Abstract
Introduction
In Alzheimer’s disease (AD), accelerated neurofibrillary tangle formation occurs which is associated with increased tau protein release into the cerebrospinal fluid (CSF). Recent studies found significantly increased CSF tau already in patients at risk of developing AD, indicating its potential as a biochemical marker of AD. Cerebral glucose metabolism is reduced in frontotemporoparietal and cingulate cortices in patients with mild AD. However, few studies have investigated CSF tau protein and cerebral glucose metabolism changes in patients at risk to develop AD.
Methods
48 patients with AD, 88 patients with aging-associated cognitive decline (AACD), and 39 healthy controls were included. In all participants, CSF levels of tau were determined by ELISA at baseline and compared between the diagnostic groups. 14 AACD patients and 14 controls underwent 18F-fluorodeoxyglucose positron emission tomography (FDG PET).
Results
AD patients showed the highest CSF tau levels compared with AACD patients and controls. AACD patients had significantly higher tau levels than the controls but lower than the AD patients. AACD patients were characterized by reduced glucose metabolism in bilateral middle temporal cortex, left posterior cingulate cortex, right angular gyrus, and right precuneus compared with controls.
Conclusion
In conclusion, our findings reflect and confirm the clinical judgment of an incipient neurodegenerative disorder in a considerable portion of AACD patients. In patients with AACD, CSF tau levels and cerebral glucose metabolism show an altered pattern comparable with that found in AD and thus may facilitate early diagnosis.
Keywords: Aging-associated cognitive decline, Alzheimer’s disease, CSF tau protein, FDG PET
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by histopathological changes such as neurofibrillary tangle formation and amyloid plaque deposition in susceptible brain regions. Epidemiological studies showed that before the onset of dementia a subtle cognitive impairment occurs which can be stable over many years (Schröder et al 1998; Elias et al 2000) but longitudinally leads to AD with an annual conversion rate of around 15% (De Carli 2003). Since treatment and monitoring of the disease requires an early diagnosis, various potential preclinical syndromes such as mild cognitive impairment (MCI) (Petersen et al 2001) have been proposed. The widely accepted amnestic MCI refers to minor, chiefly mnestic cognitive deficits in elderly individuals which exceed those of the normal physiological aging processes, but which are not severe enough to fulfil a clinical diagnosis of dementia. However, in a recent follow-up study, MCI has been found to be rather unstable compared with other concepts such as aging-associated cognitive decline (AACD), which refers also to non-mnestic deficits (Levy et al 1994; Ritchie et al 2001; Schönknecht et al 2005).
Since a long period of mild cognitive changes before the onset of dementia has to be considered, neurobiological markers to define onset and course of AD are warranted. Cerebrospinal fluid (CSF) tau protein, which is related to an increased neurofibrillary tangle formation in the brain, was found to be significantly increased in AD patients compared with controls (Schönknecht et al 2003a). Other studies demonstrated that a considerable proportion of AD patients do not show increased tau levels (Schönknecht et al 2003b). Moreover, increased tau protein release also occurred after acute cerebrovascular damage or other than AD-specific neurodegenerative pathology such as Creutzfeldt-Jakob disease (CJD) (Buerger et al 2006), making inconclusive the use of this marker to identify incipient AD cases among patients at risk of developing AD (for review see Blennow and Hampel 2003).
Therefore, brain imaging might provide an alternative diagnostic measure to reveal potential preclinical changes of AD. Previous magnetic resonance imaging (MRI) studies revealed that atrophic changes in AD patients primarily strike substructures of the medial temporal lobe but proceed to temporoparietal association cortices with disease progression (Pantel et al 1997). Using structural MRI, medial temporal lobe atrophy was confirmed both in patients with incipient AD and in subjects at risk for developing dementia (Pantel et al 2003). In comparison with controls, AD was characterized by a rapid decline of hippocampal volumes while only a moderate decrease of whole brain volumes occurred, indicating that hippocampal atrophy was not merely a function of generalized brain atrophy (Pantel et al 2002). In the cited study, progression of hippocampal atrophy but not of whole brain atrophy was significantly correlated with clinical deterioration.
Cognitive deficits in AD and AACD are hypothesized to be related to reduced cerebral glucose uptake. Furthermore, various neuropsychological deficits have been shown to be correlated with distinct patterns of cerebral glucose uptake (Schröder et al 2001; Desgranges et al 2002). Recent studies have proven 18F-fluorodeoxyglucose positron emission tomography (FDG PET) to be a sensitive tool to distinguish manifest but also incipient AD from controls (de Leon et al 2001; Schröder et al 2001; Silverman et al 2001). A large European multi-center study of 395 patients with AD and 110 controls showed reduction of temporoparietal, frontal and posterior cingulate cortical glucose metabolism in AD compared with controls (Herholz et al 2002). Drzezga et al (2003) found in a study of 22 MCI patients reduced glucose metabolism in temporoparietal and cingulate cortices which extends at follow-up to lateral prefrontal areas in those who convert to AD. However, studies that investigated FDG PET in patients with AACD are warranted. In the present study, we measured CSF tau in a large group of AD patients, AACD patients, and controls, and investigated cerebral FDG PET in a subgroup.
Methods
Patients
175 patients and controls recruited consecutively through the Section for Geriatric Psychiatry at University Hospital Heidelberg participated in the study. 48 patients diagnosed with probable Alzheimer’s disease (NINCDSADRDA) criteria: McKhann et al 1984) and 88 patients with mild cognitive impairment who met the AACD criteria were included. AACD was diagnosed according to the criteria of the International Psychogeriatric Association working party including: (1) subjective impairment reported by the individual or a reliable informant; (2) objective impairment – difficulties in any of the following cognitive domains as indicated by a neuropsychological test performance of at least one standard deviation below age and education norms: memory and learning, attention and concentration, abstract thinking (problem solving, abstraction), language, and visuospatial functioning; and (3) exclusion criteria: none of the abnormalities listed above is of a sufficient degree for a diagnosis of dementia or can be attributed to a clinically significant psychiatric disorder (in particular depression, substance abuse, psychosis). Furthermore, there should be no objective evidence from physical and neurological examination or laboratory tests and no history of cerebral disease, damage, or dysfunction, or of systemic physical disorder known to cause cognitive dysfunction (Schönknecht et al 2005).
Clinical diagnosis was based on all relevant information including history, clinical examination, blood parameters (full blood count, blood chemistry, erythrocyte sedimentation rate, thyroid function tests), neuropsychological, and neuroradiological findings. Patients were excluded if they had evidence of stroke or other neurological disorder, severe uncontrolled diabetes, or uncontrolled hypertension. The modified Hachinski ischemic score of patients and controls was less than three (Loeb and Gandolfo 1983). Severity of dementia was rated on the Mini Mental State Examination Scale (MMSE) (Folstein et al 1975).
CSF tau protein acquisition
At baseline, in all patients lumbar puncture was performed at a fixed time of the day, between 10.00 and 12.00 hours, as part of the routine diagnostic procedure to exclude inflammatory disease. The resultant CSF samples were immediately aliquoted into nonabsorbent tubes, frozen at −80°C, and stored in polypropylene tubes until examination. Tau levels were determined using the Innogenetics INNOTEST-hTau-Ag-kit3 (Vandermeeren et al 1993). Investigators were blinded to diagnosis prior to the measurement of tau. The CSF tau control group consisted of 39 individuals without cognitive impairment, psychiatric or neurological disorder from whom CSF samples were obtained during spinal anesthesia at the Department of Anesthesia, University Hospital Heidelberg.
FDG PET study protocol
In 14 of AACD patients and 14 controls, a cerebral FDG PET was performed. The FDG PET control group comprised subjects without cognitive disorder who had presented to the memory clinic requesting neuropsychological work-up. The PET protocol has been described previously (Schönknecht et al 2003c). PET was performed after at least a 6-hour fast. Before injection of 225 MBq 18F-2-fluoro-2-deoxy-D-glucose, blood glucose levels were determined and were shown to be below 110 mg/dL in all patients. From 15 minutes before injection until 45 minutes after, patients rested in a quiet room with dimmed light. Patients were told to keep their eyes closed but their ears were left unplugged, in order to avoid frontal activation as reported in total sensory deprivation studies. Then, emission scans over 20 minutes were acquired, followed by the transmission scans over 5 minutes using three 68 Ge line sources. Measurements were obtained with a whole-body PET system (ECAT EXACT HR+, CTI, Knoxville, TN, USA), covering 155 mm in the axial field of view (63 transversal slices, thickness of each slice 2.4 mm). The scanner consists of four rings each with 72 bismuth germanate detector blocks. Data were acquired in the more sensitive 3D mode without inter-slice tungsten septa, which was found to be equivalent to the 2D mode for quantification of radioactivities used in the clinical setting. The matrix size was 128 × 128 pixels. In 3D mode, the transaxial resolution is between 4.1 and 4.8 mm. Iterative image reconstruction used the ordered subsets-expectation maximisation (OSEM) algorithm, implemented in the ECAT V7.1 software (Siemens Medical Systems Inc., Knoxville, TN, USA).
Image analysis and statistics
Basic image processing was done by MEDx 3.0 (Sensor Systems, Inc.) including statistical parametric mapping (SPM) routines (Wellcome Department of Cognitive Neurology, London; Friston et al 1995) on a Silicon Graphics station. All data were spatially normalized by affine 12-parameter transformation to standard stereotactic space using the Montreal Neurological Institute (MNI) template. Normalized images were represented on a 78 × 76 × 85 matrix and smoothed by a Gaussian filter of 12 mm full width at half maximum (FWHM). PET data were analyzed at a voxel level, where a t test is performed at each voxel and a map of the corresponding Z scores is pictured. The basic statistical model for the analyses was proportional scaling (p < 0.001, uncorrected for multiple comparisons). The cerebral structures were identified by their coordinates according to the Talairach atlas (Talairach and Tournoux 1988). Data were derived from the MNI template and were transformed to Talairach atlas coordinates using the appropriate algorithms (http://www.mrc-cbu.cam.ac.uk)
Statistical analyses
For data analysis, Pearson correlation coefficients, and analyses of variance with Duncan’s post hoc test were calculated. The study was approved by the Ethics Committee of the University of Heidelberg.
Results
Means and standard deviations of the clinical variables are given in Table 1. According to the results of a Duncan’s test at the 5% level, CSF tau protein concentrations were highest in the AD patients and differed significantly from those measured in patients with AACD. In the AD patients and the AACD patients, CSF tau protein levels were significantly higher than in controls. As expected, the lowest MMSE scores were observed in the AD patients followed by the AACD patients, and both groups had significantly lower scores than controls. AD patients were significantly older than both AACD and controls.
Table 1.
Clinical characteristics of patients and controls (n = 175) according to a Duncan’s test at the 5% level
| Controls | AACD | AD | Duncan’s test | |
|---|---|---|---|---|
| a | b | c | (5%) | |
| Patients | 39 | 88 | 48 | |
| Age at baseline (years) | 57.9 ± 15.1 | 66.9 ± 11.5 | 73.5 ± 8.8 | c > b > a |
| MMSE score at baseline | 29.3 ± 0.7 | 26.7 ± 2.0 | 8.5 ± 2.9 | c < b < a |
| CSF tau at baseline (pg/ml) | 223.8 ± 97.3 | 395.0 ± 223.8 | 646.2 ± 324.4 | c > b > a |
Abbreviations: AACD, aging-associated cognitive decline; AD, Alzheimer’s disease; CSF, cerebrospinal fluid; MMSE, Mini Mental State Examination.
According to the results of a discriminant function analysis, tau levels discriminated between AD patients and controls with a sensitivity of 68% and a specificity of 97% yielding a correct reclassification rate of 73%.
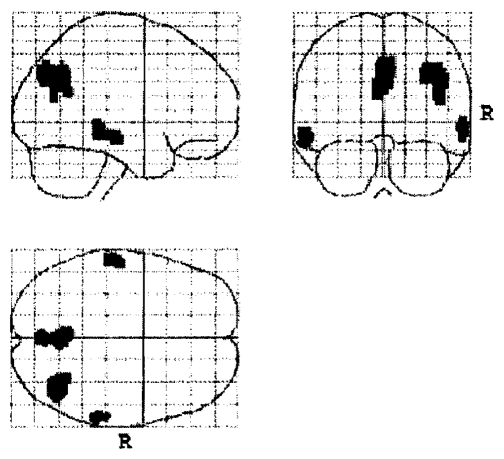
AACD patients who underwent FDG PET scanning at resting state at baseline had a significantly different pattern of cerebral glucose metabolism than the controls (Table 2). According to a SPM analysis in the AACD patients, a reduced baseline glucose metabolism occurred in the right precuneus, the right angular gyrus, the bilateral middle temporal gyri, and the left posterior cingulate cortex compared with controls (Figure 1).
Table 2.
Reduced cerebral glucose metabolism in AACD patients compared with controls at baseline
| Region | BA | x1 | y1 | z1 | Z score |
|---|---|---|---|---|---|
| R precuneus | 7 | 6 | −62 | 36 | 3.6 |
| R angular gyrus | 39 | 36 | −62 | 40 | 3.5 |
| R middle temporal gyrus | 22 | 64 | −34 | 0 | 3.3 |
| L middle temporal gyrus | 21 | −58 | −24 | −8 | 3.2 |
| R precuneus | 7 | 2 | −78 | 40 | 3.1 |
| L cingulate gyrus | 31 | −2 | −58 | 28 | 3.0 |
| R middle temporal gyrus | 39 | 46 | −68 | 24 | 2.9 |
| R angular gyrus | 39 | 48 | −66 | 32 | 2.8 |
x, y, z: Talairach coordinates of peaks.
Abbreviation: BA, Brodmann area.
Figure 1.
Spots indicating reduced cerebral glucose metabolism in aging-associated cognitive decline patients compared with controls.
Moreover, the AACD patients who underwent FDG PET scanning could be characterized by increased CSF tau protein levels (451 ± 171 pg/ml) that were similar to those found in the whole group of AACD patients.
Discussion
The study revealed two major findings. Firstly, patients with AD showed significantly increased CSF tau protein levels compared with AACD patients and controls. Secondly, AACD patients who underwent FDG PET scanning were characterized by significantly decreased temporal and cingulate cortex glucose metabolism similar to that found in patients with AD.
Our results confirm previous studies demonstrating increased total tau levels in AD patients compared with controls without cognitive deficits (Schönknecht et al 2004). The finding of significantly increased CSF tau protein baseline levels in AACD patients is in accordance with recent studies on CSF tau protein in MCI patients (Andreasen et al 2003; Herukka et al 2005). This finding fits with the hypothesis of a long and neuropsychologically stable period of cognitive impairment before the onset of AD (Schönknecht et al 2005). However, tau levels in the AD group showed a high variability. In a previous study, we could demonstrate that AD patients with tau levels below the 25%-percentile of the distribution were characterized by a significantly higher percentage of patients with presenile onset (Schönknecht et al 2003b).
Although all studies on CSF tau protein levels in AD agree with increased tau levels in AD compared with healthy controls, potential confounding factors have to be discussed. Since neuropsychiatric symptoms such as depressive symptoms and agitation are frequent in AD, one has to take into account potential psychotropic medication effects on tau protein release. In the past, medication effects were mainly investigated for beta amyloid 1–40 and 1–42 levels (Schröder et al 1997). However, recently we were able to exclude a correlation of CSF total tau levels, and dosage and type of psychotropic medication in patients with AD (Schönknecht et al 2003b).
Even if we could exclude vascular dementia (VaD) in the patients enrolled in our study, it seems pertinent to question the feasibility of separating AD from other dementias. In accordance with some (Arai et al 1998) but not all previous studies (Skoog et al 1995; Andreasen et al 1998; Tapiola et al 1998) we found previously significantly elevated tau levels in AD compared with VaD (Schönknecht et al 2003b). Discrepancies between studies may be explained by elevated tau levels in some of the investigated VaD patients following an acute damage to cerebral tissue induced by an ischemic event. The temporal relationship between an ischemic episode and the CSF examination could thus explain part of the variance of tau levels observed in VaD patients. This assumption is in line with the finding by Arai et al (1998) who observed tau levels in patients with acute cerebral infarction to be increased 2–3 weeks after the event, but to normalize several months later.
From the variability of tau levels in the AD patients it can be assumed that raised CSF tau supports the diagnosis of AD but normal values do not exclude it. Therefore, neuroimaging might provide an alternative method to distinguish potential preclinical stages of AD among AACD patients (Chetelat et al 2003). By using FDG PET as a functional neuroimaging method for reflecting reduced cerebral glucose uptake, Herholz et al (2002) found bilaterally reduced temporal glucose metabolism in patients with incipient AD compared with controls. Nestor et al (2003) demonstrated in patients with mild AD reduced glucose metabolism in posterior cingulate cortex but also in the hippocampus. Moreover, functional imaging studies demonstrate the role of precuneus and cingulate cortex in memory function (Cabeza and Nyberg 2000). Accordingly, in our study AACD patients showed reduced glucose metabolism compared with controls in right precuneus, right angular gyrus, bilateral middle temporal gyri, and left posterior cingulate gyrus. Further PET studies found the respective brain regions to be involved in manifest AD (Herholz et al 2002; Nestor et al 2003). Reduction of glucose metabolism in susceptible brain regions parallels the finding of early increased tau levels and suggests AACD as a pre-state of AD, confirming the findings of structural studies of reduced hippocampal volume in AACD (Pantel et al 2003) as well as findings of previous PET studies of reduced glucose metabolism in MCI patients who later convert to AD (Nestor et al 2003).
In conclusion, our findings reflect and confirm the clinical judgement of an incipient neurodegenerative disorder in a considerable portion of AACD patients. CSF tau levels and cerebral glucose metabolism distinguish controls from AACD and thus may facilitate early diagnosis of AD.
References
- Andreasen N, Vanmechelen E, Van de Voorde A, et al. Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer’s disease: a community follow up study. J Neurol Neurosurg Psychiatry. 1998;64:298–305. doi: 10.1136/jnnp.64.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N, Vanmechelen E, Vanderstichele H, et al. Cerebrospinal fluid levels of total-tau, p-tau and A beta 42 predicts development of Alzheimer’s disease in patients with mild cognitive impairment. Acta Neurol Scand. 2003;179(Suppl):47–51. doi: 10.1034/j.1600-0404.107.s179.9.x. [DOI] [PubMed] [Google Scholar]
- Arai H, Satoh-Nakagawa T, Higuchi M, et al. No increase in cerebrospinal fluid tau protein levels in patients with vascular dementia. Neurosci Lett. 1998;256:174–76. doi: 10.1016/s0304-3940(98)00781-2. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–13. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- Buerger K, Otto M, Teipel SJ, et al. Dissocoation between CSF total tau and tau protein phosphorylated at threonine 231 in Creutzfeldt-Jakob disease. Neurobiol Aging. 2006;27:10–15. doi: 10.1016/j.neurobiolaging.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II. An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette MD, et al. Mild Cognitive Impairment. Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–7. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- De Carli C. Mild cognitive impairment: clinical characterization and outcome. Lancet Neurol. 2003;2:15–21. [Google Scholar]
- De Leon MJ, Convit A, Wolf OT, et al. Prediction of cognitive decline in normal elderly subjects with 2-(18F) fluoro-2-deoxy-D-glucose/positron-emission-tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–71. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges B, Baron JC, Lalevee C, et al. The neural substrates of episodic memory impairment in Alzheimer’s disease as revealed by FDG-PET: relationship to degree of deterioration. Brain. 2002;125:1116–24. doi: 10.1093/brain/awf097. [DOI] [PubMed] [Google Scholar]
- Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–13. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, et al. The preclinical phase of Alzheimer disease. A 22-year prospective study of the Framingham cohort. Arch Neurol. 2000;57:808–13. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini Mental State’. A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer Dementia and Controls by automated analysis of Multicenter FDG PET. Neuroimage. 2002;17:302–16. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- Herukka SK, Hallikainen M, Soininen H, et al. CSF Aβ42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Neurology. 2005;64:1294–7. doi: 10.1212/01.WNL.0000156914.16988.56. [DOI] [PubMed] [Google Scholar]
- Loeb C, Gandolfo C. Diagnostic evaluation of degenerative and vascular dementia. Stroke. 1983;14:399–401. doi: 10.1161/01.str.14.3.399. [DOI] [PubMed] [Google Scholar]
- Levy R. Aging-associated cognitive decline. Int Psychogeriatr. 1994;6:63–8. [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–65. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Ikeda M, et al. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer’s disease) Eur J Neurosci. 2003;18:2663–7. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- Pantel J, Schröder J, Schad LR, et al. Quantitative magnetic resonance imaging and neuropsychological functions in dementia of the Alzheimer type. Psychol Med. 1997;27:221–9. doi: 10.1017/s003329179600431x. [DOI] [PubMed] [Google Scholar]
- Pantel J, Schönknecht P, Essig M, et al. Progressive medial temporal lobe changes in Alzheimer’s disease revealed by quantitative MRI – potential use for the monitoring of drug-related changes. Drug Dev Res. 2002;56:51–6. [Google Scholar]
- Pantel J, Kratz B, Essig M, et al. Parahippocampal volume deficits in subjects with aging-associated cognitive decline. Am J Psychiatry. 2003;160:379–82. doi: 10.1176/appi.ajp.160.2.379. [DOI] [PubMed] [Google Scholar]
- Petersen CP, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Arteron S, Touchon J. Classification criteria for mild cognitive impairment - a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- Schönknecht P, Pantel J, Hunt A, et al. Tau protein phosphorylated at threonine 181 is increased in incipient and manifest Alzheimer’s disease but not in vascular dementia. Neurosci Lett. 2003a;339:172–4. doi: 10.1016/s0304-3940(02)01481-7. [DOI] [PubMed] [Google Scholar]
- Schönknecht P, Henze M, Hunt A, et al. Hippocampal glucose metabolism is associated with cerebrospinal fluid estrogen levels in postmenopausal women with Alzheimer’s Disease. Psychiatry Res. 2003c;124:125–7. [PubMed] [Google Scholar]
- Schönknecht P, Pantel J, Hartmann T, et al. Cerebrospinal fluid tau levels in Alzheimer’s disease are elevated when compared with vascular dementia but do not correlate with measures of cerebral atrophy. Psychiatry Res. 2003b;120:231–8. doi: 10.1016/s0165-1781(03)00197-5. [DOI] [PubMed] [Google Scholar]
- Schönknecht P, Pantel J, Kaiser E, et al. Total and phosphotau (Thr 181) CSF levels in patients with mild cognitive impairment and Alzheimer’s disease. Polish J Old Age Psych. 2004;1:185–92. [Google Scholar]
- Schönknecht P, Pantel J, Kruse A, et al. Prevalence and natural course of aging-associated cognitive decline in a population based sample of “young-old” subjects. Am J Psychiatry. 2005;162:2071–7. doi: 10.1176/appi.ajp.162.11.2071. [DOI] [PubMed] [Google Scholar]
- Schröder J, Buchsbaum MS, Shihabuddin L, et al. Patterns of cortical activity and memory performance in Alzheimer’s disease. Biol Psychiatry. 2001;49:426–36. doi: 10.1016/s0006-3223(00)00983-5. [DOI] [PubMed] [Google Scholar]
- Schröder J, Kratz B, Pantel J, et al. Prevalence of mild cognitive impairment in a community sample. J Neural Transm Suppl. 1998;54:51–9. doi: 10.1007/978-3-7091-7508-8_5. [DOI] [PubMed] [Google Scholar]
- Schröder J, Pantel J, Ida N, et al. Cerebral changes and cerebrospinal fluid β-amyloid in Alzheimer’s disease: a study with quantitative magnetic resonance imaging. Mol Psychiatry. 1997;2:505–7. doi: 10.1038/sj.mp.4000313. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. J Am Med Assoc. 2001;286:2110–7. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- Skoog I, Vanmechelen E, Andreasson LA, et al. A population-based study of tau protein and ubiquitin in cerebrospinal fluid in 85-year-olds: relation to severity of dementia and cerebral atrophy, but not to the apolipoprotein E4 allele. Neurodegen. 1995;4:433–42. doi: 10.1006/neur.1995.0052. [DOI] [PubMed] [Google Scholar]
- Talairach JA, Tournoux P. Co-planar stereotaxic atlas of the human brain. Paris: Thieme; 1988. [Google Scholar]
- Tapiola T, Lethovirta M, Ramberg J, et al. CSF tau is related to apolipoprotein E genotype in early Alzheimer’s disease. Neurology. 1998;50:169–74. doi: 10.1212/wnl.50.1.169. [DOI] [PubMed] [Google Scholar]
- Vandermeeren M, Mercken M, Vanmechelen E, et al. Detection of tau proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem. 1993;61:1828–34. doi: 10.1111/j.1471-4159.1993.tb09823.x. [DOI] [PubMed] [Google Scholar]