Abstract
Objectives
The primary aim of this study was to determine whether 18F-FDG-PET/CT (PET/CT) scans provide additional diagnostic information in addition to the direct laryngoscopic examination (L/E) and contrast-enhanced CT (CT) in patients with glottic cancer during the initial evaluation.
Methods
Fifty-five consecutive patients with glottic cancer of the larynx that had L/E, CT and PET/CT were enrolled. The diagnostic value of each modality was compared for their accuracy in predicting the extent of the primary tumors on sub-site based analysis and the final tumor staging. The reference standards were either the surgical pathology findings or clinical/radiological follow-up outcome. Changes in patient care based on PET/CT results were compared with the treatment decisions based on L/E with CT.
Results
For primary tumor sub-site based analysis, the sensitivity was significantly higher for L/E (92.8%) than for PET/CT (79.4%, P=0.028). The comparisons between L/E vs. CT and CT vs. PET/CT did not reach statistical significance. As an initial tumor-staging method the L/E had a diagnostic accuracy of 76.4%, compared to 61.8% for CT and 41.8% for PET/CT. The L/E and CT were better than the PET/CT (P=0.0009 and 0.049) for the initial TNM staging. PET/CT scanning changed the clinical decision-making based on the L/E with CT results in 12.7% of cases, of whom 5.5% had no additional PET/CT related benefit.
Conclusion
The results of this study showed that PET/CT imaging added no clinical information benefit compared to the L/E and CT for the initial evaluation of patients with glottic cancer.
Keywords: Positron emission tomography, Tomography, X-ray computed, Laryngoscopy, Laryngeal neoplasms, Glottis
INTRODUCTION
Recent advances in technology make it possible to fuse anatomical images with functional images. The use of combined 18F-FDG PET/CT (PET/CT) fusion images has been shown to improve diagnostic accuracy (1-3). For head and neck cancers, the reported sensitivity of PET/CT is 98%, specificity of 92%, with an accuracy of 94% for the identification of a malignancy of the head and neck; this is a higher accuracy than with PET or CT alone (4). Therefore, the use of the PET/CT potentially affects the care of patients with head and neck cancer (5). Our previous study and others have also demonstrated that PET/CT is more accurate than conventional PET and contrast enhanced CT (CT) for evaluating the cervical nodes of patients with head and neck squamous cell carcinoma (6, 7).
For the evaluation of laryngeal lesions, there have been several reports on the efficacy of PET/CT. One recent report showed that the results of the PET/CT had a major impact on patient management in 59% of cases with cancer of the larynx with a higher diagnostic values than PET or CT alone (8). The subjects evaluated had supraglottic cancer (45%) and glottic cancer (55%). The major impact of the PET/CT results was on treatment planning in the post-treatment setting (86%) during evaluations of disease recurrence and response to treatment, consistent with previous reports (9, 10).
The use of PET/CT scanning has become popular for staging patients with head and neck cancer, identifying response to non-surgical therapy and allowing earlier detection of disease recurrence. However, specific recommendations for its use are needed to determine which patients are good candidates for the use of PET/CT scanning (11). Therefore, the goal of this study was to determine the diagnostic value of PET/CT for patients with glottic cancer by comparing it with direct laryngoscopic examination and CT for initial tumor staging. In addition, we evaluated the impact of the PET/CT findings on clinical decision-making for patient treatment.
MATERIALS AND METHODS
Subjects
From January 2003 to December 2005, 114 patients who were newly diagnosed with squamous cell carcinomas of the glottic larynx by biopsy were initially evaluated for inclusion in this study. Among them, 60 patients had direct laryngoscopic examinations (L/E), CT and PET/CT for tumor staging, and the remaining 54 subjects were excluded because one of the three diagnostic modalities were not performed during the initial evaluation. Five additional patients were excluded because of incomplete pathology information 55 patients with glottic cancer were enrolled in this study. The subjects included 53 men and 2 women and their age ranged from 37 to 88 year (mean age: 58.4 year). Our Institutional Review Board approved the protocol for this retrospective analysis.
Direct laryngoscopic examinations
All patients underwent laryngeal examination with a laryngeal telescope and a complete physical examination of the head and neck in the outpatient clinic; they subsequently underwent biopsy of their glottic lesions for pathological diagnosis under general anesthesia.
For the primary tumor sub-site based analysis, we divided the structure around glottis into 6 sub-sites based on the AJCC staging manual (6th edition, 2002): ipsi-lateral true vocal fold, contra-lateral true vocal fold, supraglottis, subglottis, paraglottic space, and thyroid cartilage. First, one head and neck surgeon assessed the patients with glottic cancer with the knowledge of the patient's clinical history. For suspected lesions of the glottis, a direct laryngoscopic biopsy was performed with evaluation of the extent of the primary lesions. With the information of the clinical examination but without the knowledge of the biopsy results, the physician determined the status of each sub-site and the clinical initial staging of the glottic cancer. Each sub-site was classified as malignant-positive, malignant-negative or equivocal. The information from routine chest x-rays were included in the determination of the TNM staging along with the clinical examination.
The final diagnosis of squamous cell carcinomas of the glottis was confirmed pathologically in all patients. Next, the patients had CT and PET/CT scanning within an interval of one month before the initiation of any treatment.
Contrast enhanced CT
CT scans (LightSpeed Ultra or Ultra 16, GE, Milwaukee, WI, USA) of the head and neck were performed using the following parameters: 160 mAs, 120 KeV, a section thickness of 3.75 mm, and a table feed of 8.75 mm per rotation. For contrast enhancement, 90 mL of an iodinated contrast agent (Ultravist 300, Schering, Berlin) was injected intravenously at 3 mL/sec using an automated injector. The scan delay time was 30 sec.
For the CT scans, a radiologist determined the extent of the laryngeal lesion and the status of the cervical lymph nodes; except for the fact that the patient had laryngeal cancer, the radiologist had no additional information about the clinical findings. First, the interpretation of the CT scans focused on the primary lesion of the larynx. Laryngeal lesions with abnormal enhancement patterns were evaluated to assess the extent according to the above sub-sites. The regional lymph nodes of the head and neck were also evaluated according to accepted criteria (12). The radiologist then determined the initial staging of the laryngeal lesions from the information of the standalone CT scans.
Integrated 18F-FDG PET/CT
All patients fasted for at least 6 hr prior to the PET/CT scans, which were performed using a GE Discovery LS 18F-FDG-PET/CT scanner (General Electric, Milwaukee, WI, USA). Whole body CT scanning was performed by a continuous spiral technique using an 8-slice helical CT with a gantry a rotation speed of 0.8 sec. The CT scan data were collected using the following parameters: 40-80 mAs, 140 KeV, a section width of 5 mm, and a table feed of 5 mm per rotation. No intravenous or oral contrast agents were used. Following the CT scans, and after the intravenous injection of 370 MBq 18F-FDG, an emission scan was performed from the thigh to the head for 5 min per frame for a total of 45 min. The attenuation-corrected 18F-FDG-PET images using the CT data were reconstructed with an ordered subset expectation maximization algorithm (28 subsets, 2 iterations). The images were displayed in a 128×128 matrix (pixel size=4.29×4.29 mm with a slice thickness of 4.25 mm). The separate CT and PET scan data were accurately co-registered using commercial software (eNTEGRA, Elgems, Haifa, Israel). The standardized uptake values (SUVs) were acquired using the attenuation-corrected images, the amount of injected 18F-FDG, the body weight of each patient and the cross-calibration factors between the 18F-FDG-PET and the dose calibrator.
For PET/CT interpretation, one nuclear medicine physician reviewed the fused PET/CT images without knowledge of the clinical findings except for the fact that the patient had laryngeal cancer. The nuclear medicine physician first reviewed the images to determine whether there was abnormal uptake of FDG in the larynx and neck nodes by comparing the maximal uptake values to determine whether the intensity was higher than that of the surrounding tissues. The interpretation was revised based on the anatomical information provided by the combined PET/CT images. Similar to the interpretation of the CT scans, a nuclear physician determined the status of each primary tumor sub-site and the initial staging of the laryngeal lesions from the information of the standalone PET/CT scans.
Decision making for treatment
The head and neck cancer, tumor board determined the treatment plan for the laryngeal lesions after review of the results from the clinical examination and the CT scans. Then the team reviewed the PET/CT images. The changes in the treatment decisions were recorded. The confirmed T classification of the primary lesions were 33 (60%) T1a lesions, 9 (16.4%) T1b, 6 (10.9%) T2, 5 (9.1%) T3, and 2 (3.6%) T4a lesions. There were only two patients with neck node metastases (pN2b: 2, 3.6%). The final treatment plans were determined after the available treatment options (curative surgery in 35 patients and radical radiotherapy in 20 patients as an initial treatment) were reviewed with each patient.
Analysis
As the reference standard, either the surgical pathology or the clinical/radiological follow up outcomes were used for comparisons. For the primary tumors, surgical pathology results from the laryngoscopic biopsies or surgical excision were available for all patients. However, for the neck nodes, only seven patients had neck dissections, so clinical/radiological follow up outcomes were used as the reference standard in the remaining 48 patients.
The mean follow-up period for all subjects was 21.5 months (range, 12-30 months).
We determined the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of the L/E, CT and PET/CT for predicting the extent of disease on based on the sub-site analysis. The diagnostic accuracy for the initial tumor staging among three diagnostic modalities was also compared. Statistical differences between the imaging modalities were analyzed by the McNemar test or the chi-squared test with Bonferroni's correction and the 95% confidence interval was determined by Wilson's method. Two tailed P-values less than 0.05 were considered to indicate statistical significance. The clinical treatment decisions based on L/E with CT, L/E and CT with PET/CT were recorded for each patient to determine the impact of the imaging modality on patient care.
RESULTS
Diagnostic value based on sub-site analysis of glottic cancer
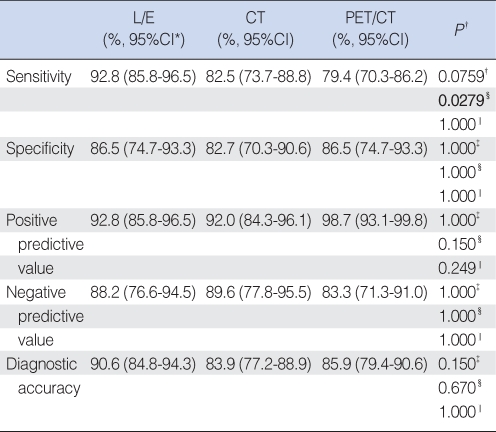
Analysis of 149 sub-sites in 55 patients were recorded; 55 ipsilateral true vocal folds, 55 contra-lateral true vocal folds, 19 adjacent supraglottis, 2 adjacent subglottis, 13 paraglottic areas, and 5 thyroid cartilages. If any of the diagnostic modalities could not predict the status of the sub-sites, we marked the status of the subsites as 'unknown'. Clinical examination with laryngoscopy (L/E) had a sensitivity of 92.8%, specificity of 86.5%, positive predictive value (PPV) 92.8%, negative predictive value (NPV) 88.2%, and a diagnostic accuracy of 90.6%, compared to 82.5%, 82.7%, 92.0%, 89.6%, 83.9 for CT scans and 79.4%, 86.5%, 98.7%, 83.3%, 85.9% for the PET/CT, respectively (Table 1).
Table 1.
Diagnostic accuracy of direct laryngoscopic examinations (L/E), contrast enhanced CT scans (CT) and PET/CT for primary tumor based on subsite analysis in patients with glottic cancer (N=149 sub-sites)
*95% CI: 95% Confidence interval using Wilson's method. †Comparison method between test modalities: McNemar test with Bonferroni's correction or chi-square test with Bonferroni's correction. ‡L/E vs. CT. §L/E vs. PET/CT. ‖CT vs. PET/CT.
The sensitivity was significantly higher in the L/E than in the PET/CT (P=0.0279, McNemar test with Bonferroni's correction). However, the differences between L/E and CT as well as CT and PET/CT did not reach statistical significance. Other parameters such as specificity, PPV, NPV and diagnostic accuracy were similar among the three diagnostic modalities.
For the neck nodes, we are unable to analyze the statistical differences between diagnostic modalities, because of the small number (two pN+) of pathologically proven regional metastases.
Diagnostic accuracy for initial tumor staging in glottic cancer
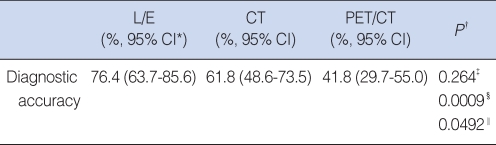
For the initial tumor staging, the L/E had a diagnostic accuracy of 76.4%, compared to 61.8% for CT scans and 41.8% for PET/CT scanning (Fig. 1; Table 2). The L/E and CT were better than the PET/CT (P=0.0009 and 0.0492) for the initial TNM staging.
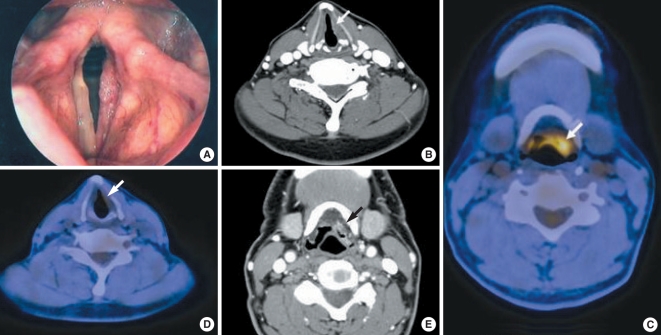
Fig. 1.
A 62-year-old man with glottic squamous cell carcinomas in the left vocal fold. (A) Preoperative laryngoscopy shows irregular surfaced mucosal change confined to the left true vocal fold, suggesting malignant lesions, T1a. (B) The CT scans were interpreted as T1a glottic cancer, based on mucosal irregularity and abnormal contrast enhancement of the left true vocal fold. (C, D) On the PET/CT images, the left epiglottis and vallecula area showed asymmetrical increased uptake of FDG (peak standard uptake value=6.3) suggesting a malignancy, but the faint uptake in the left true vocal fold (peak standard uptake value=2.3) was considered benign. (E) The CT images of the corresponding site also revealed small enhancing lesions of the vallecula, but the radiologist interpreted the lesion as benign changes of the lingual tonsil. The initial tumor staging predicted by PET/CT standalone was supraglottic cancer cT2N0M0. The final surgical pathology demonstrated malignant cells only in the left true vocal fold without extension to supraglottis (T1a).
Table 2.
Prediction of initial tumor staging by using direct laryngoscopic examination (L/E), contrast enhanced CT scans (CT), and PET/CT in patients with glottic cancer (N=55)
*95% CI: 95% Confidence interval using Wilson's method. †Comparison method between test modalities: McNemar test with Bonferroni's correction or chi-square test with Bonferroni's correction. ‡L/E vs. CT. §L/E vs. PET/CT. ‖CT vs. PET/CT.
The T and N classifications were determined by each diagnostic modality alone; however, the M classification of the TNM staging was determined by the information from routine chest x-rays, L/E and CT. In cases with PET/CT scanning, all T, N and M classifications were determined solely by the results of the PET/CT.
Changes of clinical decisions for treatment
The clinical decisions for treatment based on the L/E and CT results were changed by the added PET/CT information in seven of 55 cases (12.7%). In two patients, one additional diagnostic direct laryngoscopy procedure, under general anesthesia, was needed for re-evaluation of the primary site because of a discrepancy in the results from L/E, CT and PET/CT. In addition, the PET/CT changed an elective neck dissection to a curative neck dissection in four patients based on the hot uptake of FDG-glucose in the neck nodes. In one patient, the PET/CT changed the irradiation field and dose to cover the suspected neck nodes.
Among the seven patients, for whom the PET/CT changed the treatment planning, six patients had curative surgery for their lesions, and one had curative radiotherapy. The final surgical pathology revealed that one T classification and two N classifications, previously determined by PET/CT, were incorrect. Three patients were over-treated based on the results of the PET/CT at one primary and two neck sites. However, in four patients the results of the PET/CT were consistent with the final pathology findings and clinical follow-up data (regression of enlarged neck nodes in size and the drop of uptake value after completion of irradiation in one who had received radical radiotherapy).
DISCUSSION
The goal of this study was to determine whether PET/CT scans provided additional information that was of diagnostic benefit compared to the L/E and CT in patients with primary glottic cancer during the initial evaluation of tumors. The results from this study demonstrated that adding PET/CT scans to the conventional diagnostic modalities such as L/E and CT scans did not provide additional benefit. In addition, the impact of the PET/CT findings on clinical decision making for treatment was minimal compared to the management decisions without the PET/CT information. Some of the changes based on the PET/CT, resulted in over treating patients.
PET/CT has been reported to have promising diagnostic benefits in patients with head and neck cancer (4-7). However, malignancies at certain sites of the head and neck, for example, papillary thyroid carcinomas, have not been shown to benefit from PET/CT scanning (13). Glottic cancers have unique clinical features and natural history. Early cancers of the larynx produce hoarseness, allowing earlier recognition and a more favorable prognosis, especially for tumors developing in the glottis (14, 15).
Only one article was available in PubMed on the diagnostic value of PET/CT scanning for laryngeal cancer patients (8). However, studies on the PET/CT for the initial diagnostic work-up of patients with glottic cancer are not available. This is the first study to determine the role of PET/CT in the initial evaluation of patients with glottic cancer. In our clinical experience, PET/CT is beneficial for the diagnosis of disease recurrence after initial treatment and for the evaluation of advanced laryngeal cancer (unpublished data). For example, it can be used to differentiate the laryngeal edema of normal mucosa from recurrent tumor after radiotherapy. In addition, direct laryngoscopic biopsy is reported to have a low specificity and negative predictive value for the diagnosis of recurrence post-radiotherapy. In some cases, the conventional CT images may provide more information than the laryngoscopic evaluation. However, for patients with primary glottic cancer the situation is different. Our results showed that the PET/CT findings added to the conventional work-up for the initial evaluation of glottic cancer did not demonstrate a diagnostic advantage that would support the additional cost of the PET/CT.
The glottis can be easily evaluated by physical examination with the aide of laryngoscopy. This is another reason why the clinical examination with laryngoscopy showed good diagnostic accuracy comparable to that of the CT; it resulted in only three equivocal lesions compared to the results of the CT and PET/CT (16 lesions and 18 lesions respectively). In this study, we performed the biopsy of primary tumor site with direct laryngoscopic evaluation under general anesthesia, and then the CT and PET/CT were performed. Because glottic cancers can be accessed without difficulty, other imaging modalities are not necessary to guide the biopsy, unlike recurrent or post-radiotherapy cases.
In the present study, there were five cases where only the PET/CT erroneously diagnosed the N stage as negative for malignancy, but the lesion proved to be positive by the final pathology. In addition, it misdiagnosed one case with distant metastases that was shown to be inflammatory lesions on the follow up chest CT. For the evaluation of distant metastases in patients with glottic cancer, a simple chest x-ray, blood testing including a liver function profile or abdominal ultrasonography are all cost-effective tests. However, there is no data to suggest that the PET/CT adds additional significant information for patient management.
The results of this study also showed that the information from the L/E and CT was sufficient to make a correct diagnosis and implement an appropriate treatment plan in most patients. The results of the PET/CT affected patient care in only seven cases and correct results were confirmed in only three of the seven patients.
This study has several limitations. The study population consisted of 48 patients with early glottic cancer (T1 or T2N0M0) (87.3%); therefore the results do not apply to other stages of glottic cancer. The clinical course of patients with advanced glottic cancer differs from early glottic cancer, and these cases may benefit from PET/CT scanning; however, further study is needed for confirmation of such a benefit.
Another limitation of this study was that the CT and PET/CT were performed after biopsy of the primary tumor sites. In most of patients, the CT was performed within two weeks after the biopsy and the PET/CT at a mean of three weeks after the biopsy. The prior biopsy procedure may have confounded the evaluation of the extent of the primary lesions by CT and PET/CT, and may have resulted in the over-estimation of the T classification, although the biopsy of the primary sites removed only small representative samples from the tumors. In summary, the results of this study showed that for the initial evaluation of patients with glottic cancer, the combined PET/CT imaging did not provide additional information, useful for patient management, compared to the standard L/E and CT.
Acknowledgments
We are greatly indebted to Biostatistics Unit, Samsung Biomedical Research Institute (Chief director: Seon Woo Kim, Ph.D.) for statistical analyses and interpretation of data.
Footnotes
All authors had no conflict of interest on this study.
References
- 1.Antoch G, Saoudi N, Kuehl H, Dahmen G, Mueller SP, Beyer T, et al. Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol. 2004 Nov 01;22(21):4357–4368. doi: 10.1200/JCO.2004.08.120. [DOI] [PubMed] [Google Scholar]
- 2.Hany TF, Steinert HC, Goerres GW, Buck A, von Schulthess GK. PET diagnostic accuracy: improvement with in-line PET-CT system: initial results. Radiology. 2002 Nov;225(2):575–581. doi: 10.1148/radiol.2252011568. [DOI] [PubMed] [Google Scholar]
- 3.Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003 Jun 19;348(25):2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 4.Branstetter BF, 4th, Blodgett TM, Zimmer LA, Snyderman CH, Johnson JT, Raman S, et al. Head and neck malignancy: is PET/CT more accurate than PET or CT alone? Radiology. 2005 May;235(2):580–586. doi: 10.1148/radiol.2352040134. [DOI] [PubMed] [Google Scholar]
- 5.Schoder H, Yeung HW, Gonen M, Kraus D, Larson SM. Head and neck cancer: clinical usefulness and accuracy of PET/CT image fusion. Radiology. 2004 Apr;231(1):65–72. doi: 10.1148/radiol.2311030271. [DOI] [PubMed] [Google Scholar]
- 6.Jeong HS, Baek CH, Son YI, Chung MK, Lee DK, Choi JY, et al. Use of integrated 18F-FDG PET/CT to improve the accuracy of initial cervical nodal evaluation in patients with head and neck squamous cell carcinoma. Head Neck. 2007 Mar;29(3):203–210. doi: 10.1002/hed.20504. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz DL, Ford E, Rajendran J, Yueh B, Coltrera MD, Virgin J, et al. FDG-PET/CT imaging for preradiotherapy staging of head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005 Jan 01;61(1):129–136. doi: 10.1016/j.ijrobp.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 8.Gordin A, Daitzchman M, Doweck I, Yefremov N, Golz A, Keidar Z, et al. Fluorodeoxyglucose-positron emission tomography/computed tomography imaging in patients with carcinoma of the larynx: diagnostic accuracy and impact on clinical management. Laryngoscope. 2006 Feb;116(2):273–278. doi: 10.1097/01.mlg.0000197930.93582.32. [DOI] [PubMed] [Google Scholar]
- 9.McGuirt WF, Greven KM, Keyes JW, Jr, Williams DW, 3rd, Watson NE, Jr, Geisinger KR, et al. Positron emission tomography in the evaluation of laryngeal carcinoma. Ann Otol Rhinol Laryngol. 1995 Apr;104(4 Pt 1):274–278. doi: 10.1177/000348949510400403. [DOI] [PubMed] [Google Scholar]
- 10.Menda Y, Graham MM. Update on 18F-fluorodeoxyglucose/positron emission tomography and positron emission tomography/computed tomography imaging of squamous head and neck cancers. Semin Nucl Med. 2005 Oct;35(4):214–219. doi: 10.1053/j.semnuclmed.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Zimmer LA, Branstetter BF, Nayak JV, Johnson JT. Current use of 18F-fluorodeoxyglucose positron emission tomography and combined positron emission tomography and computed tomography in squamous cell carcinoma of the head and neck. Laryngoscope. 2005 Nov;115(11):2029–2034. doi: 10.1097/01.MLG.0000181495.94611.A6. [DOI] [PubMed] [Google Scholar]
- 12.Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol. 1992 May;158(5):961–969. doi: 10.2214/ajr.158.5.1566697. [DOI] [PubMed] [Google Scholar]
- 13.Jeong HS, Baek CH, Son YI, Choi JY, Kim HJ, Ko YH, et al. Integrated 18F-FDG PET/CT for the initial evaluation of cervical node level of patients with papillary thyroid carcinoma: comparison with ultrasound and contrast-enhanced CT. Clin Endocrinol (Oxf) 2006 Sep;65(3):402–407. doi: 10.1111/j.1365-2265.2006.02612.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang CC, Efird JT. Does prolonged treatment course adversely affect local control of carcinoma of the larynx? Int J Radiat Oncol Biol Phys. 1994 Jul 01;29(4):657–660. doi: 10.1016/0360-3016(94)90551-7. [DOI] [PubMed] [Google Scholar]
- 15.Adams GL. Malignant tumors of the larynx and hypopharynx. In: Cummings C, Fredrickson J, Harker L, Krause C, Schuller D, Richardson M, editors. Otolaryngology-head and neck surgery. St. Louis, USA: Mosby-Year Book Inc.; 1998. pp. 2130–2143. [Google Scholar]