Abstract
Objectives
Experimental models are of importance to study the pathogenesis of middle ear cholesteatoma, however, they were not established until now. We aimed to develop in vitro model of middle ear cholesteatoma using primary keratinocytes and fibroblasts isolated from cholesteatoma tissue. HaCaT cell line was used as a "skin equivalent" and to compare the grade of homogeneity between cholesteatoma keratinocytes and HaCaT cells.
Methods
Primary keratinocytes were isolated from cholesteatoma tissue, co-cultured with preliminary prepared feeder layer from cholesteatoma fibroblasts and subsequently air-exposed. The protein profile of cholesteatoma keratinocytes and HaCaT cells was evaluated by means of immunoblot using monoclonal antibody against cytokeratin (CK) 13 and 16. Tissue localization of CK 13 and 16 was accomplished with immunohistochemistry.
Results
Different protein profile and stronger expression of CK 13 and 16 were demonstrated in cholesteatoma keratinocytes in comparison with HaCaT cells. Bigger stratification was observed in the 3D-in vitro systems when both cholesteatoma keratinocytes and HaCaT cells were respectively co-cultured with fibroblasts in comparison with the corresponding control groups without fibroblasts.
Conclusion
3D-model demonstrates the significance of intercellular interaction between components of cholesteatoma tissue.
Keywords: Cholesteatoma, In vitro, Keratinocytes, Culture
INTRODUCTION
Middle ear cholesteatoma is characterized by keratinocytic hyperproliferation with bony destruction and granulation tissue by chronic inflammation. The pathogenesis of middle ear cholesteatoma has been debated for a century, but there are four basic theories: invagination, basal cell hyperplasia, epithelial ingrowth and squamous metaplasia.
During last century, etiopathogenicity of middle ear cholesteatoma raised many controversies and still a unanimous concept doesn't exist. From a histological point of view this pathological tissue is composed of 2 integrated parts - matrix and perimatrix. The interaction between cellular components is essential for the major histopathological features of cholesteatoma - hyperproliferation and bone destruction. In 1964, the American anatomist Gray referred to middle ear cholesteatoma as "skin in the wrong place" (1). This simplified misdefinition stuck into literature and has been cited profusely in many scientific papers. The metaplasia theory (2) and existence of so-called "congenital" cholesteatoma put into question the "skin origin" of middle ear cholesteatoma and gave etiopathogenetical evidences against the simplified definition "skin in the wrong place". The list of histological differences includes the absence of cutaneous adnexa and specialized dermal structures. Neither hypodermal structures, nor blood vessels, or nerve endings have been ever described in cholesteatoma. The only similarity that is left between them is the presence of multistratified squamous epithelium, but in cholesteatoma this epithelium is with hyperproliferative properties.
Experimental models are critical for studying pathogenesis of middle ear cholesteatoma. In vivo models were tried to establish by chemical injection into the middle ear (3), ligation of external auditory canal (4) and transplantation of skin (5). Keratinocytes of middle ear cholesteatoma were cultured by tissue culture (6), passaged subculture (7) and air-liquid interface system (8). However, stable experimental models of middle ear cholesteatoma are not established until now. Our study has aimed to create an "adequate" in vitro model, based on the cell-to-cell interaction between keratinocytes and fibroblasts and to assess the grade of homogeneity between cholesteatoma and skin keratinocytes by focusing on the cytokeratin profile.
MATERIALS AND METHODS
Cell culture
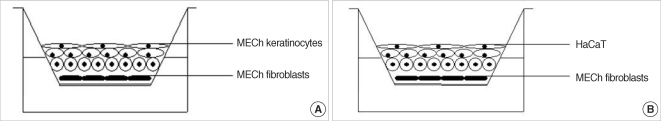
Enzymatic disaggregation protocol for simultaneous isolation of primary keratinocytes and fibroblasts from cholesteatoma tissue has been developed. The biopsy samples were harvested from patients undergoing surgical treatment in the Department of Otolaryngology, Ajou University Hospital, Korea. An informed consent form approved by the Institutional Review Board of Ajou University Hospital was signed by each patient. Cholesteatoma keratinocytes were cultured in Defined Keratinocytes Serum Free Medium (GIBCO, BRL, Gaithersburg, MD, USA) supplied with growth supplement and 5 mg/mL Gentamicin (GIBCO, BRL) according to the manufacture instructions. Cholesteatoma primary fibroblasts were successfully cultured in Dulbeco Modified Eagle Medium (GIBCO, BRL) supplemented with 10% Fetal Bovine Serum (FBS, Clonetics, Walkersville, MD, USA) and 1% Penicillin-Streptomycin mixture (GIBCO, BRL). Primary keratinocytes (passage 1) and fibroblasts (between passages 1-5) were used for establishment of the co-cultured system. HaCaT cell line was kindly provided from the Department of Dermatology in Ajou University Hospital. Feeder layer from cholesteatoma fibroblasts was developed using pretreatment with Mitomycin C (Sigma, St. Louis, MO, USA) in concentration 0.25 g/mL. Fibroblasts were incubated overnight at 37℃, 5% CO2 in humidified atmosphere. On the next day the media was exchanged and the cells were incubated additionally for another 24 hr. The feeder layer was subsequently trypsinized from the substrate and transferred on polyester membrane in Transwell (Costar, Cambridge, MA, USA) system to develop a subconfluent feeder layer. Feeder layer was incubated for 24-48 hr prior co-culturing. Cholesteatoma keratinocytes and HaCaT cells were seeded at density 2×105 cells/mL over the feeder layer, co-cultured for 3-5 days and air-exposed for a period of 14 days. Two alternative models were elaborated using primary cholesteatoma keratinocytes and HaCaT cells (Fig. 1). The control group was prepared without feeder layer.
Fig. 1.
Schematic illustrations of air-liquid interface system. (A) Middle ear cholesteatoma (MECh) keratinocytes are co-cultured with fibroblast feeder layer. (B) Alternative model with HaCaT cells is used as a control group. Upper cellular surface is exposed to the air, and the media are supported through the lower semipermeable membrane. This system induces polarity and differentiation.
Morphological study
Transwell membranes were fixed in 4% formaldehyde overnight at 4℃, dehydrated in graded alcohols, cleared in HistoClear (National diagnostics, Atlanta, Georgia) and embedded in paraffin. Six µm-thick sections were prepared and subjected to routine hematoxylin-eosin staining.
Protein profile
Polyacrylamide gel electrophoresis has been used for protein separation and staining with Coomassie blue was performed to compare the protein profile between cholesteatoma keratinocytes and HaCaT cells.
Cytokeratin profile
After separation, the proteins were transferred to a nitrocellulose membrane. Blocking of nonspecific staining was accomplished with membrane incubation with 5% non-fat skim milk for 1 hr at room temperature (RT). Membranes were then incubated with primary mouse monoclonal anti-cytokeratin antibody clone K 8.12 (Sigma) which is immunospecific for cytokeratin 13 (CK 13) and cytokeratin 16 (CK 16), with dilution factor 1:100, for 1 hr at RT. After washing membranes were incubated with goat anti-mouse horseradish peroxidase conjugate (Zymed, San Francisco, CA, USA). The positive reaction was visualized with ECL Western Blotting Detection System (Amersham, Bucks, UK).
Immunohistochemistry (IHC)
The localization of cytokeratin 13 and 16 in middle ear cholesteatoma tissue was accomplished by IHC study as described before (9). Five middle ear cholesteatoma samples were compared with the corresponding retroauricular skin samples obtained during surgery. Six µm thick formalin-fixed paraffin-embedded tissue sections were processed with ABC-method. Samples were deparaffinized in xylene and rehydrated in alcohol. Heat-induced antigen retrieval procedure was applied for antigen unmasking. Primary mouse monoclonal anticytokeratin antibody (Sigma) at concentration 1:20 was incubated overnight at RT. Vectorlab Elite ABC kit (Vector Laboratories, Burlingame, CA, USA ) was used for detection of positive reaction using diaminobenzidine substrate.
RESULTS
Cell culture
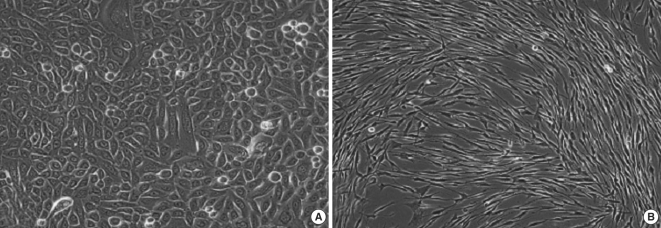
Enzymatic disaggregation protocol yield a high number of separated cells. The low selectivity and potential damages of cells in case of prolonged incubation were major disadvantages of the procedure. As an inflammatory tissue, cholesteatoma posed some difficulties in isolation of primary keratinocytes, because of the attendant inflammation and possible contamination, which requires an adequate decontamination. The first primary keratinocytes appeared usually 7-10 days after trypsinization and generally within the next 10 days keratinocytes reached a confluence. The individual variations in proliferative activity of cholesteatoma keratinocytes were related to cholesteatoma localization and sex of the patient. For instance, attic cholesteatoma frequently resulted in isolation of primary keratinocytes with bigger proliferative ability. Another interesting correlation was the fact that cholesteatoma samples obtained from young female patients were characterized by higher proliferative activity in vitro. The above-mentioned dependencies were not statistically significant. Primary cholesteatoma keratinocytes were uniform in shape and arranged in a typical monolayer pattern (Fig. 2A). In passage 2 an abrupt deceleration in proliferation was established, accompanied by morphological alterations such as enlargement and flattening of the cells, as well as vacuolization of the cytoplasm. In passage 3 cells were practically incapable of proliferation. Primary cholesteatoma fibroblasts were steadily maintained without significant changes of doubling time until passage 10. Primary cholesteatoma fibroblasts displayed typical spindle-shaped morphology upon confluence (Fig. 2B). No spontaneous transformation was detected in any of the cultures.
Fig. 2.
(A) Confluent monolayer of primary middle ear cholesteatoma (passage I) keratinocytes shows a polygonal appearance. (B) Primary middle ear cholesteatoma fibroblasts show typical spindle-shaped morphology (passage 1) upon reaching a confluence. Original magnification ×400.
Air-liquid interface (ALI)
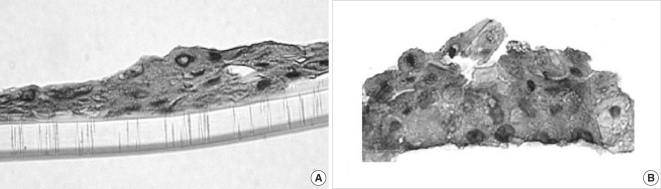
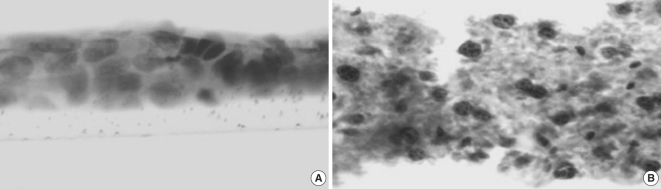
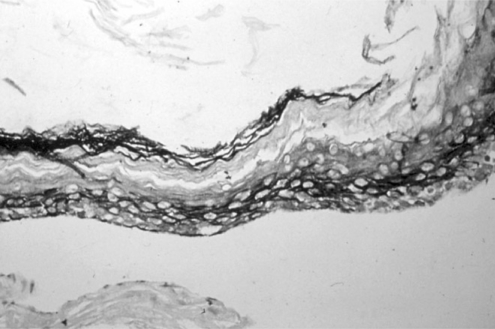
Bigger stratification and proliferation have been observed in the proposed 3D in vitro system when primary cholesteatoma keratinocytes were co-cultured in the presence of fibroblasts (Fig. 3B) in comparison with the control group without fibroblasts (Fig. 3A). Similar morphological landmarks were established in the control group of HaCaT cells (Fig. 4A). Disrupted differentiation in the suprabasal layers was revealed when cholesteatoma keratinocytes were air-exposed in the presence of fibroblasts mimicking the parakeratosis state, which is typical for this pathological tissue in vivo (Fig. 4B).
Fig. 3.
Light microscopic observation on 3D-in vitro middle ear cholesteatoma model developed without fibroblasts (A) and in the co-cultured system with middle ear cholesteatoma fibroblasts (B). Middle ear cholesteatoma keratinocytes show bigger stratification and increased cellular size in the presence of fibroblasts. Hematoxylin-eosin staining, Original magnification ×400.
Fig. 4.
Light microscopic observation on the alternative 3D-in vitro model utilizing HaCaT cells cultured without fibroblasts (A) and air-exposed co-cultured system in the presence of middle ear cholesteatoma fibroblasts (B). HaCaT cells show bigger stratification when co-cultured with fibroblasts. Hematoxylin-eosin staining, Original magnification ×400.
Protein and cytokeratin profile
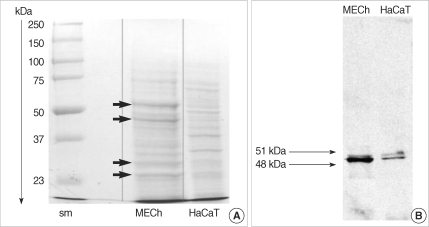
Coomassie blue staining of the protein extracts from primary cholesteatoma keratinocytes and HaCaT cells revealed significantly different protein profiles (Fig. 5A). Stronger expression of CK 16 (48 kDa) and CK 13 (51 kDa) was established in cholesteatoma keratinocytes in comparison with HaCaT cells (Fig. 5B).
Fig. 5.
Comparative study on total protein profile (A) and cytokeratin profile (B) between primary middle ear cholesteatoma (MECh) keratinocytes and HaCaT cells. Middle ear cholesteatoma keratinocytes show different protein profile (arrows) in comparison with HaCaT cells. Blotting with anti-cytokeratin antibody shows the expression of cytokeratin 13 and 16 in both middle ear cholesteatoma keratinocytes and HaCaT cells.
Immunohistochemistry
Using mouse monoclonal antibody clone K 8.12, tissue localization of CK 13 and CK 16 was verified both in the basal and suprabasal layers of cholesteatoma (Fig. 6). The described staining patterns were established in all 5 cholesteatoma samples. The intensity of the CK 13, 16-positive staining varied in different areas of the same cholesteatoma sample. Specific positive reaction was not detected in retroauricular skin samples used as a control. The positive control revealed specific expression of CK 13 and 16 confined to the islands of neoplastic squamous cells in laryngeal carcinoma specimens.
Fig. 6.
Immunohistochemical observation on cytokeratin 13 and 16 expression in middle ear cholesteatoma tissue. Cytokeratin 13 and 16 are markers of active proliferative keratinocytes. Immunolabeling shows the expression of them in the whole layer of middle ear cholesteatoma.
DISCUSSION
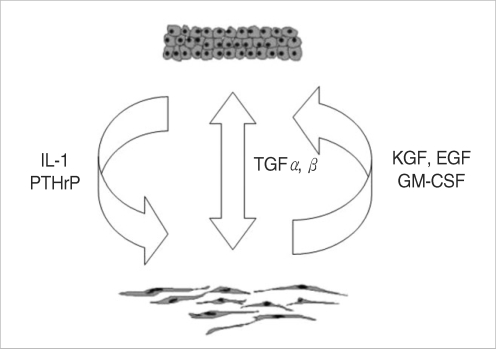
The putative interactions between major cellular components of cholesteatoma tissue have been extensively studied in the literatures. We demonstrate a summarized schematic model of possible intercellular "cross-talking" between epithelial-mesenchymal components of cholesteatoma in Fig. 7. Both paracrine and autocrine regulatory mechanisms have been proposed for maintenance of tissue homeostasis. Pro-inflammatory cytokines such as interleukin-1α and interleukin-1β, (IL-1α, IL-1β) released from matrix keratinocytes stimulate keratinocytes growth factor (KGF) expression, which in turn induced proliferation of the cholesteatoma keratinocytes (10). Granulocyte-macrophage colony stimulating factor (GM-CSF) is another fibroblasts-derived growth factor induced by IL-1, with acknowledged stimulating effects both on proliferation and terminal differentiation of keratinocytes (11). Keratinocytes-derived parathyroid hormone-related protein (PTHrP) induces expression of KGF. KGF is then released by perimatrix fibroblasts and accelerates the proliferative response in matrix keratinocytes. A possible connection between PTHrP and bone destructive lesions have also been reported (12). Transforming growth factor alpha (TGF-α) is constitutively expressed in keratinocytes and regulates keratinocyte proliferation and differentiation in an autocrine manner (13). On the other hand, a possible synergism between TGF-α and epidermal growth factor (EGF) was proposed with possible effect on keratinocytes proliferation through epidermal growth factor receptor (EGF-R), indicating existence of an alternative paracrine regulatory mechanism (14). Transforming growth factor-beta (TGF-β) is expressed in hyperproliferative epithelium and granulation tissue (15) and influences the epithelial morphogenesis both in an autocrine and paracrine fashion.
Fig. 7.
Summarized schematic model of putative autocrine and paracrine regulatory mechanisms, responsible for maintaining of middle ear cholesteatoma tissue homeostasis. IL: interleukin; PTHrP: parathyroid hormone-related protein; TGF: transforming growth factor; KGF: keratinocytes growth factor; EGF: epidermal growth factor; GM-CSF: granulocyte-macrophage colony stimulating factor.
The above statements about cellular interactions were taken into consideration for creating an adequate in vitro middle ear cholesteatoma model. As it was mentioned before, the proliferative capacity of primary cholesteatoma keratinocytes in vitro is limited and expires within 2 passages. To improve their plating efficiency and survival rate at low density we create a growth-arrested feeder layer from primary middle ear cholesteatoma fibroblasts. 3T3-mouse embryo fibroblasts that used routinely in cell culture practice display different morphological patterns (cobble-stone morphology), which differs from in vivo state and complicates result interpretation. Feeder layer secretes nutrients, growth factors and matrix constituents. As a positive control in our study we choose HaCaT cell line in order to avoid donor-to-donor and site-to-site variations in proliferative capacity of normal skin keratinocytes. HaCaT cells are spontaneously immortalized human adult skin keratinocytes raised in reduced calcium level and elevated temperature conditions (16). HaCaT found an extensive application in tissue engineering for development of artificial skin equivalents (17). The proposed organotypic in vitro model revealed stronger proliferation and bigger stratification, when HaCaT cells were co-cultured with fibroblasts, in comparison with the control group without fibroblasts. Similar patterns have been established with primary cholesteatoma keratinocytes. Histopathological diagnosis of cholesteatoma includes: unrestrained proliferation, uncoordinated differentiation, migration, and invasion (18). Consistent with the above-mentioned observation, the proposed 3D-model disclosed disrupted differentiation and presence of nucleated cells in the upper layers of the cholesteatoma ALI with fibroblasts. The following data corresponds to the parakeratotic properties in cholesteatoma tissue in vivo.
Squamous metaplasia of middle ear epithelium is considered to be an adaptive tissue response towards persistent pathological stimuli such as chronic inflammation and possible etiopathogenetical mechanism for middle ear cholesteatoma. An experiment has been reported on the confirmation of a possible transformation of differentiated middle ear epithelium towards functionally inferior multilayered squamous epithelium (19). Epithelial cells are characterized by the presence of a specific set of intermediate protein filaments named cytokeratins, specific for different epithelial types. One of our primary objectives was to assess the grade of homogeneity between HaCaT cells and cholesteatoma keratinocyte. CK 16 was suggested in the literature as a proliferation marker, while CK 13 is considered as a marker of differentiation (20). Immunohistochemical investigation in our study disclosed non-uniform patterns of expression of CK 13 and CK 16 in middle ear cholesteatoma tissue. Variation in intensity of the positively stained areas between different cholesteatoma samples as well as between different areas in the same sample was probably related to the different proliferative capacity of cholesteatoma. No specific staining was detected in the retroauricular skin used as a control. The expression of nonepidermal cytokeratins in middle ear cholesteatoma tissue gives arguments against the probable skin origin of MECh keratinocytes. Protein separation and Coomassie blue staining revealed significantly different protein profile between cholesteatoma and HaCaT cells. Precised Western-blotting analysis demonstrated comparatively stronger expression of CK 13 and 16 in cholesteatoma keratinocytes with regard to HaCaT cell line.
CONCLUSIONS
The different protein and cytokeratin profiles established between cholesteatoma keratinocytes and HaCaT cells favors metaplasia as a possible cause for development of MECh. The proposed in vitro model underlines the importance of fibroblasts as a major component of 3D-in vitro system. Our results emphasize the integrity of cholesteatoma as a separated pathological tissue, composed of two closely related functional and structural parts: the matrix and the perimatrix.
Footnotes
This study was presented at the 8th International Conference on Cholesteatoma and Ear Surgery which was held in Antalya, Turkey through June 15-20, 2009, and received the cholesteatma prize.
References
- 1.Gray JD. The chronic ear: the treatment of cholesteatoma in children. Proc R Soc Med. 1964 Sep;57:769–771. [PMC free article] [PubMed] [Google Scholar]
- 2.Sadé J, Babiacki A, Pinkus G. The metaplastic and congenital origin of cholesteatoma. Acta Otolaryngol. 1983 Jul–Aug;96(1-2):119–129. doi: 10.3109/00016488309132882. [DOI] [PubMed] [Google Scholar]
- 3.Vassalli L, Harris DM, Gradini R, Applebaum EL. Propylene glycol-induced cholesteatoma in chinchilla middle ears. Am J Otolaryngol. 1988 Jul–Aug;9(4):180–188. doi: 10.1016/s0196-0709(88)80026-7. [DOI] [PubMed] [Google Scholar]
- 4.McGinn MD, Chole RA, Henry KR. Cholesteatoma. Experimental induction in the Mongolian Gerbil, Meriones Unguiculaus. Acta Otolaryngol. 1982 Jan–Feb;93(1-2):61–67. doi: 10.3109/00016488209130853. [DOI] [PubMed] [Google Scholar]
- 5.Park K, Chun YM, Kim SM, Lee DH. Experimental cholesteatoma by transplanting a free skin graft in the bulla of Mongolian gerbil. Korean J Otolaryngol - Head Neck Surg. 1996 Jul;39(7):1138–1143. [Google Scholar]
- 6.Proops DW, Parkinson EK. Tissue culture of human cholesteatomatous keratinocytes. Clin Otolaryngol Allied Sci. 1983 Jun;8(3):165–170. doi: 10.1111/j.1365-2273.1983.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheshire IM, Blight A, Proops DW. An in vitro growth study on cholesteatoma and normal skin. Clin Otolaryngol Allied Sci. 1995 Oct;20(5):453–460. doi: 10.1111/j.1365-2273.1995.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 8.Albers-op t' Hof BM, Peek FA, Huisman MA, Grote JJ. Air-exposed tissue culture of human middle ear epithelium and meatal epidermis: a method to study the advancing front of cholesteatoma. Acta Otolaryngol. 2002 Oct;122(7):720–725. doi: 10.1080/00016480260349782. [DOI] [PubMed] [Google Scholar]
- 9.Park K, Park HJ, Chun YM. Immunohistochemical study of cytokeratin expression in experimental cholesteatoma. In: Lim DJ, Casselbrant M, Klein JO, Ogra PL, editors. Recent advances in otitis media, Sixth International Symposium. Hamilton: B.C. Decker Inc.; 1996. pp. 275–277. [Google Scholar]
- 10.Tanaka Y, Kojima H, Miyazaki H, Koga T, Moriyama H. Roles of cytokines and cell cycle regulating substances in proliferation of cholesteatoma epithelium. Laryngoscope. 1999 Jul;109(7 Pt 1):1102–1107. doi: 10.1097/00005537-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Huang T, Yan SD, Huang CC. Colony-stimulating factor in middle ear cholesteatoma. Am J Otolaryngol. 1989 Nov–Dec;10(6):393–398. doi: 10.1016/0196-0709(89)90034-3. [DOI] [PubMed] [Google Scholar]
- 12.Cheshire IM, Blight A, Ratcliffe WA, Proops DW, Heath DA. Production of parathyroid-hormone-related protein by cholesteatoma cells in culture. Lancet. 1991 Oct;338(8774):1041–1043. doi: 10.1016/0140-6736(91)91902-7. [DOI] [PubMed] [Google Scholar]
- 13.Schulz P, Bujía J, Holly A, Shilling V, Kastenbauer E. Possible autocrine growth stimulation of cholesteatoma epithelium by transforming growth factor alpha. Am J Otolaryngol. 1993 Mar–Apr;14(2):82–87. doi: 10.1016/0196-0709(93)90044-8. [DOI] [PubMed] [Google Scholar]
- 14.Ergün S, Zheng X, Carlsöö B. Expression of transforming growth factor-alpha and epidermal growth factor receptor in middle ear cholesteatoma. Am J Otol. 1996 May;17(3):393–396. [PubMed] [Google Scholar]
- 15.Lang S, Schilling V, Wollenberg B, Mack B, Nerlich A. Localization of transforming growth factor-beta-expressing cells and comparison with major extracellular components in aural cholesteatoma. Ann Otol Rhinol Laryngol. 1997 Aug;106(8):669–673. doi: 10.1177/000348949710600810. [DOI] [PubMed] [Google Scholar]
- 16.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boelsma E, Verhoeven MC, Ponec M. Reconstruction of a human skin equivalent using a spontaneously transformed keratinocyte cell line (HaCaT) J Invest Dermatol. 1999 Apr;112(4):489–498. doi: 10.1046/j.1523-1747.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 18.Choufani G, Mahillon V, Decaestecker C, Lequeux T, Danguy A, Salmon I, et al. Determination of the levels of expression of sarcolectin and calcyclin and of the percentages of apoptotic but not proliferating cells to enable distinction between recurrent and nonrecurrent cholesteatomas. Laryngoscope. 1999 Nov;109(11):1825–1831. doi: 10.1097/00005537-199911000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Kuijpers W, Vennix PP, Peters TA, Ramaekers FC. Squamous metaplasia of the middle ear epithelium. Acta Otolaryngol. 1996 Mar;116(2):293–298. doi: 10.3109/00016489609137844. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki H, Huang CC. Expression of cytokeratins 13 and 16 in middle ear cholesteatoma. Otolaryngol Head Neck Surg. 1994 Mar;110(3):310–317. doi: 10.1177/019459989411000309. [DOI] [PubMed] [Google Scholar]