Abstract
OBJECTIVES
To assess the relationship between cognitive function, socioeconomic status, and neighborhood deprivation (lack of local resources of all types, financial and otherwise).
DESIGN
Nationally representative cross-section.
SETTING
The English Longitudinal Study of Ageing (ELSA).
PARTICIPANTS
Seven thousand one hundred twenty-six community-dwelling individuals aged 52 and older and resident in urban areas.
MEASUREMENTS
Individual cognitive function score and index of multiple deprivation (IMD) at the Super Output Area level, adjusting for health, lifestyle, and sociode-mographic confounders. Analyses were conducted separately according to sex and age group (52–69 and ≥70).
RESULTS
IMD affected cognitive function independent of the effects of education and socioeconomic status. For example, in fully adjusted models, women aged 70 and older had a standardized cognitive function score (z-score) that was 0.20 points (95% confidence interval (CI) = 0.01–0.39) lower in the bottom 20% of wealth than the top 20%, 0.44 points (95% CI = 0.20–0.69) lower in the least-educated group than in the most educated, and 0.31 points (95% CI 0.15–0.48) lower if resident lived in an area in the bottom 20% of IMD than in the top 20%.
CONCLUSION
In community-based older people in urban neighborhoods, neighborhood deprivation—living in a neighborhood with high levels of deprivation, compared with national levels—is associated with cognitive function independent of individual socioeconomic circumstances. The mechanisms underlying this relationship are unclear and warrant further investigation.
Keywords: deprivation, education, socioeconomic status, cognitive function, cognition
The characteristics of the places people inhabit influence many aspects of their health and well-being. Living in a deprived neighborhood has been shown to be associated with risky health behaviors,1 poor cardiovascular health,2 higher mortality,3 and greater depression.4 Various mechanisms to account for neighborhood effects on health have been proposed, including access to resources such as primary care and stores selling healthy food5,6 and relationships with the built environment.7,8
Older people are at heightened risk of being affected by such neighborhood effects,9 and mental health10,11 and physical function12,13 have been found to be poorer in older people living in deprived urban neighborhoods. Previous studies also suggest a relationship between neighborhood characteristics and cognitive function in older adults, indicating that differences between neighborhoods explain ethnic differences in Mini-Mental State Examination (MMSE) scores14 and that there is a relationship between mean levels of educational attainment in U.S. census tracts and the cognitive status of adults aged 70 and older living in them.15 Cognitive function is known to be associated with level of education16 and with socioeconomic status, including wealth and income,17-19 but an association between neighborhood socioeconomic factors and cognitive function additional to the effect of individual circumstances has been suggested.20
This study examined the relationship between cognitive function and individual socioeconomic circumstances, level of education, and neighborhood deprivation. Neighborhoods are defined here in terms of small areas identified as socially homogeneous using national census data. The measure of deprivation used covers several aspects of neighborhood deprivation, such as low income, poor living environment, and crime levels. Independently assessed differences in individual health and health behaviors, including physical activity, were also taken into account. Data were from a population-based survey of older people in England, and analyses were conducted separately for those younger than 70 and those aged 70 and older and for men and women. The hypothesis tested was that neighborhood socioeconomic deprivation would be associated with cognitive function scores independent of the effect of individual socioeconomic circumstances and health behaviors.
METHODS
Participants
The English Longitudinal Study of Ageing (ELSA) is a national panel study established to enable the study of the dynamic relationships between health, functioning, and socioeconomic factors as people age beyond 50. The ELSA sample was drawn from households responding to the Health Survey for England (HSE), an annual government-funded study of households in England in 1998, 1999, and 2001. Households were included in ELSA if one or more individuals living there were aged 50 and older. There were 19,924 individuals in eligible households who would have been aged 50 by the time the ELSA sample was taken in 2002. Two thousand five hundred ninety-six of these older individuals died or were ineligible for follow-up; of the remainder, 11,392 (65.7%) became ELSA respondents. Comparison of sociodemographic characteristics and census results indicated that the ELSA sample remained population representative.21
In 2004, 9,324 of these individuals were still alive and responded to the second wave of the ELSA survey. In keeping with the theory that the effects of neighborhood deprivation apply primarily to those living in nonrural environments,22 only the 8,102 respondents who lived in urban (n = 6,972) or suburban (n = 1,130) areas were included. Of these, 626 had incomplete individual socioeconomic status data and were omitted; a further 260 had missing information on the cognitive function tests. The remaining 7,216 respondents were included in the analyses.
Measures of Neighborhood Deprivation
The Index of Multiple Deprivation (IMD) 2004 is a measure based on distinct dimensions of deprivation that can be measured separately at the small-area level. Seven dimensions of deprivation are included: income deprivation; employment deprivation; health deprivation and disability; education, skills, and training deprivation; barriers to housing and services; living environment deprivation; and crime. IMD 2004 has been used to examine the association between socioeconomic deprivation and outcomes such as equity of access to care,23 life expectancy,24 preterm birth rates,25 and postsurgical mortality.26 Full details of the theoretical and practical implementation of the IMD measure, including discussion of its reliability and validity, are available.27
Using information from the national Census of 2001, the UK Office for National Statistics calculated IMD scores at the Super Output Area (SOA) level. SOAs, developed by the Office for National Statistics for use in small-area statistics and reporting after the 2001 Census, contain a minimum of 1,000 individuals and a mean of 1,500 individuals. There are 34,378 SOAs in England.28 Because information about IMD scores at the SOA level is potentially disclosive, IMD information in ELSA is only available divided according to quintiles, and data on the seven separate dimensions are currently unavailable. In this study, IMD divided according to quintiles was used to represent the level of socioeconomic deprivation of the neighborhoods in which respondents lived.
Measures of Individual Socioeconomic Status and Education
Separate measures of individual income, wealth, and education were used. These came from individual responses in ELSA and are independent of the IMD information used. Income included total income from employment, self-employment, private or state pension, benefits, assets, and other sources and was divided by quintiles; wealth included total financial, physical, and housing wealth but not pension wealth and was also divided by quintiles. Education was classified according to the age at which the respondent reported having completed full-time schooling. This was classified as (having left school at age) 14 or younger and then by year of age up to 19 or older. An additional category was included for those who reported that they had not yet finished their education.
Outcome Measures
The outcome measure was a standardized cognitive function score. The neuropsychological tests incorporated in ELSA to assess cognitive function are summarized below and described in detail elsewhere.17 Time orientation was assessed using questions relating to day and date from the Mini-Mental State Examination (MMSE).29 Immediate and delayed verbal memory were assessed using a 10-word learning task from the Health and Retirement Study.30 Ten common words are presented aurally by computer at a rate of one word every 2 seconds. The sound level is adjusted to meet the requirements of each participant. Participants are then asked to recall as many words as possible immediately and again after a short delay during which they complete other cognitive tests. Four different randomly assigned word lists are used, and members of the same household are given different versions. Prospective memory (also called “remembering to remember”) was assessed by asking participants to remember to carry out a prior instruction at a specified point later in the session (writing their initials in the top left-hand corner of a page attached to a clipboard when it is handed to them). This prospective memory test is closely based on a task incorporated in the UK Medical Research Council Cognitive Function and Aging Study (MRC CFAS).31
The verbal fluency task examines how readily participants are able to think of words from a particular category, in this case naming as many animals as possible in 1 minute. The same task has been used in several other studies, including MRC CFAS. Attention and mental speed were assessed using a letter cancellation task from the National Study of Health and Development, also known as the 1946 birth cohort study.32 Participants are asked to cross out as many of the 65 target letters (P and W) as possible in 1 minute on a page incorporating 780 letters in a grid. The total number of letters searched provides a measure of processing speed. The ratio of correctly identified target letters to all target letters scanned provides a measure of search accuracy. Because the scoring of each individual test varies, test scores were standardized according to sex and age group to give a mean of 0 and a standard deviation of 1 (z-scores). Scores representing overall cognitive function were obtained by averaging individual standardized scores on all tests, with high scores representing high levels of cognitive function.
Statistical Analysis
Ordinary least squares regression was used to estimate the effects of individual wealth, income, and education level and neighborhood IMD quintile on cognitive function. The primary sampling unit in HSE is the household; cluster correction was used to take into account anticipated similarity between individuals living in the same household, and survey weights were used. Analyses were conducted using Stata SE Version 9.2 (StataCorp, College Station, TX).
The following factors known to relate to cognitive function were included: age, sex; smoking,33 alcohol consumption,34,35 having being told by a doctor that they had diabetes mellitus36 or other vascular problems (hypertension or high blood pressure, angina pectoris, heart murmur, arrhythmia),37 visual problems,38 and self-reported hearing loss39 or health37 or depressive symptoms40 measured using a version of the Center for Epidemiologic Studies Depression Scale (CES-D).41 A subset of eight of the original 20 CES-D items was used in the ELSA study, as in the Established Populations for Epidemiologic Study of the Elderly survey.42 Those who reported having had a stroke were excluded from the main analysis but included in a sensitivity analysis.43 All these data were gathered as part of the ELSA study.
RESULTS
Table 1 shows the baseline characteristics of study participants. For men and women, the modal level of education was higher in the younger than the older age group. This is in line with secular changes in levels of education in England and is also linked to statutory changes in school leaving age that occurred within the lifetimes of the respondents. (In 1947, the age a child could legally leave school in England rose from 14 to 15.) Subjects in the older age group reported poorer eyesight, higher levels of stroke and diabetes mellitus, and lower levels of diagnosed psychological or emotional problems. than those in the younger age group.
Table 1.
Sample Characteristics According to Age and Sex
| <70 | ≥70 | |||
|---|---|---|---|---|
| Men (n = 2,065) | Women (n = 2,449) | Men (n = 1,109) | Women (n = 1,503) | |
| Characteristic | n (%) | |||
| Age when finished full-time education | ||||
| ≤14 | 88 (4.3) | 89 (3.6) | 601 (54.0) | 763 (50.5) |
| 15 | 932 (45.1) | 1,105 (45.1) | 173 (15.6) | 229 (15.2) |
| 16 | 407 (19.7) | 529 (21.6) | 130 (11.7) | 218 (14.5) |
| 17 | 121 (5.9) | 214 (8.7) | 46 (4.1) | 106 (7.1) |
| 18 | 111 (5.4) | 136 (5.6) | 39 (3.5) | 59 (3.9) |
| ≥19 | 350 (17.0) | 322 (13.2) | 96 (8.7) | 90 (6.0) |
| Still being educated | 56 (2.7) | 54 (2.2) | 24 (2.2) | 38 (2.5) |
| Current smoker | 405 (19.6) | 457 (18.7) | 115 (10.4) | 169 (11.2) |
| Alcohol consumption, drinks/d* | ||||
| 0 | 104 (5.2) | 224 (9.6) | 94 (8.8) | 243 (17.0) |
| <1 | 902 (45.1) | 1,686 (71.9) | 607 (57.1) | 1,004 (70.3) |
| 1 to <2 | 481 (24.1) | 326 (13.9) | 207 (19.5) | 148 (10.4) |
| ≥2 | 512 (25.6) | 110 (4.7) | 156 (14.7) | 34 (2.4) |
| Health conditions | ||||
| Stroke | 66 (3.2) | 56 (2.3) | 99 (8.9) | 107 (7.1) |
| Hypertension/high blood pressure | 718 (34.5) | 769 (31.4) | 469 (42.3) | 739 (49.2) |
| Angina pectoris | 164 (7.9) | 108 (4.4) | 195 (17.6) | 209 (13.9) |
| Heart murmur | 68 (3.3) | 117 (4.8) | 63 (5.7) | 115 (7.7) |
| Heart arrhythmia | 145 (7.0) | 141 (5.8) | 125 (11.3) | 169 (11.2) |
| Diabetes mellitus | 66 (3.2) | 56 (2.3) | 99 (8.9) | 107 (7.1) |
| Self-rated health | ||||
| Excellent | 290 (14.0) | 353 (14.4) | 96 (8.7) | 117 (7.8) |
| Very good | 603 (29.2) | 735 (30.0) | 255 (23.0) | 372 (24.8) |
| Good | 639 (30.9) | 755 (30.8) | 379 (34.2) | 502 (33.4) |
| Fair | 371 (18.0) | 454 (18.5) | 282 (25.4) | 361 (24.0) |
| Poor | 162 (7.9) | 152 (6.2) | 97 (8.8) | 151 (10.1) |
| Eyesight | ||||
| Excellent | 361 (17.5) | 362 (14.8) | 121 (10.9) | 133 (8.9) |
| Very good | 726 (35.2) | 820 (33.5) | 338 (30.5) | 459 (30.5) |
| Good | 780 (37.8) | 992 (40.5) | 471 (42.5) | 596 (39.7) |
| Fair | 169 (8.2) | 231 (9.4) | 134 (12.1) | 251 (16.7) |
| Poor | 29 (1.5) | 44 (1.8) | 45 (4.1) | 64 (4.3) |
| Hearing | ||||
| Excellent | 351 (17.0) | 662 (27.0) | 117 (10.6) | 256 (17.0) |
| Very good | 583 (28.2) | 750 (30.6) | 217 (19.6) | 386 (25.7) |
| Good | 689 (33.4) | 742 (30.3) | 369 (33.3) | 490 (32.6) |
| Fair | 354 (17.1) | 246 (10.0) | 299 (27.0) | 281 (18.7) |
| Poor | 88 (4.3) | 49 (2.0) | 107 (9.7) | 90 (6.0) |
Note: Percentages may not sum to 100 because of rounding.
1 drink = 14 g of alcohol.
Figure 1 shows mean standardized cognition scores according to age group and sex, in relation to the IMD score of the neighborhood in which respondents lived, divided into quintiles. Across all the age and sex groups, there is a clear downward trend in mean cognitive function score from the least-deprived 20% of areas to the most deprived (P <. 001 for each group). There is little apparent difference between the groups in terms of the effects of living in a more-deprived area; in all groups, it is statistically significantly worse to live in an area with high levels of deprivation than an area with low levels of deprivation. (Standardized scores were calculated separately according to sex and age group, so comparisons of levels of cognitive function across groups are not possible in Figure 1.)
Figure 1.
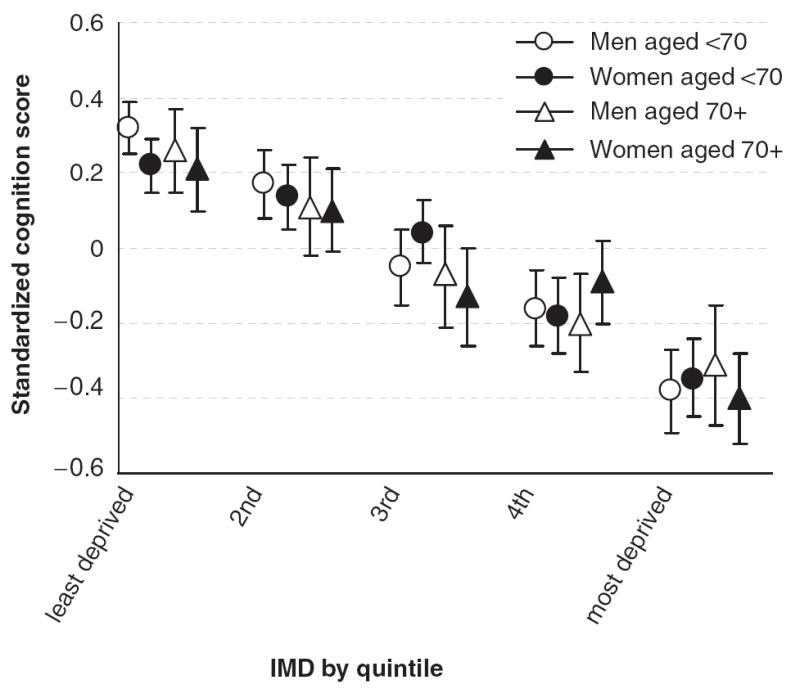
Mean standardized cognition score according to neighborhood index of multiple deprivation (IMD) divided into quintiles.
The outcomes of regressing standardized cognition scores on wealth, income, level of education, and neighborhood IMD, separately according to sex and age group and adjusted for possible confounders as described above, are shown in Table 2. For clarity, the estimates associated with the demographic and health variables are not shown; these are available from the authors on request. Reference categories are indicated.
Table 2.
Outcomes of Multiple Regressions of Standardized Cognitive Function Score on Wealth, Income, Education, and Neighborhood Deprivation According to Age and Sex
| <70 | ≥70 | ||||
|---|---|---|---|---|---|
| Men (n = 1,996) | Women (n = 2,392) | Men (n = 1,006) | Women (n = 1,391) | All Respondents | |
| Variable | Differences in Z-Scores of Cognitive Function (95% Confidence Interval) | ||||
| Wealth | |||||
| Lowest 20% | — | — | — | — | — |
| 2nd | 0.11 (−0.04–0.26) | 0.20 (0.06–0.34) | 0.10 (−0.08–0.27) | 0.14 (−0.01–0.29) | 0.14 (0.06–0.21) |
| 3rd | 0.19 (0.04–0.34) | 0.31 (0.17–0.45) | 0.07 (−0.10–0.25) | 0.13 (−0.03–0.29) | 0.19 (0.12–0.26) |
| 4th | 0.20 (0.05–0.36) | 0.35 (0.21–0.49) | 0.09 (−0.11–0.30) | 0.26 (0.10–0.42) | 0.24 (0.17–0.31) |
| Highest 20% | 0.23 (0.07–0.40) | 0.38 (0.23–0.52) | 0.02 (−0.20–0.24) | 0.26 (0.06–0.47) | 0.26 (0.18–0.34) |
| Income | |||||
| Lowest 20% | — | — | — | — | — |
| 2nd | − 0.04 (−0.22–0.13) | 0.14 (−0.01–0.28) | 0.17 (−0.01–0.35) | 0.06 (−0.07–0.19) | 0.06 (−0.01–0.13) |
| 3rd | − 0.05 (−0.21–0.10) | 0.14 (0.01–0.28) | 0.25 (0.06–0.44) | 0.18 (0.04–0.31) | 0.09 (0.02–0.16) |
| 4th | 0.06 (−0.09–0.20) | 0.12 (−0.00–0.25) | 0.39 (0.18–0.60) | 0.15 (−0.02–0.32) | 0.10 (0.03–0.17) |
| Highest 20% | 0.17 (0.03–0.32) | 0.10 (−0.04–0.23) | 0.58 (0.32–0.84) | 0.01 (−0.25–0.27) | 0.12 (0.05–0.20) |
| Age when finished full-time education | |||||
| ≤14 | — | — | — | — | — |
| 15 | 0.25 (0.03–0.46) | 0.18 (−0.05–0.42) | 0.00 (−0.16–0.16) | 0.05 (−0.09–0.18) | 0.08 (0.02–0.15) |
| 16 | 0.53 (0.30–0.76) | 0.50 (0.25–0.74) | 0.22 (0.04–0.40) | 0.21 (0.03–0.38) | 0.32 (0.24–0.40) |
| 17 | 0.57 (0.30–0.85) | 0.48 (0.21–0.74) | 0.37 (0.06–0.67) | 0.38 (0.20–0.56) | 0.38 (0.29–0.48) |
| 18 | 0.45 (0.18–0.73) | 0.52 (0.23–0.80) | 0.17 (−0.10–0.44) | 0.05 (−0.22–0.32) | 0.26 (0.15–0.37) |
| ≥19 | 0.73 (0.50–0.96) | 0.74 (0.47–1.00) | 0.31 (0.09–0.53) | 0.36 (0.10–0.62) | 0.47 (0.38–0.56) |
| Not finished | 0.32 (−0.01–0.65) | 0.26 (−0.06–0.58) | 0.36 (0.00–0.71) | 0.11 (−0.19–0.27) | 0.18 (0.04–0.33) |
| Index of multiple deprivation | |||||
| 20% least deprived | — | — | — | — | — |
| 2nd 20% | 0.00 (−0.11–0.11) | 0.02 (−0.08–0.12) | − 0.03 (−0.19–0.13) | −0.04 (−0.18–0.11) | −0.01 (−0.04–0.05) |
| 3rd 20% | −0.11 (−0.24–0.01) | 0.01 (−0.09–0.12) | −0.17 (−0.33 to −0.01) | −0.20 (−0.36 to −0.04) | −0.10 (−0.16 to −0.04) |
| 4th 20% | −0.11 (−0.24–0.03) | −0.12 (−0.23 to −0.01) | −0.19 (−0.37 to −0.01) | −0.11 (−0.27–0.04) | −0.12 (−0.19 to −0.05) |
| 20% most deprived | −0.11 (−0.26–0.04) | −0.16 (−0.30 to −0.02) | −0.28 (−0.48 to −0.07) | −0.29 (−0.46 to −0.12) | −0.18 (−0.25 to −0.10) |
Analyses were controlled for age, sex, smoking, alcohol consumption, doctor-diagnosed diabetes mellitus, hypertension, high blood-pressure, angina pectoris, heart murmur, arrhythmia, visual problems, self-reported hearing loss, self-reported health, and depressive symptoms. Subjects who reported having had a stroke were excluded.
These results show differing relationships with cognitive function according to sex and age group. Wealth was statistically significantly associated with cognitive function (comparing the upper and lower categories of wealth) for men and women younger than 70 and for women aged 70 and older. Income was statistically significantly associated with cognitive function in men (in both age groups) but not in women. Level of education was statistically significantly associated with cognitive function (comparing those who left school at 14 or younger with those who left school at 19 or older) in all sex and age groups. Neighborhood IMD score was statistically significantly associated with cognitive function in women but not men younger than 70 and in men and women aged 70 and older.
Sensitivity Analyses
A number of sensitivity analyses were conducted. To explore the effects of vascular problems on cognitive function, analyses were repeated with the inclusion of measured systolic blood pressure (these data were not available for all respondents) and (separately) with an indicator of whether a doctor had ever told respondents that they had high blood pressure. In analyses with these measures included, the shape of the response in relation to neighborhood deprivation was unchanged, although the smaller numbers meant that the confidence intervals were wider. In analyses controlling for (rather than excluding) those who reported having had a stroke, there was little difference from the association between deprivation and cognitive function found in the main models.
As a way of exploring the causality issues associated with the use of cross-sectional data, data on how many years before respondents had moved into their current accommodation were used. Including this variable in analyses made little difference in the overall relationships observed, and there were no significant interactions between years of residence and IMD group. Excluding respondents who reported having moved within the previous 5 or 10 years, who might have moved to escape a more-deprived neighborhood, also made no difference to the relationships observed. Analyses were repeated with a measure indicating highest educational qualification achieved rather than age of completion of formal schooling. Results with this alternative education variable were almost identical to those using the original variable.
An additional variable was used to assess the effect of access to resources, because poor access to key resources such as medical facilities and local shops or stores might account for some of the difference in cognitive function related to deprived neighborhoods.44 Respondents were asked to score, on a scale from 1 (very easy) to 4 (very difficult), how difficult they found it to get to a number of facilities: bank or cash machine, dentist, family doctor, hospital, local shops, post office, shopping center, and supermarket. A summary variable was produced by adding the scores on these variables and dividing the total into quintiles. Including this variable in analyses accounted for some additional variance but did not alter the shape of the association between neighborhood deprivation and cognitive function.
For those who reported living with a spouse or partner, analyses were repeated with the addition of a variable for that other person’s level of education. There was no statistically significant association between a respondent’s cognitive function and his or her partner’s or spouse’s level of education. Whether or not a respondent reported living with a spouse or partner made no difference to the relationship between cognitive function and level of neighborhood deprivation.
The results of all these sensitivity analyses are available from the authors on request.
DISCUSSION
These results suggest that neighborhood deprivation in urban areas, measured according to IMD score, is associated with cognitive function in older adults independent of their individual socioeconomic circumstances and level of education. This finding is in line with studies that have found a higher risk of depression in older people who live in more-deprived urban areas10,11 and poorer cognitive function in older people living in U.S. census tracts with low mean levels of education.15 Results were robust to adjustment for the effects of systolic blood pressure and of having had a stroke, suggesting that some other mechanism underlies the association between neighborhood deprivation and cognitive function.
This study is the first to use data from a nationally representative survey to assess the effects of neighborhood deprivation in urban areas on cognitive function in older adults. IMD scores, calculated based on national census data, are an objective measure of neighborhood deprivation, and the use of a similar approach has been proposed in the United States.45 IMD scores take into account a range of social factors and capture a broad range of factors about the neighborhoods in which respondents live. These deprivation scores were calculated at the level of the SOA, and SOAs, with a mean population of 1,500 individuals, are smaller than U.S. Census tracts, which have a population of between 2,500 and 8,000. This means that the data provided in relation to UK SOAs relate to smaller areas than those associated with U.S. Census tracts.
This study was not based on specific locales, as similar studies have been,15 but on the overall level of deprivation in the area in which an individual lived, and analysis involving locally specific data would add to what has been done here. SOAs represent administrative rather than natural or community-defined neighborhoods, but they were constructed using national census data with the express purpose of maximizing internal social homogeneity.28 Although deprivation and deprived neighborhoods are features of all societies, replication of these findings in other countries would be useful.
Other methodological issues ought to be borne in mind in assessing these findings. One relates to the problem of differentiating between members of the group of older individuals who have, according to contemporary standards, relatively low levels of education. More than 50% of those in the older age group in this study reported having left school at age 14 or younger. The differences in their levels of wealth and income will have captured some of the socioeconomic difference between them, but in terms of years of education and of highest level of qualification (as in the sensitivity analysis), there is no way to differentiate them.
Another methodological concern is that the sample used here includes only community-dwelling individuals and excludes those residing in institutions, those for whom cognitive function data were missing, and those for whom only proxy responses were available. This will tend to bias the results toward a population with relatively good cognitive function, although raw scores on the summary cognitive measure were normally distributed, indicating that the sample represented the full range of cognitive ability. Controlling for depressive symptoms suggests that depression, although associated with neighborhood deprivation in urban areas,10,11 does not account for the observed association between neighborhood deprivation and cognitive function, although it is not possible to eliminate the possibility that differences in cognitive function scores reflect depression or pseudo-dementia.
The suggestion that cognitive function in older people is lower in those living in deprived areas is consistent with the idea that older people may be particularly susceptible to neighborhood factors because many age in place9—that is, they are long-term residents in communities that are in decline—and are more directly exposed to neighborhood factors.46 It is possible that older people with impaired cognitive function lack the resources, mentally and otherwise, to move out of neighborhoods in which levels of deprivation are increasing. The sensitivity analysis including duration of residence suggests that length of stay in a particular location is not an important factor in these results, but longitudinal data are necessary to address this directly.
A key unanswered question concerns the mechanisms by which neighborhood deprivation affects cognitive function in older people, mechanisms that are likely to be complex. Only aggregate IMD scores are available for the ELSA data set, and it would be useful to assess the effects of the different dimensions of neighborhood deprivation that make up the IMD; as has been suggested,47 examining specific features of areas would help to assess the relationship between neighborhood deprivation and individual out-comes. The sensitivity analysis reported suggests that access to resources, although of consequence, does not account for the difference in cognitive function associated with neighborhood deprivation. This is consistent with recent findings that socially disadvantaged neighborhoods do not necessarily have poorer access to health-related community resources,48 and similar analyses using geocoded data to assess levels of access to resources will be useful here. Including the effect of spouse’s or partner’s level of education made no difference to the respondent’s cognitive function, although a related factor that may explain the findings of this study relates to “the advantages of advantaged neighbors.”49 Living among people who are well educated and well off, rather than poorly educated and deprived, may affect one’s cognitive function in a way that operates over and above the effects of one’s own household socioeconomic circumstances.
The cross-sectional nature of the data used limits the conclusions that can be drawn. Analysis of longitudinal data might clarify some aspects of these findings. For example, the effect of neighborhood deprivation on cognition in men younger than 70 is less marked than in the other groups, and it is unclear whether, for example, biological factors, such as differing levels of subclinical cerebrovascular disease, or socioeconomic factors, such as the possibility that men in this age group work outside of the immediate neighborhood and thus experience less exposure to neighborhood factors, may account for this difference. In the absence of such data, it impossible to comment on causality; further research is necessary to identify suitable interventions to address the public health issues identified here.
This study has identified a number of aspects of neighborhood deprivation that are, and a number that are not, related to cognitive function in elderly people. Epidemiological work remains to be done in investigating the relationship between the micro- and macro-level factors impinging on health.50 In relation to these results, observing that living in a deprived neighborhood is associated with poor cognitive functioning is the first step toward identifying and confirming the specific aspects of neighborhood deprivation connected with this outcome and designing appropriate interventions to improve public health.
CONCLUSION
Neighborhood deprivation in urban areas is associated with cognitive function in older adults independent of the effects of individual and household socioeconomic factors. The mechanisms by which neighborhood deprivation influences cognitive function remain unclear and require further investigation. Recognizing and identifying the effects on cognitive health of urban neighborhood deprivation, in societies in which the vast majority of people live in urban or suburban areas, is important.
Acknowledgments
ELSA is cofunded by the U.S. National Institute on Aging and by UK Government departments involved in areas relating to the aging process.
Sponsor’s Role: The funders supported the ELSA study but played no other role in the preparation or submission of this manuscript.
Footnotes
Conflict of Interest: Iain A. Lang is an Academic Specialist Trainee in Public Health supported by the NHS South-West Region Public Health Training Scheme. David J. Llewellyn was supported by grants from the Health Foundation (543/2216) and the Isaac Newton Trust. The authors have no financial disclosure to report.
Author Contributions: Iain A. Lang: analysis and interpretation of data, preparation of manuscript. David J. Llewellyn, Kenneth M. Langa, and David Melzer: interpretation of data, preparation of manuscript. Robert B. Wallace and Felicia A. Huppert: study concept and design, interpretation of data, preparation of manuscript.
References
- 1.Stimpson JP, Ju H, Raji MA, et al. Neighborhood deprivation and health risk behaviors in NHANES III. Am J Health Behav. 2007;31:215–222. doi: 10.5555/ajhb.2007.31.2.215. [DOI] [PubMed] [Google Scholar]
- 2.Winkleby M, Sundquist K, Cubbin C. Inequities in CHD incidence and case fatality by neighborhood deprivation. Am J Prev Med. 2007;32:97–106. doi: 10.1016/j.amepre.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davey Smith G, Hart C, Watt G, et al. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: The Renfrew and Paisley study. J Epidemiol Community Health. 1998;52:399–405. doi: 10.1136/jech.52.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galea S, Ahern J, Nandi A, et al. Urban neighborhood poverty and the incidence of depression in a population-based cohort study. Ann Epidemiol. 2007;17:171–179. doi: 10.1016/j.annepidem.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose D, Richards R. Food store access and household fruit and vegetable use among participants in the US food stamp program. Public Health Nutr. 2004;7:1081–1088. doi: 10.1079/PHN2004648. [DOI] [PubMed] [Google Scholar]
- 6.Pearce J, Blakely T, Witten K, et al. Neighborhood deprivation and access to fast-food retailing: A national study. Am J Prev Med. 2007;32:375–382. doi: 10.1016/j.amepre.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Jackson RJ. The impact of the built environment on health: An emerging field. Am J Public Health. 2003;93:1382–1384. doi: 10.2105/ajph.93.9.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellaway A, Macintyre S, Bonnefoy X. Graffiti, greenery, and obesity in adults: Secondary analysis of European cross sectional survey. BMJ. 2005;331:611–612. doi: 10.1136/bmj.38575.664549.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cagney KA, Browning CR, Wen M. Racial disparities in self-rated health at older ages: What difference does the neighborhood make? J Gerontol B Psychol Sci Soc Sci. 2005;60:S181–S190. doi: 10.1093/geronb/60.4.s181. [DOI] [PubMed] [Google Scholar]
- 10.Aneshensel CS, Wight RG, Miller-Martinez D, et al. Urban neighborhoods and depressive symptoms among older adults. J Gerontol B Psychol Sci Soc Sci. 2007;62:S52–S69. doi: 10.1093/geronb/62.1.s52. [DOI] [PubMed] [Google Scholar]
- 11.Walters K, Breeze E, Wilkinson P, et al. Local area deprivation and urban-rural differences in anxiety and depression among people older than 75 years in Britain. Am J Public Health. 2004;94:1768–1774. doi: 10.2105/ajph.94.10.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schootman M, Andresen EM, Wolinsky FD, et al. Neighborhood conditions and risk of incident lower-body functional limitations among middle-aged African Americans. Am J Epidemiol. 2006;163:450–458. doi: 10.1093/aje/kwj054. [DOI] [PubMed] [Google Scholar]
- 13.Balfour JL, Kaplan GA. Neighborhood environment and loss of physical function in older adults: Evidence from the Alameda County study. Am J Epidemiol. 2002;155:507–515. doi: 10.1093/aje/155.6.507. [DOI] [PubMed] [Google Scholar]
- 14.Espino DV, Lichtenstein MJ, Palmer RF, et al. Ethnic differences in Mini-Mental State Examination (MMSE) scores: Where you live makes a difference. J Am Geriatr Soc. 2001;49:538–548. doi: 10.1046/j.1532-5415.2001.49111.x. [DOI] [PubMed] [Google Scholar]
- 15.Wight RG, Aneshensel CS, Miller-Martinez D, et al. Urban neighborhood context, educational attainment, and cognitive function among older adults. Am J Epidemiol. 2006;163:1071–1078. doi: 10.1093/aje/kwj176. [DOI] [PubMed] [Google Scholar]
- 16.White L, Katzman R, Losonczy K, et al. Association of education with incidence of cognitive impairment in three established populations for epidemiologic studies of the elderly. J Clin Epidemiol. 1994;47:363–374. doi: 10.1016/0895-4356(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 17.Huppert FA, Gardener EA, McWilliams B. Cognitive function. In: Banks J, Breeze E, Lessof C, et al., editors. Retirement, Health and Relationships of the Older Population in England: The 2004 English Longitudinal Study of Ageing (Wave 2) London: Institute for Fiscal Studies; 2006. pp. 217–242. [Google Scholar]
- 18.Cagney KA, Lauderdale DS. Education, wealth, and cognitive function in later life. J Gerontol B Psychol Sci Soc Sci. 2002;57:163–172. doi: 10.1093/geronb/57.2.p163. [DOI] [PubMed] [Google Scholar]
- 19.Gallacher JEJ, Elwood PC, Hopkinson C, et al. Cognitive function in the Caerphilly study: Associations with age, social class, education and mood. Eur J Epidemiol. 1999;15:161–169. doi: 10.1023/a:1007576324313. [DOI] [PubMed] [Google Scholar]
- 20.George LK, Landerman LR, Blazer DG. Cognitive impairment. In: Robbins LN, Regier DA, editors. Psychiatric Disorders in America. New York: Free Press; 1991. pp. 291–327. [Google Scholar]
- 21.Taylor R, Conway L, Calderwood L, et al. Methodology. In: Marmot M, Banks J, Blundell R, et al., editors. Health, Wealth and Lifestyles of the Older Population in England: The 2002 English Longitudinal Study of Ageing. London: Institute for Fiscal Studies; 2003. pp. 357–374. [Google Scholar]
- 22.Raudenbush SW. The quantitative assessment of neighborhood social environments. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. New York: Oxford University Press; 2003. pp. 112–131. [Google Scholar]
- 23.Maunder P, Landes DP, Steen N. The equity of access to primary dental care for children in the North East of England. Community Dent Health. 2006;23:116–119. [PubMed] [Google Scholar]
- 24.Woods LM, Rachet B, Riga M, et al. Geographical variation in life expectancy at birth in England and Wales is largely explained by deprivation. J Epidemiol Community Health. 2005;59:115–120. doi: 10.1136/jech.2003.013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith LK, Draper ES, Manktelow BN, et al. Socioeconomic inequalities in very preterm birth rates. Arch Dis Child Fetal Neonatal Ed. 2007;92:F11–F14. doi: 10.1136/adc.2005.090308. Epub April 4, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leigh Y, Seagroatt V, Goldacre M, et al. Impact of socio-economic deprivation on death rates after surgery for upper gastrointestinal tract cancer. Br J Cancer. 2006;95:940–943. doi: 10.1038/sj.bjc.6603315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Office of the Deputy Prime Minister. The English Indices of Deprivation 2004 (Revised) London: ODPM Publications; 2004. [on-line]. Available at http://www.communities.gov.uk/pub/446/Indicesofdeprivation2004revisedPDF2198Kb_id1128446.pdf. [Google Scholar]
- 28.Bates C. Methodology used for producing ONS’s small area population estimates. Popul Trends. 2006;125:30–36. [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHug PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Ofstedal MB, Fisher GG, Herzog AR. Documentation of Cognitive Functioning Measures in the Health and Retirement Study. HRS Documentation Report DR-006. 2005 March; [Google Scholar]
- 31.Huppert FA, Johnson T, Nickson J. High prevalence of prospective memory impairment in the elderly and in early-stage dementia: Findings from a population-based study. Appl Cogn Psychol. 2000;14(Special Issue) [Google Scholar]
- 32.Richards M, Kuh D, Hardy R, et al. Lifetime cognitive function and timing of the natural menopause. Neurology. 1999;53:308–314. doi: 10.1212/wnl.53.2.308. [DOI] [PubMed] [Google Scholar]
- 33.Launer LJ, Feskens EJM, Kalmijn S, et al. Smoking, drinking, and thinking: The Zutphen elderly study. Am J Epidemiol. 1996;143:219–227. doi: 10.1093/oxfordjournals.aje.a008732. [DOI] [PubMed] [Google Scholar]
- 34.Lang I, Guralnik JM, Wallace RB, et al. What level of alcohol consumption is hazardous for older people? Functioning and mortality in U.S. and English national cohorts. J Am Geriatr Soc. 2007;55:49–57. doi: 10.1111/j.1532-5415.2006.01007.x. [DOI] [PubMed] [Google Scholar]
- 35.Lang I, Wallace RB, Huppert FA, et al. Moderate alcohol consumption in older adults is associated with better cognition and well-being than abstinence. Age Ageing. 2007;36:256–261. doi: 10.1093/ageing/afm001. [DOI] [PubMed] [Google Scholar]
- 36.Barnes DE, Cauley JA, Lui LY, et al. Women who maintain optimal cognitive function into old age. J Am Geriatr Soc. 2007;55:259–264. doi: 10.1111/j.1532-5415.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- 37.Hendrie HC, Albert MS, Butters MA, et al. The NIH cognitive and emotional health project. Alzheimer Dementia. 2006;2:12–32. doi: 10.1016/j.jalz.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 39.Li SC, Dinse HR. Aging of the brain, sensorimotor, and cognitive processes. Neurosci Biobehav Rev. 2002;26:729–732. doi: 10.1016/s0149-7634(02)00059-3. [DOI] [PubMed] [Google Scholar]
- 40.Roberts RE, Kaplan GA, Shema SJ, et al. Does growing old increase the risk for depression? Am J Psychiatry. 1997;154:1384–1390. doi: 10.1176/ajp.154.10.1384. [DOI] [PubMed] [Google Scholar]
- 41.Fechner-Bates S, Coyne JC, Schwenk TL. The relationship of self-reported distress to depressive disorders and other psychopathology. J Consult Clin Psychol. 1994;62:550–559. doi: 10.1037//0022-006x.62.3.550. [DOI] [PubMed] [Google Scholar]
- 42.Cornoni-Huntley J, Ostfeld A, Taylor J, et al. Established populations for epidemiological studies in the elderly: Study design and methodology. Aging Clin Exp Res. 1993;1993:27–37. doi: 10.1007/BF03324123. [DOI] [PubMed] [Google Scholar]
- 43.Ferrucci L, Guralnik JM, Salive ME, et al. Cognitive impairment and risk of stroke in the older population. J Am Geriatr Soc. 1996;44:237–241. doi: 10.1111/j.1532-5415.1996.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 44.Catalano R, Pickett KE. A taxonomy of research concerned with place and health. In: Albrecht GL, Fitzpatrick R, Scrimshaw SC, editors. Handbook of Social Studies in Health and Medicine. Thousand Oaks, CA: Sage Publications; 2000. pp. 64–83. [Google Scholar]
- 45.Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83:1041–1062. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glass TA, Balfour JL. Neighborhoods, aging, and functional limitations. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. Oxford: Oxford University Press; 2003. pp. 303–334. [Google Scholar]
- 47.Diez Roux AV, Mujahid MS, Morenoff JD, et al. Respond to “Beyond the Metrics for Measuring Neighborhood Effects”. Am J Epidemiol. 2007;165:872–783. [Google Scholar]
- 48.Pearce J, Witten K, Hiscock R, et al. Are socially disadvantaged neighbourhoods deprived of health-related community resources? Int J Epidemiol. 2007;36:348–355. doi: 10.1093/ije/dyl267. [DOI] [PubMed] [Google Scholar]
- 49.Jencks C, Mayer SE. The social consequences of growing up in a poor neighborhood. In: Lynn JLE, McGeary MHH, editors. Inner City Poverty in the United States. Washington, DC: National Academy Press; 1990. pp. 111–186. [Google Scholar]
- 50.Galea S, Ahern J. Invited commentary: Considerations about specificity of associations, causal pathways, and heterogeneity in multilevel thinking. Am J Epidemiol. 2006;163:1079–1082. doi: 10.1093/aje/kwj177. [DOI] [PubMed] [Google Scholar]


