Abstract
The large intestine is a major site of infection and disease yet little is known about how immunity is initiated within this site and the role of dendritic cells (DCs) in this process. We used the well-established model of Trichuris muris infection to investigate the innate response of colonic DCs in mice that are inherently resistant or susceptible to infection. One day post-infection, there was a significant increase in the number of immature colonic DCs in resistant but not susceptible mice. This increase was sustained at day 7 post-infection in resistant mice when the majority of the DCs were mature. There was no increase in DC numbers in susceptible mice until day 13 post-infection. In resistant mice, most colonic DCs were located in or adjacent to the epithelium post-infection. There were also marked differences in the expression of colonic epithelial chemokines in resistant mice and susceptible mice. Resistant mice had significantly increased levels of epithelium-derived CCL2, CCL3, CCL5 and CCL20 compared with susceptible mice. Furthermore, administering neutralizing CCL5 and CCL20 antibodies to resistant mice prevented DC recruitment. This study provides clear evidence of differences in the kinetics of DC responses in hosts inherently resistant and susceptible to infection. DC responses in the colon correlate with resistance to infection. Differences in the production of DC chemotactic chemokines by colonic epithelial cells in response to infection in resistant versus susceptible mice may explain the different kinetics of the DC response.
Keywords: Rodents, dendritic cells, parasite-helminth, mucosa
Introduction
Although diseases of the large intestine are amongst the most prevalent in the world, surprisingly little is known about how immune responses are initiated, activated and regulated in the large intestine. The epithelium lining the large intestine is comprised of a single layer of cells that act as a physical barrier between commensal bacteria in the gut lumen and the immune cells in the underlying lamina propria. The interaction between the gut flora and the epithelium is complex and important for epithelial homeostasis (1, 2). Colonic epithelial cells can actively sense and respond to commensal bacteria microbiota and pathogens via expression of pattern recognition receptors (PRRs) (3, 4). Ligation of PRRs in epithelial cells drives secretion of effector molecules such as chemokines and cytokines (3, 4) which have the potential to recruit and activate immune cells including dendritic cells (DCs) from the colonic lamina propria to the epithelial layer.
DCs direct immune responses by responding to pathogen and directing the appropriate T cell responses. DCs activate and direct T cell bias (Th1, Th2) and the type of immune response (inflammation versus tolerance) but little is known about how DCs function in the tissue microenvironment of the large intestine. This contrasts with the considerable body of literature on tissue-resident DCs in other regions of the gastrointestinal tract including the small intestine (5-7). Whilst it is possible that there are some commonalities between mucosal responses and DC function in the small and large intestine, this seems unlikely given the clear differences in their anatomy, physiology and function. For example, the large intestine has a distinct and more complex microbiota several magnitudes greater in number than that of the small intestine. In the small intestine, DCs are found primarily in two compartments; the Peyer’s patches and the lamina propria. The colon lacks conventional Peyer’s patches and has few M cells although structures such as colonic patches and isolated lymphoid follicles may be analogous to Peyer’s patches and represent primary sites of antigen uptake in the colon (8). Within the Peyer’s patches, DCs access luminal antigens indirectly via specialised intestinal epithelial M cells (5-7) whereas in the lamina propria, CX3CR1 expressing DC may directly sample lumen antigens via the extension of transepithelial dendrites (9, 10). DCs with transepithelial dendrites are not found constitutively in the terminal ileum. However, they can be induced in response to infection (11). In contrast there are few DCs in the naïve large intestine (12). Also, the ability of colonic DCs to extend dendrites into the intestinal lumen has not been demonstrated for colonic DCs suggesting that this may be a rare or non-event (13). The importance of colonic DCs is however demonstrated in infection in which clearance of bacteria is dependent upon caecal DCs (13). There is therefore significant regional diversity in DC subset and function and most likely in epithelial/DC crosstalk throughout the gastrointestinal tract.
The enteric pathogen Trichuris muris has been used as a model large intestinal infection to address fundamental questions about how immune responses are initiated in the colon and caecum. T.muris specifically invades colonic and caecal epithelial cells within the first 24 h of ingesting embryonated eggs (14, 15) eliciting either a Th1 (susceptible) or Th2 (resistant) response depending upon the level of infection or strain of host (14). Here we compared the magnitude and kinetics of DC responses in mice resistant or susceptible to T. muris and present evidence for differences in the kinetics and magnitude of the DC response that distinguish between susceptible and resistant animals.
Materials and Methods
Mice and Infections
BALB/c and AKR mice (Harlan UK, Bicester, UK) were infected orally with 150 embryonated eggs or 20 eggs (low dose) at 6-8 weeks (14, 16). BALB/c given a high dose of T. muris are resistant to infection and termed HD BALB/c or resistant BALB/c throughout this paper. BALB/c given a low dose of T.muris eggs are susceptible to infection and termed LD BALB/c or susceptible BALB/c throughout this paper. AKR are referred to as susceptible AKR throughout. At least 3 mice were used per time-point studied. Worm burden counts (D10-14 post-infection) taken from each series of infections. For each series of infections, AKR and BALB/c mice were analysed simultaneously to reduce intra-experimental variation. In a series of infections BALB/c mice (n=16) were infected with 150-200 embryonated eggs prior to intravenous injection with either 100μg/mouse Rat IgG (R&D Systems, Abingdon, UK (n=8)) or 100μg/mouse CCL5 and CCL20 (R&D systems) neutralising antibodies (n=8).
Flow Cytometry
DC-enriched preparations of colonic lamina propria mononuclear cells (12, 17) were stained with anti-CD11c, CD45, CD80, CD86, MHC II, CD11b, CD8α, CCR7, CCR5, CD103 (Becton Dickinson), TLR2, TLR4, CCR2 and CCR5 (provided by Matthias Mack), CCR6 (Insight Biotechnology Ltd, Wembley, UK) and PDCA (Miltenyi Biotech Inc, Bisley, Surrey, UK) antibodies. Isotype matched antibodies of irrelevant specificity were used to determine the level of non-specific staining. Stained cells were analysed on a FACSCalibur™ flow cytometer using CellQuest software (BD). Endocytosis was assessed by measuring the uptake of FITC-dextran (Sigma, Dorset, UK) by flow cytometry as described previously (12).
Immunohistochemistry
Frozen sections (5-20μm) of caecum and proximal colon were air dried, fixed in ice-cold acetone, rehydrated in PBS and incubated with anti-CD11c, -MAC-1, -CD4, -B220, -CD8α, −F4/80 (Becton Dickinson, Oxford, UK), -cytokeratin (Sigma, Poole, Dorset, UK), -claudin 3 (Panomics, Redwood City, CA), -occludin (Zymed Laboratories, San Francisco, CA), -Fractalkine receptor (Cambridge Bioscience, UK), laminin (Abcam, Cambridge, UK) antibodies followed by tyramide amplification reagents (Perkin Elmer, Berkshire, UK) (12) or secondary antibodies conjugated to Texas Red (Abcam) or AF633 (Molecular Probes). Sections were counterstained with the nuclear counterstains, DAPI or TOPRO3 (Molecular Probes), mounted with Fluoromount G (Southern Biotechnology Associates) and viewed using a Zeiss Axiovert 200M microscope (Zeiss, Welwyn Garden City, UK) with Axiovision software (Figure 2A-F, Figure 2 J) or a Zeiss upright LSM 510 META confocal microscope (Figure 2G-I, Figure 2K, Figure 3 and Figure 4). Specific band pass filter sets for DAPI, FITC and Texas Red were used to prevent bleed through from one channel to the next. Images were then processed and analysed using Axiovision software or LSM Zeiss software. Three-dimensional data sets (Figure 3, 4) were taken from 20μm thick sections and then analysed with Imaris v4.0.4 software (Bitplane AG, Zurich, Switzerland). Negative controls of secondary antibody only and appropriate control IgGs together with a secondary antibody were include in each experiment. For enumeration of DCs in association with the crypt epithelial cells only DCs in or immediately adjacent to the epithelial layer (which was identified by either cytokeratin or claudin 3) with evident nuclei (DAPI or TOPRO3 stained) were counted. All sections were counted blind and each section was counted 2-3 times. A minimum of 15 sections per mouse were counted and a minimum of 3 mice were analysed per timepoint.
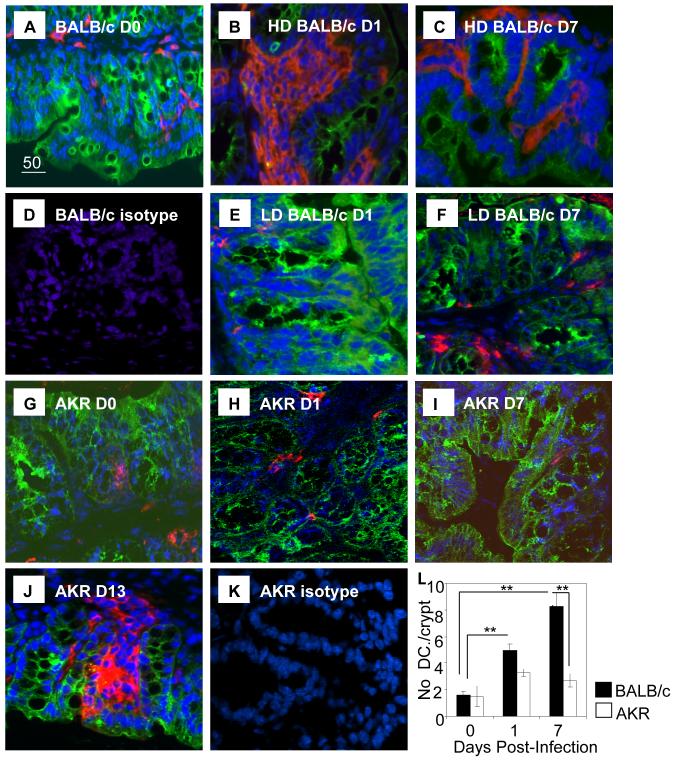
Figure 2. Altered distribution of Colonic DCs in Resistant but not Susceptible Mice.
Colon from resistant BALB/c mice (HD BALB/c: A-D), susceptible BALB/c mice (LD BALB/c; E, F) susceptible AKR (G-K) at D0 (A and G) and D1 (B, E and H), D7 (C, F and I) and D13 post-infection (J) were stained for DCs (CD11c, red) and epithelial cells (cytokeratin, green) and counterstained with TOPRO3 or DAPI (blue). Isotype controls for BALB/c (D) and AKR (K) tissue are shown. Magnification x 400. (L) The numbers of DCs/crypt associated with the epithelium of AKR and BALB/c mice pre- and post-infection was quantified.
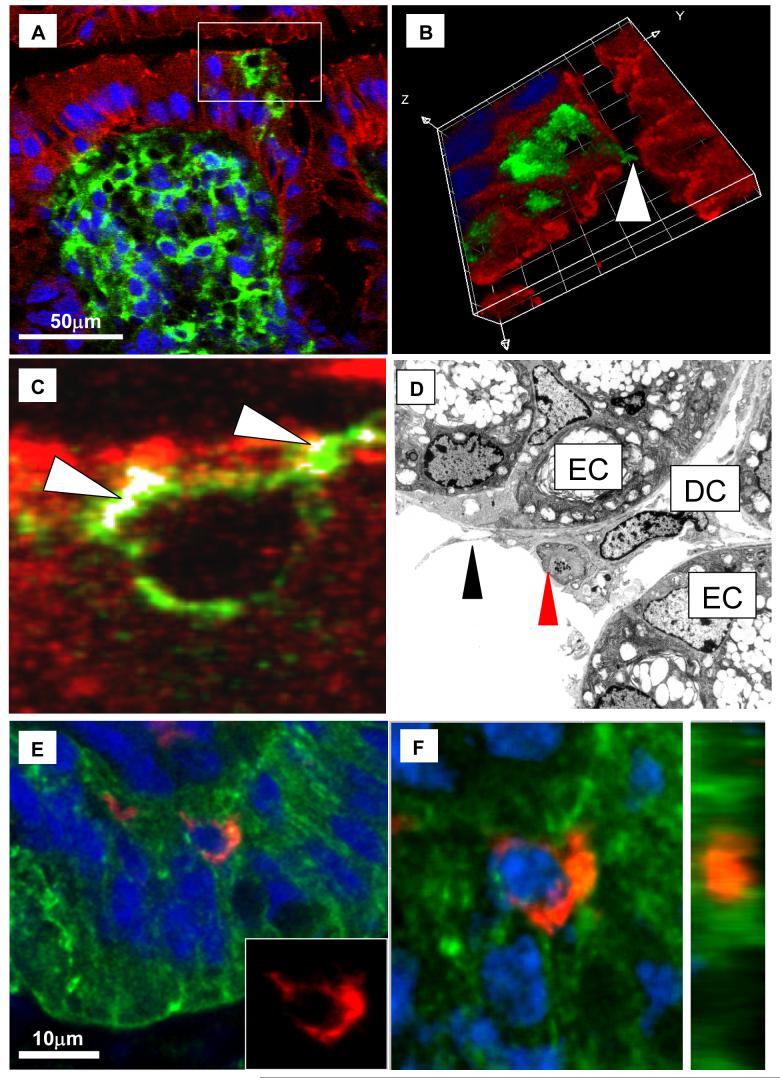
Figure 3. DC -Epithelial Cell Interactions in Infected Resistant Mice.
Immunofluorescent confocal images (A, B, C, E and F) and electron micrograph images (D) of colon from resistant BALB/c mice at D1 (A, B, C, E and F) and D7 (D) post-infection (BALB/c HD) showing DCs within the colonic epithelium. (A-C) Tissue was stained with claudin 3 (red) and CD11c (green) and counterstained with TOPRO3 (Blue). (A) identifies a large DC aggregate underneath the colonic epithelium with two DCs in the epithelial layer (boxed area), (B) is a 3D render of a Z-stacked image of the boxed area in (A) showing the X, Y and Z dimensions and identifies a dendrite extending through the epithelium and into the lumen (arrowed). The points of co-localisation between the DC and claudin 3 are highlighted in white in (C) and are arrowed. (D) Electron microscope photomicrographs of T. muris infected BALB/c colon showing a DC localized to epithelial tight junctions between adjacent epithelial cells (EC) and extending dendrites (arrowed) into the lumen. In (D) a DC appears to be taking up cellular antigen (red arrow), possibly apoptotic cell material (Mag. x 6900). (E and F) Confocal images of colon from resistant BALB/c mice stained with cytokeratin (green, epithelial cells), CD11c (red, DCs) and counterstained with TOPRO3 (blue). (E) is the original unmanipulated confocal image in (E) the inset box shows the DC in isolation with the green and blue fluorescence subtracted out to reveal DC morphology. (F) is an orthogonal view of a full thickness (Z-stack) image of the (right hand panel) showing the DC touching adjacent epithelial cells (Grid size 5 μm2).
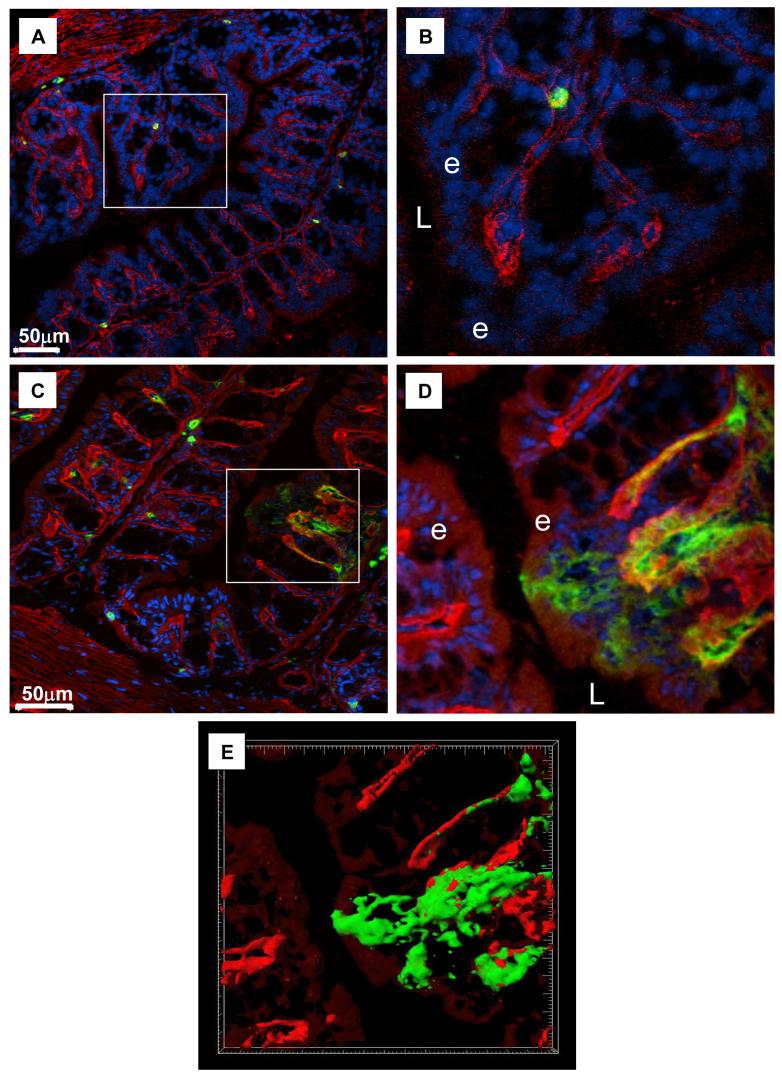
Figure 4. In Response to Infection DCs Cross the Epithelial Basement Membrane.
Immunofluorescent images of sections of colon from resistant BALB/c mice before (A, B) and 1 day post-infection (C-E) with a high antigen dose of T. muris (BALB/c HD). Sections of colon were stained for DC (CD11c, green), basement membrane (laminin, red) and counterstained with DAPI (blue) (A-D). In (A and C) are the original images over-exposed to visualise autofluorescent epithelial cells (red). (B and D) are higher magnification of the areas boxed in A,C showing DCs beneath the epithelial basement membrane prior to infection (B) but after infection they are breaching the basement membrane and are in the epithelial layer (D, E). L= lumen, e-epithelium. (E) shows iso-surface volume rendering of the confocal z-sections from (C). Grid squares = 5μm2. Iso-surface volume rendering was carried out with the Surpass module within Imaris v5.7 (Bitplane AG, Switzerland).
Electron Microscopy
Segments of intestine recovered at D1 and D7 post-peroral infection with T.muris were fixed in 1% osmium tetroxide, washed and stained hours in 2% uranyl acetate. After washing the samples were dehydrated using acetone, embedded in araldite resin and cured at 65°C. Ultrathin sections (100nm thick) were cut on a Reichert–Jung Ultracut E microtome and collected on a 200 mesh copper grid. After staining with Reynold’s lead citrate, the grid was carbon coated and visualised using a JEOL 1200 EX electron microscope.
MLN Cultures
MLN cells were prepared from naïve AKR and BALB/c mice or mice at D7 post-infection with either a high dose (HD BALB/c, AKR) or low dose of T.muris (LD BALB/c). Cells were plated at 5×106cells/ml in a 24 plate in the presence or absence of T.muris excretory secretory antigen (ES antigen) as described previously (18). After 48 h supernatants were taken and analysed on a flow cytometer using the CBA Flex set (BD) to measure IL10, IFNγ, IL12 p70 and IL4.
PCR and qPCR
The large intestine was excised from AKR, BALB/c mice D0, D1 and D7 post T. muris infection separated into caecum, proximal colon and mid-distal colon for epithelial cell isolation (3, 4). Briefly, sections of gut (caecum, proximal colon or mid-distal colon) were cut longitudinally, cleaned to remove faecal debris, cut into 5mm segments and incubated in 1mg/ml dispase 1 in media for 90 min at 37°C under gentle agitation. The resultant cell suspension was washed and purity of the resultant cells was confirmed by flow cytometry. RNA was extracted using Tri-reagent and 1μg used to generate cDNA using Superscript II (Invitrogen, Paisley, UK). cDNA was amplified with Reddy-mix Taq polymerase (Abgene, Epsom, UK) and qPCR performed using SensiMixPlus SYBR (Quantace, London, U.K.) on an OPTICON DNA engine with OPTICON Monitor software version 2.03 (Real-Time systems; MJ Research). β-actin was used to control for the starting amount of cDNA. Expression levels of genes of interest are shown as fold change over that in naïve animals after normalisation to β-actin using the ΔΔCt method.
Primer sequences used were: β-actin Forward: ACGATGCTCCCCGGGCTGTATTC, β-actin Reverse: TTCTCCATGTCGTCCCAGTTGG; CCL2 Forward: TCTGGGCCTGCTGTTCACA, CCL2 Reverse: CTGTCACACTGGTCACTCCTA: CCL3 Forward: ATCACTGACCTGGAACTGAATG, CCL3 Reverse: CAAGTGAAGAGTCCCTCGATG: CCL5 Forward: GGGTACCATGAAGATCTCTGCA, CCL5 Reverse: TTGGCGGTTCCTTCGAGTGA: CCL20 Forward: AATGGCCTGCGGTGGCAACCL20 Reverse: CATCGGCCATCTGTCTTGTGA: Thymic Stromal Lymphopoietin (TSLP) Forward: AGCAAGCCAGCTTGTCTCCTG 3 Reverse: TGTGCCATTTCCTGAGTACCGTCA
Statistics
Data are represented as ± SEM. Data was analysed using a Kruskal-Wallis test with a Dunns multiple comparison test.
Results
Rapid Recruitment of Colonic DCs in Resistant but not Susceptible Mice following T. muris Infection
BALB/c given a high dose of T.muris are resistant to infection and are termed HD BALB/c or resistant BALB/c whereas BALB/c given a low dose of T.muris eggs are susceptible to infection and are termed LD BALB/c or susceptible BALB/c. AKR are referred to as susceptible AKR throughout.
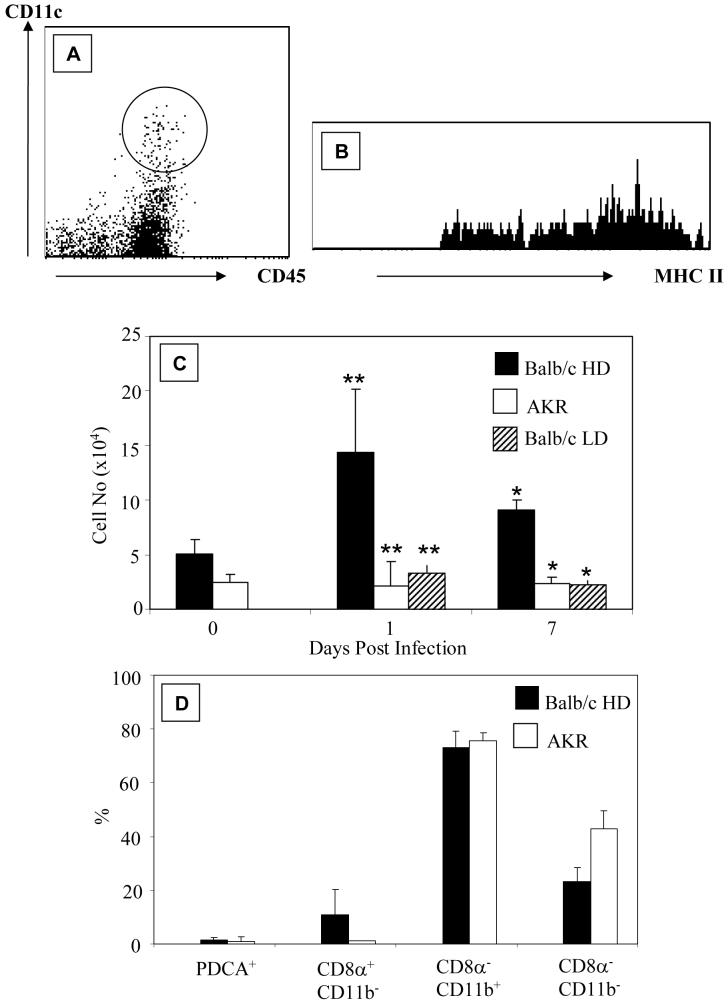
Colonic DCs (CD45+, CD11c+, MHCII+ cells) (Figure 1A and B) were enumerated in AKR and BALB/c mice before and at D1 and D7 post-infection with 150-200 (high dose) or 20 (low dose) embryonated eggs. The total number of colonic DCs in naïve BALB/c mice (~5×104) was higher than in naïve AKR mice (~2.5×104, Figure 1C) although this did not reach statistical significance. Twenty-four hours after T. muris infection (Figure 1C), there were almost 7 times as many DCs (~14.4×104) in resistant BALB/c mice as in susceptible AKR mice (~2.2×104, p<0.001). At D7 post-infection the number of DCs in the colon of resistant mice was still higher (~9.1×104) than susceptible AKR mice in which DC numbers remained unchanged (~2.3×104, p<0.01). Differences in DC number were not due to mouse strain differences, since susceptible LD BALB/c mice showed no increase in DC numbers at D1 (~3×104, p<0.001) and D7 (~2.5×104, p<0.01) compared with resistant BALB/c post-infection (Figure 1C).
Figure 1. Rapid Recruitment of Colonic DCs in T. muris infected Resistant Mice.
Colonic lamina propria cells were identified by flow cytometry using CD45, CD11c and MHC II. Data shows representative profile of CD11c and CD45 expression (A). The region drawn around CD45+CD11c+ cells was used to determine MHCII expression (B). (C) shows the number of DCs in BALB/c and AKR mice pre (0) and post-infection with 150 (HD BALB/c; AKR) or 20 embryonated T.muris eggs (LD BALB/c). (D) shows the subtypes of colonic DCs in AKR and BALB/c mice. The data is from 4-9 experiments. *p<0.01, **p<0.001 comparing resistant versus susceptible mice at the same time point.
As seen previously in C57BL/6 mice (12), the majority of colonic DCs (~75%) in both AKR and BALB/c mice were myeloid (CD11b+ CD11c+) DCs (Figure 1D). No lymphoid (CD8α+ CD11b− CD11c+) colonic DCs were detected in AKR mice with <10% in BALB/c mice (Figure 1D). Plasmacytoid (PDCA+ CD11c+) DCs represented a minor subset (1-2%) of colonic DCs (Figure 1D). In both strains of mice the remaining DCs were CD11b−CD8α−CD11c+ (Figure 1D). At D1 post-infection there was a decrease in the proportion of myeloid DCs (~55%) and a relative increase of CD8α+ CD11b− CD11c+ colonic DCs (~30%, data not shown) in resistant BALB/c mice. The proportions of lymphoid and plasmacytoid DCs were unchanged throughout infection.
DCs Localise to the Epithelium in Resistant but not Susceptible Mice
In the colon of naïve AKR and BALB/c mice, DCs were rare and localised primarily to the colonic lamina propria with only occasional DCs adjacent to the epithelium (typically 1 or none /crypt; Figure 2 A, G). In the caecum a similar distribution and proportion of DCs as colon was seen in naïve BALB/c and AKR mice (data not shown). Similar to the flow cytometry data (Figure 1), fewer DCs were detected in the colon and caecum of naïve AKR mice (Figure 2G) compared to naïve BALB/c mice (Figure 2A).
As observed by flow cytometry, in resistant BALB/c mice at D1 post-infection more colonic DCs were detected compared with susceptible BALB/c and AKR mice (Figure 2B, E, H). This trend was also seen in the caecum (data not shown). In addition, DC localization was also altered in resistant BALB/c post-infection with the majority residing in large aggregates within lymphoid follicle-like aggregates or, in close proximity to the epithelium along the full length of the crypt. Indeed, quantification of colonic DCs revealed significantly more DCs (p<0.0001) were found in close association with colonic epithelial cells (CECs) (5 DC±0.4/crypt, Figure 2L) D1 after T. muris infection compared with non-infected mice or susceptible mice (typically 1 or none /crypt). Also, consistent with flow cytometry analysis (Figure 1), there were more DCs in the large intestine of resistant BALB/c mice (Figure 2C) compared with susceptible AKR mice (Figure 2I, 2L) or susceptible BALB/c (Figure 2F) at D7 post infection. In these animals, colonic DCs were clustered around the epithelium although they tended to be in aggregates associated with the epithelium rather than single cells, as seen at D1 post-infection. Overall, the number and size of the lymphoid follicles increased during infection in resistant BALB/c mice but not in susceptible AKR mice, consistent with previous reports of lymphoid aggregates developing later after infection in AKR mice (19). Tissue from resistant and susceptible mice was also assessed at D2, D3 and D4 post-infection with a similar trend showing increased colonic DCs in resistant but not susceptible mice (data not shown). To rule out the possibility of a general defect in DC recruitment in AKR mice, a later time point post-infection was assessed. At D13 post-infection in AKR mice there was an increase in the number of DCs with many clustering around the epithelium (Figure 2J) similar to that in BALB/c mice day 1 post-infection (Figure 2B).
Collectively this data demonstrates there is a delay in the DC response in susceptible mice compared with resistant mice. Furthermore, DC expansion correlates with an increase in the proportion of the DCs adjacent to the epithelium.
DC-Epithelial Cell Interactions in Resistant Mice Post-Infection
Electron microscopy, confocal imaging and three-dimensional tissue reconstruction were used to investigate the possibility that DCs make contact with epithelial cells during T. muris infection in resistant BALB/c mice. A small proportion (<5%) of DCs appeared to be in contact with epithelial cells (Figure 3 E, F). Analysis of the tight junction proteins claudin 3 and occludin revealed that the cell surface of these DCs co-localised with or co-expressed tight junctions proteins (Figure 3C and data not shown). Among the DCs in the epithelial layer, the majority had long cellular dendritic processes extending between adjacent epithelial cells (Figure 3). In some instances the dendritic processes extended into the lumen (Figure 3A-D) with some containing electron dense material resembling apoptotic cellular debris (Figure 3D).
Transepithelial dendritic processes among epithelia-associated colonic DCs were only observed in resistant BALB/c mice after infection. In non-infected mice or susceptible BALB/c and AKR mice, very few DCs were seen adjacent to the epithelium with the majority residing deeper within the lamina propria. From >50 sections of 6 or more non-infected mice (BALB/c and AKR) only one DC was found to have short dendrite projections that extended between epithelial cells. This suggests that the formation of transepithelial dendrites is associated with a post-infection event and too rare to be observed in naïve mice.
Post-infection, a large proportion of DCs were found directly underneath the epithelial layer (Figure 3). In order to assess whether DC were in the epithelial layer, the epithelial basement membranes were stained using anti-laminin antibodies (Figure 4). Prior to infection, no DCs had breached the epithelial basement membrane and crossed the epithelial layer (Figure 4A-B). In contrast, post-infection, several DCs were observed to have breached the basement membrane and were in direct contact with the epithelium (Figure 4C-E). This data confirms the confocal and electron microscope images in Figure 3 and demonstrates that post-infection DCs are recruited into the epithelium, many of which had dendrite processes extending between epithelial cells (Figure 3A-D). However, although DCs were clearly seen in the epithelium, the laminin staining showed that post-infection the majority of DCs were adjacent to the epithelial basement membrane and had not crossed it.
Distinctive Colonic DCs in Infected Resistant and Susceptible Mice
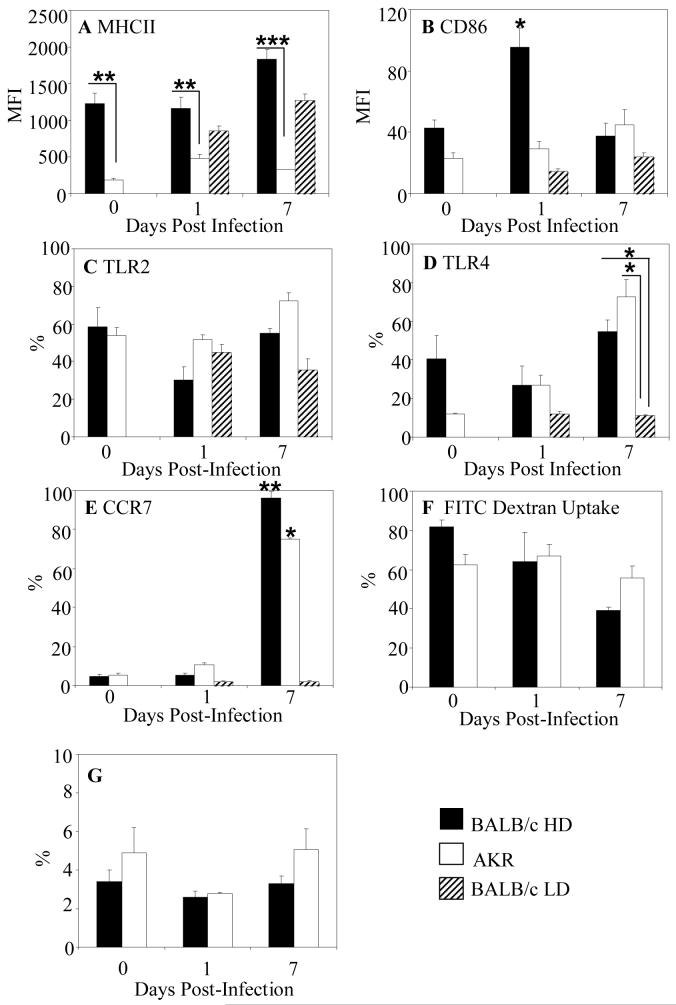
Colonic DCs from BALB/c mice expressed higher levels of CD80, CD86 and MHC II than DCs from AKR mice (Figure 5A and B and data not shown). Only expression of MHC II was statistically significantly higher in naïve BALB/c mice compared with AKR mice (p<0.01). In susceptible mice expression of CD80 and CD86 by colonic DCs was unchanged during infection (Figure 5B and data not shown). By D1 post-infection CD86 expression increased significantly on colonic DCs from resistant BALB/c mice (Figure 5B, p<0.05) whereas MHCII expression remained unchanged (Figure 5A). Consistent with an immature phenotype, DCs at D0 and D1were endocytic (Figure 5F) with <5% expressing the chemokine receptor CCR7 (Figure 5E). At D7 post-infection, MHC II expression increased almost two-fold on colonic DCs in resistant BALB/c mice but changed little among DCs from susceptible mice (p<0.01) (Figure 5A). Consistent with colonic DCs from D7 infected HD BALB/c mice having a mature phenotype the majority expressed CCR7 (Figure 5E) and had relatively low levels of endocytic activity, with 50% fewer DCs taking up FITC-dextran (p<0.05) compared with naïve animals (Figure 5F). In contrast, DCs from susceptible BALB/c mice showed no upregulation of MHCII (Figure 5A) during infection with <5% of DCs expressing CCR7 (Figure 5E) consistent with them being immature. DCs from AKR mice also had low levels of MHC II and costimulatory molecules throughout infection and endocytic activity was unaltered throughout infection (Figure 5F). However, at D7 post-infection DCs from AKR mice did upregulate CCR7 expression (Figure 5E) suggesting that these DCs represent “semi-mature” DCs. The proportion of MLN DCs was assessed at D0, D1 and D7 post-infection (Figure 5G). There was no increase in the proportion of DCs in the nodes of BALB/c mice and in AKR mice post-infection (Figure 5G). The DCs in the MLNs were MHCII high and expressed moderate levels of CD80 and CD86 consistent with a mature phenotype. To investigate the possibility that DCs in MLNs at D7 post-infection promote T cell mediated immunity the ability of MLN cells to respond to T.muris-derived ES antigen was investigated. In cultures of MLN cells stimulated with ES antigen there was no induction of either Th1 (IFNγ and IL12) or Th2 cytokines (IL4) or IL10 (data not shown). These observations support the finding that at Day 7 post-infection, DCs are not being recruited to the MLNs to promote T cell activation in either the AKR or BALB/c mice.
Figure 5. Distinctive Colonic DCs in infected Resistant and Susceptible Mice.
MHCII (A), CD86 (B), TLR2 (C), TLR4 (D), CCR7 (E) expression and FITC Dextran uptake (F) was analysed by flow cytometry by colonic DCs from BALB/c (filled bars) and AKR (open bars) mice pre (0) and post-infection with 150 or 20 embryonated T. muris eggs (hatched bars). (G) The proportion of CD11c+MHCII+ DCs were analysed in the MLNs of BALB/c and AKR mice during the time course of infection. The data is from 4-9 experiments. *p<0.05; **p<0.01;***p<0.001 P values comparing resistant versus susceptible mice at the same timepoint and in E and F comparing naïve mice and D7 post-infection.
Expression of TLR2 and TLR4 was analysed on DCs pre- and post-infection in BALB/c and AKR mice. TLR2 was constitutively expressed by approximately 60% of DCs (Figure 5C) in both strains of mice. Following infection, TLR2 expression did not change significantly in resistant or susceptible animals. In contrast, TLR4 expression was expressed by 2-fold more DCs in naïve BALB/c mice compared with AKR mice (Figure 5D) although this was not statistically significant. TLR4 expression was unchanged in all mice strains at D1 post-infection. Up to D7 post-infection, there was dramatic increase in the number of DCs expressing TLR4 in both resistant BALB/c and susceptible AKR mice (Figure 5D, p<0.05 compared with naïve animals).
Chemokines are major triggers for DC recruitment therefore we investigated the expression of chemokine receptors on colonic DCs. The majority of colonic DCs expressed CCR5 (65±2.96%) with approximately half expressing CCR6 (51.1±2.2%). CCR2 was only expressed on a subset (22.2±3.07%) of DCs. Given the intra-epithelial nature of T. muris, the possibility that epithelial-derived signals (chemokines) trigger DC mobilisation was explored next.
Increased Production of DC chemoattractants by Colonic Epithelial Cells in Resistant Mice
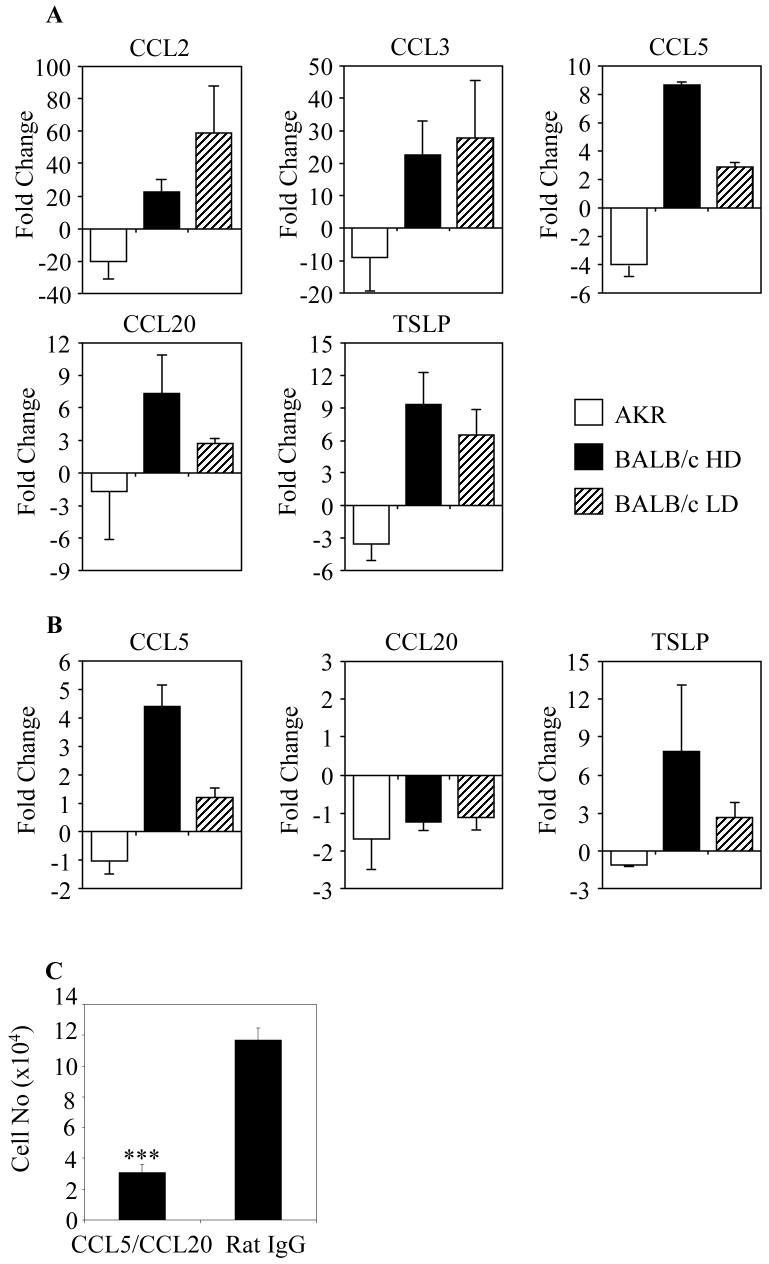
There were marked differences in colonic epithelial cell chemokine profiles in resistant BALB/c mice and susceptible AKR mice at D1 post-infection. In the epithelium of the proximal colon of resistant BALB/c there was an increase in CCL2 (22-fold), CCL3 (22-fold), CCL5 (9-fold) and CCL20 (7-fold) compared with the levels in the colonic mucosa of naïve animals (Figure 6A). In contrast, there was a decrease in the level of CCL2 (20-fold), CCL3 (9-fold), CCL5 (4-fold) and CCL20 (two-fold) in the proximal colonic epithelium of susceptible AKR mice (Figure 6A). A similar trend in chemokine expression was seen in epithelial cells taken from the caecum of susceptible AKR and resistant BALB/c mice at D1 post-infection (data not shown). Although the chemokine profile of the epithelium of the mid-distal colon was similar to the rest of the large intestine, the magnitude of change compared with naïve tissue was much less (data not shown). The Th2 polarising cytokine TSLP was upregulated 10-fold at D1 post-infection in resistant BALB/c mice compared with a decrease in susceptible AKR mice (Figure 6A). Surprisingly, the pattern of CCL2, CCL3 and TSLP expression was similar in resistant BALB/c versus susceptible BALB/c mice at D1 post-infection, with high levels of all three chemokines (Figure 6). However, in contrast, CCL20 and CCL5 were not significantly upregulated (p<0.001, p<0.05 respectively) in the colon of susceptible BALB/c mice with high expression only observed in mice resistant to infection (Figure 6A). At D7 post-infection, CCL20 had returned to levels in naive mice (Figure 6B). By contrast, levels of CCL5 remained elevated in resistant mice compared with susceptible AKR and BALB/c mice at D7 post-infection (Figure 6B). TSLP levels remained high at D7 post-infection in resistant BALB/c compared with susceptible BALB/c in which the levels were reduced with no increase seen in susceptible AKR mice.
Figure 6. Increased Production of DC chemoattractants by Colonic Epithelial Cells in Resistant Mice Promote DC recruitment.
Epithelial cells (n=4) from the proximal colons of resistant BALB/c, susceptible AKR and susceptible BALB/c mice were analysed for expression of CCL2, CCL3, CCL5, CCL20 and TSLP mRNA 0, and 24 hours (A) and 7 days post-infection (B) by q-PCR. The data is shown as the relative expression of mRNA compared to samples from naïve non-infected animals. (C) In order to assess whether CCL5 and CCL20 were necessary for DC recruitment in resistant BALB/c mice, BALB/c mice were infected with T.muris and given intravenous neutralising antibodies to CCL5 and CCL20 (left hand column) or control rat IgG (right hand column). After 24 hours the DCs were enumerated by flow cytometry. In antibody treated mice there was a complete ablation of DC recruitment (***p=0.0001, n=8).
Overall, these results demonstrate dramatic differences in the chemokine profiles of colonic epithelial cells in mice that are susceptible or resistant to infection with T. muris. To assess the potential involvement of CCL5 and CCL20 in the recruitment of DCs in the colons of resistant mice, BALB/c mice were infected with 150-200 embryonated eggs and given intravenous CCL5 and CCL20 blocking antibodies or control IgG antibodies. After 24 hours DCs in the colon were enumerated by flow cytometry (Figure 6C). In mice treated with the neutralising CCL5 and CCL20 antibodies there was a significant decrease in the numbers of DCs in the colon compared with control antibody treated mice (p=0.0001). This data demonstrates the requirement for CCL5 and/or CCL20 in the recruitment of DCs to the colonic mucosa in T.muris infection.
Discussion
We have identified striking differences in the kinetics and magnitude of the DC response in the colon and caecum early in the host response to helminth infection in resistant versus susceptible animals. The Trichuris muris model is well defined with resistance and worm expulsion being dependent upon a Th2 CD4 T cell response and susceptibility to infection and inability to shed worms being linked to a Th1 response (20). The mechanisms underlying different Th cell responses are not known. Using two different models of Th1-mediated susceptibility and a resistant mouse model we found evidence of a delayed DC response in susceptible AKR mice. The lack of an early DC response in AKR mice was not strain specific, since BALB/c mice rendered susceptible to infection by administration of a low dose of eggs also lacked an early DC response. The early DC response in resistant mice was associated with an upregulation in the epithelial cell-derived chemokines CCL5 and CCL20 suggesting that the DC response may be driven by epithelial-mediated factors. Little is known about the initial immune response to T. muris and significant CD4+ T infiltrates are only seen at day 14 post-infection (19). No mouse strain has been found to expel a primary infection before 10 days post-infection. Thus, the early recruitment of DCs in resistant mice occurring several days prior to the development of adaptive immune responses (20) may facilitate the generation of effector immune responses.
Chieppa et al have shown that DC are recruited to the small intestinal epithelium following epithelial TLR stimulation (11). It is tempting to speculate that recognition of T. muris via epithelial TLRs may contribute to the recruitment of DCs in the large intestine. However, to date no TLRs for T. muris have been identified. We have previously shown that ligation of colonic epithelial TLRs and the interaction of epithelial cells with T.muris ES antigen both result in secretion of chemokines known to be DC chemo-attractants (3, 4, 21). Since chemokines are the major mediators of DC migration (22, 23) we explored the possibility that epithelial-derived chemokines in the proximal colon and caecum mediate DC recruitment in resistant mice. In resistant BALB/c mice there was a dramatic increase of epithelial derived CCL2, CCL3, CCL5 and CCL20 24 hours post-infection compared with susceptible AKR mice. The secretion of chemokines was highest in the regions of the gut in which there are T. muris larvae reside (i.e the caecum and proximal colon) while the magnitude of induction of chemokine expression was lower elsewhere in the large intestine. The induction of CCL2 seen in BALB/c contrasts with our earlier published findings in which we found no difference in CCL2 secretion (21). This apparent discrepancy may be due to the different isolation strategies used to isolate epithelial cells (EDTA versus dispase) and the fact that we analysed specific regions of the large intestinal epithelium (caecum, proximal colon and mid-distal colon) versus the intact large intestine. We saw differential expression of chemokines between the different intestinal regions with the most significant changes being in the caecum and proximal colon such that any differences may be masked when analysing the entire gut epithelium in our earlier study(21). EDTA isolation is associated with reduced cell viability compared with dispase isolation has been shown to specifically enrich for cells from the base of the epithelial crypts (2). The major difference between resistant and susceptible mice was epithelial-derived CCL5 and CCL20. Using C57BL/6 mice we have shown a number of chemokines including CCL5 are chemotactic for colonic DCs in vitro (Cruickshank and Carding, unpublished findings and manuscript submitted). Furthermore, we now show that blocking CCL5 and CCL20 prevented DC recruitment to the colonic mucosa in vivo, emphasising the importance of these chemokines in DC mobilisation. Thus, the differences in epithelial chemokine secretion in resistant versus susceptible mice may be a factor in the enhanced DC mobilisation to the epithelium seen in vivo in resistant animals. Accelerated DC responses may contribute to host resistance by facilitating antigen uptake from the epithelium/lumen.
How antigens are taken up by DCs in the large intestine is not known. In the small intestine two major routes of antigen uptake are indirectly via specialised epithelial M cells within the Peyers patches (5, 6) and directly via the extension of transepithelial dendrites on CX3CR1 expressing lamina propria DCs residing immediately beneath the epithelium (6, 7, 9). Colonic DCs are rarely found in the epithelial layer of naive animals as seen here and previously (12). Also, in contrast to the large resident population of DCs found within the lamina propria of the small intestine, few DCs reside in the colon of naïve mice. The appearance of DCs with transepithelial dendrites, some of which extended into the colon lumen of T. muris infected resistant BALB/c mice demonstrates that colonic DCs can form transepithelial dendrites. These transepithelial dendrites may be analogous to those described among DCs in the small intestine, enabling direct sampling of luminal antigens by colonic DCs. Alternatively, dendrites may facilitate antigen transfer from pinocytic CECs. Thus, the rapid recruitment of DCs to the epithelium in BALB/c mice may enhance epithelial /dendritic cell interactions facilitating more rapid or efficient antigen uptake thereby contributing to an effective immune response. Transepithelial dendrites have not been reported in the large intestine before. The fact that transepithelial dendrites were only observed in mice that develop an effective immune response suggests they may be of importance in resistance to infection. The factors underlying the formation of transepithelial dendrites in the large intestine and their function will form the basis for future investigations.
Before DCs can prime T cells they must undergo a well-characterised process of maturation. There a number of DC maturation factors including host cytokines and pathogen-derived factors. Maturation of DCs outside the large intestine is associated with induction of CCR7 which facilitates migration to the lymph nodes, increased expression of MHC II and reduced phagocytic function. In resistant mice there was clear evidence of earlier maturation of colonic DCs whereas DCs from susceptible BALB/c mice remained immature. Although DCs from susceptible AKR mice had increased expression of CCR7 post-infection it is unlikely that they are mature as MHC II expression and endocytic function was unaltered. Since CCR7 expression has been described on a population of semi-mature DCs (24) it is possible that DCs in infected AKR mice are not fully mature. Although TLR4 expression was increased on DCs from resistant mice, it is not known if TLRs are involved in recognition of T. muris. TLR4 deficient mice (25) are resistant to T. muris infection suggesting that TLR4 expression on DCs is not of primary importance in resistance to infection.
The increased association of DCs with epithelial cells may facilitate epithelial-DC interactions. There is increasing evidence to support the importance of epithelial cells in shaping the ability of DCs to polarise T cell responses. Most notably, epithelial associated-TSLP has been shown to be important for the generation of Th2 immunity and worm expulsion (26). In keeping with this observation we found a marked and rapid upregulation of epithelial-derived TSLP in resistant BALB/c compared with susceptible AKR mice. Interestingly this rapid upregulation in TSLP expression was also seen in BALB/c mice made susceptible to infection suggesting that there may be other factors involved in polarising Th2 immunity and that increased production of TSLP is an innate feature of BALB/c mice in response to injury or infection. Consistent with this observation we found a marked and rapid upregulation of epithelial-derived TSLP in resistant BALB/c compared with susceptible AKR mice in which there was downregulation of TSLP. As TSLP is important for the generation of Th2 immunity (26, 27) it is possible that its downregulation promotes skewing to a Th1 immune response. However, in BALB/c mice made susceptible to infection and that do not develop Th2 immunity there was also a rapid upregulation of TSLP expression suggesting there may be other factors involved in polarising Th2 immunity and that increased production of TSLP is an innate feature of BALB/c mice in response to injury or infection.
In summary, we have described for the first time key differences in the kinetics of the early innate immune response of epithelial cells and dendritic cells of the large intestine to T.muris that might underlie qualitative differences in the subsequent adaptive immune response.
Acknowledgments
Grant Support: This work was in part supported by Wellcome Trust grants awarded to SRC, SMC and KJE, Action Medical Research Grant awarded to SRC and SMC and a grant funded from The Royal Society to SMC.
References
- 1.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Cruickshank SM, Wakenshaw L, Cardone J, Howdle PD, Murray PJ, Carding SR. Evidence for the involvement of CARD15/NOD2 in regulating colonic epithelial cell growth and survival. World J Gastroenterol. 2008;14:5834–5841. doi: 10.3748/wjg.14.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lan JG, Cruickshank SM, Singh JC, Farrar M, Lodge JP, Felsburg PJ, Carding SR. Different cytokine response of primary colonic epithelial cells to commensal bacteria. World Journal of Gastroenterology. 2005;11:3375–3384. doi: 10.3748/wjg.v11.i22.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh JC, Cruickshank SM, Newton DJ, Wakenshaw L, Graham A, Lan J, Lodge JP, Felsburg PJ, Carding SR. Toll-like receptor-mediated responses of primary intestinal epithelial cells during the development of colitis. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2005;288:G514–524. doi: 10.1152/ajpgi.00377.2004. [DOI] [PubMed] [Google Scholar]
- 5.Uhlig HH, Powrie F. Dendritic cells and the intestinal bacterial flora: a role for localized mucosal immune responses. Journal of Clinical Investigation. 2003;112:648–651. doi: 10.1172/JCI19545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milling SW, Cousins L, MacPherson GG. How do DCs interact with intestinal antigens? Trends in Immunology. 2005;26:349–352. doi: 10.1016/j.it.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Iliev ID, Matteoli G, Rescigno M. The yin and yang of intestinal epithelial cells in controlling dendritic cell function. J Exp Med. 2007;204:2253–2257. doi: 10.1084/jem.20062535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kweon MN, Yamamoto M, Rennert PD, Park EJ, Lee AY, Chang SY, Hiroi T, Nanno M, Kiyono H. Prenatal blockage of lymphotoxin beta receptor and TNF receptor p55 signaling cascade resulted in the acceleration of tissue genesis for isolated lymphoid follicles in the large intestine. Journal of Immunology. 2005;174:4365–4372. doi: 10.4049/jimmunol.174.7.4365. [DOI] [PubMed] [Google Scholar]
- 9.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunology. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 10.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 11.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruickshank SM, English NR, Felsburg PJ, Carding SR. Characterization of colonic dendritic cells in normal and colitic mice. World Journal of Gastroenterology. 2005;11:6338–6347. doi: 10.3748/wjg.v11.i40.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hapfelmeier S, Muller AJ, Stecher B, Kaiser P, Barthel M, Endt K, Eberhard M, Robbiani R, Jacobi CA, Heikenwalder M, Kirschning C, Jung S, Stallmach T, Kremer M, Hardt WD. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J Exp Med. 2008;205:437–450. doi: 10.1084/jem.20070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deschoolmeester ML, Else KJ. Cytokine and chemokine resposnes underlying acute and chronic Trichuris muris infection. International Reviews in Immunology. 2002;21:439–467. doi: 10.1080/08830180213278. [DOI] [PubMed] [Google Scholar]
- 15.Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Advances in Parasitology. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- 16.Bancroft AJ, Else KJ, Humphreys NE, Grencis RK. The effect of challenge and trickle Trichuris muris infections on the polarisation of the immune response. Int J Parasitol. 2001;31:1627–1637. doi: 10.1016/s0020-7519(01)00281-8. [DOI] [PubMed] [Google Scholar]
- 17.Ashcroft AJ, Cruickshank SM, Croucher PI, Perry MJ, Rollinson S, Lippitt JM, Child JA, Dunstan C, Felsburg PJ, Morgan GJ, Carding SR. Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity. 2003;19:849–861. doi: 10.1016/s1074-7613(03)00326-1. [DOI] [PubMed] [Google Scholar]
- 18.Taylor MD, Betts CJ, Else KJ. Peripheral cytokine responses to Trichuris muris reflect those occurring locally at the site of infection. Infect Immun. 2000;68:1815–1819. doi: 10.1128/iai.68.4.1815-1819.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little MC, Bell LV, Cliffe LJ, Else KJ. The characterization of intraepithelial lymphocytes, lamina propria leukocytes, and isolated lymphoid follicles in the large intestine of mice infected with the intestinal nematode parasite Trichuris muris. Journal of Immunology. 2005;175:6713–6722. doi: 10.4049/jimmunol.175.10.6713. [DOI] [PubMed] [Google Scholar]
- 20.Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.deSchoolmeester ML, Manku H, Else KJ. The innate immune responses of colonic epithelial cells to Trichuris muris are similar in mouse strains that develop a type 1 or type 2 adaptive immune response. Infect Immun. 2006;74:6280–6286. doi: 10.1128/IAI.01609-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 23.Calle Y, Burns S, Thrasher AJ, Jones GE. The leukocyte podosome. Eur J Cell Biol. 2006;85:151–157. doi: 10.1016/j.ejcb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Sanchez N, Riol-Blanco L, Rodriguez-Fernandez JL. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J Immunol. 2006;176:5153–5159. doi: 10.4049/jimmunol.176.9.5153. [DOI] [PubMed] [Google Scholar]
- 25.Helmby H, Grencis RK. Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection. European Journal of Immunology. 2003;33:2974–2979. doi: 10.1002/eji.200324264. [DOI] [PubMed] [Google Scholar]
- 26.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 27.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]