Abstract
Tibolone is a synthetic molecule used extensively for the management of menopausal symptoms, with the proposed additional advantage of enhanced mood and libido. Tibolone, after oral administration, is rapidly converted into 3 major metabolites: 3α-hydroxytibolone and 3β-hydroxytibolone, which have estrogenic effects, and the Δ4-isomer, which has progestogenic and androgenic effects. The tissue-selective effects of tibolone are the result of metabolism, of enzyme regulation, and of receptor activation which vary in different tissues. Tibolone seems to be effective on estrogen-withdrawal symptoms such as hot flushes, sweating, insomnia, headache, and vaginal dryness, with results generally comparable to the effects exerted by estrogen-based treatments, and the additional property of a progestogenic activity on the endometrium. As well as relieving vasomotor symptoms, tibolone has positive effects on sexual well-being and mood, and improves dyspareunia and libido. These effects may depend on both estrogenic and androgenic actions exerted at the genital level and in the central nervous system, and on a reduction of sex-hormone-binding globulin and an increase of free testosterone, without affecting Δ-5 androgens levels. Based on the evidence available, tibolone is a valuable treatment option to relieve menopausal complaints, especially in women suffering persistent fatigue, blunted motivation, and loss of sexual desire despite an adequate estrogen replacement.
Keywords: tibolone, menopause, mood, well-being and sexuality
Introduction
The climateric period and the postmenopause are characterized by several endocrine, neuroendocrine, and metabolic modifications that produce a variety of short-term and long-term symptoms. The short-term consequences are represented by vasomotor instability (hot flushes and sweats), psychiatric or neurological disturbances (depression, anxiety, migraine–headhaches, insomnia), and dyslipidemia. Long-term consequences are represented by vaginal dryness dyspareunia, decrease of libido, urinary disturbances (stress incontinence, nicturia), osteoporosis, and increased risk of cardiovascular and cerebral diseases (Sherwin 1997; Genazzani et al 2005; Lauritzen and Studd 2005).
In women, quality of life (QoL) can be seriously affected during the menopausal transition. Since physical and mental health, well-being, social role, and physical capacity are essential dimensions of QoL, the physiological changes that occur around the time of menopause are likely to alter a woman’s perceived QoL (Kuh et al 1997; Short 2003). A large body of evidence suggests that estrogens, progestins, and androgens can induce several effects in different brain areas of the central nervous system (CNS) through binding with specific receptors. The actions of sex hormones are not limited to the regulation of endocrine functions and mating behaviour: experimental and clinical evidence confirms an involvement of sex hormones in controlling well- being, cognitive functions, and memory processes in physiological as well as in pathological conditions (Genazzani et al 1996; McEwen 1999; McEwen and Alves 1999).
Changes in female sex steroids and especially changes in estrogen concentration are thought to be responsible for the occurrence of vasomotor symptoms and other menopause-related complaints. As a result, hormone replacement therapy (HRT) has been suggested to relieve these symptoms and therefore affect QoL (Genazzani 2004).
Although estrogen–progestin replacement therapy is considered the most effective approach to manage menopausal symptoms, several studies have focused on androgen replacement therapy, especially for patients suffering from sexual disorders, loss of pubic and axillary hair, loss of well-being and energy, mood disorders, and metabolic and bone mass effects despite apparently adequate estrogen replacement. All these symptoms are part of the androgen deficiency syndrome (Sherwin 1991; Davis and Burger 1996; Davis 1999, 2002b; Davis and Tran 2001; Rosen et al 2002). Tibolone (Livial®; Organon, Oss, the Netherlands) is a synthetic steroid with estrogenic, progestagenic, and androgenic properties. It is structurally different from estradiol and selective oestrogen-receptor modulators (SERMs) and, unlike the latter compounds, its metabolites play a key role in its mechanism of action. Tibolone is used extensively in Europe and Asia for the management of menopausal symptoms with the proposed additional benefit of improved mood and libido (Albertazzi et al 1998; Davis 2002a; Modelska and Cummings 2002; Kenemans and Speroff 2005). The mechanism of action of tibolone and its effect on mood, well-being, cognition, and sexuality, from basic science to clinical trials, are reviewed.
Pharmacology of tibolone
Tibolone has a 3-keto-Δ5–10 steroid structure with 17α-ethynyl and 7α-methyl groups. It is very rapidly metabolized to 3α-hydroxy tibolone and 3β-hydroxy tibolone by hepatic and intestinal 3α-hydroxysteroid dehy-drogenase and 3β-hydroxysteroid dehydrogenase (HSD). Both hydroxy-metabolites have a half-life of approximately 7 hours, but circulatory levels of the 3α-hydroxy metabo-lite are about 4 times higher than that of the 3β-hydroxy metabolite. These two metabolites are responsible for the estrogenic activity of tibolone. A third metabolite, the Δ4-isomer of tibolone, has progestogenic and androgenic properties (Kloosterboer 2004). It is formed directly from tibolone by 3-HSD-isomerase, for which the 3α-hydroxy metabolite is also a potential substrate. Tibolone is not a substrate for 3β-HSD type I or type II, and the conversion is therefore most likely due to 3β-HSD activity residing in 17β-HSD type II (Suzuki et al 2000). Like the parent compound, the Δ4-isomer is rapidly removed from the circulation. This metabolite can also be formed locally from tibolone in the endometrium, where it prevents estrogenic stimulation (Tang et al 1993).
Tibolone has structural similarities to norethynodrel and norethisterone, but these compounds lack the 7α-methyl group of tibolone that is a key substituent in determining steroid metabolism. The presence of the 7α-methyl group means that the Δ4-isomer is not subject to 5α-reduction and therefore it retains its progestogenic activity in the endometrium for considerably longer (Zdravkovic et al 2001; Timmer and Houwing 2002).
Most metabolites of tibolone (approximately 80%) are in the inactive mono-sulfated and di-sulfated forms, from which active estrogenic 3-hydroxy metabolites may be continuously formed via the sulfatase enzyme, depending on the local enzyme activity level. The 3α-hydroxy sulfated tibolone is the main sulfated metabolite (de Gooyer et al 2001). In transactivation studies, using cells transfected with human estrogen receptor and a –reporter construct, the two 3-hydroxy metabolites of tibolone appear to be full agonists at the human estradiol receptor. Although the 3-hydroxy metabolites are weaker agonists than estradiol, a full estrogen response can be obtained due to the high circulating levels (de Gooyer et al 2003). The 3-hydroxy metabolites of tibolone show a selectivity for the classical estrogen receptor α over the β isoform. Tibolone itself has been shown to slightly activate the estradiol receptor, despite lacking a hydroxyl group and an aromatic A-ring (de Gooyer et al 2003). The Δ4-isomer of tibolone activates the progesterone and androgen receptors but not the estrogen receptor, and has a higher affinity for progesterone receptor type B than for type A. Tibolone and its metabolites have no affinity for the glucocorticoid receptor and indeed show no anti-hormonal activity at any steroid receptors (Schoonen et al 2000). The androgenic activity of the Δ4-isomer may play a role in the liver and brain. In the liver, favorable effects are seen on hemostasis, although the effects on lipids, particularly the decrease in high-density lipoprotein (HDL)-cholesterol, remain controversial. The monkey studies of Clarkson’s group indicate, however, that the tibolone-induced reduction in HDL-cholesterol does not translate into unfavorable effects on the cardiovascular system (Register et al 2002). The favorable effects on the brain, such as improvements in mood and libido, may be a reflection of the androgenicity of the Δ4-metabolite.
In contrast to its actions in other tissues such as bone, in the breast tibolone inhibits the sulfatase enzyme, and the sulfated metabolites in breast tissue are not activated. Furthermore, this tissue-specific sulfatase inhibition may reduce desulfation of estrone sulfate by breast tissue. Tibolone inhibits human breast cell proliferation and stimulates apoptosis (Gompel et al 1997). The incidence of breast tenderness is low and mammographic density does not increase with tibolone, which is unlike classical hormone replacement (Hammar et al 1998; Valdivia and Ortega 2000).
The conclusion is that tibolone has a selective mode of action, and that it belongs to a separate class of drugs. Tibolone can be characterized as a selective tissue-estrogenic-activity regulator (STEAR). In the term STEAR, the focus is on the estrogenic activity, which is expressed in a tissue-selective manner and in which steroid metabolism plays a pivotal role in determining ligand availability for the receptor (Reed and Kloosterboer 2004).
Tibolone, mood, and well-being
Menopause-associated symptoms impair QoL for many women. More than 75% of postmenopausal women experience hot flushes and sweating. Other symptoms, such as insomnia, headache, or fatigue, as well as changes in mood and libido, may result directly from menopause or indirectly, such as effects of hot flushes on sleep disturbances. Throughout the climacteric period, the decline in ovarian sex steroid production is associated with a change in the turnover of various neurotransmitters and neuropeptides, which is improved by estrogen administration. Estrogen administration positively affects not only vasomotor instability, thus reducing the number and intensity of hot flushes and sweats, but also seems to ameliorate the psychological disturbances of menopause, such as depression, and sexual and affective behavior disorders (Genazzani 2004).
The effects of estrogen on mood in postmenopausal women are mostly related to the direct effect of estrogen on neural activity and to the modulation of adrenergic, dopaminergic, and serotoninergic tone (McEwen and Alves 1999). This estrogen action increases also the action of drugs that act on serotonin, such as selective serotonin reuptake inhibitors (SSRIs) (Stahl 2001).
No data are available on the direct effect of tibolone on central neurotransmitter levels. However, tibolone did not enhance the antidepressant effect of the SSRI fluoxetine in a group of postmenopausal patients with major depressive episodes (Berlanga et al 2003).
Estrogens directly modulate endogenous opioid activity as well as directly stimulating opioid receptor expression and by acting on the opiatergic pathway. Circulating modifications of β-erythropoietin (Ep) levels may be considered markers of neuroendocrine function. A decrease in plasma β-Ep levels has been detected in postmenopausal women after surgical or spontaneous menopause (Aleem and McIntosh 1985), and this decrease has been suggested to have a role in the mechanisms of hot flushes and sweats episodes (Linghtman et al 1981; Genazzani et al 1984). The decrease in plasma β-Ep levels has also been related to the pathogenesis of mood, behaviour, and nociceptive disturbances occurring in this period (Petraglia et al 1993). Indeed, a positive role of HRT on vasomotor and subjective psychobehavioral symptoms may be mediated by acting on the opiatergic pathway (Genazzani et al 1990; Petraglia et al 1993). In fact, oral estrogen replacement therapy (ERT) after spontaneous or surgically induced menopause is followed by a significant increase in circulating β-endorphin levels (Genazzani et al 1988; Stomati et al 1997). It has been demonstrated that the oral administration of tibolone in ovariectomized rats is able to increase the β-endorphin levels in pituitary and plasma similarly to the administration of estradiol benzoate (Genazzani et al 1987).
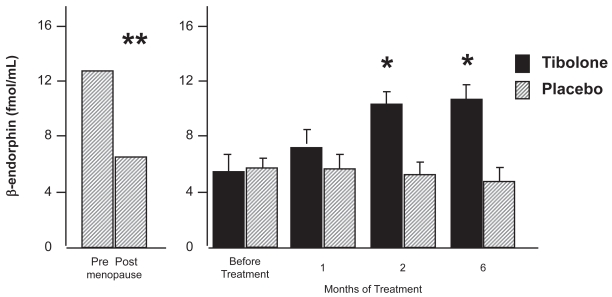
The effects of tibolone on β-endorphin and β-lipotropin (LPH) have been compared with conjugated equine estrogens (CEE) and placebo in symptomatic postmenopausal women. After 2 months of treatment both groups of patients who underwent steroid supplementation showed circulating levels of β-Ep and β-LPH higher than basal levels. Tibolone treatment increased β-Ep and β-LPH levels more than conjugated estrogens at the 2nd month of therapy. No further change was found after 4 months (Genazzani et al 1988) (Figure 1). Both treatments alleviated hot flushes and physical and psychological symptoms, but the effects of tibolone may involve not only estrogenic action but also androgenic effects, because testosterone modulates the amount of β-endorphin in the anterior pituitary in castrated male rats, and women with polycystic ovarian disease, which is characterized by hyperandrogenism, have elevated plasma β-endorphin levels (Aleem and McIntosh 1984; Forman et al 1985).
Figure 1.
β-endorphin plasma level during tibolone administration. Adapted from Genazzani et al (2005).
*p < 0.05; **p < 0.001 among groups.
While steroid-metabolizing enzymes induce the CNS able to modify circulating steroids, the CNS is also able to synthesize steroids from cholesterol, at least in part, independently of peripheral steroidogenic gland secretion (Baulieu 1991), leading to the production of a series of potent steroidal compounds. These brain-produced steroids have been named “neurosteroids”, and have been found to exert important regulatory actions on neurons and glial cells (Baulieu 1997; Baulieu et al 2001).
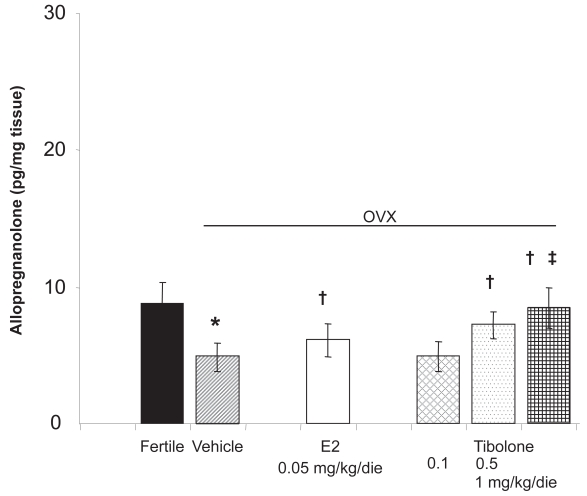
Several studies have shown that some psychological functions and symptoms such as depression, anxiety, irritability, and affectivity can affect the fluctuation of the synthesis and the release of the neurosteroids and in particular of allopreg-nanolone and dehydroepiandrosterone (DHEA) (Majewska 1992). Allopregnanolone acts as an agonist on γ-aminobutyric acidA (GABAA) receptors, modulating stress, mood, and behavior with anxiolytic, sedative, and antiepileptic effects (Genazzani et al 1995). Ovariectomy significantly decreases allopregnanolone levels both in serum and in the CNS while it increases the adrenal content of allopregnanolone. On the other hand, when 17β-estradiol is administered to castrated female rats allopregnanolone levels increase in the hippocampus, hypothalamus, pituitary, and serum, while they decrease in adrenals (Genazzani et al 1995; Stomati et al 2002). Tibolone administration in ovariectomized rats induces a significant increase in CNS allopregnanolone content particularly in the frontal lobe, parietal lobe, hippocampus, and hypothalamus (Figure 2). Similarly, the serum levels of allopregnanolone increase while its levels in adrenal glands decrease significantly after treatment (Genazzani et al in press). The effects of tibolone on allopregnanolone levels have been compared with different hormonal treatments in postmenopausal women. After 1 month, all patients who underwent tibolone and estrogen-based supplementation showed circulating levels of allopregnanolone higher than basal levels. After 1 year of treatment, tibolone increased allo-pregnanolone levels, but to a lesser extent than transdermal estradiol, CEE+medroxyprogesterone acetate (MPA), and estradiol (E2)+norethisterone acetate (NETA). These results let us speculate that mood changes described after HRT as well as after tibolone administration might depend also on the increased levels of allopregnanolone, a neurosteroid with sedative and anxiolytic properties (Pluchino et al 2005).
Figure 2.
Hippocampal levels of allopregnanolone during tibolone and estradiol administration in ovariectomized (OVX) rats. Adapted from Genazzani et al (2006).
*p < 0.05 vs fertile; † p < 0.05 vs vehicle; ‡ p < 0.05 vs estradiol.
Several other clinical studies have examined the impact of tibolone administration on mood scales and QoL-measuring questionnaires. In a placebo-controlled, crossover study of 256 postmenopausal women, total weekly mood score improved, measured using a 16-item visual analog scale (Tax et al 1987). In a randomized, double-blind study Crona et al (1988) found that tibolone and E2 valerate reduced hot flushes and improved mood to a similar degree (Crona et al 1988). In another randomised, double-blind, placebo-controlled study in healthy postmenopausal women, assessing QoL with the Nottingham Health Profile, tibolone-treated women showed a positive trend compared with placebo, especially for sleep and physical mobility. However, the sample size of this study was not sufficient to show statistical significance, considering the high individual variation in QoL measures (Meeuwsen 2002).
Ross et al (2000) investigated the psychological effects of tibolone versus CEE 0.625 mg/day with cyclical norgestrel 150 μg using the Women’s Health Questionnaire and the Irritability, Depression, and Anxiety Scale. Both treatments improved psychological symptoms to a similar extent. In conclusion, basic science and clinical studies have reported mainly beneficial effects of tibolone on mood and well-being generally, comparable to those of estrogen-based treatments.
Tibolone and cognition
The term cognition encompasses the spectrum of intellectual abilities, including attention, learning and memory, language, perception, abstract reasoning, and judgment. There is much evidence that estrogen may affect cognitive skills. However, even if one assumes the validity of positive findings, it is still important to consider whether the estrogen effects might be mediated indirectly. Both depression and anxiety can affect cognitive performance in older persons, and estrogen may mediate mood and reduce stress. It is likely that estrogen does influence cognitive skills independently of mood, but this caveat should be heeded in interpreting clinical data.
Pan et al compared cognitive function in postmenopausal women receiving continuous HRT CEE (0.625 mg/day) and MPA (5 mg/day), and women receiving tibolone (2.5 mg/day) in a prospective, single-blind, randomized study. The Cognitive Abilities Screening Instrument (CASI) and Mini-Mental State Examination (MMSE) scores of the CEE+MPA group and of the tibolone group after 3 and 6 months of treatment showed an increasing trend in CASI and MMSE levels after treatment, although the increases were not statistically significant. The rate of increase of both CASI and MMSE scores in the CEE+MPA group was greater than in the tibolone group, though again the difference was not significant. The authors conclude that tibolone can preserve cognitive function and may be able to prevent cognitive decline in postmenopausal women during short-term treatment (Pan et al 2003). In an open study, Fluck et al investigated the cognitive performance of 25 women who had been taking tibolone (2.5 mg/day) for approximately 10 years. Each woman in this group was pair-matched to one in the control group on age, years since menopause, IQ, years of secondary education, and occupation. The tibolone group did not show the increase in stress, anxiety, and mental slowness that the control group showed, after performing the Paced Auditory Serial Addition Test (PASAT). These effects resulted in an enhanced cortisol response to the stress of cognitive testing. It has been demonstrated that young women and postmenopausal women taking estrogens show a marked increase in anxiety after the PASAT, whereas this is not shown by young men. This raises the interesting possibility that the protective effect of tibolone against stress might be due to the activation of the androgen receptors by the Δ4-isomer (Fluck et al 2002). In addition, the group taking tibolone had significantly better semantic memory (memory for facts), as assessed in a category generation task, but they did not differ in tests of episodic memory (memory for events). Semantic memory represents our knowledge of the world and is an elaborated form of memory that is not tied to a specific place or time. The improvement in this function that was found in the women treated with tibolone could have very real benefits in everyday life. An unexpected finding was that the tibolone group performed significantly worse on a sustained attention task and a planning task, properties that are associated with frontal lobe function (Fluck et al 2002).
Similar results have been reported by another randomized study that investigated the effects of tibolone and E2+NETA administration on cognitive function in post-menopausal women. Both groups showed an improvement of semantic memory, but only the E2+NETA treatments showed an effect on recognition memory (Albertazzi et al 2000).
In conclusion, results from these studies suggest that tibolone potentially confers benefits on cognitive performance. However, several variables can affect cognition in postmenopausal women such as the type of HRT, the duration of treatment, the nature of the cognitive test, and the underlying brain structure mediating the particular cognitive function. Further long-term and specifically designed studies are needed of such effects.
Tibolone and sexuality
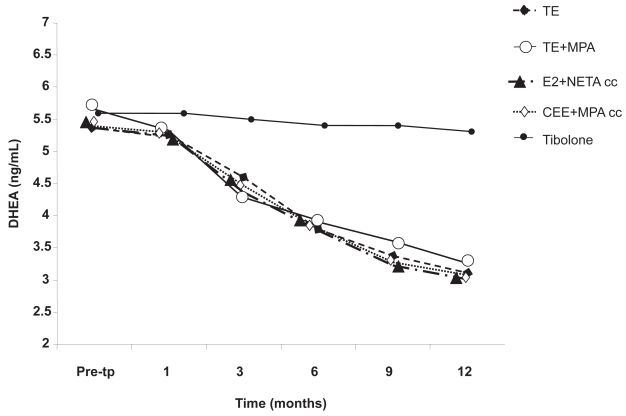
Sex hormones play a crucial role in maintaining the anatomical and functional integrity of all the structures involved in women’s sexual response. While the role of estrogen on the activity of neuroendocrine circuitries and on the trophism of genital organs has been well established, the contribution of androgens to female physical and mental well-being is still a matter of debate (Davis and Burger 1996). A large number of studies suggest that tibolone may improve sexual function. This is plausible because testosterone (T) has been shown to increase libido and frequency of sexual activities, and tibolone has androgenic activity. Specifically, as noted earlier, the Δ4-isomer of tibolone stimulates the androgen receptor. In addition, tibolone may act indirectly to decrease sex-hormone-binding globulin (SHBG) concentrations and thereby increase the availability of T. Postmenopausal women have, on the average, 30% lower circulating T levels than those measured in premenopausal women (Davis and Burger 1996; Davis and Tran 2001). Dören and colleagues found that women treated with tibolone had higher free T levels and lower SHBG levels than women treated with E2+NETA (Dören et al 2001). Recent data have demonstrated that circulating levels of DHEA decrease progressively and significantly during 12 months of treatment using various estrogen or estrogen-progestin molecules, regimens, and routes of administration. On the contrary, tibolone does not induce any modification in circulating levels of DHEA. As consequence, unlike estrogen-based therapy, tibolone does not affect the androgen milieu in postmenopausal women and does not aggravate the physiological androgen deficiency syndrome (Pluchino et al 2005) (Figure 3).
Figure 3.
Dehydroepiandrosterone (DHEA) serum in postmenopausal women treated with different estrogen or estrogen–progestin therapies and tibolone after 1, 3, 6, 9, and 12 months of treatment. Adapted from Pluchino et al (2005).
Abbreviations: CEE, conjugated equine estrogens; E2, estradiol; MPA, medroxyprogesterone acetate; NETA, norethisterone acetate; TE, transdermal estradiol.
A recent double-blind, placebo-controlled trial by Laan and van Lunsen (2001) showed that treatment with tibolone significantly improved the physiological aspects of sexual function in postmenopausal women and subjective measures such as sexual desire and arousability. There was no difference in frequency of sexual intercourse, nonpenetrative sexual activity, or initiation and rejection of sexual activity between women who were taking tibolone versus those receiving placebo. However, the small sample size in the subgroups may have diminished the power to detect statistical differences between these variables. In another trial, an improvement in sexual function with regard to frequency, satisfaction, and enjoyment compared with baseline was recognized (Nathorst-Böös and Hammar 1997). Castelo-Branco et al, in a prospective study, enrolled 120 surgical postmenopausal women who received oral E2 valerate (4 mg/day) with dihydroandrosterone enanthate (monthly, intramuscularly), transdermal 17β-estradiol (50 μg/day), tibolone (2.5 mg/day), or placebo for 12 months. Sexuality improved significantly with all treatments, but the combined regimen of androgens and ERT increased sexual activity in postmenopausal women equal to that of tibolone and to a greater extent than ERT alone (Castelo-Branco et al 2000). These data, together with the recent observation that tibolone significantly increases vaginal pulse amplitude at baseline and following erotic stimulation compared with placebo (Laan and van Lunsen 2001), further supports the notion that such a tissue-specific compound is a good therapeutic option to relieve low libido, arousability, and lubrication at menopause because of both its estrogenic and androgenic properties.
Conclusion
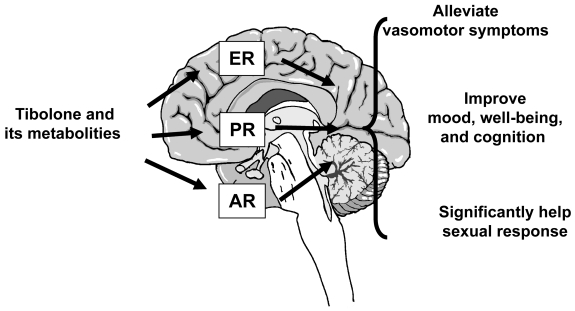
Data originating from basic science and from clinical trials highlight that tibolone exerts encouraging effects on climacteric symptoms. These effects have been attributed to its unique molecular profile and to the tissue-related metabolism into estrogenic, progestogenic, and androgenic metabolities (Figure 4). In particular, tibolone seems to be effective on estrogen-withdrawal symptoms such as hot flushes, sweating, and vaginal dryness, with results generally comparable to the effects exerted by estrogen-based treatments. In addition, during postmenopause women commonly report symptoms related to both estrogen and androgen deprivations such as low libido, persistent fatigue, blunted motivation, and a general reduced sense of well-being. Evidence implies that tibolone is able to increase arousal and libido in postmenopausal women to a greater extent than estrogen therapy alone. These effects may be dependent on both estrogenic and androgenic actions exerted at the genital level and in the CNS, and to a reduction of SHBG and an increase of free T, without affecting Δ-5 androgens levels. Data on the effects of tibolone on cognitive function are not sufficient to establish if estrogen-based therapies are more effective than tibolone in improving cognitive processes in postmenopausal women. Further long-term and specifically designed studies are needed on such effects.
Figure 4.
Central effects of tibolone administration.
Abbreviations: AR, androgen receptors; ER, estradiol receptors; PR, progesterone receptors.
In conclusion, the pharmacological profile and the clinical effects of tibolone make this synthetic steroid an additional option for clinicians in selecting the most appropriate treatment to counteract individually the physical and psychological symptoms of each postmenopausal woman.
Disclosures
Dr Andrea R Genazzani is a Full Professor of Obstetrics and Gynecology at the University of Pisa, Italy. He has received and receives research support, grants, and occasional honoraria from Bracco, Eli Lilly and Company, Igea, Lunar Corporation, MSD, Novartis, Novo Nordisk, Organon, Pfizer, Procter & Gamble Pharmaceuticals, Schering, Solvay, and Wyeth.
References
- Albertazzi P, Di Micco R, Zanardi E. Tibolone: a review. Maturitas. 1998;30:295–305. doi: 10.1016/s0378-5122(98)00059-0. [DOI] [PubMed] [Google Scholar]
- Albertazzi P, Natale V, Barbolini C, et al. The effect of tibolone versus continuous combined norethisterone acetate and oestradiol on memory, libido and mood of postmenopausal women: a pilot study. Maturitas. 2000;36:223–9. doi: 10.1016/s0378-5122(00)00147-x. [DOI] [PubMed] [Google Scholar]
- Aleem F, McIntosh T. Elevated levels of plasma beta-endorphin in a group of women with polycystic ovarian disease. Fertil Steril. 1984;42:686–9. [PubMed] [Google Scholar]
- Aleem FA, McIntosh T. Menopausal syndrome: plasma levels of – endorphin in postmenopausal women using a specific radioim-munoassay. Maturitas. 1985;13:76–84. doi: 10.1016/0378-5122(85)90056-8. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a new function in the brain. Biol Cell. 1991;71:3–10. doi: 10.1016/0248-4900(91)90045-o. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- Baulieu EE, Robel P, Schumacher M. Neurosteroids: beginning of the story. Int Rev Neurobiol. 2001;46:1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- Berlanga C, Mendieta D, Alva G, et al. Failure of tibolone to potentiate the pharmacological effect of fluoxetine in postmenopausal major depression. J Womens Health (Larchmt) 2003;12:33–9. doi: 10.1089/154099903321154121. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco C, Vicente JJ, Figueras F, et al. Comparative effects of estrogens plus androgens and tibolone on bone, lipid pattern and sexuality in postmenopausal women. Maturitas. 2000;34:161–8. doi: 10.1016/s0378-5122(99)00096-1. [DOI] [PubMed] [Google Scholar]
- Crona NGL, Samsioe UB, Silfverstolpe G. Treatment of climacteric complaints with Org OD 14: a comparative study with oestradiol valerate and placebo. Maturitas. 1988;9:303–8. doi: 10.1016/0378-5122(88)90095-3. [DOI] [PubMed] [Google Scholar]
- Davis S. Androgen replacement therapy: a commentary. J Clin Endocrinol Metab. 1999;84:1886–91. doi: 10.1210/jcem.84.6.5802. [DOI] [PubMed] [Google Scholar]
- Davis SR. The effects of tibolone on mood and libido. Menopause. 2002a;9:162–70. doi: 10.1097/00042192-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Davis SR. When to suspect androgen deficiency other than at menopause. Fertil Steril. 2002b;77:S68–71. doi: 10.1016/s0015-0282(02)02977-1. [DOI] [PubMed] [Google Scholar]
- Davis SR, Burger H. Androgen and postmenopausal women. J Clin Endocrinol Metab. 1996;81:2759–63. doi: 10.1210/jcem.81.8.8768824. [DOI] [PubMed] [Google Scholar]
- Davis S, Tran J. Testosterone influences libido and well-being in women. Trends Endocrinol Metab. 2001;12:33–7. doi: 10.1016/s1043-2760(00)00333-7. [DOI] [PubMed] [Google Scholar]
- de Gooyer ME, Deckers GH, Schoonen WGEJ, et al. Receptor profiling and endocrine interactions of tibolone. Steroids. 2003;68:21–30. doi: 10.1016/s0039-128x(02)00112-5. [DOI] [PubMed] [Google Scholar]
- de Gooyer ME, Overklift Vaupel Kleyn GT, Smits KC, et al. Tibolone: a compound with tissue specific inhibitory effects on sulfatase. Mol Cell Endocrinol. 2001;183:55–62. doi: 10.1016/s0303-7207(01)00606-2. [DOI] [PubMed] [Google Scholar]
- Dören M, Ruebig A, Holzgreve W. Differential effects on the androgen status of postmenopausal women treated with tibolone and continuous combined estradiol and norethindrone acetate replacement therapy. Fertil Steril. 2001;75:554–8. doi: 10.1016/s0015-0282(00)01768-4. [DOI] [PubMed] [Google Scholar]
- Fluck E, File SE, Rymer J. Cognitive effects of 10 years of hormone-replacement therapy with tibolone. J Clin Psychopharmacol. 2002;22:62–7. doi: 10.1097/00004714-200202000-00010. [DOI] [PubMed] [Google Scholar]
- Forman L, Hartwell M, Cater J. Beta-endorphin in the male rat pituitarytestosterone influences the effects of cocaine. Brain Res Bull. 1985;25:65–8. doi: 10.1016/0361-9230(90)90253-v. [DOI] [PubMed] [Google Scholar]
- Genazzani AR. HRT In Climacteric and aging brain. London: The Parthenon Publishing Group; 2004. [Google Scholar]
- Genazzani AR, Bernardi F, Pluchino N, et al. Endocrinology of menopausal transition and its brain implications. CNS Spectr. 2005;10:449–57. doi: 10.1017/s1092852900023142. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Bernardi F, Pluchino N, et al. Effect of tibolone administration on central and peripheral levels of allopregnanolone and β-endorphin in female rats. Menopause. 2006;13:57–64. doi: 10.1097/01.gme.0000191372.79052.d3. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Palumbo MA, de Micheroux AA, et al. Evidence for a role for the neurosteroid allopregnanolone in the modifications of reproductive function in female rats. Eur J Endocrinol. 1995;133:375–80. doi: 10.1530/eje.0.1330375. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Facchinetti F, et al. Increase of proopiomelanocortin-related peptides during subjective menopausal flushes. Am J Obstet Gynecol. 1984;149:775–9. doi: 10.1016/0002-9378(84)90121-2. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Facchinetti F, et al. Effects of Org OD 14 on pituitary and peripheral beta-endorphin in castrated rats and post-menopausal women. Maturitas. 1987;(Suppl 1):35–48. doi: 10.1016/0378-5122(87)90041-7. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Facchinetti F, et al. Steroid replacement increase beta-endorphin and beta-lipotropin plasma levels in postmenopausal women. Gynecol Obstet Invest. 1988;26:153–9. doi: 10.1159/000293687. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Mercuri N, et al. Effect of steroid hormones and antihormones on hypothalamic beta-endorphin concentrations in intact and castrated female rats. J Endocrinol Invest. 1990;13:91–6. doi: 10.1007/BF03349515. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Purdy RH. The brain: source and target for sex steroid hormones. London: The Parthenon Publishing Group; 1996. [Google Scholar]
- Gompel A, Kandouz M, Siromachkova M, et al. The effects of tibolone on proliferation, differentiation and apoptosis in human breast cells. Gynecol Endocrinol. 1997;11(Suppl 1):77–9. [Google Scholar]
- Hammar M, Christau S, Nathorst-Boos J, et al. A double blind randomised trial comparing the effects of tibolone and continuous combined hormone replacement therapy in postmenopausal women with menopausal symptoms. Br J Obstet Gynaecol. 1998;105:904–11. doi: 10.1111/j.1471-0528.1998.tb10237.x. [DOI] [PubMed] [Google Scholar]
- Kenemans P, Speroff L for the International Tibolone Consensus Group. Tibolone: Clinical recommendations and practical guidelines. A report of the International Tibolone Consensus Group 2005. Maturitas. 2005;16(51):21–8. doi: 10.1016/j.maturitas.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Kloosterboer HJ. Tissue-selectivity: the mechanism of action of tibolone. Maturitas. 2004;48(Suppl 1):S30–40. doi: 10.1016/j.maturitas.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Kuh D, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstet Gynaecol. 1997;104:923–33. doi: 10.1111/j.1471-0528.1997.tb14352.x. [DOI] [PubMed] [Google Scholar]
- Laan E, van Lunsen RH. The effects of tibolone on vaginal blood flow, sexual desire and arousability in postmenopausal women. Climacteric. 2001;4:28–41. [PubMed] [Google Scholar]
- Lauritzen C, Studd J. Current management of menopause. London: Taylor and Francis; 2005. [Google Scholar]
- Linghtman SL, Jacobs HS, Maguire AK. Climateric flushing: clinical and endocrine response to infusion of naloxone. Br J Obstet Gynaecol. 1981;88:919–24. doi: 10.1111/j.1471-0528.1981.tb02229.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABA A receptors. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–95. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SH. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279– 307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Clinical review 108: The molecular and neuroanatomical basis for estrogen effects in the central nervous system. J Clin Endocrinol Metab. 1999;84:1790–7. doi: 10.1210/jcem.84.6.5761. [DOI] [PubMed] [Google Scholar]
- Meeuwsen IB, Samson MM, Duursma SA, et al. The influence of tibolone on quality of life in postmenopausal women. Maturitas. 2002;41:35–43. doi: 10.1016/s0378-5122(01)00251-1. [DOI] [PubMed] [Google Scholar]
- Modelska K, Cummings S. Tibolone for Postmenopausal Women: Systematic review of randomized trials. J Clin Endocrinol Metab. 2002;87:16–23. doi: 10.1210/jcem.87.1.8141. [DOI] [PubMed] [Google Scholar]
- Nathorst-Böös J, Hammar M. Effect on sexual life–a comparison between tibolone and a continuous estradiol-norethisterone acetate regimen. Maturitas. 1997;26:15–20. doi: 10.1016/s0378-5122(96)01069-9. [DOI] [PubMed] [Google Scholar]
- Pan HA, Wang ST, Pai MC, et al. Cognitive function variations in postmenopausal women treated with continuous, combined HRT or tibolone. A comparison. J Reprod Med. 2003;48:375–80. [PubMed] [Google Scholar]
- Petraglia F, Comitini G, Genazzani AR. Endorphin in human reproduction. In: Herz A, editor. Opioids II. Berlin: Springer-Verlag; 1993. pp. 763–80. [Google Scholar]
- Pluchino N, Genazzani AD, Bernardi F, et al. Tibolone, transdermal estradiol or oral estro-progestin therapies: effects on circulating allopregnanolone, cortisol and DHEA levels. Gynecol Endocrinol. 2005;20:144–9. doi: 10.1080/09513590400021169. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Kloosterboer HJ. Tibolone: a selective tissue estrogenic activity regulator (STEAR) Maturitas. 2004;48(Suppl 1):S4–6. doi: 10.1016/j.maturitas.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Register TC, Wagner JD, Zhang L, et al. Effects of tibolone and conventional hormone replacement therapies on arterial and hepatic cholesterol accumulation and on circulating endothelin-1, vascular cell adhesion molecule-1, and E-selectin in surgically menopausal monkeys. Menopause. 2002;9:411–21. doi: 10.1097/00042192-200211000-00006. [DOI] [PubMed] [Google Scholar]
- Rosen R, et al. Androgen Insufficiency in women: the Princeton Conference. Fertil Steril. 2002;77(Suppl 4):S1–99. [PubMed] [Google Scholar]
- Ross R, Paganni Hill A, Wan P, et al. Effect of hormone replacement therapy on breast cancer risk; estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328–32. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- Schoonen WG, Deckers GH, de Gooijer ME, et al. Hormonal properties of norethisterone, 7alpha-methyl-norethisterone and their derivatives. J Steroid Biochem Mol Biol. 2000;74:213–22. doi: 10.1016/s0960-0760(00)00125-4. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. J Clin Endocrinol Metab. 1991;72:336–43. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen effects on cognition in menopausal women. Neurology. 1997;48:S21–6. doi: 10.1212/wnl.48.5_suppl_7.21s. [DOI] [PubMed] [Google Scholar]
- Short M. Menopause, mood and management. Climacteric. 2003;6(Suppl 2):33–6. [PubMed] [Google Scholar]
- Stahl SM. Sex and psychopharmacology. Is natural estrogen a psychotropic drug in women? Arch Gen Psychiatry. 2001;58:537. doi: 10.1001/archpsyc.58.6.537. [DOI] [PubMed] [Google Scholar]
- Stomati M, Bernardi F, Luisi S, et al. Conjugated equine estrogens, estrone sulphate and estradiol valerate oral administration in ovariectomized rats: effects on central and peripheral allopregnanolone and beta-endorphin. Maturitas. 2002;43:195–206. doi: 10.1016/s0378-5122(02)00205-0. [DOI] [PubMed] [Google Scholar]
- Stomati M, Bersi C, Rubino S, et al. Neuroendocrine effects of different estradiol-progestin regimens in postmenopausal women. Maturitas. 1997;28:127–35. doi: 10.1016/s0378-5122(97)00073-x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Andersson S, et al. 3ß-Hydroxysteroid dehydrogenase/~5 ‡4-isomerase activity associated with the human 17ß-hydroxysteroid dehydrogenase type II isoform. J. Clin Endocrino Metab. 2000;85:3669–72. doi: 10.1210/jcem.85.10.6918. [DOI] [PubMed] [Google Scholar]
- Tang B, Markiewicz L, Kloosterboer HJ, et al. Human endometrial 3 beta-hydroxysteroid dehydrogenase/isomerase can locally reduce intrinsic estrogenic/progestagenic activity ratios of a steroidal drug (Org OD 14) J Steroid Biochem Mol Biol. 1993;45:345–51. doi: 10.1016/0960-0760(93)90003-f. [DOI] [PubMed] [Google Scholar]
- Tax L, Goorissen E, Kicovic P. Clinical profile of Org OD 14. Maturitas. 1987;(Suppl 1):3–13. doi: 10.1016/0378-5122(87)90038-7. [DOI] [PubMed] [Google Scholar]
- Timmer C, Houwing NS. Dose proportionality of three different doses of tibolone. Pharmacotherapy. 2002;22:6–13. doi: 10.1592/phco.22.1.6.33495. [DOI] [PubMed] [Google Scholar]
- Valdivia I, Ortega D. Mammographic density in postmenopausal women treated with tibolone, estriol or conventional hormone replacement therapy. Clin Drug Inv st. 2000;20:101–7. doi: 10.2165/00044011-200020020-00005. [DOI] [PubMed] [Google Scholar]
- Zdravkovic M, Mueller M, Larsen S, et al. Bioequivalence and relative bioavailability of three estradiol and norethisterone acetate-containing hormone replacement therapy tablets. Int J Clin Pharmacol Ther. 2001;39:41–6. doi: 10.5414/cpp39041. [DOI] [PubMed] [Google Scholar]