Abstract
Obstructive sleep apnea (OSA) is a highly significant condition based both on the high prevalence in community and significant consequences. Obstructive sleep apnea syndrome (OSAS), OSA together with hypersomnolence, is seen in 4% of middle-aged men and 2% of middle-aged women. OSA is associated with impaired quality of life and increased risks of motor vehicle accidents, cardiovascular disease (including hypertension and coronary artery disease), and metabolic syndrome. There is some evidence for the use of conservative interventions such as weight loss and position modification. CPAP remains the mainstay of treatment in this condition with high-level evidence supporting its efficacy. Continuous positive airway pressure (CPAP) is an intrusive therapy, with long-term adherence rates of less than 70%. Dental appliances have been shown to be effective therapy in some subjects but are limited by the inability to predict treatment responders. Alternative treatments are discussed but there is little role for upper airway surgery (except in a select few experienced institutions) or pharmacological treatment. The current levels of evidence for the different treatment regimens are reviewed.
Keywords: obstructive sleep apnea, treatment, review
Introduction
Obstructive sleep apnea (OSA) is a condition characterized by repetitive upper airway obstruction during sleep, resulting in arousal from sleep, sleep fragmentation, and variable arterial oxygen desaturation (Figure 1). The condition occurs in up to 24% of men and 9% of women (Young et al 1993) and is increased in prevalence in males, increasing age and obesity. Obstructive sleep apnea syndrome (OSAS), the combination of respiratory disturbance during sleep and symptoms, particularly daytime hypersomnolence, occurs in 4% of men and 2% of women based on large population studies (Young et al 1993).
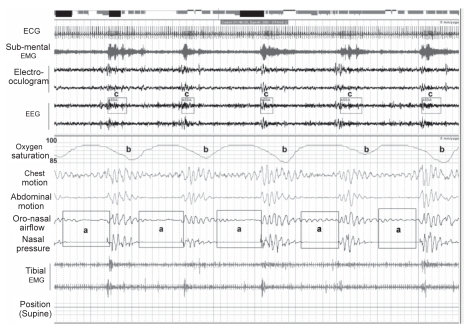
Figure 1.
Five-minute epoch of supine Stage II sleep in a subject with severe OSA. Cessation (apnea) in breathing (a) result in arterial oxygen desaturation (b) and EEG arousal from sleep (c). Chest wall motion continues during the apneas indicating that the events are due to upper airway obstruction.
The upper airway obstruction (apneas) or narrowing (hypopneas) occurs in the muscular pharynx with airway narrowing occurring predominantly in the lateral dimension (Bijaoui et al 2002; Akan et al 2004). The pathophysiology of OSA is thought to involve the loss of compensatory pharyngeal muscle dilator activity during sleep in subjects with anatomically compromised pharyngeal airways. Subjects with OSA tend to have smaller airway dimensions (Bradley et al 1986; Isono et al 1997). Subjects with OSA compared with those without have been shown to have a lower-set hyoid bone even after correction for obesity (Riha et al 2005a). Pharyngeal dilator muscle activity, best studied in the genioglossus and tensor palatini muscles, is increased in wakefulness in subjects with OSA (Mezzanotte et al 1992; Fogel et al 2001) but this compensatory augmented activity is lost at sleep onset (Mezzanotte et al 1996; Fogel et al 2003, 2005) leading to upper airway obstruction during sleep. The control of dilator muscle activity is complex and may demonstrate phasic activity (increased activation during inspiration) or tonic activity (similar activation throughout inspiration and expiration) or both. Both phasic and tonic genioglossus activity is increased in subjects with OSA (Fogel et al 2001) and is dependent upon both local mechanisms, most likely as a reflex response to negative airway pressure (Malhotra et al 2000; Fogel et al 2001), and central activation by medullary respiratory neurons shortly before diaphragmatic activation (Horner 2000). There have been some recent insights into the neurochemical control of the upper airway dilator muscles. Most focus has been on serotonin, which exerts an excitatory effect on upper airway motor dilator neurons via serotonin receptor subtypes 2A and 2C in animal models (Fenik and Veasey 2003). Endogenous serotonin, however, appears to have minimal effect on genioglossus activity across sleep-wake states unless augmented by the upper airway reflex inputs (Sood et al 2005).
Significance of OSA
The cardinal symptoms of OSAS are sleepiness–fatigue or unrefreshing sleep as a consequence of the sleep fragmentation due to upper airway obstruction during sleep. The correlation between the frequency of respiratory events or EEG arousals from sleep and reported sleepiness is not tight (Gonsalves et al 2004), indicating that other factors than sleep disturbance are involved, which may include subcortical arousals, poor perception of sleepiness, differences in the biological susceptibility to sleep disturbance, and sleep habits. Chervin et al (2005) recently reported the presence of respiratory cycle related electro-encephalographic changes, brief changes to cortical activity that occur on a breath-by-breath basis in non-apneic sleep, in 38 adults evaluated by polysomnography. The variation in sigma EEG power within the respiratory cycle was most predictive of next day sleepiness, particularly in those subjects with underlying OSA. The authors speculated that the changes reflected numerous inspiratory micro-arousals that may be involved in the pathogenesis of hypersomnolence.
The cost of OSA to the community includes both direct costs, such as cost of health care utilization, and indirect costs, such as the costs of impaired productivity, work-related accidents, motor-vehicle accidents and the health-care costs of diseases associated with OSA, particularly cardiovascular disease. Case-controlled studies do show increased health-care utilization in patients with untreated OSA (Kryger et al 1996; Bahammam et al 1999; Kapur et al 1999) and that health-care utilization progressively increases in the years before diagnosis and falls following effective treatment with continuous positive airway pressure (CPAP) (Albarrak et al 2005). Kapur et al (1999) estimated that the cost in 1999 of untreated moderate to severe OSA per year in the US was US$3.4 billion. Data from a large population study have shown than men with even mild OSA have a significantly increased risk for a motor vehicle accident but both men and women with at least moderate OSA have an odds ratio (OR) for multiple accidents of 7.3 (95% confidence interval [CI] 2–>25) (Young et al 1997a). Several studies have reported increased mortality rates in subjects with untreated OSA but the methodology of these cohort studies is flawed with potential for selection and lead-time bias (Lavie et al 1995; Marti et al 2002). Most of the deaths are related to cardiovascular disease.
Association with cardiovascular disease
The impact of OSA extends well beyond simply causing hypersomnolence. OSA has been known to be associated with cardiovascular disease since the 1970s, including hypertension, myocardial infarction, and cerebrovascular disease, but it was not clear to what degree the relationship was cause and effect or related to confounding factors such as obesity, gender, and age. There have been several population-based epidemiological studies which now suggest a direct association between OSA and cardiovascular disease.
A recent randomized, double-blind, placebo-controlled study suggests that OSA has direct effects on cardiac responses to exercise (Alonso-Fernandez et al 2006). Subjects with OSA have been shown to have reduced exercise-related increases in cardiac output and stroke volume compared with normal controls, despite normal resting left ventricular function. These depressed responses improve after 3 months of therapy with CPAP.
Hypertension
The Wisconsin Sleep Cohort Study is a prospective study of over 1100 subjects from a stratified random cohort of employees from 4 Wisconsin state agencies. In the first 4–8 years of follow-up of 709 subjects, the study reported a corrected OR of 2.89 (95% CI 1.46–5.64) for the development of hypertension (defined as a blood pressure of at least 140/90 or the use of antihypertensive medications) in subjects with an Apnea-Hypopnea Index (AHI, the number of apneas and hypopneas occurring per hour of sleep) of ≥15 at baseline (normal <5, ≥15 indicating moderate OSA) compared with an AHI of zero at baseline (Peppard et al 2000b). It was also surprising that the data demonstrated a dose response based on increasing AHI with an OR of 1.42 (95% CI 1.13–1.78) for the development of hypertension in subjects with an AHI of 0.1–4.9 at baseline (that is still within the normal range for this measurement). The Sleep Heart Health Study also demonstrated a relationship between OSA and hypertension (also defined as a blood pressure of at least 140/90 or the use of antihypertensive medications) in cross-sectional analysis of 6132 subjects recruited from ongoing population studies. The OR (corrected for demographics, anthropometric variables, alcohol, and smoking) was 1.37 (95% CI 1.03–1.83) for the presence of hypertension comparing the highest AHI quartile (AHI ≥ 30) to the lowest (AHI < 1.5) (Nieto et al 2000). Data from the Wisconsin Sleep Cohort Study raises the possibility that there may be synergistic associations between OSA and angiotensin-converting enzyme (ACE) gene insertion/ deletion polysmorphisms and hypertension (Ling et al 2004). In cross-sectional analysis of 1100 subjects, there was a strong association between subjects with mild to moderate OSA with ACE gene deletions and the presence of hypertension. The authors hypothesized that ACE gene deletion was only sufficient to cause hypertension in the presence of sleep-disordered breathing but the effect of severe OSA on blood pressure overwhelms this association between gene deletion and hypertension.
Coronary artery disease
Numerous studies have demonstrated an increased prevalence of OSA at the time of diagnosis of coronary artery disease (Mooe et al 1996; Peker et al 1999) but these studies demonstrate the association between OSA and coronary artery disease at the point of time of a cardiac event rather than in the years leading up to the event when the coronary artery disease was developing. Many studies, including the larger population-based epidemiological studies, collate coronary artery disease with other cardiovascular outcomes as a composite outcome measure to achieve adequate statistical power (Shahar et al 2001). Longitudinal studies do suggest that OSA is a risk factor for coronary artery disease in both men and women. A 7-year prospective study of a sleep clinic cohort of 182 middle-aged men showed that untreated OSA was associated with the development of coronary artery disease, with an OR of 5.4 (95% CI 1.4–20.6) relative to non-OSA subjects (Peker et al 2002). In a prospective study of 103 women with repeated coronary angiography with an average interval of 3.25 years, the presence of snoring and tiredness was associated with larger progression of atherosclerosis after correction for other cardiac risk factors (Leineweber et al 2004).
Stroke
The association between stroke and OSA is complex. Self-reported snoring was associated with an increased risk of stroke in a large, prospective study with 8 years follow-up of 71 779 women, The Nurses Health Study (Hu et al 2000). The age-adjusted relative risk for stroke was 1.60 (95% CI 1.21–2.12) in occasional snorers and 1.88 (1.29–2.74) in regular snorers, although this relationship was no longer significant after correction for other cardiovascular risk factors such as body mass index and smoking. Cross-sectional data from the Sleep Heart Health Study (6424 subjects) also failed to show a significant association between OSA and the prevalence of stroke once corrected for all known cardiovascular risk factors (Shahar et al 2001). Cross-sectional data from the Wisconsin Sleep Cohort study (1475 subjects) did show a significant association after correction for confounding factors between at least moderate OSA (AHI of ≥20 events per sleep hour) and the prevalence of stroke with an OR of 3.83 (95% CI 1.17–12.56) but no association with milder OSA (Artz et al 2005). However, 4-year longitudinal data from the same study did not demonstrate a significant relationship between OSA and the incidence of stroke. OSA has been shown to be a risk factor for a composite end-point of stroke or death from any cause in a prospective observational cohort study of 1022 subjects referred to a sleep clinic (68% of which were subsequently diagnosed with OSA) with a reported hazard ratio for stroke or death from any cause of 1.97 (95% CI 1.12–3.48) after adjustment for other cardiovascular confounding risk factors (Yaggi et al 2005).
OSA is observed in up to 62%–77% of patients acutely following a stroke (Dyken et al 1996; Bassetti and Aldrich 1999; Parra et al 2000) although causality (in either direction) has not been proven. The presence of OSA has also been reported as an independent factor associated with increased mortality following first ever stroke or transient ischaemic attack (Parra et al 2004). Further prospective studies (including further longitudinal data from the current large population-based epidemiological studies) are required to clarify the association between sleep-disordered breathing and stroke without the use of composite end-points.
Mechanisms
There are a number of possible mechanisms for the relationship between OSA and cardiovascular disease. Hypertension, independently associated with OSA, certainly will increase rates of cardiovascular disease. There is also evidence of increased sympathetic nervous system activity in subjects with OSA (Carlson et al 1993) and that at least daytime sympathetic nervous activity is correlated with the severity of OSA (Ziegler et al 2001). Treatment with CPAP reduces the increased sympathetic activity (Ziegler et al 2001; Heitmann et al 2004), suggesting a causal relationship. There is some evidence for a primary endothelial dysfunction. Kato et al (2000) have demonstrated impaired endothelial function in subjects with OSA who had blunted vasodilatation in response to acetylcholine. Ip et al (2004) also demonstrated impaired endothelium-dependent vasodilatation in subjects which OSA and showed improvement following treatment with CPAP. Nitric oxide has been implicated in the pathogenesis of vascular disease in OSA. Circulating nitric oxide levels, which have potent effects on endothelial relaxation, have been reported to be suppressed in OSA subjects (Ip et al 2000) and increase with treatment.
There are also a number of inflammatory changes seen in OSA which may contribute to atherosclerosis and vascular remodeling. OSA is associated with increased reactive oxygen species production in some leukocyte populations and increased adhesion molecules expression and increased monocyte adherence to human endothelial cells in vitro with reductions by treatment with CPAP (Dyugovskaya et al 2002; El-Solh et al 2002). Tumor necrosis factor-alpha (TNF-α), involved in the pathogenesis of atherosclerosis, is also elevated in patients with moderate to severe OSA and falls with treatment with CPAP, suggesting a causal relationship (Minoguchi et al 2004). Riha et al (2005b) have recently demonstrated that OSA is associated with the TNF-α-308A gene polymorphism, suggesting that the increased TNF-α production associated with this allele may be involved in the pathogenesis of OSA. This hypothesis is supported by the significant improvement in objective sleepiness and the small but significant improvement seen in AHI following treatment with etanercept, a TNF-α antagonist, compared with placebo (44.3 ± 10.3 vs 52.8 ± 9.1 compared with a baseline of 55.9 ± 11.6 events per sleep hour) (Vgontzaz et al 2004). Patients with OSA may also have heightened coagulability, although the studies have not used well matched control populations. Robinson et al (2004) have demonstrated increased plasma levels of activated coagulation factors XIIa (a marker of endothelial activation or dysfunction) and VIIa, thrombin-antithrombin complex (a marker of thrombin turnover) and soluble P-selectin in subjects with OSA compared with unmatched controls, although the levels did not fall following one month of therapeutic CPAP suggesting that confounding factors such as obesity may be involved. Patients with OSA compared with those without have been shown to have increased morning fibrinogen levels and blood viscosity in a recent cohort study of patients undergoing polysomnography (Steiner et al 2005). D-dimer, a degradation product of fibrin, is elevated in OSA indicating increased fibrinolytic activity with higher levels seen in patients with greater hypoxia (Shitrit et al 2005). CRP, known to be a risk factor for cardiovascular events, is elevated in patients with OSA but does not fall with treatment, again suggesting the relationship is due to the confounding association with obesity rather than OSA per se (Guilleminault et al 2004).
Association with impaired glucose homeostasis
There is increasing evidence supporting a causal relationship between OSA and metabolic syndrome, including hypertension, insulin resistance, impaired glucose tolerance, and dyslipidemia, which are significant cardiac risk factors. OSA appears independently associated with insulin resistance and dyslipidemia (Ip et al 2002; Coughlin et al 2004; Pujabi et al 2004) even after correction for obesity. Cross-sectional data from the Wisconsin Sleep Cohort Study (1387 participants) has shown a greater prevalence of diabetes with increasing severity of OSA (Reichmuth et al 2005). The OR (corrected for gender, age and body habitus) for a physician diagnosis of diabetes in subjects with an AHI of 15 or greater compared with less than 5 was 2.30 (95% CI 1.28–4.11). Longitudinal data from the same study did not demonstrate a significant relationship with an OR for developing diabetes within a 4-year period with an AHI of 15 or more compared with less than 5 was 1.62 (95% CI 0.67–3.65). CPAP has been shown to improve insulin resistance within two days of treatment with CPAP (Harsch et al 2004). Multiple studies have demonstrated improvements in glucose levels and significant falls in hemoglobin A1C in subjects with Type 2 diabetes and OSA treated with CPAP (Babu et al 2005; Hassaballa et al 2005). There may also be a dose-response relationship between the improvement in hemoglobin A1C and CPAP usage (Babu et al 2005). These data support a causal relationship between the sleep-disordered breathing and impaired glucose homeostasis and indeed presents glucose dysregulation as another potential indication for the treatment of OSA with CPAP.
Thus, OSA is a highly prevalent condition which is likely to increase in prevalence associated with the epidemic of obesity in Western societies and is associated with significant sequelae. As such, OSA presents a substantial burden of disease and a major public health issue. However, large population-based epidemiological studies demonstrate that at least 80% of all subjects with moderate to severe OSA remain undiagnosed and untreated, particularly women (Young et al 1997b; Kapur et al 2002).
Treatment
Conservative treatment
Patients with OSAS are frequently advised to avoid supine sleep, minimize alcohol intake, and reduce weight. Most studies report that moderate alcohol consumption increases respiratory disturbance and heart rate during sleep in both non-snoring (Hertzog and Riemann 2004) and snoring men (Scanlon et al 2000; Hertzog and Riemann 2004) but some studies have not demonstrated exacerbation of OSA by alcohol (Teschler et al 1996). There have been no systematic reviews of the effectiveness of the advice to reduce alcohol intake but the data from studies assessing the impact of additional alcohol on sleep-disordered breathing would suggest that this advice is appropriate.
Avoidance of supine sleep can be achieved using a number of inexpensive devices such as a backpack containing a soft ball. This treatment can be effective in reducing time spent supine (Jokic et al 1999) and can improve AHI in positional OSA (Cartwright et al 1985, 1991) although less effectively than with CPAP (Jokic et al 1999). This treatment has been shown to have similar effects to CPAP in positional OSA to improve sleep architecture, subjective sleepiness, ability to maintain wakefulness during the day, psychometric test performance, mood scales, and quality of life measures in a single prospective cross-over study (Jokic et al 1999). In contrast, modifying posture using a shoulder-head elevation pillow was significantly less effective than CPAP in one small study of subjects with variable-severity OSA, although 2/14 subjects did normalize AHI with this therapy, including one subject with underlying severe OSA (Skinner et al 2004).
Few studies have evaluated the effect of major weight loss on the severity of OSA. Dixon et al investigated the effect of laparoscopic gastric banding on severely obese subjects with severe OSA (Dixon et al 2005). Weight loss of 44.9 ± 22 kg resulted in a significant fall in AHI from 61.6 ± 34 to 13.4 ± 13 and improvement in sleep architecture and subjective sleepiness. Longitudinal data of 690 subjects from the Wisconsin Sleep Cohort Study (Peppard et al 2000a) demonstrated that lesser weight loss will also influence severity of OSA, with a 10% weight loss associated with a 26% (95% CI 18%–34%) fall in AHI. Conversely, a 10% weight gain resulted in a 32% (20%–45%) increase in the AHI.
Continuous positive airway pressure (CPAP)
CPAP, first described in 1981, remains the mainstay of treatment in this condition (Sullivan et al 1981). The treatment consists of a flow generator attached by tubing to a nasal or oronasal mask from which the air is vented (Figure 2). The resistance of air flow across the vent leads to the generation of positive pressure in the mask which is transferred to the upper airway. Positive airway pressure in the pharyngeal lumen overcomes the tendency of the airway to collapse with the loss of dilator muscle activity at sleep onset, therefore acting as a pneumatic splint. The treatment itself is conceptually simple; however, considerable effort on the part of the patient is required to wear the therapy each night.
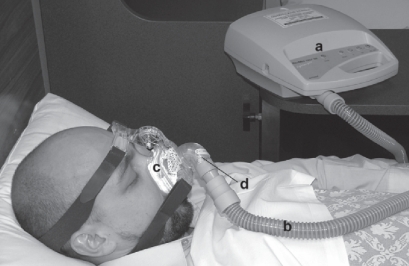
Figure 2.
Subject demonstrating the principle of nasal CPAP. The flow generator (a) delivers air through a smooth tube (b) to the mask (c) from which the air is vented (d) generating pressure in the mask which is transmitted to the upper airway.
Optimal CPAP settings must be individually determined. Conventionally, patients require labor-intensive laboratory based CPAP titration polysomnography. CPAP settings are adjusted during the study to control apneas, hypopneas, flow limitation, oxygen saturation, and arousals. There are no agreed standards on techniques of CPAP titration and it is likely there is considerable variability between laboratories. Furthermore, increased upper airway resistance may persist despite increases in CPAP settings to abolish features of airflow limitation (Monsterrat 1995). The demand for laboratory investigations greatly outstrips supply (National-Institutes-of-Health-Report 1994) leading to log waiting times for polysomnography in many services.
The lack of standardization of CPAP titration and the resource-intense nature of laboratory CPAP titration has led to alternative techniques to determine optimal CPAP settings. Fitzpatrick et al (2003) suggested that patients could effectively self-titrate CPAP without laboratory-titration based on the comfort and response to the treatment. Hukins (2005) demonstrated that the use of arbitrary-pressure CPAP according to body mass index resulted in similar subjective sleepiness, adherence to CPAP and quality of life measures to laboratory-determined CPAP pressure. However, the commonest technique used as an alternative to laboratory polysomnography is auto-titrating positive airway pressure (APAP). APAP devices use algorithms to estimate upper airway resistance and automatically adjust the delivered airway pressure without the need for an attending technician. The principles of this treatment are discussed below. These devices are capable of storing data including pressures delivered (usually mean or median pressure, maximal pressure and the 95th percentile pressure or the pressure limit that is exceeded for only 5% of the study time), system leaks (mask and/or mouth leaks), and an estimation of the frequency of persistent respiratory events. Some devices also allow for integration of oximetry data to the other stored data. The physician can then review the data stored by the APAP device to decide optimal CPAP settings. The pressure recommended by APAP devices correlates well to laboratory-determined pressures (Stradling et al 1997; Teschler et al 1997) but there is evidence that different devices using different algorithms and technology can recommend quite different settings. Kessler et al (2003) found considerable differences in the 95th percentile pressure between a device adjusting settings according to measurements of airflow compared with a device using the forced-oscillation technique to estimate upper airway impedance, with a mean difference of 2.9 cm H2O between the devices. The other area of concern with the use of this technology is the potential effect on long-term compliance which may arise by transferring patient contact from the laboratory to the home. Means et al (2004) reported that patients who underwent home CPAP titration had lower nightly CPAP usage than those who underwent laboratory titration (3.9 vs 5.0 hours per night). The authors hypothesized that the loss of support and education provided by sleep technologists may explain this finding.
A 1997 systematic review of the health consequences of OSA and effectiveness of CPAP concluded that the pubic health risk of OSA was exaggerated and was highly critical of the paucity of high quality evidence supporting this therapy (Wright et al 1997). This condemning review stimulated numerous randomized–controlled trials to document the efficacy of CPAP in this condition.
Effect of CPAP on sleep architecture
There is randomized–controlled evidence that CPAP improves sleep parameters in subjects with OSA. McArdle and Douglas (2001) reported that CPAP resulted in a significant improvement in arousals during sleep and redistribution of Stage 1 sleep to deeper Stage 3/4 (slow wave) sleep compared with oral placebo. The change in the frequency of arousals during sleep and increased proportion of slow wave sleep correlated to improvements in subjective sleepiness. An earlier study by Loredo et al (1999) comparing the effects of therapeutic with sham CPAP also showed a reduction in arousals during sleep, improved oxygen saturation and reduced respiratory disturbance but the sleep stage proportions did not differ.
Effect of CPAP on daytime function
A Cochrane collaboration review (White et al 2001) concluded that CPAP resulted in significant improvements in objective and subjective sleepiness and several quality of life and depression measures relative to placebo. The placebo used in the randomized-controlled studies takes the form of either oral placebo tablets (Engleman et al 1998, 1999; Barbe et al 2001; Barnes et al 2002, 2004) or sham CPAP (CPAP at ineffective pressure) (Loredo et al 1999; Montserrat et al 2001). There has been considerable debate about which is the better placebo. CPAP significantly improves both objective sleepiness (Engleman et al 1998) measured by the Multiple Sleep Latency Test and subjective sleepiness (Engleman et al 1998; Montserrat et al 2001) assessed by the Epworth Sleepiness Score (Johns 1991), a self-reported scale based on patient’s tendency to doze during 8 different passive activities. Some quality of life measures improve with CPAP relative to placebo. The disease-specific Functional Outcomes Sleep Questionnaire (Weaver et al 1997b) showed improved social function, activity level, and vigilance with 6 weeks of CPAP therapy relative to placebo in 45 patients with severe OSA (Montserrat et al 2001). A number of studies have shown improvements both in the short term (Montserrat et al 2001; Parish and Lyng 2003; Kawahara et al 2005) and after up to 18 months (Sin et al 2002a; Pichel et al 2004) of CPAP therapy in some of the sub-scales of the generic SF-36 quality of life questionnaire. The Vitality scale may show the largest effect size with CPAP therapy (Sin et al 2002a; Pichel et al 2004). Patients with more severe OSA appear to show the greatest improvements in quality of life with CPAP (Sin et al 2002a). Improvements are not only seen in the patient. An interesting finding from Parish et al (2003) was that there is improvement in the quality of life of the bed partners of patients with OSA after treatment with CPAP.
A randomized–controlled study of 45 subjects with severe OSA demonstrated that short-term CPAP therapy improved some but not all neuropsychological assessments (namely Digit Span Test Forward and Backward, Complex Figure Recall, SteerClear, and Digit Symbol tests – refer to original paper for details) relative to placebo (Henke et al 2001). Other studies have not shown improvements in neurocognitive function (Engleman et al 1993; Bardwell et al 2001; Monastero et al 2001; Barnes et al 2002), but many of these studies were conducted in mild OSA where baseline tests showed only minimal abnormality and therefore the effect size of CPAP is likely to be quite small. High intelligence may have some protective effect against the neurocognitive effects of OSA. Alchanatis et al (2005) demonstrated that patients of normal intelligence with OSA show deficits in attention and alertness compared with controls that reversed with prolonged CPAP treatment. These CPAP-responsive deficits were not seen in highly intelligent patients, possibly reflecting increased “cognitive reserve”.
In patients with mild to moderate OSA, CPAP was shown to be no more effective than placebo in improving subjective or objective sleepiness, quality of life measures or neurobehavioral function in a randomized–controlled trial of 28 subjects, although self-reported symptoms including snoring, restless sleep, and irritability did improve (Barnes et al 2002). Another randomized placebo-controlled study of mild to moderate OSA by Engleman did show improvement in subjective sleepiness, some sub-scales of the generic SF-36 quality of life assessment and improvement in 2 of 7 cognitive tests but objective sleepiness did not improve (Engleman et al 1999). CPAP has been compared with a mandibular advancement splint (MAS) in mild OSA in several studies. CPAP is more effective in reducing AHI and arousals during sleep than the MAS (Ferguson et al 1996; Engleman et al 2002; Barnes et al 2004) but the effects on subjective sleepiness and quality of life are variable. Some studies have demonstrated patient preference for CPAP (Engleman et al 2002; Barnes et al 2004) and others for the dental appliance (Ferguson et al 1996).
Barbe et al (2001) explored the role of CPAP therapy in subjects with severe OSA but who were free of daytime sleepiness. Fifty-five subjects with severe OSA were randomized to either 6 weeks of effective CPAP or sham CPAP. There were no differences between the groups in subjective or objective sleepiness, quality of life, blood pressure or neurocognitive assessment in the short term. The authors concluded that CPAP is not indicated in non-sleepy patients with significant OSA but long-term studies are required given the association between OSA and cardiovascular disease.
Effect of CPAP on cardiovascular risk factors
The evidence shows that CPAP improves sleep parameters, daytime symptoms, quality of life and possibly neuropsychological function. In view of the increasing evidence of an association between OSA and cardiovascular disease, does CPAP reduce the risk of cardiovascular complications?
There have been methodological problems for many studies assessing the effect of CPAP on blood pressure, including selection of adequate controls. The effect of CPAP on blood pressure remains controversial. Short-term, randomized studies have shown reduction in daytime blood pressure compared with placebo. Faccenda et al (2001) demonstrated a small fall in diastolic blood pressure in 68 normotensive subjects with OSA treated with CPAP compared with oral placebo and a fall in systolic blood pressure only in the group with more frequent nocturnal desaturation. Pepperell et al demonstrated falls in both systolic and diastolic blood pressure with therapeutic CPAP compared with sham CPAP with a difference in mean blood pressure of 3.3 mmHg between the groups (Pepperell et al 2002). Becker et al (2003) showed much larger falls in diurnal and nocturnal blood pressure of about 10 mmHg (diastolic, systolic and mean) in patients with moderate to severe OSA treated with effective CPAP compared with a sub-therapeutic CPAP setting which reduced respiratory disturbance by only 50%. There is a suggestion that effects on blood pressure are greater with more severe OSA (Faccenda et al 2001; Pepperell et al 2002). Other studies have shown no significant difference between the effect CPAP and placebo on diurnal blood pressure (Engleman et al 1996; Dimsdale et al 2000), although one study did show improvement in daytime blood pressure with CPAP only in the sub-group of patients whose blood pressure did not fall during sleep (Engleman et al 1996). Several studies have shown a fall in nocturnal but not diurnal blood pressure (Dimsdale et al 2000; Hla et al 2002).
CPAP leads to reductions in plasma norepinephrine levels by both increases in norepinephrine clearance and decreases in diurnal and nocturnal excretion compared with sham CPAP or oxygen supplementation alone (Mills et al 2006). Treatment of OSA with CPAP has also been shown to reduce daytime muscle sympathetic nerve activity in patients with heart failure (Usui et al 2005). The improvement in sympathetic activity is one likely mechanism for the changes in blood pressure seen with CPAP.
There have been no randomized–controlled trials to assess the impact of CPAP on cardiovascular disease. Several cohort studies suggest that CPAP may reduce cardiovascular outcomes, although these are usually assessed as a composite outcome of several cardiovascular events. Marin et al (2005) followed 1387 sleep clinic patients (including simple snorers, untreated mild–moderate OSA, untreated severe OSA, and 372 treated OSA subjects) and 264 healthy controls over 10.1±1.6 (SD) years and compared fatal cardiovascular events (death from myocardial infarction or stroke) and non-fatal cardiovascular events (non-fatal myocardial infarction, non-fatal stroke, or invasive coronary artery procedures). Patients with untreated severe OSA had significantly higher incidence of both fatal and non-fatal cardiovascular events than the other cohorts with no difference between the normal control and treated OSA cohorts. The study did report excellent follow-up rates but analysis was not intention to treat. Milleron et al (2004) prospectively assessed 54 patients with both OSA and coronary artery disease over a mean of 86.5 months and demonstrated that the cohort receiving treatment (21 with CPAP and 4 with upper airway surgery) had a significantly lower incidence of a composite endpoint of cardiovascular death, acute coronary syndrome, hospitalization for heart failure, or need for coronary revascularization. Doherty et al (2005) prospectively followed 107 subjects with OSA treated with CPAP compared with 61 subjects non-tolerant of CPAP over an average 7.5 years with a follow-up rate of 75%. There was a significant difference in deaths from cardiovascular disease in the untreated cohort (14.8% compared with 1.9%) but there was no difference in the incidence of new cardiovascular events (hypertension, cardiac disorder or stroke).
Effect of CPAP on mortality
Campos-Rodriguez et al (2005) retrospectively reviewed the mortality of patients with OSA comparing those compliant with CPAP to those non-compliant. There was a dose-response relationship between 5-year cumulative survival and CPAP adherence (comparing average nightly CPAP use of <1 hour, 1–6 hours and >6 hours). The major cause of death was related to cardiovascular disease.
CPAP adherence
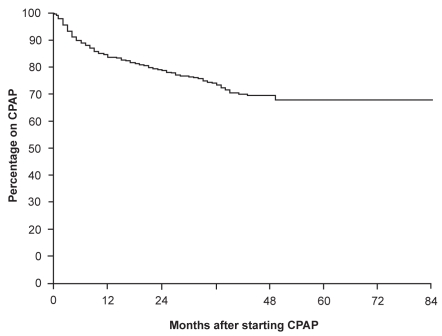
The eternal challenge in sleep medicine is maximizing the adherence of patients to CPAP. Weaver et al (1997a) reported a bimodal distribution of use in the first three months of treatment with about half of the subjects using the therapy consistently (use on >90% of nights with an average usage of 6.22±1.21 hours/night) and the other half using therapy intermittently with an average use of only 3.45±1.94 hours/night on the nights the therapy was used. The authors could not identify factors predictive of usage pattern. Other studies have reported objective nightly use of CPAP for 4.5–5.8 hours/night (Kribbs et al 1993; Rauscher et al 1993; Engleman et al 1994; Sin et al 2002b; Drake et al 2003). Usage of CPAP is over-reported by patients compared with objective measurements based on the CPAP device’s in-built hour-meter (Rauscher et al 1993). McArdle et al (1999) reviewed the adherence to CPAP in 1103 patients in up to 84 months of treatment with CPAP. Eighty-four per cent of subjects continued to use CPAP 12 months after commencing the therapy and the use of CPAP plateaued only after 4 years, with 68% continuing to adhere to therapy (Figure 3). The independent variables influencing continued use of CPAP were CPAP usage at 3 months (with a relative risk [RR] of stopping CPAP of 13.8 comparing <2 hours vs ≥2 hours), AHI (RR of 2.48 AHI < 15 vs AHI ≥ 15), presence of subject sleepiness (RR of 1.92), and snoring status (RR of 2.76 non-snorer vs snorer).
Figure 3.
Proportion of patients continuing CPAP over time. The proportion adhering to CPAP continues to fall over the first 4 years of therapy. Reprinted from McArdle N, Devereux G, Heinarnejad H, et al. 1999. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med, 159:1108–14. Copyright © 1999, with permission from American Thoracic Society.
Auto-titrating positive airway pressure (APAP)
A number of APAP devices have been developed either for home use or for supervised or unsupervised CPAP titration. I will discuss here only the chromic treatment of OSA in the home with APAP. These devices automatically adjust delivered airway pressure according to algorithms to estimate upper airway resistance. These algorithms utilize different parameters including snoring, apnea, hypopnea, airflow limitation, and impedance by the forced oscillation technique. The algorithms used vary between devices, and as a result the devices have different response characteristics (Kessler et al 2003; Hussain et al 2004; Peveragie et al 2004; Stammnitz et al 2004). There are a number of theoretical benefits of APAP over conventional CPAP. APAP does not require laboratory pressure-determination polysomnography which may reduce the delay to initiation of treatment and health-related costs (Planes et al 2003). As the delivered pressure varies according to “instantaneous” need, APAP may be preferable in subjects who have great variations in pressure requirement, for example subjects with predominantly REM sleep or supine-related OSA. Furthermore, APAP may address longer-term changes in pressure requirement that may occur with changes in weight without the need for repeat polysomnography.
There have been a number of randomized–controlled trials evaluating APAP, although most of these have been short term (generally 2 months or less of APAP) and crossover studies. APAP appears to be equally effective as fixed pressure CPAP in improving respiratory disturbance and sleep architecture (Ficker et al 1998; Konerman et al 1998; D’Ortho et al 2000; Randerath et al 2001). APAP has been consistently shown to deliver lower average airway pressures relative to conventional fixed-pressure CPAP (Konerman et al 1998; D’Ortho et al 2000; Hudgel and Fung 2000; Teschler et al 2000; Randerath et al 2001; Massie et al 2003; Hukins 2004), with APAP delivering a lower mean pressure of 0.9–4.2 cm H2O. Some studies have also shown fewer leaks (mask and/or mouth) recorded by the device (Teschler et al 2000; Hukins 2004) as a direct consequence of lower airway pressures. Most studies have not demonstrated improved compliance with APAP compared with fixed-pressure CPAP (D’Ortho et al 2000; Hudgel et al 2000; Teschler et al 2000; Randerath et al 2001; Hukins 2004) but some studies have shown increased proportion of nights used (Konerman et al 1998) or longer use of APAP on the nights patients used treatment (Hudgel et al 2000). Massie et al (2003) reported higher average nightly use by a mean of 35 minutes in patients using APAP. One study (Hukins 2004) reported improved compliance with APAP in patients who had complained of any side-effect with CPAP, including pressure intolerance, bloating, or leaks, but this was based only on post hoc analysis and on small numbers. The one study comparing APAP with fixed-pressure CPAP specifically in subjects with high within-night variability in pressure requirement (related to sleep position, sleep stages, or other factors such as alcohol) did not show improved compliance, although subjective sleepiness assessed by the Epworth Sleepiness Score was significantly lower statistically but not clinically with APAP (Noseda et al 2004). Other studies have not shown differences in subjective sleepiness (Ficker et al 1998; D’Ortho et al 2000; Hudgel et al 2000; Teschler et al 2000; Massie et al 2003; Planes et al 2003; Hukins 2004). The other potential benefit of APAP devices is the ability to record data on technical factors such as leaks and persistent respiratory disturbance, although the impact of these data has not been systematically assessed.
Based on current knowledge, it can be said that APAP seems comparable to fixed-pressure CPAP. There are potential benefits in avoiding laboratory sleep studies but further research is required to determine the role of APAP in the management of OSA.
Mandibular advancement splints (MAS)
Mandibular advancement splints or MAS (also referred to as mandibular advancement devices [MAD] or mandibular repositioning devices in the literature) are the main alternative treatment to CPAP in the management of OSA. These dental devices are worn during sleep to advance the mandible (Figure 4). Not all subjects are suitable for this type of therapy. Oral appliances cannot be used in those who are edentulous, have inadequate mouth opening, a strong gag, or temporo-mandibular joint (TMJ) instability (such as TMJ clicking). The devices have been shown to increase oropharyngeal dimensions in wakefulness, particularly increasing the lateral diameter of the velopharynx (Ryan et al 1999; Kyung et al 2005) and reducing the curvature of the anterior velopharyngeal wall (corresponding to the posterior border of the soft palate) (Tsuiki et al 2004). MAS have been shown to reduce upper airway collapsibility in sleeping subjects with OSA (Ng et al 2003).
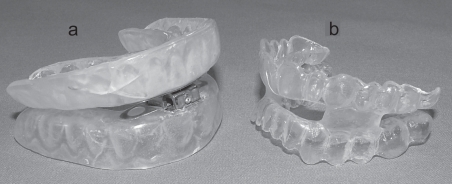
Figure 4.
Mandibular advancement splints showing both adjustable appliance (a) which uses a hinge to adjust mandibular advancement or one-piece mono-block appliance (b) with fixed mandibular advancement.
The efficacy of oral appliances in OSA has been assessed in many randomized–controlled trials. A systematic review of the evidence for the efficacy of these devices has been performed by the Cochrane Collaboration (Lim et al 2004). MAS therapy improves subjective sleepiness and improves AHI relative to controls but is less effective than CPAP in reducing AHI. Subjective sleepiness improves in 82%–96% of subjects with moderate–severe OSA (Mehta et al 2001; Gotsopoulos et al 2002) but objective sleepiness based on mean sleep latency during a Multiple Sleep Latency Test does not differ from controls (Gotsopoulos et al 2002). Most studies have not shown a difference between CPAP and MAS therapy in subjective sleepiness (Tan et al 2002; Barnes et al 2004) although CPAP is more effective in reducing AHI (Ferguson et al 1997; Barnes et al 2004). MAS therapy has been compared with uvulopalatopharygoplasty (UPPP) in several randomized–controlled studies. MAS are superior in improving polysomnographic parameters in a randomized cohort assessed at 1 year (Walker-Engstrom et al 2000) and 4 years (Walker-Engstrom et al 2002) of treatment.
The greatest limitation to the use of dental appliances in OSA is the unpredictable response to therapy. Complete responses in OSA (defined as an AHI less than 5–10 and control of symptoms) with dental appliances are reported in only 34%–63% of subjects (Ferguson et al 1997; Walker-Engstrom et al 2002; Gotsopoulos et al 2004; Marklund et al 2004) with at least partial responses (defined as a ≥50% reduction in AHI) seen in 60%–81% (Ferguson et al 1997; Walker-Engstrom et al 2002; Gotsopoulos et al 2004). Selection of patients for MAS therapy is controversial but critical given the variable response rates to this therapy. There are no uniform predictors of success in the literature but some authors have reported increased success in mild OSA or habitual snoring (Mehta et al 2001; Marklund et al 2004), supine-exacerbated OSA in men (Marklund et al 2004), lower neck circumference, and some cephalometric measurements (Mehta et al 2001). Most studies found no predictors of success with MAS.
Mild side-effects with the dental appliances are seen in up to 86% of subjects, including mucosal drying, hyper-salivation, temporo-mandibular joint, myo-facial, or tooth discomfort (Pantin et al 1999; Fritsch et al 2001). The most serious side-effect of oral appliances is malocclusion, which may occur in up to 14% of subjects and is poorly perceived by the patient (Pantin et al 1999). Studies of long-term compliance of this therapy are limited by the lack of objective measures and the dependence on patient self-reports. Studies show long-term continuation rates with this therapy in the order of 62%–76% (Pantin et al 1999; Walker-Engstrom et al 2002; Marklund et al 2004).
MAS therapy can be an effective treatment for OSA but it has lower efficacy in improving polysomnographic variables and has lower response rates than CPAP. At this time, the treatment is regarded as second-line therapy in patients intolerant of CPAP or perhaps first-line in habitual snorers or minimally symptomatic mild OSA. Further research is required to direct optimal selection of patients who would benefit from this therapy.
Surgical treatment of OSA
Surgical intervention by tracheostomy was the first effect treatment of OSA but the role of surgery in this condition has greatly diminished with the development of more acceptable or effective treatments, in particular CPAP. Although patients are attracted by the option of curative therapy, there is a paucity of high quality evidence for the use of surgery in OSA. The major surgical interest is in palatal surgery, either uvulopalatopharyngoplasty (UPPP) or laser-assisted uvuloplasty (LAUP). This review will not consider the role of tonsillectomy in individuals with tonsillar enlargement.
Palatal surgery
A Cochrane Collaboration systematic review of this therapy did not support the widespread use of surgical treatment for unselected patients with OSA (Sundaram et al 2005). Several studies have explored the role of LAUP in the treatment of OSA. Ferguson et al (2003) randomized 45 subjects with mild OSA to LAUP or no treatment (control) and reported only a small improvement in AHI in the treatment group (from 18.6 ± 4.3 to 14.7 ± 7.5) (Ferguson et al 2003). Only 17% of subjects achieved a near normal AHI of <10 with treatment but 48% reported a subjective improvement in snoring. Larrosa et al (2004) compared 28 patients with snoring and mild to moderate OSA randomized to either LAUP or to a surgical placebo involving the injection of saline to the base of the uvula under local anesthesia followed by a weekly oral placebo for 12 weeks (Larrosa et al 2004). There were no differences in the AHI or the Epworth Sleepiness Scale (subjective sleepiness) between groups or from baseline when evaluated after 3 months.
There are serious methodological problems in the large number of observational studies in the literature, including small sample size with low study power, lack of control groups, selection bias, incomplete follow-up, and poor selection of end-points (for example defining success as only AHI < 20 and at least a 50% reduction in AHI, which as best represents partial response) (Pepin et al 1996). Sher et al (1996) performed a meta-analysis of surgical interventions for OSA and reported a success rate of 41%, defined as a 50% fall in AHI to an AHI < 20. This end-point represents partial response only and will overestimate the true success rate. Weighted mean results from 19 studies showed only UPPP resulted in only a 38.2% fall in AHI from a baseline of 60.0. The addition of procedures such as surgical tongue base suspension to the UPPP procedure may improve treatment response rates but normalization of sleep disordered breathing is still unlikely (Omur et al 2005). Even the optimal surgical technique is not clear. Data are inconclusive whether the lower morbidity LAUP is equivalent to UPPP because of methodological flaws (Littner et al 2001). The American Academy of Sleep Medicine published practice parameters on the use of LAUP in 2001 and concluded that LAUP is not recommended for treatment of sleep-related breathing disorders (Littner et al 2001).
Maxillofacial surgery
The Stanford University School of Medicine (Li et al 1999a, 1999b) have proposed a step-wise surgical approach to OSA. Phase I surgery involves UPPP and genioglossus advancement with hyoid myotomy-suspension. Patients not responsive to this intervention proceed to Phase II treatment of maxillary-mandibular advancement osteotomy. Riley et al have reviewed 306 consecutively treated patients and reported a fall in AHI from 55.8 ± 26.7 at baseline to 9.2 ± 7.5 post-operatively (Riley et al 1993). However, there were major methodological problems including lack of a control group, potential for selection bias, and incomplete follow-up. Reports from other centers, although promising, are also uncontrolled, non-randomized before and after studies (Conradt et al 1998; Lee et al 1999). Surgical expertise for this approach is available only in a few centers at this time. Randomized–controlled studies are required to validate the treatment.
Radiofrequency tissue volume reduction
Radiofrequency (RF) tissue volume reduction of the tongue was proposed as a potential intervention for OSA in 1997 by Powell et al (1997) based on the results of an animal model. RF energy is delivered into the tongue by a needle electrode causing a localized thermal lesion that results in scarring and a 26% volume reduction at the treatment site. Observational studies have shown only a partial response in AHI to RF treatment (Li et al 2002; Stuck et al 2002). Woodson et al (2003) reported on the outcomes of RF tissue ablation applied to both the tongue base and palate in subjects with mild to moderate OSA and hypersomnolence. Subjects were randomized to active intervention with three RF lesions in each of the tongue and soft palate, sham-RF (where the electrodes were introduced into the tongue and soft palate but RF not delivered), or CPAP. The AHI was significantly lower with CPAP than with RF treatment or placebo (4.6 vs 16.8 and 13.6 respectively). There were similar improvements in subjective sleepiness and the disease-specific Functional Outcomes of Sleep Quality of Life Questionnaire in both treatment arms. The lack of difference between RF volume reduction and CPAP may be explained by the suboptimal adherence with CPAP, averaging only 2.4 hours per night. This intervention at this time does not appear to be adequately effective as a management of OSA.
Hypoglossal nerve stimulation
As discussed, the loss of upper airway dilator muscle compensation for anatomically compromised airways at sleep onset appears important in the pathogenesis of OSA. Therefore, electrical stimulation of the dilator muscles during sleep may be a therapeutic option for this condition. Hypoglossal nerve stimulation in animal models has been show to increase pharyngeal airway size by ventral displacement of the ventral and lateral pharyngeal walls (Brennick et al 2004) and to reverse inspiratory flow limitation and snoring as effectively as CPAP (Bellemare et al 2005). In human studies, genioglossus muscle or hypoglossal nerve stimulation results in reduced upper airway collapsibility (the critical airway pressure at which obstruction occurs) and reduces airflow limitation (Oliven et al 2003). Schwartz et al (2001) have also demonstrated improvement in sleep indices with unilateral hypoglossal nerve stimulation in 8 subjects with moderate to severe OSA (mean AHI improving in non-REM sleep from 52.0 ± 20.4 to 22.6 ± 12.1 events per hour and in REM sleep from 48.2 ± 30.5 to 16.6 ± 17.1 per hour) (Schwartz et al 2001). Electrical nerve stimulation of upper airway dilator muscles has strong potential as a therapeutic option for OSA but at this time remains experimental.
Drug treatment of OSA
Just as a surgical cure, pharmaceutical treatment for OSA is an attractive option for the patient facing treatment with CPAP. A review by Smith et al (2001) did not support the use of a number of drugs compared with placebo, including acetazolamide, protriptyline, medroxyprogesterone, clonidine, and aminophylline–theophylline.
In theory, the emerging knowledge on the neurochemistry of upper airway dilator muscle control would support a role for pharmacotherapy in the management of OSA. Serotoninergic agents particularly are an attractive pharmacotherapy in theory in view of the excitatory effect of serotonin on hypoglossal motor neurons. Serotonin applied directly to the hypoglossal motor neuron in rats does significantly increase tonic genioglossus activity (Jelev et al 2001) but does not abolish the phasic genioglossus excitation and suppression, indicating the roles for other transmitters. Serotonin does not cross the blood–brain barrier and therefore is not a suitable therapy for humans. Ritanserin, a serotonin 2A/2C receptor antagonist, has been shown to reduce pharyngeal critical pressure (the pharyngeal pressure at which upper airway collapse occurs which is a measure of airway collapsibility) in rats but has not been evaluated in human studies (Ogasa et al 2004). Selective serotonin reuptake inhibitors (SSRI) have been investigated in human OSA. Paroxetine at doses of 20–40 mg daily in short-term studies in small numbers of subjects with severe OSA does augment genioglossus EMG activity but has little (Kraiczi et al 1999) to no (Berry et al 1999) effect on frequency of respiratory events during sleep.
Cross sectional data from the large Sleep Heart Health population study (2852 subjects) demonstrated a significantly lower prevalence of sleep-disordered breathing, defined as an AHI of ≥15 per sleep hour, in post-menopausal subjects using hormonal replacement therapy (HRT) with an OR adjusted for age, body mass index, and neck circumference of 0.55 (95% CI 0.41–0.75) (Shahar et al 2003). Interventional studies assessing the effect of HRT on OSA severity have involved only very small patient numbers (up to 21 subjects only). Not surprisingly, the outcomes have been inconsistent, with some studies showing no effect on AHI (Block et al 1981; Cistulli et al 1994) and others demonstrating a reduction in respiratory events (Pickett et al 1989; Keefe et al 1999). More recent studies have been reported as “pilot studies” involving small numbers but have also shown reduction in respiratory disturbance during sleep. Manber et al (2003) reported a reduction in AHI from 22.7 to 12.2 in just six post-menopausal subjects with estrogen monotherapy but no benefit from baseline with the combination of estrogen and progesterone. Wesstrom et al (2005) also reported an improvement in AHI (14.9 improving to 3.6) in 5 subjects with mild OSA with treatment with estradiol and gestagen. Neither study included normal control subjects or placebo. There is a need for well designed, randomized–controlled studies to clarify the role of HRT in the management of OSA in poist-menopausal women.
The role of modafinil, a unique wakefulness-promoting agent used in narcolepsy, in patients with OSA and residual sleepiness despite treatment with CPAP has been evaluated by several randomized–controlled trials. Early results were contradictory, some showing improvements in subjective and objective sleepiness with modafinil compared with placebo (Pack et al 2001) which continued in an open label extension of the study (Schwartz et al 2003), but another showed no benefit (Kingshot et al 2001). More recently, the benefits of modafinil therapy have been demonstrated by Black and Hirschkowitz (2005) who assessed 305 subjects with OSA and residual sleepiness on CPAP in a 12-week, double-blind, randomized, controlled trial comparing modafinil 200 mg or 400 mg daily and placebo. Subjective sleepiness improved with modafinil on the Epworth Sleepiness Scale by 4.5 points compared with a 1.8-point decrease with placebo. Objective sleepiness, assessed by mean sleep latency on the Maintenance of Wakefulness Test, improved in the modafinil groups (by 1.6 minutes for the 200-mg dose cohort and 1.5 minutes on the 400-mg dose cohort) but deteriorated in the placebo group (shorter sleep latency by 1.1 minutes). Modafinil is now approved in the United States for treatment of residual sleepiness in patients with OSA treated with CPAP.
Golbart et al (2005) reported that the oral leukotriene receptor antagonist montelukast resulted in significant reductions in adenotonsillar size radiologically in children with mild OSA. There were statistically significant improvements in EEG arousals due to respiratory events, apnea index, and obstructive AHI compared with controls but the absolute improvements were only small and of questionable clinical significance. The effects of the montelukast would appear to be due to direct antagonism of the abundant leukotriene receptors in adenoid tissue. These findings are worthy of further evaluation by appropriately blinded, randomized–controlled studies.
Summary
Obstructive sleep apnea is a significant condition, both in terms of its prevalence and significant sequelae. There is increasing evidence of a causal relationship between OSA and cardiovascular and metabolic sequelae. CPAP remains the mainstay of treatment for this condition and has high-level evidence supporting its efficacy. However, the treatment is intrusive and not tolerated by all patients. There is evidence that dental appliances are effective in OSA but the difficulty is predicting those patients who may respond to this therapy. There is also evidence to support the recommendation of conservative interventions, particularly weight loss and avoidance of supine sleep in selected patients. The role of surgical interventions is not established in OSA but probably is not indicated in the majority of cases. Drug therapy plays little role.
The future direction in the management of OSA must address the poor patient acceptance of the current gold standard of treatment (CPAP). Auto-titrating CPAP will continue to evolve such that laboratory-based investigations will not be required for the majority of patients with OSAS. The role of dental appliances needs to be determined, with better predictive ability to select suitable candidates for this therapy. Research is striving for a cure to OSA. A curative therapy must address either the anatomical deficiencies of the upper airway utilizing techniques less highly specialized than the current maxillomandibular advancement procedures or must address the sleep-related loss of pharyngeal dilator muscle activity, pharmacologically, or by direct electrical stimulation.
References
- Akan H, Aksoz T, Belet U, et al. Dynamic upper airway soft-tissue and caliber changes in healthy subjects and snoring patients. Am J Neuroradiol. 2004;25:1846–50. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Albarrak M, Banno K, Sabbagh A, et al. Utilization of healthcare resources in obstructive sleep apnea syndrome:a 5-year follow-up study in men using CPAP. Sleep. 2005;28:1306–11. doi: 10.1093/sleep/28.10.1306. [DOI] [PubMed] [Google Scholar]
- Alchanatis M, Zias N, Deligiorgis N, et al. Sleep apnea-related cognitive deficits and intelligence:an implication of cognitive reserve theory. J Sleep Res. 2005;14:69–75. doi: 10.1111/j.1365-2869.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- Alonso-Fernandez A, Garcia-Rio F, Arias MA, et al. Obstructive sleep apnoea-hypopnoea syndrome reversibly depresses cardiac response to exercise. Eur Heart J. 2006;27:207–15. doi: 10.1093/eurheartj/ehi621. [DOI] [PubMed] [Google Scholar]
- Artz M, Young T, Finn L, et al. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu AR, Herdegen J, Fogelfeld L, et al. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Int Med. 2005;165:447–52. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- Bahammam A, Delaive K, Ronald J, et al. Health care utilization in males with obstructive sleep apnea syndrome two years after diagnosis and treatment. Sleep. 1999;22:740–7. doi: 10.1093/sleep/22.6.740. [DOI] [PubMed] [Google Scholar]
- Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. Ann Int Med. 2001;134:1015–23. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- Bardwell WA, Ancoli-Israel S, Berry CC, et al. Neuropsychological effects of one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea:a placebo-controlled study. Psychosom Med. 2001;63:579–84. doi: 10.1097/00006842-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:773–80. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–64. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22:217–223. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Pecchiari M, Bandini M, et al. Reversibilty of airflow obstruction hy hypoglossus nerve stimulation in anesthetized rabbits. Am J Respir Crit Care Med. 2005;172:606–12. doi: 10.1164/rccm.200502-190OC. [DOI] [PubMed] [Google Scholar]
- Berry RB, Yamaura EM, Gill K, et al. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep. 1999;22:1087–92. doi: 10.1093/sleep/22.8.1087. [DOI] [PubMed] [Google Scholar]
- Bijaoui EL, Champagne V, Baconnier PF, et al. Mechanical properties of the lung and upper airways in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:1055–61. doi: 10.1164/ajrccm.165.8.2107144. [DOI] [PubMed] [Google Scholar]
- Black JE, Hirschkowitz M. Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep. 2005;28:464–71. doi: 10.1093/sleep/28.4.464. [DOI] [PubMed] [Google Scholar]
- Block AJ, Wynne JW, Boysen PG, et al. Menopause, medroxyprogesterone and breathing during sleep. Am J Med. 1981;70:506–10. doi: 10.1016/0002-9343(81)90572-6. [DOI] [PubMed] [Google Scholar]
- Bradley TD, Brown IG, Grossman RF, et al. Pharyngeal size in snorers, nonsnorers and patients with obstructive sleep apnea. N Eng J Med. 1986;315:1327–31. doi: 10.1056/NEJM198611203152105. [DOI] [PubMed] [Google Scholar]
- Brennick MJ, Pickup S, Dougherty L, et al. Pharyngeal airway wall mechanics using tagged magnetic resonance imaging during medial hypoglossal nerve stimulation in rats. J Physiol. 2004;561:597–610. doi: 10.1113/jphysiol.2004.073502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman SA, Dunn KM, Ducharme F. Surgery for obstructive sleep apnoea. The Cochrane Database of Systematic Reviews. 1998;(1) doi: 10.1002/14651858.CD001004. Art. No.:CD001004. [DOI] [PubMed] [Google Scholar]
- Campos-Rodriguez F, Pina-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–33. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- Carlson JT, Hedner J, Elam M, et al. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–8. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- Cartwright R, Lloyd S, Lilie J, et al. Sleep position training as treatment for sleep apnea syndrome:a preliminary study. Sleep. 1985;8:87–94. doi: 10.1093/sleep/8.2.87. [DOI] [PubMed] [Google Scholar]
- Cartwright R, Ristanovic R, Diaz F, et al. A comparative study of treatments for positional sleep apnea. Sleep. 1991;14:546–52. doi: 10.1093/sleep/14.6.546. [DOI] [PubMed] [Google Scholar]
- Chervin RD, Burns JW, Ruzicka DL. Electroencephalographic changes during respiratory cycles predict sleepiness in sleep apnea. Am J Respir Crit Care Med. 2005;171:652–8. doi: 10.1164/rccm.200408-1056OC. [DOI] [PubMed] [Google Scholar]
- Cistulli PA, Barnes DJ, Grunstein RR, et al. Effect of short term hormonal replacement in the treatment of obstructive sleep apnoea in postmenopausal women. Thorax. 1994;49:699–702. doi: 10.1136/thx.49.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt R, Hochban W, Heitman J, et al. Sleep fragmentation and daytime vigilance in patients with OSA treated by surgical maxillomandibular advancement compared with CPAP therapy. J Sleep Res. 1998;7:217–23. doi: 10.1046/j.1365-2869.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure. A placebo trial. Hypertension. 2000;35:144–7. doi: 10.1161/01.hyp.35.1.144. [DOI] [PubMed] [Google Scholar]
- Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes. 2005;29:1048–54. doi: 10.1038/sj.ijo.0802960. [DOI] [PubMed] [Google Scholar]
- Doherty LS, Kiely JL, Swan V, et al. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–84. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- D’Ortho MP, Grillier-Lanoir V, Levy P, et al. Constant vs automatic continuous positive airway pressure therapy. Home evaluation. Chest. 2000;118:1010–17. doi: 10.1378/chest.118.4.1010. [DOI] [PubMed] [Google Scholar]
- Drake CL, Day R, Hudgel D, et al. Sleep during titration predicts continuous positive airway pressure compliance. Sleep. 2003;26:308–11. doi: 10.1093/sleep/26.3.308. [DOI] [PubMed] [Google Scholar]
- Dyken ME, Somers VK, Yamada T, et al. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27:401–7. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–9. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- El-Solh AA, Mador MJ, Sikka P, et al. Adhesion molecules in patients with coronary artery disease and moderate-severe obstructive sleep apnea. Chest. 2002;121:1541–7. doi: 10.1378/chest.121.5.1541. [DOI] [PubMed] [Google Scholar]
- Engleman HM, Cheshire KE, Deary IJ, et al. Daytime sleepiness, cognitive performance and mood after continuous positive airway pressure for the sleep apnoea/hypopnoea syndrome. Thorax. 1993;48:911–14. doi: 10.1136/thx.48.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman HM, Gough K, Martin SE, et al. Ambulatory blood pressure on and off continuous positive airway pressure therapy for sleep apnea/hypopnea syndrome:effects in “non-dippers”. Sleep. 1996;19:387–81. doi: 10.1093/sleep/19.5.378. [DOI] [PubMed] [Google Scholar]
- Engleman HM, Kingshot RN, Wraith PK, et al. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:461–7. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax. 1994;49:263–6. doi: 10.1136/thx.49.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman HM, Martin SE, Kingshot RN, et al. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998;53:341–5. doi: 10.1136/thx.53.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman H, McDonald JP, Graham D, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome. Continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166:855–9. doi: 10.1164/rccm.2109023. [DOI] [PubMed] [Google Scholar]
- Faccenda JF, Mackay TW, Boon NA, et al. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–8. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–9. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- Ferguson KA, Heighway K, Ruby RRF. A randomized trial of laser-assisted uvuloplasty in the treatment of mild obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:15–9. doi: 10.1164/rccm.2108050. [DOI] [PubMed] [Google Scholar]
- Ferguson KA, Ono T, Lowe AA, et al. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax. 1997;52:362–8. doi: 10.1136/thx.52.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KA, Ono T, Lowe AA, et al. A randomised crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996;109:1269–75. doi: 10.1378/chest.109.5.1269. [DOI] [PubMed] [Google Scholar]
- Ficker JH, Wiest GH, Lehnert G, et al. Evaluation of an auto-CPAP device for treatment of obstructive sleep apnea. Thorax. 1998;53:643–8. doi: 10.1136/thx.53.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick M, Alloway C, Wakeford T, et al. Can patients with obstructive sleep apnea titrate their own continuous positive airway pressure? Am J Respir Crit Care Med. 2003;167:716–22. doi: 10.1164/rccm.200204-360OC. [DOI] [PubMed] [Google Scholar]
- Fogel RB, Malhotra A, Pillar G, et al. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med. 2001;164:2025–30. doi: 10.1164/ajrccm.164.11.2102048. [DOI] [PubMed] [Google Scholar]
- Fogel RB, Trinder J, Malhotra A, et al. Within-breath control of genioglossal muscle activation in humans:effect of sleep-wake state. J Physiol. 2003;550:899–910. doi: 10.1113/jphysiol.2003.038810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel RB, Trinder J, White DP, et al. The effect of sleep onset on upper airway muscle activity in patients with sleep apnea versus controls. J Physiol. 2005;564:549–62. doi: 10.1113/jphysiol.2005.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch KM, Iseli A, Russi EW, et al. Side effects of mandibular advancement devices for sleep apnea treatment. Am J Respir Crit Care Med. 2001;164:813–8. doi: 10.1164/ajrccm.164.5.2003078. [DOI] [PubMed] [Google Scholar]
- Golbart AD, Goldman JL, Veling MC, et al. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med. 2005;172:364–70. doi: 10.1164/rccm.200408-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves MA, Paiva T, Ramos E, et al. Obstructive sleep apnea syndrome, sleepiness and quality of life. Chest. 2004;125:2091–6. doi: 10.1378/chest.125.6.2091. [DOI] [PubMed] [Google Scholar]
- Gotsopoulos H, Chen C, Qian J, et al. Oral appliance therapy improves symptoms in obstructive sleep apnea. A randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:743–8. doi: 10.1164/rccm.200203-208OC. [DOI] [PubMed] [Google Scholar]
- Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea:a randomized, controlled trial. Sleep. 2004;27:934–41. doi: 10.1093/sleep/27.5.934. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep. 2004;27:1507–11. doi: 10.1093/sleep/27.8.1507. [DOI] [PubMed] [Google Scholar]
- Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous Positive Airway Pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- Hassaballa HA, Tulaimat A, Herdegen JJ, et al. The effect of continuous positive airway presure on glucose control in diabetic patients with severe obstructive sleep apnea. Sleep Breath. 2005;9:176–80. doi: 10.1007/s11325-005-0033-y. [DOI] [PubMed] [Google Scholar]
- Heitmann J, Ehlenz K, Penzel T, et al. Sympathetic activity is reduced by nCPAP in hypertensive obstructive aleep apnoea patients. Eur Respir J. 2004;23:255–62. doi: 10.1183/09031936.04.00015604. [DOI] [PubMed] [Google Scholar]
- Henke KG, Grady JJ, Kuna ST. Effect of nasal continuous positive airway pressure on neuropsychological function in sleep apnea-hypopnea syndrome. A randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2001;163:911–7. doi: 10.1164/ajrccm.163.4.9910025. [DOI] [PubMed] [Google Scholar]
- Hertzog M, Riemann R. Alcohol ingestion influences the nocturnal cardio-respiratory activity in snoring and non-snoring males. Eur Arch Otorhinolaryngol. 2004;261:459–62. doi: 10.1007/s00405-003-0704-x. [DOI] [PubMed] [Google Scholar]
- Hla KM, Skatrud JB, Finn L, et al. The effect of correction of sleep-disordered breathing on BP in untreated hypertension. Chest. 2002;122:1125–32. doi: 10.1378/chest.122.4.1125. [DOI] [PubMed] [Google Scholar]
- Horner RL. Impact of brainstem sleep mechanisms on pharyngeal motor control. Respir Physiol. 2000;119:113–21. doi: 10.1016/s0034-5687(99)00106-1. [DOI] [PubMed] [Google Scholar]
- Hu FB, Willett WC, Manson JE, et al. Snoring and risk of cardiovascular disease in women. J Am Coll Cardiol. 2000;35:308–13. doi: 10.1016/s0735-1097(99)00540-9. [DOI] [PubMed] [Google Scholar]
- Hudgel DW, Fung C. A long-term randomized, cross-over comparison of auto-titrating and standard nasal continuous airway pressure. Sleep. 2000;23:645–8. [PubMed] [Google Scholar]
- Hukins CA. Comparative study of autotitrating and fixed-pressure CPAP in the home:a randomized, single-blind cross-over study. Sleep. 2004;27:1512–17. doi: 10.1093/sleep/27.8.1512. [DOI] [PubMed] [Google Scholar]
- Hukins CA. Arbitrary-pressure continuous positive airway pressure for obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2005;171:500–5. doi: 10.1164/rccm.200401-019OC. [DOI] [PubMed] [Google Scholar]
- Hussain SF, Love L, Burt H, et al. A randomized trial of auto-titrating CPAP and fixed CPAP in the treatment of obstructive sleep apnea-hypopnea. Respir Med. 2004;98:330–3. doi: 10.1016/j.rmed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed by obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–71. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- Ip M, Lam B, Ng M, et al. Obstructive sleep apnoea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- Ip MS, Tse HF, Tsang KWT, et al. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- Isono S, Remmers JE, Tanaka A, et al. Anatomy of pharynx in patients with obstructive sleep apnea and normal subjects. J Appl Physiol. 1997;82:1319–26. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- Jelev A, Sood S, Lui H, et al. Microdialysis perfusion of 5-HT into the hypoglossal motor nucleus differentially modulates genioglossal motor nucleus activity across natural sleep-wake states in rats. J Physiol. 2001;532:467–81. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Jokic R, Klimaszewski A, Crossley M, et al. Positional treatment vs Continuous Positive Airway Pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115:771–81. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22:749–55. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- Kapur V, Strohl KP, Redline S, et al. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–10. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- Kawahara S, Akashiba T, Akahoshi T, et al. Nasal CPAP improves the quality of life and lessens the depressive symptoms in patients with obstructive sleep apnea syndrome. Internal Medicine. 2005;44:422–7. doi: 10.2169/internalmedicine.44.422. [DOI] [PubMed] [Google Scholar]
- Keefe DL, Watson R, Naftolin N. Hormonal replacement therapy may alleviate sleep apnea in menopausal women:a pilot study. Menopause. 1999;6:196–200. doi: 10.1097/00042192-199906030-00004. [DOI] [PubMed] [Google Scholar]
- Kessler R, Weitzenblum E, Chaouat A, et al. Evaluation of unattended automated titration to determine therapeutic continuous positive airway pressure in patients with obstructive sleep apnea. Chest. 2003;123:704–10. doi: 10.1378/chest.123.3.704. [DOI] [PubMed] [Google Scholar]
- Kingshot RN, Vennelle M, Coleman EL, et al. Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of residual excessive daytime sleepiness in the sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:918–23. doi: 10.1164/ajrccm.163.4.2005036. [DOI] [PubMed] [Google Scholar]
- Konerman M, Sanner BM, Vyleta M, et al. Use of conventional and self-adjusting nasal continuous positive airway pressure for treatment of severe obstructive sleep apnea syndrome. A comparative study. Chest. 1998;113:714–18. doi: 10.1378/chest.113.3.714. [DOI] [PubMed] [Google Scholar]
- Kraiczi H, Hedner J, Dahlof P, et al. Effect of serotonin uptake inhibition on breathing during sleep and daytime symptoms in obstructive sleep apnea. Sleep. 1999;22:61–7. [PubMed] [Google Scholar]
- Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- Kryger MH, Roos L, Delaive K, et al. Utilization of health care services in patients with severe obstructive sleep apnea. Sleep. 1996;19(Suppl 9):S111–6. doi: 10.1093/sleep/19.suppl_9.s111. [DOI] [PubMed] [Google Scholar]
- Kyung SH, Park YC, Pae EK. Obstructive sleep apnea patients with the oral appliance experience pharyngeal size and shape changes in three dimensions. Angle Orthod. 2005;75:15–22. doi: 10.1043/0003-3219(2005)075<0015:OSAPWT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Larrosa F, Hernandez L, Morello A, et al. Laser-assisted uvuloplasty for snoring:does it meet the expectations? Eur Respir J. 2004;24:66–70. doi: 10.1183/09031936.04.00082903. [DOI] [PubMed] [Google Scholar]
- Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients:a multivariate analysis of risk factors. Sleep. 1995;18:149–57. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- Lee NR, Givens CD, Wilson J, et al. Staged surgical treatment for obstructive sleep apnea syndrome:a review of 35 patients. J Oral Maxillofac Surg. 1999;57:382–5. doi: 10.1016/s0278-2391(99)90272-0. [DOI] [PubMed] [Google Scholar]
- Leineweber C, Kecklund G, Janszky I, et al. Snoring and progression of coronary artery disease:the Stockholm Female Coronary Angiography Study. Sleep. 2004;27:1344–9. doi: 10.1093/sleep/27.7.1344. [DOI] [PubMed] [Google Scholar]
- Li KK, Powell NB, Riley RW, et al. Temperature-controlled radiofrequncy tongue base reduction for sleep-disordered breathing:Long-term outcomes. Otolaryngol Head Neck Surg. 2002;127:230–4. doi: 10.1067/mhn.2002.126900. [DOI] [PubMed] [Google Scholar]
- Li KK, Powell NB, Riley RW, et al. Overview of phase I surgery for obstructive sleep apnea syndrome. Ear Nose Throat J. 1999a;78:841–5. [PubMed] [Google Scholar]
- Li KK, Powell NB, Riley RW, et al. Overview of phase II surgery for obstructive sleep apnea syndrome. Ear Nose Throat J. 1999b;78:854–7. [PubMed] [Google Scholar]
- Lim J, Lasserson TJ, Fleetham J, et al. Oral appliances for obstructive sleep apnoea. The Cochrane Database of Systematic Reviews. 2004;4 doi: 10.1002/14651858.CD004435.pub2. Art. No. CD004435.pub2. [DOI] [PubMed] [Google Scholar]
- Ling L, Finn L, Zhang J, et al. Angiotensin-converting enzyme, sleep-disordered breathing, and hypertension. Am J Respir Crit Care Med. 2004;170:1349–53. doi: 10.1164/rccm.200405-616OC. [DOI] [PubMed] [Google Scholar]
- Littner M, Kushida CA, Hartse K, et al. Practice parameters for the use of laser-assisted uvuloplasty:an update for 2000. Sleep. 2001;24:603–19. doi: 10.1093/sleep/24.5.603. [DOI] [PubMed] [Google Scholar]
- Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure vs placebo continuous positive airway pressure on sleep quality in obstructive sleep apnea. Chest. 1999;116:1545–9. doi: 10.1378/chest.116.6.1545. [DOI] [PubMed] [Google Scholar]
- McArdle N, Devereux G, Heinarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;164:1459–63. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Fogel RB, Edwards JK, et al. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1746–9. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- Manber R, Kuo TF, Cataldo N, et al. The effects of hormone replacement therapy on sleep-disordered breathing in post-menopausal women:A pilot study. Sleep. 2003;26:163–8. [PubMed] [Google Scholar]
- Marin JM, Carizzo SJ, Vincete E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure:an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring. Tolerability and predictors of treatment success. Chest. 2004;125:1270–8. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- Marti S, Sampol G, Munoz X, et al. Mortality in severe sleep apnoea/ hypopnoea syndrome patients:impact of treatment. Eur Respir J. 2002;20:1511–1518. doi: 10.1183/09031936.02.00306502. [DOI] [PubMed] [Google Scholar]
- Massie CA, McArdle N, Hart RW, et al. Comparison between automatic and fixed pressure positive airway pressure therapy in the home. Am J Respir Crit Care Med. 2003;167:20–3. doi: 10.1164/rccm.200201-022OC. [DOI] [PubMed] [Google Scholar]
- Means MK, Edinger JD, Husain AM. CPAP compliance in sleep apnea patients with and without laboratory CPAP titration. Sleep Breath. 2004;8:7–14. doi: 10.1007/s11325-004-0007-5. [DOI] [PubMed] [Google Scholar]
- Mehta A, Qian J, Petocz P, et al. A randomized. controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal EMG in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–9. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153:1880–7. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease:a long-term follow-up study. Eur Heart J. 2004;25:728–34. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Kennedy BP, Loredo JS, et al. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100:343–8. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–9. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- Monastero C, Vidal S, Duran J, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;164:939–43. doi: 10.1164/ajrccm.164.6.2008010. [DOI] [PubMed] [Google Scholar]
- Monsterrat JM. Time-course of stepwise CPAP titration. Behaviour of respiratory and neurological variables. Am J Respir Crit Care Med. 1995;152:1854–9. doi: 10.1164/ajrccm.152.6.8520746. [DOI] [PubMed] [Google Scholar]
- Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome. A randomized controlled study with an optimized placebo. Am J Respir Cit Care Med. 2001;164:608–13. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- Mooe T, Rabben T, Wikland U, et al. Sleep disordered breathing in men with coronary artery disease. Chest. 1996;109:659–63. doi: 10.1378/chest.109.3.659. [DOI] [PubMed] [Google Scholar]
- National-Institutes-of-Health-Report. Wake up America:A national sleep alert. Vol. 2. Washington DC: US Government Printing Office; 1994. National Commission on Sleep Disorders Research. Working group reports. [Google Scholar]
- Ng AT, Gotsopoulos H, Qian J, et al. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;168:238–41. doi: 10.1164/rccm.200211-1275OC. [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1880–1. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Noseda A, Kempenaers C, Kerkofs M, et al. Constant vs auto-continuous positive airway pressure in patients with sleep apnea hypopnea syndrome and a high variability in pressure requirement. Chest. 2004;126:31–7. doi: 10.1378/chest.126.1.31. [DOI] [PubMed] [Google Scholar]
- Ogasa T, Ray AD, Michlin CP, et al. Systemic adminstration of serotonin 2A/2C agonist improves upper airway stability in Zucker rats. Am J Respir Crit Care Med. 2004;170:804–10. doi: 10.1164/rccm.200312-1674OC. [DOI] [PubMed] [Google Scholar]
- Oliven A, O’Hearn DJ, Boudewyns A, et al. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol. 2003;95:2023–9. doi: 10.1152/japplphysiol.00203.2003. [DOI] [PubMed] [Google Scholar]
- Omur M, Ozturan D, Elez F, et al. Tongue base suspension combined with UPPP in severe OSA patients. Otolaryngol Head Neck Surg. 2005;133:218–23. doi: 10.1016/j.otohns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Pack AI, Black JE, Schwartz JRL, et al. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164:1675–81. doi: 10.1164/ajrccm.164.9.2103032. [DOI] [PubMed] [Google Scholar]
- Pantin CC, Hillman DR, Tennant M. Dental side effects of an oral device to treat snoring and obstructive sleep apnea. Sleep. 1999;22:237–40. doi: 10.1093/sleep/22.2.237. [DOI] [PubMed] [Google Scholar]
- Parish JM, Lyng PJ. Quality of life in bed partners of patients with obstructive sleep apnea or hypopnea after treatment with positive airway pressure. Chest. 2003;124:942–7. doi: 10.1378/chest.124.3.942. [DOI] [PubMed] [Google Scholar]
- Parra O, Arboix A, Garcia-Eroles L, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161:375–80. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- Parra O, Arboix A, Montserrat JM, et al. Sleep-related breathing disorders:impact on mortality of cerebrovascular disease. Eur Respir J. 2004;24:195–6. doi: 10.1183/09031936.04.00061503. [DOI] [PubMed] [Google Scholar]
- Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea. A 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- Peker Y, Kraiczi H, Hedner J, et al. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14:179–84. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- Pepin JL, Veale D, Mayer P, et al. Critical analysis of the results of surgery in the treatment of snoring, upper airway resistance syndrome (UARS), and obstructive sleep apnea (OSA) Sleep. 1996;19:S90–100. doi: 10.1093/sleep/19.suppl_9.s90. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000a;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Eng J Med. 2000b;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea:a randomised parallel trial. Lancet. 2002;359(9302):204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- Peveragie DA, Proot PM, Hertegonne KB, et al. Efficacy of flow- vs impedance-guided autoadjustable continuous positive airway pressure. Chest. 2004;126:25–30. doi: 10.1378/chest.126.1.25. [DOI] [PubMed] [Google Scholar]
- Pichel F, Zamarron C, Magan F, et al. Health-related quality of life in patients with obstructive sleep apnea:effects of long-term positive airway pressure treatment. Respir Med. 2004;98:968–76. doi: 10.1016/j.rmed.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Pickett CK, Regensteiner JG, Woodard WD, et al. Progestin and estrogen reduce sleep-disordered breathing in post-menopausal women. J Appl Physiol. 1989;66:1656–61. doi: 10.1152/jappl.1989.66.4.1656. [DOI] [PubMed] [Google Scholar]
- Planes C, d’Ortho MP, Foucher A, et al. Efficacy and cost of home-initiated auto-nCPAP versus conventional CPAP. Sleep. 2003;26:156–60. doi: 10.1093/sleep/26.2.156. [DOI] [PubMed] [Google Scholar]
- Powell NB, Riley RW, Troell RJ, et al. Radiofrequency volumetric reduction of the tongue. A porcine pilot study for the treatment of obstructive sleep apnea syndrome. Chest. 1997;111:1348–55. doi: 10.1378/chest.111.5.1348. [DOI] [PubMed] [Google Scholar]
- Pujabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance:the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- Randerath WJ, Schraeder O, Galetke W, et al. Autoadjusting CPAP therapy based on impedance. Efficacy, compliance and acceptance. Am J Respir Crit Care Med. 2001;163:652–657. doi: 10.1164/ajrccm.163.3.2006168. [DOI] [PubMed] [Google Scholar]
- Rauscher H, Formanek D, Popp W, et al. Self-reported vs measured compliance with nasal CPAP for obstructive sleep apnea. Chest. 1993;103:1675–80. doi: 10.1378/chest.103.6.1675. [DOI] [PubMed] [Google Scholar]
- Reichmuth KJ, Austin D, Skratrud JB, et al. Association of sleep apnea and Type II diabetes. A Population based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha R, Brander P, Vennelle M, et al. A cephalometric comparison of patients with the sleep apnea/hypopnea syndrome and their siblings. Sleep. 2005a;28:315–20. [PubMed] [Google Scholar]
- Riha RL, Brander P, Vennelle M, et al. Tumour necrosis factor-alpha (-308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J. 2005b;26:673–8. doi: 10.1183/09031936.05.00130804. [DOI] [PubMed] [Google Scholar]
- Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome:a review of 306 consecutively treated surgical patients. Otolaryngol Head Neck Surg. 1993;108:117–25. doi: 10.1177/019459989310800203. [DOI] [PubMed] [Google Scholar]
- Robinson GV, Pepperell JCT, Segal HC, et al. Circulating cardiovascular risk factors in obstructive sleep apnoea:data from randomised controlled trials. Thorax. 2004;59:777–82. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CF, Love LL, Peat D, et al. Mandibular advancement oral appliance therapy for obstructive sleep apnoea:effect on awake calibre of the velopharynx. Thorax. 1999;54:972–7. doi: 10.1136/thx.54.11.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon MF, Roebuck T, Little PJ, et al. Effect of moderate alcohol upon obstructive sleep apnoea. Eur Respir J. 2000;16:909–13. doi: 10.1183/09031936.00.16590900. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Bennett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2001;127:1216–23. doi: 10.1001/archotol.127.10.1216. [DOI] [PubMed] [Google Scholar]
- Schwartz JRL, Hirshkowitz M, Erman MK, et al. Modafinil for adjunct therapy for daytime sleepiness in obstructive sleep apnea. A 12-week, open label study. Chest. 2003;124:2192–9. doi: 10.1378/chest.124.6.2192. [DOI] [PubMed] [Google Scholar]
- Shahar E, Redline S, Young T, et al. Hormonal replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–92. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease. Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Sher AE, Schechttman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–77. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- Shitrit D, Peled N, Shitrit AB, et al. An association between oxygen saturation and D-dimer in patients with obstructive sleep apnea syndrome. Thromb Haemost. 2005;94:544–7. [PubMed] [Google Scholar]
- Sin DD, Mayers I, Man GCW, et al. Can continuous positive airway pressure therapy improve the general health status of patients with obstructive sleep apnea? Chest. 2002a;122:1679–85. doi: 10.1378/chest.122.5.1679. [DOI] [PubMed] [Google Scholar]
- Sin DD, Mayers I, Man GCW, et al. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea. A population based study. Chest. 2002b;121:430–5. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- Skinner MA, Kingshot RN, Jones DR, et al. Elevated posture for the management of obstructive sleep apnea. Sleep Breath. 2004;8:193–200. doi: 10.1007/s11325-004-0193-1. [DOI] [PubMed] [Google Scholar]
- Smith I, Lasserson TJ, Wright J. Drug treatments for obstructive sleep apnoea. The Cochrane Database of Systematic Reviews. 2001;(4) doi: 10.1002/14651858.CD003002. Art. No.:CD003002. [DOI] [PubMed] [Google Scholar]
- Sood S, Morrison JL, Liu H, et al. Role of endogenous serotonin in modulating genioglossus activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–47. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- Stammnitz A, Jerrentrup A, Penzel T, et al. Automatic CPAP titration with different self-setting devices in patients with obstructive sleep apnoea. Eur Resp J. 2004;24(2):273–8. doi: 10.1183/09031936.04.00074304. [DOI] [PubMed] [Google Scholar]
- Steiner S, Jax T, Evers S, et al. Altered blood rheology in obstructive sleep apnea as a mediator of cardiovascular risk. Cardiology. 2005;104:92–6. doi: 10.1159/000086729. [DOI] [PubMed] [Google Scholar]
- Stradling JR, Barbour C, Pitson DJ, et al. Automatic nasal continuous positive airway pressure titration in the laboratory:patient outcomes. Thorax. 1997;52:72–5. doi: 10.1136/thx.52.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuck BA, Maurer JT, Verse T, et al. Tongue base reduction with temperature-controlled radiofrequency volumetric tissue reduction for treatment of obstructive sleep apnea syndrome. Acta Otolaryngol. 2002;122:531–6. doi: 10.1080/00016480260092354. [DOI] [PubMed] [Google Scholar]
- Sullivan CE, Berthan-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- Sundaram S, Bridgeman SA, Lim J, et al. Surgery for obstructive sleep apnoea. The Cochrane Database of Systematic Reviews. 2005;(4) doi: 10.1002/14651858.CD001004. pub2. Art. No.:CD001004.pub2. [DOI] [PubMed] [Google Scholar]
- Tan YK, L’Estrange PR, Luo YM, et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea:a randomized cross-over trial. Eur J Orthod. 2002;24:239–49. doi: 10.1093/ejo/24.3.239. [DOI] [PubMed] [Google Scholar]
- Teschler H, Berthon-Jones M, Wessendorf T, et al. Influence of moderate alcohol consumption on obstructive sleep apnoea with and without Autoset nasal CPAP therapy. Eur Respir J. 1996;9:2371–7. doi: 10.1183/09031936.96.09112371. [DOI] [PubMed] [Google Scholar]
- Teschler H, Farhat AA, Exner V, et al. AutoSet nasal CPAP titration:constancy of pressure, compliance and effectiveness at 8-month follow-up. Eur Respir J. 1997;10:2073–8. doi: 10.1183/09031936.97.10092073. [DOI] [PubMed] [Google Scholar]
- Teschler H, Wessendorf TE, Farhat AA, et al. Two months auto-adjusting versus conventional CPAP for obstructive sleep apnea syndrome. Eur Respir J. 2000;15:990–5. doi: 10.1034/j.1399-3003.2000.01503.x. [DOI] [PubMed] [Google Scholar]
- Tsuiki S, Lowe AA, Almeida FR, et al. Effects of mandibular advancement on airway curvature and obstructive sleep apnoea severity. Eur Respir J. 2004;23:263–8. doi: 10.1183/09031936.04.00094304. [DOI] [PubMed] [Google Scholar]
- Usui K, Bradley TD, Spaak J, et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol. 2005;45:2008–11. doi: 10.1016/j.jacc.2004.12.080. [DOI] [PubMed] [Google Scholar]
- Vgontzaz AN, Zoumakis E, Lin HM, et al. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor - alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- Walker-Engstrom ML, Tegelberg A, Wilhelmsson B, et al. 4-year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea. A randomized study. Chest. 2002;121:739–46. doi: 10.1378/chest.121.3.739. [DOI] [PubMed] [Google Scholar]
- Walker-Engstrom ML, Wilhelmsson B, Tegelberg A, et al. Quality of life assessment of treatment with dental appliance or UPPP in patients with mild to moderate obstructive sleep apnoea. A prospective, randomized 1-year follow-up study. J Sleep Res. 2000;9:303–8. doi: 10.1046/j.1365-2869.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- Weaver TE, Barone-Kribbs N, Pack AI, et al. Night-to night variability in CPAP use over the first three months of treatment. Sleep. 1997a;20:278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- Weaver TE, Lainzer A, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997b;20:835–43. [PubMed] [Google Scholar]
- Wesstrom J, Ulfberg J, Nilsson S. Sleep apnea and hormone replacement therapy:A pilot study and literature review. Acta Obstet Gynecol Scand. 2005;84:54–7. doi: 10.1111/j.0001-6349.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- White J, Cates C, Wright J. Continuous positive airways pressure for obstructive sleep apnoea. The Cochrane Database of Systematic Reviews. 2001;(4) doi: 10.1002/14651858. CD001106. Art No.:CD001106. [DOI] [PubMed] [Google Scholar]
- Woodson BT, Stewart DL, Weaver EM, et al. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2003;128:848–61. doi: 10.1016/S0194-59980300461-3. [DOI] [PubMed] [Google Scholar]
- Wright J, Johns R, Watt I, et al. Health effects of obstructive sleep apnoea and the effectiveness of continuous positive airways pressure:a systematic review of the research evidence. BMJ. 1997;314:851–60. doi: 10.1136/bmj.314.7084.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- Young TB, Blustein J, Finn L, et al. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep. 1997a;20:608–13. doi: 10.1093/sleep/20.8.608. [DOI] [PubMed] [Google Scholar]
- Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997b;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- Ziegler MG, Mills PJ, Loredo JS, et al. Effect of Continuous Positive Airway Pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest. 2001;120:887–93. doi: 10.1378/chest.120.3.887. [DOI] [PubMed] [Google Scholar]