Abstract
Objectives
Sinonasal mucosal melanoma is a rare and aggressive disease. The aim of this study was to analyze the clinical features of patients with sinonasal mucosal melanoma and to determine the role of Ki67 antigen as a predictor of prognosis in sinonasal mucosal melanoma.
Methods
This was a retrospective case-series study at a single institution, an academic tertiary referral center. From 1995 to 2007, 27 patients with sinonasal mucosal melanoma were reviewed retrospectively, and the expression of Ki67 antigen was assessed by immunohistochemistry.
Results
The overall 5-yr survival rate was 33.9%. No significant differences were observed in 5-yr survival according to age, sex, stage, or the presence of melanin. The rates of local failure, regional failure, and distant failure were 37.0%, 14.8%, and 11.1%, respectively. Patients with spindle or mixed cell types had better prognoses than those with other cell types. At a cut-off value of 35%, patients with lower Ki67 scores showed better survival than those with higher Ki67 scores.
Conclusion
The presence of spindle or mixed cell types may indicate a better prognosis than other cell types. Ki67 immunostaining may be a useful predictor of prognosis in patients with mucosal malignant melanoma of the sinonasal tract.
Keywords: Ki67 antigen, Melanoma, Nasal cavity, Paranasal sinuses
INTRODUCTION
Mucosal malignant melanoma (MMM) of the sinonasal tract accounts for 1% of all mucosal melanomas and 3-4% of malignant neoplasms of the sinonasal tract (1). In MMM, most lesions are limited to the nasal cavity and paranasal sinuses without nodal or distant metastasis. However, this disease has a unique, multifocal characteristic which makes it difficult to obtain a clear resection margin (2-4). For this reason, MMM of the sinonasal tract has a high recurrence rate and a poor prognosis. Our previous report also demonstrated a poor 5-yr overall survival rate of approximately 27.2% (5).
Angiolymphatic invasion, tumor thickness, and a wide margin of surgical resection are widely accepted predictors of survival rate (6, 7). The Ki67 antigen is an indicator of proliferative activity, and numerous studies have shown that Ki67 immunohistochemical staining is an effective method to predict prognosis in various tumors, including cutaneous melanoma (8, 9). However, the association between immunohistochemical staining for Ki67 and prognosis has not yet been studied in MMM of the sinonasal tract.
Thus, our primary objectives were to analyze the clinical features of sinonasal MMM and to determine whether Ki67 expression is correlated with prognosis.
MATERIALS AND METHODS
Subjects
From March 1995 through January 2007, the medical records of 27 patients who were diagnosed and treated for malignant melanoma of the sinonasal tract at our hospital were reviewed retrospectively. The patients were diagnosed as having malignant melanoma based on the presence of melanin pigment in the tumor cells and immunohistochemical staining for HMB-45 and S-100. The population in the present study consisted of 15 males and 12 females with a median age of 62 yr (range, 41 to 87 yr). The average follow-up period was 28.2 months (range, 3 to 104 months). We used Stern's staging system to classify lesions as follows; Stage I confined to the primary site (local disease), Stage II with regional lymph node metastasis, Stage III distant metastasis (10).
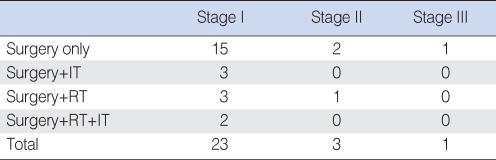
All patients underwent surgery. In 18 patients an en bloc excision was performed with a sufficient margin of resection, regardless of stage. Three patients received additional immunotherapy with interferon (IFN)-α after surgery. Surgery and postoperative radiation therapy was performed in four patients. Two patients was underwent surgery followed by both immunotherapy and radiation therapy (Table 1). We evaluated the survival rates of 27 patients with MMM according to age, sex, and stage. The presence of melanin and histological cell type were evaluated in 21 patients. Regarding these factors, we could not evaluate 6 patients because preoperative slides were not available.
Table 1.
Initial treatment modalities
IT: Immunotherapy; RT: radiotherapy.
Immunohistochemical staining
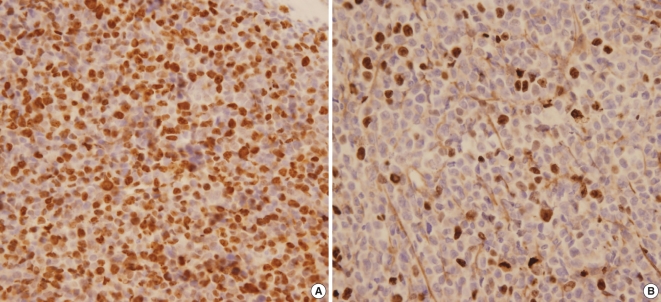
The Ki67 immunohistochemical staining was performed in available 13 tissues which were preserved in paraffin block and the others 14 were preserved on slide only. Paraffin-embedded sections were cut to a thickness of 4 µm, deparaffinized in xylene for 30 min, and then rehydrated in 100%, 95%, and 70% ethanol for 3 min each. The sections were then washed in distilled water for 3 min. For antigen retrieval, the sections were placed in 0.1% citrate buffer (pH 6.0) and heated in a microwave oven for 30 min. Endogenous peroxidase activity was blocked with 3% H2O2 for 5 min, and the slides were washed in phosphate-buffered saline (PBS, 0.1 mol/L, pH 7.4). The tissues were incubated in 10% fetal bovine serum (FBS) and TBST (1% TBS and Tween 20) at room temperature for 20 min to prevent non-specific protein binding. The tissues were then incubated with the primary antibody (anti-Ki67, clone MIB-1; DAKO, Glostrup, Denmark) at a dilution of 1:40 for 12 hr, followed by three washes in PBS. The secondary antibody (biotinylated IgG, mouse/rabbit; DAKO), was applied for 25 min, followed by one wash in PBS. Finally, the sections were incubated with HRP-conjugated streptavidin (DAKO) for 20 min, followed by two washes in PBS. The sections were counterstained with Mayer's hematoxylin. A pathologist with no background knowledge of the present study performed the immunohistochemical analysis. Melanin staining was detected in the cytoplasm, whereas Ki67 was detected in the nuclei of tumor cells. In areas with definite nuclear staining, the number of Ki67-positive tumor cells was counted per 300 tumor cells and the percentage was calculated (Fig. 1).
Fig. 1.
(A) Ki67 staining was detected in 80% of tumor cell nuclei under a light microscope (original magnification ×400). (B) Ki67 staining was detected in 10% of tumor cell nuclei under a light microscope (original magnification ×400).
Statistical analysis
The survival rate and the correlation between Ki67 expression and each factor were assessed using the Kaplan-Meier method and the log-rank test using the SPSS software (SPSS Inc., Chicago, IL, USA). A P-value <0.05 was considered to be statistically significant.
RESULTS
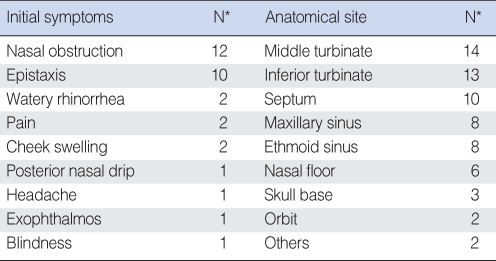
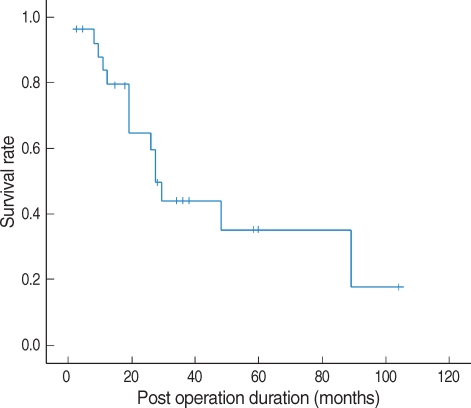
The most common presenting symptoms are nasal obstruction followed by epistaxis (Table 2). The 5-yr survival rate based on the Kaplan-Meier method was 35.3% for MMM of the sinonasal tract (Fig. 2). In the follow-up period, 17 patients (62.9%) showed recurrence; 10 local recurrences (37.0%), four regional recurrences (14.8%), and three distant metastases (11.1%). In the cases of distant metastasis, cancerous cells invaded the brain, lung, and lower abdomen. The mean interval between surgical excision and recurrence was 6.9 months.
Table 2.
Initial symptoms and anatomic site at initial presentation
*Numbers are not mutually exclusive.
Fig. 2.
The 5-yr survival rate for patients with mucosal malignant melanoma of the sinonasal tract is 35.3%.
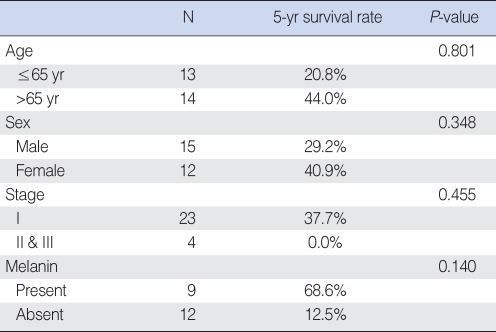
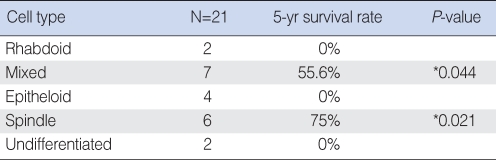
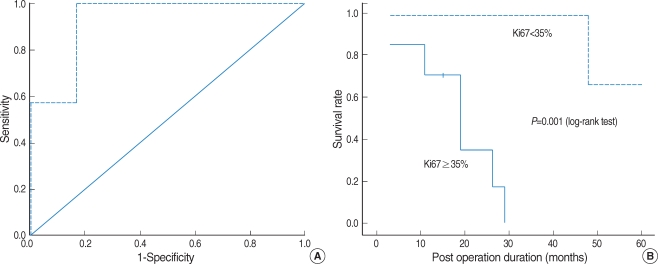
No significant differences in the 5-yr survival rate were observed with regard to age, sex, stage or the presence of melanin (Table 3). However, histological cell type had an impact on prognosis. Patients with spindle or mixed cell types showed significantly higher 5-yr survival rates than those with other cell types (Table 4). Patients with scores of <35% for Ki67 positive staining in tumor cells also had significantly higher 5-yr survival rates than those with scores ≥35% (Fig. 3).
Table 3.
Survival rate according to age, sex, stage, and the presence of melanin
Table 4.
Survival rate according to cell type
*When compared to patients with epitheloid cells, patients with mixed and spindle cells showed significantly higher 5-yr survival rates.
Fig. 3.
(A) We determined cut-off value at 35% by ROC curve. (B) Patients with scores of <35% for Ki67 staining have a distinct survival advantage over those patients with scores of ≥35%.
DISCUSSION
Cutaneous melanoma is more common in Caucasians than in Asians, whereas mucosal melanoma is relatively common in Asians (11). More than one-third of patients are diagnosed as having amelanotic MMM in Western, in which diagnosis tends to be delayed because of atypical symptoms and signs (12). From our series of patients, amelanotic MMM was found in twelve patients (44.4%) which is slightly higher when compared to the previous reports (13). Furthermore, a precursor lesion, such as the nevus in cutaneous malignant melanoma, has not yet been identified (12).
The symptoms at diagnosis depend on the site of the primary lesion. Since the most common sites are the turbinate and septum, patients frequently present with unilateral nasal obstruction and epistaxis (14). However, in advanced stages, pain and facial deformity may also be present (3). Our results are consistent with previous reports regarding the age, sex, and symptoms presented at the initial diagnosis.
It has been reported that 81% of primary MMMs arise from the nasal cavity and 19% from the sinuses (15). We found that 75% of primary MMMs originated from the nasal cavity and 25% from the sinuses. The present study demonstrates that the turbinate and septum are the most common anatomic sites for mucosal melanoma.
Stern's classification is universally used to stage tumors (10). However, many studies have reported on stage I tumors at the initial diagnosis and have found that stage is not associated with survival. Likewise, 85.2% of the tumors in our patients were at stage I, and there was no significant difference in survival according to stage. Recently, other staging systems have been suggested for primary MMM (2, 16). However, these staging systems are also irrelevant with survival in our data. These outcomes indicate that a modified staging system is needed.
Local recurrence is a common problem in MMM. Previous studies have shown that the local recurrence rate is greater than 50% (6), and this study showed a relatively high rate of local recurrence (37.0%). This high rate of local recurrence may result from the non-apparent diffusion of tumor cells via the submucosal lymphatic route or the presence of multifocal lesions (6). Unlike squamous cell carcinoma, MMM of the sinonasal tract metastasizes to distant regions, such as the lung and brain, more frequently than it undergoes cervical nodal metastasis (17). However, in our series the regional metastasis rate is similar to the distant metastasis rate.
Previous studies have reported that the 5-yr survival rate for malignant mucosal melanoma of the head and neck ranges from 0% to 44% (1, 3, 7, 12), which is consistent with our value of 35.3%. The 5-yr survival rate was not correlated with age, sex, stage, or the presence of melanin.
We also analyzed the survival rate with respect to histological cell type and found that patients with spindle or mixed cell types had a better prognosis. However, the number of patients available for the histological study was small and future large-scale studies are required to confirm our results.
Intense immunohistochemical staining for Ki67 is correlated with poor prognosis in various malignancies, including cutaneous malignant melanoma (8, 9, 18, 19). One small series reported that scores of ≤40% for Ki67 and ≤80% for PCNA were correlated with good prognosis in anorectal malignant melanoma (17). Another study reported that scores of ≤20% for Ki67 and ≤35% for PCNA were correlated with good prognosis in cutaneous malignant melanoma (18). However, there is no information available regarding the association between survival and Ki67 staining in MMM. This study showed that Ki67 staining in <35% of tumor cells is statistically correlated with a good prognosis, which indicates that Ki67 antigen may be useful in a prognostic factor for MMM.
CONCLUSIONS
MMM of the sinonasal tract shows frequent local recurrence and poor prognosis. Even though the number of participants in this study is small, we demonstrate that patients with spindle cells or mixed cell types generally have better prognoses than patients with other cell types and that Ki67 immunohistochemical staining may be a useful marker to predict the prognosis for MMM of the sinonasal tract.
References
- 1.Holdcraft J, Gallagher JC. Malignant melanomas of the nasal and paranasal sinus mucosa. Ann Otol Rhinol Laryngol. 1969 Feb;78(1):5–20. doi: 10.1177/000348946907800101. [DOI] [PubMed] [Google Scholar]
- 2.Thompson LD, Wieneke JA, Miettinen M. Sinonasal tract and nasopharyngeal melanomas: a clinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol. 2003 May;27(5):594–611. doi: 10.1097/00000478-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Freedman HM, DeSanto LW, Devine KD, Weiland LH. Malignant melanoma of the nasal cavity and paranasal sinuses. Arch Otolaryngol. 1973 Apr;97(4):322–325. doi: 10.1001/archotol.1973.00780010332008. [DOI] [PubMed] [Google Scholar]
- 4.Berthelsen A, Andersen AP, Jensen TS, Hansen HS. Melanomas of the mucosa in the oral cavity and the upper respiratory passages. Cancer. 1984 Sep 01;54(5):907–912. doi: 10.1002/1097-0142(19840901)54:5<907::aid-cncr2820540526>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Hong SL, Kim SW, Won TB, Shim WS, Kim YM, Kim JW, et al. Clinical features and treatment outcomes of mucosal malignant melanomas of nasal cavity and paranasal sinuses. Korean J Otolaryngol-Head Neck Surg. 2006 Dec;49(12):1176–1180. [Google Scholar]
- 6.Patel SG, Prasad ML, Escrig M, Singh B, Shaha AR, Kraus DH, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002 Mar;24(3):247–257. doi: 10.1002/hed.10019. [DOI] [PubMed] [Google Scholar]
- 7.Shah JP, Huvos AG, Strong EW. Mucosal melanomas of the head and neck. Am J Surg. 1977 Oct;134(4):531–535. doi: 10.1016/0002-9610(77)90393-2. [DOI] [PubMed] [Google Scholar]
- 8.Linden MD, Torres FX, Kubus J, Zarbo RJ. Clinical application of morphologic and immunocytochemical assessments of cell proliferation. Am J Clin Pathol. 1992 May;97(5 Suppl 1):S4–S13. [PubMed] [Google Scholar]
- 9.Cuevas E, Jones DB, Wright DH. Immunohistochemical detection of tumour growth fraction (Ki-67 antigen) in formalin-fixed and routinely processed tissues. J Pathol. 1993 Apr;169(4):477–478. doi: 10.1002/path.1711690415. [DOI] [PubMed] [Google Scholar]
- 10.Stern SJ, Guillamondegui OM. Mucosal melanoma of the head and neck. Head Neck. 1991 Jan–Feb;13(1):22–27. doi: 10.1002/hed.2880130104. [DOI] [PubMed] [Google Scholar]
- 11.Takagi M, Ishikawa G, Mori W. Primary malignant melanoma of the oral cavity in Japan. With special reference to mucosal melanosis. Cancer. 1974 Aug;34(2):358–370. doi: 10.1002/1097-0142(197408)34:2<358::aid-cncr2820340221>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Kingdom TT, Kaplan MJ. Mucosal melanoma of the nasal cavity and paranasal sinuses. Head Neck. 1995 May–Jun;17(3):184–189. doi: 10.1002/hed.2880170303. [DOI] [PubMed] [Google Scholar]
- 13.Chiu NT, Weinstock MA. Melanoma of oronasal mucosa. Population-based analysis of occurrence and mortality. Arch Otolaryngol Head Neck Surg. 1996 Sep;122(9):985–988. doi: 10.1001/archotol.1996.01890210057013. [DOI] [PubMed] [Google Scholar]
- 14.Manolidis S, Donald PJ. Malignant mucosal melanoma of the head and neck: review of the literature and report of 14 patients. Cancer. 1997 Oct 15;80(8):1373–1386. doi: 10.1002/(sici)1097-0142(19971015)80:8<1373::aid-cncr3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Batsakis JG, Regezi JA, Solomon AR, Rice DH. The pathology of head and neck tumors: mucosal melanomas, part 13. Head Neck Surg. 1982 May–Jun;4(5):404–418. doi: 10.1002/hed.2890040509. [DOI] [PubMed] [Google Scholar]
- 16.Prasad ML, Patel SG, Huvos AG, Shah JP, Busam KJ. Primary mucosal melanoma of the head and neck: a proposal for microstaging localized, Stage I (lymph node-negative) tumors. Cancer. 2004 Apr 15;100(8):1657–1664. doi: 10.1002/cncr.20201. [DOI] [PubMed] [Google Scholar]
- 17.Rinaldo A, Shaha AR, Patel SG, Ferlito A. Primary mucosal melanoma of the nasal cavity and paranasal sinuses. Acta Otolaryngol. 2001 Dec;121(8):979–982. [PubMed] [Google Scholar]
- 18.Ben-Izhak O, Bar-Chana M, Sussman L, Dobiner V, Sandbank J, Cagnano M, et al. Ki67 antigen and PCNA proliferation markers predict survival in anorectal malignant melanoma. Histopathology. 2002 Dec;41(6):519–525. doi: 10.1046/j.1365-2559.2002.01444.x. [DOI] [PubMed] [Google Scholar]
- 19.Niezabitowski A, Czajecki K, Ryś J, Kruczak A, Gruchala A, Wasilewska A, et al. Prognostic evaluation of cutaneous malignant melanoma: a clinicopathologic and immunohistochemical study. J Surg Oncol. 1999 Mar;70(3):150–160. doi: 10.1002/(sici)1096-9098(199903)70:3<150::aid-jso2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]