0.016, P=0.009, respectively) (Table 1).
Table 1.
Univariate analysis for factors related to regional recurrence
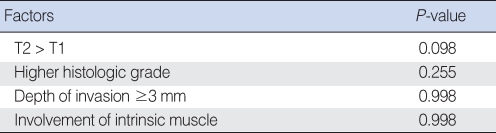
In multivariate analysis using logistic regression, T stage, histologic grade, depth of invasion, and intrinsic muscle involvement which were the significant related factors to regional recurrence on univariate analysis were introduced, but there was no statistical significance. This result was considered as because these factors had a close relation to each others, that is, multicollinearity, especially between T stage, depth of invasion and intrinsic muscle involvement. When performed only about T stage and histologic grade, T stage showed a strong relationship to regional recurrence significantly (P=0.011), which means that T stage can be a single most powerful prognostic factor of regional recurrence in early stage SCC of the oral tongue (Table 2, 3).
Table 2.
Multivariate analysis for factors related to regional recurrence (I)
Table 3.
Multivariate analysis for factors related to regional recurrence (II)
Neck dissection in clinical T1N0
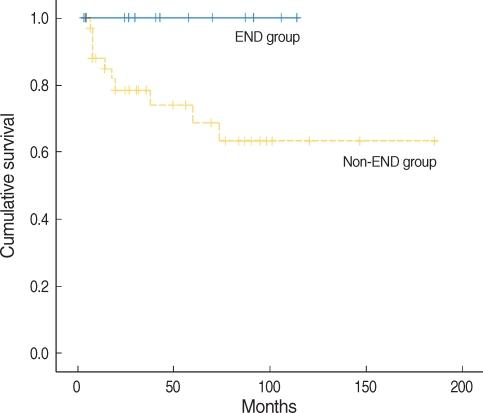
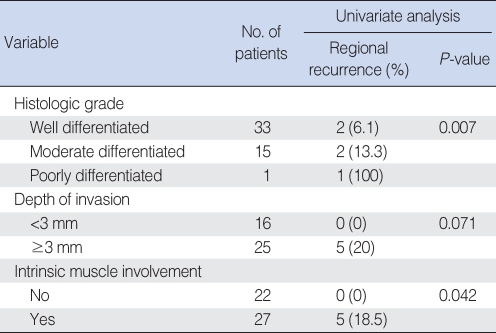
All 49 patients in stage I underwent partial glossectomy with or without END, and divided into two groups: END group (n=13) and non-END group (n=36). The non-END group showed 13.9% of regional recurrence rate, while END group did not have any regional recurrence (Fischer's Exact Test, OR=0.861, P=0.198), however this difference did not reach statistical significance. Five year disease free survival rates of non-END and END groups showed a significant difference (68.7% and 100%, respectively, P=0.045) (Fig. 3). However, 5-yr overall survival rates had no difference (96% and 100%, respectively, P=0.527), because, all 5 patients with regional recurrence were successfully salvaged by modified radical neck dissection with/without postoperative radiotherapy. The related factors for regional recurrence in stage I were also evaluated as previously studied. Histologic grade and intrinsic muscle involvement were significantly related (P=0.007, P=0.042, respectively) and depth of invasion ≥3 mm had a tendency to increase the incidence of regional recurrence (P=0.071) (Table 4).
Fig. 3.
Five year disease free survival rates of non-END and END groups. END: elective neck dissection.
Table 4.
Univariate analysis for factors related to regional recurrence in T1N0
DISCUSSION
In our institute, 5-yr overall survival rate was 97.1% in stage I and 76.2% in stage II for early stage SCC of the oral tongue. Overall rate of regional recurrence was 15.9%. Regional recurrence was the most common type of recurrence and related to lowest survival rate. Overall salvage rate after regional recurrence was 70% in our series, whereas it was about 30-40% in many other studies (3, 6, 12, 13). Our better outcome may result from thorough physical examination with every month follow-up till 1 yr after surgery and regular imaging follow up with CT. Friedlander et al. presented that patients younger than 40 yr with SCC of the oral tongue had a higher rate of locoregional recurrence rate than did older patients (14), whereas Davison et al. concluded that increasing age predicted worse disease-specific survival (15). Fukano et al. demonstrated that depth of invasion exceeding 5 mm was statistically significantly correlated with cervical metastases (16) and O'Brient et al. presented that prognosis changed significantly at a cutoff of 4 mm of depth of invasion (17). Sparano et al. reported that greater tumor thickness, greater depth of muscle invasion, T2 stage, poorly differentiated tumors, infiltrating-type front, presence of perineural invasion and presence of angiolymphatic invasion correlated positively to occult neck metastasis (10). Factors related to neck recurrence in early stage SCC of the oral tongue were found to be T stage, depth of invasion, intrinsic muscle involvement and histologic grade, while age, gender, perineural invasion and lymphovascular emboli, resection margin and pathologic nodal stage were not significantly related in our series.
The management of the N0 neck in early stage SCC of the oral tongue has been the subject of heated debate over the years. Keski-Santti et al. presented that for stage I and II of oral tongue SCC, there were significantly fewer regional recurrences in patients with END, but statistical differences in overall or disease specific survival between END and non-END group were not found. They concluded that the risk for occult cervical metastasis was high in patients with early tongue tumors and only carefully selected patients could be left without prophylactic neck treatment (3). It is generally recommended that for SCC of the upper aerodigestive tract, the clinically N0 neck should be treated when the incidence of occult metastasis is greater than 20% (18). In our series, rate of occult nodal metastasis was 42.9% in stage II, and T2 stage was the most important factor for regional recurrence through univariate and multivariate analysis. Therefore patients with clinical stage T2N0 were justified in undergoing END to reduce regional recurrence and improve survival. Then, when END should be performed in T1N0 SCC of the oral tongue? Fernando et al. (13) found in T1N0 SCC patients of the oral tongue and mouth floor that 24% of non-END group developed regional recurrences in comparison with 4% of END group, and that END group had a 23% higher disease-free survival rate than non-END group. In T1N0 SCC patients of oral tongue in our series, the rate of occult nodal metastasis was 15.4%, and the END group showed a lower incidence of regional recurrence and higher disease free survival rate than END group. However, 5-yr overall survival rates had no difference between two groups, because all 5 patients with regional recurrence were successfully salvaged by modified radical neck dissection with/without postoperative radiotherapy. Despite of high success rate of salvage treatment, recurrence of cancer is a fear to patients and regular follow-up is difficult for some patients. Therefore patients with higher possibility of regional recurrence, which means those with factors related to regional recurrence as described above, had better undergo END.
In conclusion, we obtained successful survival rate in early stage SCC of the oral tongue treated by, mainly, surgery. And we should consider performing the END in following cases: T2 stage, and T1 stage with higher histologic grade, depth of invasion more than 3 mm, or presence of intrinsic muscle involvement.
References
- 1.Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head Neck. 1997 Oct;19(7):583–588. doi: 10.1002/(sici)1097-0347(199710)19:7<583::aid-hed4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Haddadin KJ, Soutar DS, Oliver RJ, Webster MH, Robertson AG, MacDonald DG. Improved survival for patients with clinically T1/T2, N0 tongue tumors undergoing a prophylactic neck dissection. Head Neck. 1999 Sep;21(6):517–525. doi: 10.1002/(sici)1097-0347(199909)21:6<517::aid-hed4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Keski-Säntti H, Atula T, Törnwall J, Koivunen P, Mäkitie A. Elective neck treatment versus observation in patients with T1/T2 N0 squamous cell carcinoma of oral tongue. Oral Oncol. 2006 Jan;42(1):96–101. doi: 10.1016/j.oraloncology.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Teichgraeber JF, Clairmont AA. The incidence of occult metastases for cancer of the oral tongue and floor of the mouth: treatment rationale. Head Neck Surg. 1984 Oct;7(1):15–21. doi: 10.1002/hed.2890070105. [DOI] [PubMed] [Google Scholar]
- 5.Lim YC, Ahn JY, Koo BS, Lee JS, Chun JY, Park YM, et al. Occult lymph node metastasis in early oral tongue squamous cell carcinoma. Korean J Otolaryngol-Head Neck Surg. 2006 Apr;49(4):407–410. [Google Scholar]
- 6.Yii NW, Patel SG, Rhys-Evans PH, Breach NM. Management of the N0 neck in early cancer of the oral tongue. Clin Otolaryngol Allied Sci. 1999 Feb;24(1):75–79. doi: 10.1046/j.1365-2273.1999.00224.x. [DOI] [PubMed] [Google Scholar]
- 7.Spiro RH, Strong EW. Surgical treatment of cancer of the tongue. Surg Clin North Am. 1974 Aug;54(4):759–765. doi: 10.1016/s0039-6109(16)40379-8. [DOI] [PubMed] [Google Scholar]
- 8.Kligerman J, Lima RA, Soares JR, Prado L, Dias FL, Freitas EQ, et al. Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. Am J Surg. 1994 Nov;168(5):391–394. doi: 10.1016/s0002-9610(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 9.Kaya S, Yilmaz T, Gürsel B, Saraç S, Sennaroğlu L. The value of elective neck dissection in treatment of cancer of the tongue. Am J Otolaryngol. 2001 Jan–Feb;22(1):59–64. doi: 10.1053/ajot.2001.20681. [DOI] [PubMed] [Google Scholar]
- 10.Sparano A, Weinstein G, Chalian A, Yodul M, Weber R. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004 Oct;131(4):472–476. doi: 10.1016/j.otohns.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Keski-Säntti H, Atula T, Tikka J, Hollmén J, Mäkitie AA, Leivo I. Predictive value of histopathologic parameters in early squamous cell carcinoma of oral tongue. Oral Oncol. 2007 Nov;43(10):1007–1013. doi: 10.1016/j.oraloncology.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Fakih AR, Rao RS, Borges AM, Patel AR. Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. Am J Surg. 1989 Oct;158(4):309–313. doi: 10.1016/0002-9610(89)90122-0. [DOI] [PubMed] [Google Scholar]
- 13.Dias FL, Kligerman J, Matos de Sá G, Arcuri RA, Freitas EQ, Farias T, et al. Elective neck dissection versus observation in stage I squamous cell carcinomas of the tongue and floor of the mouth. Otolaryngol Head Neck Surg. 2001 Jul;125(1):23–29. doi: 10.1067/mhn.2001.116188. [DOI] [PubMed] [Google Scholar]
- 14.Friedlander PL, Schantz SP, Shaha AR, Yu G, Shah JP. Squamous cell carcinoma of the tongue in young patients: a matched-pair analysis. Head Neck. 1998 Aug;20(5):363–368. doi: 10.1002/(sici)1097-0347(199808)20:5<363::aid-hed1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Davidson BJ, Root WA, Trock BJ. Age and survival from squamous cell carcinoma of the oral tongue. Head Neck. 2001 Apr;23(4):273–279. doi: 10.1002/hed.1030. [DOI] [PubMed] [Google Scholar]
- 16.Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997 May;19(3):205–210. doi: 10.1002/(sici)1097-0347(199705)19:3<205::aid-hed7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien CJ, Lauer CS, Fredricks S, Clifford AR, McNeil EB, Bagia JS, et al. Tumor thickness influences prognosis of T1 and T2 oral cavity cancer--but what thickness? Head Neck. 2003 Nov;25(11):937–945. doi: 10.1002/hed.10324. [DOI] [PubMed] [Google Scholar]
- 18.Wei WI, Ferlito A, Rinaldo A, Gourin CG, Lowry J, Ho WK, et al. Management of the N0 neck--reference or preference. Oral Oncol. 2006 Feb;42(2):115–122. doi: 10.1016/j.oraloncology.2005.04.006. [DOI] [PubMed] [Google Scholar]