Abstract
Objective
The authors report three cases of neuroleptic malignant syndrome (NMS) induced by atypical antipsychotics (olanzapine and clozapine) which showed classic features of NMS including muscular rigidity and prominent fever.
Method
Case reports.
Results
A 66-year-old man with dementia and alcohol abuse developed NMS while on olanzapine for agitation and combativeness. A 62-year-old man with schizophrenia developed NMS 6 days after starting clozapine. A 43-year-old man with bipolar disorder developed NMS 14 days after starting clozapine. All three cases showed classic features of NMS including muscular rigidity and fever. Resolution of fever and muscular rigidity occurred within 72 hours with discontinuation of neuroleptics, supportive care, and lorazepam. The NMS rating scale reflected daily clinical improvement.
Conclusion
Classic NMS characterized by muscular rigidity and prominent fever may occur with atypical neuroleptics. Our cases suggest recovery from NMS associated with atypical neuroleptics may be hastened by lorazepam, as was previously reported for NMS from typical neuroleptics. Also, the NMS rating scale was sensitive to clinical improvement.
Keywords: atypical antipsychotics, neuroleptic malignant syndrome, lorazepam, catatonia, rating scale
Introduction
Neuroleptic malignant syndrome (NMS) is a rare but potentially life-threatening adverse reaction to antipsychotic drugs and other dopamine-modulating agents (Caroff and Mann 1993). The syndrome is characterized by motor, behavioral, autonomic, and laboratory abnormalities (Caroff et al 1993; Francis et al 2000). Several clinical and research diagnostic criteria for NMS are available. Most require muscular rigidity and fever (Caroff et al 1993; APA 1994) but some permit a diagnosis without rigidity (eg, Levenson 1985).
Almost all typical neuroleptics have been associated with NMS (Caroff et al 1993). Some workers predicted that atypical neuroleptics would not cause NMS since intense dopaminergic blockade is one hypothesized basis for NMS. However, numerous cases of apparent NMS related to atypical neuroleptics have been reported (Caroff et al 2000, 2003), and most of these are associated with muscular rigidity and fever. In one review, however, 20% of reported cases of clozapine-related NMS lacked muscle rigidity as part of clinical picture (Caroff et al 2000).
We report three cases of NMS related to atypical neuroleptics (olanzapine and clozapine). All had classic features of NMS, including fever and muscle rigidity and met both APA (1994) and Caroff-Mann (1993) criteria. Lorazepam appeared to be beneficial in hastening recovery. In all three cases, the NMS rating scale showed improvement in all four clinical domains (motor, behavioral, autonomic, and laboratory).
NMS rating scale
The NMS rating scale (Yacoub et al 2004; Appendix) includes 23 items encompassing motor, behavioral, autonomic, and laboratory domains of NMS. In conjunction with the present report, reliability was determined using records from 10 well-defined cases of NMS (Francis et al 2000). All cases met APA (1994) or Caroff-Mann (1993) criteria on the day of presentation. Two independent raters determined scores at presentation and on the third and fifth day of treatment, yielding 30 pairs of ratings. Overall reliability was high (r = 0.99 for total score and r = 0.98 for number of signs present; range of scores 0–53, range of signs 0–16). The kappa statistic for agreement (presence/absence) averaged 0.85 (range 0.59–1.00) for all 23 items. The Cronbach alpha statistic was 0.48 calculated from 19 of 23 scale items which were present in ≥20% of cases.
Case 1
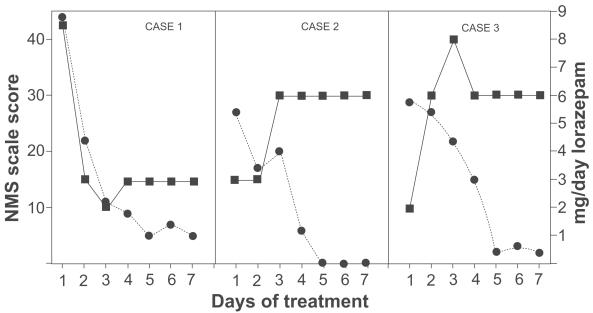
A 66-year-old white male with a history of dementia and alcohol abuse was hospitalized for increased aggressive behavior. He had no prior psychiatric admissions. On the day of admission after he sustained a fall, a CT scan of brain showed subarachnoid hemorrhage at the right superior sulcus and possible hemorrhagic contusion at the left frontal lobe. Over the course of hospitalization, he had serial CT scans to observe resolution of the hemorrhage. He was started on olanzapine for intermittent periods of agitation and combativeness. Olanzapine was titrated to 7.5 mg daily. Ten days after initiation of olanzapine, he became abruptly somnolent with body temperature 39.7ºC (rectal) and severe muscle rigidity in both upper and lower extremities. He had severe diaphoresis and fluctuation of blood pressure and pulse. Laboratory values showed elevation of white blood cells (WBC) (14800 K/L), elevation of creatine phosphosphkinase (CPK) 2800 U/L (normal < 174 U/L), and mild elevation in serum alanine aminotransferase and aspartate aminotransferase. Brain CT showed resolution of the previous subarachnoid hemorrhage and no new cerebrovascular abnormalities. An MRI of the brain was unremarkable, as were CSF studies and chest X-ray. Blood, urine, and sputum cultures showed no growth in 48 hours. A presumptive diagnosis of NMS was made. Olanzapine was immediately discontinued and supportive care initiated (IV hydration and as-needed acetaminophen). The NMS rating scale showed 44 on the first day. Intravenous lorazepam was given as needed every 4 hours for behavioral agitation along with a fixed 0.5 mg intravenous push twice daily. He received a total of 8.5 mg lorazepam in the first 24 hours and 3 mg lorazepam in the next day. Fever and muscular rigidity resolved within 48 hours. All other NMS manifestations resolved in 9 days (Figure 1, Case 1). He was not rechallenged with neuroleptic medication. He was discharged to a nursing home in stable condition on lorazepam 2 mg, divaloproex sodium 2000 mg, and trazodone 300 mg (daily doses).
Figure 1.
Neuroleptic malignant syndrome (NMS) scale scores (•) and daily dose of lorazepam (▪) in patients with NMS.
Case 2
A 62-year-old man with a history of schizophrenia was admitted to the hospital for worsening of paranoid ideation, non-commanding auditory hallucinations, and agitation. For years, he had been on various neuroleptics with partial response. Prior to this hospitalization, he was partially stable on thiothixene but adherence to this medication was questionable. On admission, he was taking thiothixene and benztropine. Within few days, the dose of thiothixene was increased to 15 mg daily. He became less agitated but was paranoid and had bizarre ideas (“I feel a razor cutting me up”). Gradually, thiothixene and benztropine were discontinued and clozapine started. Clozapine was titrated to 100 mg daily. By the sixth day, he became febrile (38.1ºC oral) with increased muscular rigidity of both upper extremities. He was confused and had urinary incontinence. Laboratory studies showed CPK of 412 U/L and WBC of 7.6 K/L. Blood and urinary cultures were negative and the chest X ray was unremarkable. During the first 24 hours, he had tremors in both upper extremities and autonomic instability with diaphoresis and fluctuation of blood pressure and pulse. A presumptive diagnosis of NMS was made. The initial NMS rating scale score was 27. Clozapine was discontinued, and supportive care initiated (oral hydration and as_needed acetaminophen). During the first two days, he received lorazepam 3 mg daily. On the third day, he received a total of 6 mg lorazepam and remained on this dose for a week. Gradually, lorazepam was tapered to 3 mg daily. Muscular rigidity and fever abated within 72 hours after onset of NMS. All other features of NMS resolved on the fifth day (Figure 1, Case 2). He was rechallenged with quetiapine two weeks after the resolution of NMS without recurrence of symptoms. He was discharged from the hospital in stable condition on quetiapine 200 mg and lorazepam 2 mg (daily doses).
Case 3
A 43-year-old man with a history of bipolar disorder was hospitalized for manic symptoms with psychotic features. Upon admission to the hospital, he was taking daily doses of perphenazine 8 mg, divaloproex sodium 2000 mg, and aripriprazole 20 mg. During the hospitalization, aripriprazole was discontinued and perphenazine titrated to 48 mg in divided doses. Divaloproex sodium was increased to 3000 mg but later decreased to 1750 mg for excessive sedation and fatigue. He was maintained on these medications with minimal response, showing paranoid delusions and periods of agitated behavior. Perphenazine was gradually discontinued. Clozapine was started and titrated up to 175 mg daily. Fourteen days after initiation of clozapine, he developed fever 38.3ºC (oral) with rigidity of both upper extremities. He was confused with fluctuating orientation and sensorium, and showed diaphoresis and tachypnea. His blood pressure was stable, his gait was unsteady, and he had an unwitnessed fall. A head CT scan showed mild cortical atrophy. Urine and blood cultures were negative as was the chest X-ray. CPK was 312 U/L, WBC were 12800 K/L, serum iron level decreased to 16 μg/dL (normal range 30–160 μg/dL), and transaminases remained normal. A clinical diagnosis of NMS was made. Both clozapine and divaloproex sodium were discontinued and intravenous fluids started. During the first day of NMS, he received 2 mg lorazepam intramuscularly for severe agitation. The NMS rating scale score was 27. He received total of 6 mg lorazepam on the second day and 8 mg on the third day. From day 4, he was on lorazepam 6 mg daily for 5 more days, then it was gradually discontinued. Within 72 hours of NMS onset, both fever and muscular rigidity resolved. All other NMS features resolved in 9 days (Figure 1, Case 3). He was discharged from the hospital in stable condition 12 days after the onset of NMS on divaloproex sodium 2000 mg daily. He was not rechallenged with any neuroleptic medication.
Discussion
Our three cases with atypical neuroleptics (clozapine and olanzapine) demonstrated classic presentations of NMS, which met Levenson (1985), APA (1994), and Caroff-Mann (1993) criteria. In Case 2, cessation of benztropine may have promoted NMS as well. Each case showed prominent fever and muscular rigidity as well as a variety of behavioral, autonomic, and laboratory abnormalities. In each case, NMS remitted rapidly with cessation of the antipsychotic followed by supportive care. Lorazepam was administered in all cases, which may have hastened recovery as has been reported for NMS due to typical neuroleptics (Francis et al 2000; Khaldarov 2000).
In a review of NMS cases associated with atypical neuroleptics, Sachdev et al (1995) concluded that NMS may present with fewer motor manifestations and milder CPK elevation. Caroff et al (2000) reviewed all NMS cases published related to atypical neuroleptics, and applied various diagnostic criteria sets retrospectively. They concluded that although all NMS cases associated with atypical antipsychotics met broad criteria for NMS, some patients present only with a partial or incomplete form of classic NMS such as lack of rigidity, fever, or other primary features. Nevertheless, they found most published cases of NMS associated with olanzapine and clozapine included prominent muscular rigidity.
The standard approach to manage NMS includes recognizing the diagnosis early, excluding alternative causes of the symptoms, discontinuing suspected triggering drugs, and providing supportive care to reduce temperature, ensure fluid balance, and prevent complications (Caroff 2003). There is limited consensus and inconsistent evidence on the comparative efficacy of specific treatments for NMS. Davis et al (2000) concluded that, in view of heterogeneity of cases diagnosed as NMS, lack of prospective, controlled trials, and standardized dosing, it is difficult to consider any specific pharmacological intervention or electroconvulsive therapy (ECT) to be superior.
Benzodiazepines have been recommended for managing agitation and reversing catatonic symptoms of NMS (Fink 1996). In some cases, benzodiazepines were found to be effective when other medications failed (Miyaoko 1997). Some workers have advocated benzodiazepines as specific treatments for NMS, based on clinical similarities between catatonia and NMS (Fink 1996; Davis et al 2000; Francis et al 2000). Francis el at (2000) reported robust resolution of NMS symptoms with high potency benzodiazepines in 16 patients meeting APA (1994) or Caroff-Mann (1993) criteria whose NMS was precipitated by typical antipsychotic agents. Muscular rigidity and fever abated in 24–48 hours with lorazepam at daily doses of ~3 mg. Khaldarov (2000) reported two additional cases of NMS from typical anti-psychotics that appeared to respond similarly to lorazepam treatment with prompt resolution of NMS. In the present three cases, NMS was precipitated by atypical neuroleptic agents. We employed a similar management in our three patients, using supportive care and lorazepam. Both fever and muscular rigidity improved and resolved in 24–72 hours. These findings indicate that lorazepam may be useful for management of NMS precipitated by both typical and atypical antipsychotic agents.
A review of the literature showed several diagnostic criteria for NMS. All encompass a combination of motor, behavioral, autonomic, and laboratory domains. One prior attempt to construct a scale for NMS included only one item in each domain (Hynes and Victar 1996). Yacoub et al (2004) designed an NMS rating scale (Appendix) to aid recognition and quantify the severity and clinical course of NMS once a diagnosis has been established. The scale includes 23 items with anchored scoring according to presence, absence, or severity. The scale assesses 5 motor, 2 behavioral, 10 autonomic, and 6 laboratory domains of NMS, and was found to be of high reliability. The Francis-Yacoub NMS rating scale was applied to all three cases from day one to day seven, and the scores reflected rapid clinical improvement (Figure 1).
Further research and clinical data are needed in terms of risk factors, nosological issues, and more effective and specific treatment options of NMS.
Acknowledgment
We thank Drs. Izchak Kohen and Angel Caraballo for assistance in performing ratings.
Appendix: Francis-Yacoub NMS Rating Scale
This scale is designed to rate the severity of neuroleptic malignant syndrome [NMS] and should not be used for diagnosis. The diagnosis of NMS can be made by a variety of criteria including the APA [1994] and Caroff-Mann [1993]. Patients considered for a diagnosis of NMS are typically quite ill and other etiologies of fever and autonomic disturbance should be sought concurrently.
Ratings can be made at regular intervals, eg, daily or q8 hours; express results as score obtained/scored items
Rate peak observation or most extreme value for interval
Interpolate laboratory values as appropriate
0. [Not scored] Proper Pharmacological Setting
History of administration of oral neuroleptics 7 days prior to onset [4 weeks for depot formulations] or other agents associated with NMS, eg, cessation of dopamine agonists, adjustments in antiparkinsonian medications, etc.
Yes. Specify agent, dosage, and route of administration
No. Pursue alternative explanations for rigidity, fever, autonomic signs, etc. May complete scale pending further history.
1. Extrapyramidal features: rigidity of extremities
Preferably examined on passive motion of large joints while patient relaxes in sitting posture [lying posture acceptable].
0 Absent
1 Minimal or found only with distraction by patient moving other limbs
2 Mild to moderate
3 Marked, full range of motion can be obtained
4 Severe, restricted range of motion, or “lead pipe”
2. Extrapyramidal features: rigidity of neck and/or upper trunk
Examined as per extremities. Upper trunk examined by [passive and active] flexion.
0 Absent
1 Minimal or only with distraction by patient moving limbs
2 Mild to moderate
3 Marked, but full range of motion obtained
4 Severe, restricted range of motion, or “lead pipe”
3. Extrapyramidal features: pharyngeal/swallowing
Rate by observation or reports from interval of evaluation.
0 Normal
1 Rare disturbed swallowing or choking episodes
2 Occasional disturbed swallowing or choking episodes
3 Dietary change required, eg, soft food
4 Tube or gastrostomy feedings required
4. Extrapyramidal features: pharyngeal/speech
Rate by observation or reports from interval of evaluation. Rate “0” if mute or intubated.
0 Normal
1 Mildly affected. No difficulty being understood; slight loss of expression, clarity, or loudness
2 Moderately affected. Slurred or monotonous, or must repeat some statements; can communicate
4 Severely affected. Frequently must repeat statements or impaired ability to communicate
5. Extrapyramidal features: tremors at rest
Observe limbs, trunk, and head. Note and rate region of highest severity.
0 Absent
1 Slight: low amplitude or intermittent
2 Moderate: persistent; easily noticeable, bothers patient
3 Marked: prominent, disrupts some activities
4 Severe: disrupts most activities
6. Consciousness/mental status changes
Attempt to engage in conversation and assess orientation.
0 Normal, full consciousness/oriented to time, person and place; normal verbal communication
4 Delirium [fluctuation of consciousness or orientation], or somnolent/drowsy, or oriented to 1–2 out of 3
8 Coma or complete disorientation
7. Catatonia
Observe and elicit catatonic signs, preferably using Bush-Francis Catatonia Scale. Alternatively, assess for DSM-IV catatonic signs other than rigidity: immobility, catalepsy, waxy flexibility, excitement, negativism, mutism, posturing, stereotypy, mannerisms, grimacing, and echopraxia/echolalia. At least 2 signs define presence of catatonia.
0 absent
8 present
8. Body temperature
Oral measurement is convenient, but rectal measurement more closely reflects the core body temperature. Rectal temperature is ~0.5 C higher than oral. Rate peak value in interval of evaluation.
0 ≤ 37.0ºC [98.6 ºF] [Rectal ≤ 37.5ºC]
4 > 37.0 and < 37.5ºC [98.6–99.5 ºF] [Rectal > 37.5 and < 38ºC]
8 ≥ 37.5 and < 39ºC [99.5–102.2 ºF] [Rectal ≥ 38 and < 39.5ºC]
12 ≥ 39ºC and < 40ºC [102.2–104 ºF] [Rectal ≥ 39.5ºC and < 40.5ºC]
16 ≥ 40ºC [104 ºF] [Rectal ≥ 40.5ºC]
9. Systolic blood pressure
Record peak value during interval being rated.
0 < 140 mmHg
1 ≥ 140 and < 160
2 ≥ 160 and < 180
4 ≥ 180
10. Diastolic blood pressure
Record peak value during interval being rated.
0 < 90 mmHg
1 ≥ 90 and < 100
2 ≥ 100 and < 110
4 ≥ 110
11. Pulse
Record peak value during interval being rated.
0 < 90/min
1 ≥ 90 and < 100
2 ≥ 100 and < 120
4 ≥ 120
12. Labile blood pressure [systolic or diastolic]
Record difference between peak and lowest values during interval being rated, for both systolic and diastolic. Record larger value.
0 +/− 10 mmHg
1 +/− 20
2 +/− 30
4 +/− 40 or greater
13. Labile pulse
Record difference between peak and lowest values during interval being rated.
0 +/− 10/min
1 +/− 20
2 +/− 30
4 +/− 40 or more
14. Diaphoresis
Observe for diaphoresis on face, trunk, and extremities and check clothing or bedding.
0 Absent
2 Mild/slight
4 Prominent or bedding/clothing moist
15. Respiration
Ask for subjective complaints, or observe respiration.
0 No dyspnea, normal respiration
2 Shortness of breath or tachypnea [rate ≥ 20] with minimal exertion [eg, talking]
4 Shortness of breath or tachypnea [rate ≥ 20] at rest or accessory respiratory muscle use
8 Supplemental oxygen or ventilator use even if intermittent
16. Dehydration
Evidence of dehydration based on clinical or laboratory examination.
0 Absent
2 [A] Poor skin turgor and/or [B] dry skin; normal laboratory values
4 [A] or [B] with laboratory evidence of dehydration, eg, increased serum BUN [blood urea nitrogen]/creatinine ratio > 20, increased hematocrit, increased urine osmolality, urine sodium < 20 mEq/L, weight loss > 0.25 kg/day, etc.
17. Incontinence
Urinary or fecal incontinence in the period of rating. If urinary catheter in use, rate “0.”
0 Absent
2 One episode
4 More than one episode
18. Serum CPK
Record peak value. If not repeated, record most recent. Values based on normal < 200 IU/L. Adjust for local normal value
0 Normal
1 200–500 [2.5 x normal]
2 501–1000 [5 x normal]
3 1001–5000 [25 x normal]
4 More than 5000
19. WBC
Record peak value. If not repeated, record most recent measurement.
0 Normal [< 11000]
4 > 11000 and < 15000
8 ≥15000
20. Serum transaminase (AST or ALT)
Record peak value. If not repeated, record most recent measurement. Values based on normal < 50 IU/L. Adjust for local normal value. Rate “0” if pre-existing liver disease.
0 Normal [≤50]
1 51–125 [2.5 x normal]
2 126–250 [5 x normal]
3 251–1250 [25 x normal]
4 > 1250 [>25 x normal]
21. Serum iron level
Laboratory values based on local normal.
0 normal
2 decreased levels
22. Myoglobinemia/myoglobinuria
Laboratory evidence of muscle breakdown
0 Absent or normal
4 Present or elevated
23. Acid-base balance
Laboratory evidence of acid/base disturbance. Metabolic acidosis is likely to be the most common finding. Best determined with arterial blood gases [ABG].
0 Absent [normal pH, normal CO2, normal HCO3]
4 Present with compensation, or reduced HCO3 without ABG determination
8 Present, inadequate compensation [eg, metabolic acidosis with low pH]
Total Score __________
Date __________
Time __________
Rater __________
References
- [APA] American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th ed. APA; 1994. [Google Scholar]
- Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77:185–202. doi: 10.1016/s0025-7125(16)30278-4. [DOI] [PubMed] [Google Scholar]
- Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatr Ann. 2000;30:314–21. [Google Scholar]
- Caroff SN. Neuroleptic malignant syndrome [Still a risk but which patient may be in danger?] Curr Psychiatr. 2003;12:36–42. [Google Scholar]
- Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatr Ann. 2000;30:325–31. [Google Scholar]
- Fink M. Neuroleptic malignant syndrome and catatonia: one entity or two? Biol Psychiatry. 1996;39:1–4. doi: 10.1016/0006-3223(95)00552-8. [DOI] [PubMed] [Google Scholar]
- Francis A, Chandragiri S, Rizivi S, et al. Lorazepam a treatment of neuroleptic malignant syndrome? CNS Spectr. 2000;5:54–7. doi: 10.1017/s1092852900013407. [DOI] [PubMed] [Google Scholar]
- Hynes A, Victar E. Case study: neuroleptic malignant syndrome without pyrexia. J Am Acad Child Adolesc Psychiatry. 1996;35:959–61. doi: 10.1097/00004583-199607000-00024. [DOI] [PubMed] [Google Scholar]
- Khaldarov V. Benzodiazepines as treatment for neuroleptic malignant syndrome. Hosp Physician. 2000;6:51–5. [Google Scholar]
- Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry. 1985;142:1137–45. doi: 10.1176/ajp.142.10.1137. [DOI] [PubMed] [Google Scholar]
- Miyaoko H, Shishikura K, Otsubo T, et al. Diazepam- responsive neuroleptic malignant syndrome: a diagnostic subtype? Am J Psychiatry. 1997;1997;154:882. doi: 10.1176/ajp.154.6.882. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Kruk J, Kneebone M, et al. Clozapine induced neuroleptic malignant syndrome: review and report of new cases. J Clin Psychopharmacol. 1995;15:365–71. doi: 10.1097/00004714-199510000-00010. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Kohen I, Caraballo A, et al. Rating Scale of Neuroleptic Malignant Syndrome [poster] American Psychiatric Association; 2004. May 2004. poster 684. [Google Scholar]