Abstract
This paper reviews the results of placebo-controlled trials on topiramate (TPM) in the prophylaxis of migraine, focusing particularly on efficacy and tolerability of the target dose (100 mg/day). Data from well-conducted trials and analyses of pooled data show that TPM is effective against migraine, confirming the experience of physicians in various countries. High responder rate and good tolerability following slow titration suggest TPM as a first-line option for migraine prophylaxis. Patient acceptability may be enhanced by lack of weight gain, lack of major contraindications, and positive effects on quality of life.
Keywords: migraine, topiramate, prophylaxis, clinical trials, tolerability, acceptability
Introduction
Migraine is a chronic neurological disorder characterized by recurrent attacks of headache and other symptoms which may last up to 3 days. The pain is moderate to severe, and is associated with other symptoms, such as phonophobia, photophobia, nausea, and vomiting. Prodromes like somnolence and mood changes may also be present. In some patients the headache phase is preceded by aura, a complex of focal neurological symptoms, which may include visual disturbances, numbness, paresthesia, and speech difficulties (Headache Classification Subcommittee of the International Headache Society 2004; Lipton et al 2000).
Migraine is a widespread disorder and tends to be present for long periods in patients’ lives producing a wide range of impacts on personal and social functioning (Stewart et al 1994; Lipton, Hamelsky, et al 2001). The prevalence rate of migraine with or without aura in the US in 1999 was 12.6% overall and 18.2% in women compared with 6.5% in men (Lipton, Stewart, et al 2001). Similar estimates have been reported by various population-based studies from other countries (Launer et al 1999; Roncolato et al 2000; Lipton, Hamelsky, et al 2001; Henry et al 2002; Steiner et al 2003). Such surveys indicate that migraine prevalence increases steadily with age, particularly in women, and peaks between the mid thirties and mid forties – the peak years of personal and professional activity.
About a half of migraineurs in the general population surveyed in a Canadian study (Edmeads et al 1993) and in the second American Migraine Study (Lipton, Stewart, et al 2001) reported that their severe headaches led to substantial impairment or discontinuation of their daily activities, and required bed rest in many cases. These finding are supported by population and clinical studies which show that migraineurs are affected in work and nonwork activities, and may manifest not only as absence from the workplace but also as substantially reduced productivity in paid work and household work, as well as disruption of relations with family and friends, and social and leisure activities (von Korff et al 1998; Brandes 2002; Bigal et al 2003; D’Amico, Genco, et al 2004; D’Amico, Usai, et al 2004; Dueland et al 2004; MacGregor et al 2004).
Migraine also has a pervasive negative influence on patient well-being during headache-free periods, as manifested by compromised physical, mental, emotional, and social functioning. These health-related quality of life (HRQOL) effects have been extensively studied (Dahlöf and Dimenäis 1995; Solomon and Santanello 2000; Lipton et al 2003; D’Amico, Usai, et al 2004; Dueland et al 2005). HRQOL in migraineurs is poor in comparison with people without migraine, and it seems to be worse than that experienced by people with several other chronic disorders (myocardial infarction, diabetes, hypertension, and asthma) (Osterhaus et al 1992; Terwindt et al 2000).
The prevalence figures and the results of studies assessing the functional consequences of migraine indicate that the disorder has major negative effects on individuals and on society as a whole, so that effective migraine management is an important priority of both general practitioners and neurologists.
Acute treatments should be taken during migraine attacks to reduce the severity and duration of the episodes. Migraine patients with high attack frequency, unsatisfactory response to acute treatment, or who overuse acute medications are candidates for prophylactic treatments (Silberstein 2000; Silberstein et al 2001; Dowson et al 2002), which are taken daily with the aim of reducing the frequency, duration, and severity of attacks, and ultimately improving quality of life and ability to function in daily activities.
Various drugs are currently used for migraine prophylaxis, including β-blockers, antidepressants, calcium channel antagonists, serotonin antagonists, and anti-epileptics (Gray et al 1999; Silberstein et al 2001). However, many of the studies conducted with these drugs did not adhere to the criteria proposed by the International Headache Society (International Headache Society Clinical Trials Subcommittee 2000), and by the US Headache Consortium guidelines (Silberstein 2000) for conducting trials with preventive migraine compounds and validating their use in migraine patients. These criteria require that preventive treatments should be validated using evidence-based standards, and in particular that efficacy should be supported by data from large, well-designed, placebo-controlled trials. Such trials should assess prophylactic efficacy (which must be sustained), safety, and tolerability. Furthermore the treatment should not worsen comorbid conditions, and side-effects should not impede compliance.
Topiramate (TPM) is a neuromodulatory drug with unique pharmacological and clinical profiles. TPM has emerged relatively recently as a treatment for migraine, and is approved for migraine prevention in several countries including the US.
We review here the pharmacology of TPM and also the results of large well-conducted trials of TPM in migraine patients, focusing particularly on efficacy and tolerability of the target dose of this drug in migraine prevention (100 mg/day). We also discuss data and issues pertaining to patient satisfaction (quality of life and acceptability).
Pharmacology and mode of action
TPM bioavailability after oral assumption is greater than 80%. Maximum plasma levels (Cmax) are reached 1.3–1.7 hours after oral administration, and half-life is 19–23 hours. Protein binding is around 15%; 50%–80% of the drug is excreted unchanged in the urine.
TPM possesses a broad clinical spectrum of activity. Animal studies and clinical trials have led to its indication in epilepsy, as adjunctive therapy and now also as monotherapy (Silberstein et al 2005). Pilot studies and small controlled trials have assessed its efficacy in several psychiatric conditions, including binge-eating disorders (McElroy et al 2003) and alcohol dependence (Johnson et al 2003).
TPM was introduced as a treatment for migraine prophylaxis on the basis of results from controlled trials (Brandes et al 2004; Diener et al 2004; Silberstein et al 2004). Although the exact mechanisms by which it is effective in migraine have not been established, several effects of TPM may contribute to its anti-migraine action. Migraine is a neurovascular disorder characterized by a state of neuronal hyperexcitability, with abnormal modulation involving several receptors and ion channels at several sites including the cerebral cortex, the trigeminovascular system, and brainstem nuclei (Goadsby 2005; Welch 2005). TPM inhibits the excitatory effect of glutamate at α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid–kainite receptor subtypes; it enhances chloride flux mediated by the inhibitory γ-aminobutyric acid receptor A; it inhibits voltage-gated sodium channels and high-voltage-gated calcium channels; and it also inhibits some subtypes of the enzyme carbonic anhydrase (Shank et al 2000). TPM may therefore exert a variety of pharmacodynamic effects in migraineurs that could explain its efficacy such as reducing cortical hyperexcitability that leads to cortical spreading depression, inhibiting glutamatergic signaling by trigeminal afferent nerves, or modulating nociceptive signaling through GABA-receptors in the trigeminal nucleus caudalis or in descending brain pathways (Shank et al 2000).
Efficacy
The two pivotal studies conducted to determine the efficacy of TPM in migraine prevention are MIGR-001, conducted at 49 sites in the US (Silberstein et al 2004), and MIGR-002, conducted at 52 US and Canadian centers (Brandes et al 2004). Both these studies were randomized, double-blind, placebo-controlled trials. The treatment period was 26 weeks, divided into an 8-week titration phase and an 18-week maintenance phase. Three daily doses were tested: 50, 100, or 200 mg. In all cases, TPM was started at 25 mg/day and titrated to target dose or maximum tolerated dose at the rate of 25 mg/week. Efficacy was assessed throughout the double-blind period, including the titration period. The primary efficacy measure was change in mean monthly migraine frequency compared with baseline, assessed using migraine periods, ie, migraine headache that started and ended or recurred and ended within 24 hours. Secondary efficacy endpoints included the percentage of responders (proportion with ≥ 50% reduction in the monthly migraine frequency), and the time to onset of action. The intent-to-treat populations were 469 patients in MIGR-001 and 468 patients in MIGR-002.
The results of these trials showed that TPM treatment was associated with significant improvements in the 100 mg/day and in the 200 mg/day arms. Reductions in monthly migraine frequency were significantly higher than in the placebo arm (p < 0.001 for both doses in MIGR-001; p = 0.008 in the 100 mg/day arm, and p = 0.001 in the 200 mg/day arm in MIGR-002). Furthermore there were significantly more responders in both dosage arms than in the placebo arm (p < 0.001 for both dosages, MIGR-001 and MIGR-002 studies).
Thus it emerged that 200 mg/day TPM was not more effective than 100 mg/day TPM. For example, in MIGR-001, migraine frequency decreased from 5.4 ± 2.2 days/month at baseline to 3.3 ± 2.9 days/month during the double-blind phase with 100 mg/day TPM, and from 5.6 ± 2.6 to 3.3 ± 2.9 days/month with 200 mg/day TPM; the responder rate was 54% for 100 mg/day, and 52.3% for 200 mg/day.
In both MIGR studies, patients in the 50 mg/day arm had reductions in migraine frequency but responders were fewer than with higher daily doses, and did not differ significantly from those found in the placebo arm.
The MIGR-003 trial was a randomized, double-blind, multicenter comparative trial (61 sites, 13 countries) evaluating 575 migraine patients as the intent-to-treat population. The trial was designed to assess the efficacy and safety of TPM vs placebo in migraine prophylaxis, and used propranolol as active control (Diener et al 2004). Patients were randomized to TPM (100 mg/day or 200 mg/day), propranolol (160 mg/day), or placebo. The trial results were substantially in agreement with those of the two previous MIGR trials: TPM was superior to placebo in reducing monthly migraine frequency and in increasing responder rate. The 100 mg/day TPM and propranolol groups were characterized by similar reductions in migraine frequency, responder rate, and daily use of rescue medication.
The overall results of these three studies indicate that 100 mg/day is more effective than the 50 mg/day, and that 200 mg/day does not provide a clear additional benefit. Based on these results it was concluded that 100 mg/day of TPM is the target dose in migraine prevention.
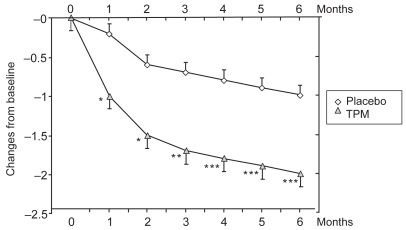
The protocols of the three trials ( Brandes et al 2004; Diener et al 2004; Silberstein et al 2004) were similar in design and had the same primary and secondary endpoints. The data from these studies have been pooled to evaluate the consistency of efficacy of 100 mg/day TPM (386 patients) vs placebo (372 patients) (Bussone et al 2005; Silberstein et al 2005). These analyses showed that TPM is superior to placebo, and that efficacy always emerged irrespective of the assessment method (migraine periods, number of migraine attacks, or number of days with migraine). Thus, the decrease in mean monthly number of migraine periods from baseline to endpoint was significant (mean change − 2.0 ± 0.16 in the TPM arm, − 1.0 ± 0.13 in the placebo arm, p < 0.001) (Figure 1). Similarly, TPM treatment was associated with a significant reduction in migraine attack frequency (− 1.7 ± 0.16 vs − 0.8 ± 0.13; p < 0.001), and in monthly migraine days (− 2.4 ± 0.18 vs − 1.2 ± 0.16; p < 0.001). Monthly migraine duration also decreased during the treatment periods in all the three clinical trials, with a mean reduction of − 0.9 ± 0.09 hours in the TPM arms compared with − 0.5 ± 0.08 hours in patients who received placebo (p < 0.05).
Figure 1.
Changes from baseline in migraine period frequency during controlled clinical trials (Brandes et al 2004; Diener et al 2004; Silberstein et al 2004): pooled data from patients receiving topiramate (TPM) 100 mg/day (Bussone et al 2005).
*p < 0.05; **p < 0.01; ***p < 0.001.
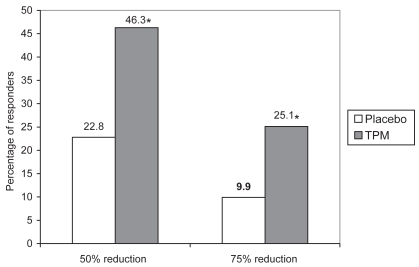
Although about half of the patients receiving TPM achieved at least a 50% reduction in monthly number of migraine periods, it is important to note that one in four patients obtained the greater clinical benefit of at least a 75% reduction (Figure 2); furthermore 5.8% of patients became free of migraine periods and migraine days.
Figure 2.
Proportions of patients achieving at least 50% reduction and at least 75% reduction in monthly migraine period frequency during controlled clinical trials (Brandes et al 2004; Diener et al 2004; Silberstein et al 2004): pooled data from patients receiving topiramate (TPM) 100 mg/dayay (Bussone et al 2005).
*p < 0.001.
Other important findings of the pooled analysis were that monthly migraine frequency declined progressively over time, as shown in Figure 1: a significant difference between TPM and placebo was achieved as early as the first month (by which time the target dose of 100 mg/day had been reached) and the improvement continued steadily throughout the double-blind period. Improvement rates were similar in males and females.
Tolerability
Pooled data from the above-mentioned controlled studies (Bussone et al 2005; Silberstein et al 2005) show that paresthesia is the most common adverse event, being reported in 51% of patients on TPM 100 mg/day, in 49% of those on 200 mg/day, and by 6% of patients on placebo.
Fatigue was the second most common adverse event, being reported by 11% of patients on placebo, and slightly more patients on TPM (15% for TPM 100 mg/day, and 19% for in TPM 200 mg/day).
The incidence of adverse central nervous system events in migraine patients was also low: somnolence, insomnia, memory or concentration difficulties, language problems, and mood changes were present in 2%–5% of patients on placebo, 6%–7% of patients on TPM 100 mg/day, and 7%–12% of those on TPM 200 mg/day; while 8% of patients on TPM 100 mg/day and 12% of those on 200 mg/day TPM complained of taste perversion, compared with 1% of patients on placebo. The rates of these adverse events were generally lower in patients receiving 50 mg/day TPM.
The most common adverse events in patients receiving different doses of TPM in controlled clinical trials (pooled data from Silberstein et al 2005) are shown in Table 1.
Table 1.
Most common adverse events in patients receiving different doses of topiramate (TPM) in controlled clinical trials (Brandes et al 2004; Diener et al 2004; Silberstein et al 2004): pooled data (Silberstein et al 2005)
| TPM 50 mg/day
|
TPM 100 mg/day
|
TPM 200 mg/day
|
Placebo
|
|||||
|---|---|---|---|---|---|---|---|---|
| Adverse eventsa | Led to withdrawalb | Adverse events | Led to withdrawal | Adverse events | Led to withdrawal | Adverse events | Led to withdrawal | |
| Paresthesia | 35 | 3 | 51 | 8 | 49 | 7 | 6 | 1 |
| Fatigue | 14 | 3 | 15 | 5 | 19 | 5 | 11 | 1 |
| Anorexia | 9 | 1 | 15 | 2 | 14 | 6 | 3 | <1 |
| Nausea | 9 | 3 | 13 | 2 | 14 | 6 | 8 | 1 |
| Dizziness | 8 | 1 | 9 | 2 | 12 | 3 | 10 | 2 |
| Taste perversion | 15 | 0 | 8 | 0 | 12 | 0 | 1 | 0 |
| Insomnia | 6 | 2 | 7 | 3 | 6 | 3 | 5 | 1 |
| Somnolence | 8 | 1 | 7 | 2 | 10 | 2 | 5 | 2 |
| Difficulty with memory | 7 | 1 | 7 | 3 | 11 | 2 | 2 | 1 |
| Language problems | 7 | 2 | 6 | 2 | 7 | 2 | 2 | <1 |
Adverse events: percentage of patients reporting each adverse event.
Led to withdrawal: percentage of patients who withdrew from clinical trials due to each adverse event.
Overall discontinuation rates were quite high: about 25% in patients on 100 mg/day TPM and about 40% in those on 200 mg/day. The percentages of patients who withdrew from clinical trials because of each adverse event are shown in Table 1.
In patients on 100 mg/day TPM, discontinuation was for paresthesia in 8%, fatigue in 5%, insomnia in 3%, and for other symptoms (nausea, anorexia, dizziness, and concentration difficulties) in about 2% each.
Other side-effects may occur rarely after TPM exposure (Brandes 2005). Kidney stone formation is a rare adverse event. Metabolic acidosis is a possibility when predisposing conditions are present. Such conditions include kidney disease, severe respiratory disorder, status epilepticus, diarrhea, surgery, ketogenic diet, and use of other carbonic anhydrase inhibitors. Hyperthermia may occur, especially in children doing physical exercise, or after exposure to high ambient temperature. Patients should be advised that adequate hydration is necessary when taking TPM to minimize the risk of these unusual side-effects. It is also advisable to periodically measure serum bicarbonate levels in patients predisposed to acidosis.
Acute myopia and secondary angle-closure glaucoma syndrome are other possibilities (Fraunfelder et al 2004): these bilateral manifestations are rare, easily recognizable, and reversible following TPM withdrawal; prescreening is not recommended.
Effect on bodyweight
Weight gain is a common problem and concern in patients receiving treatments such as valproate, flunarizine, and propranolol for migraine prevention. By contrast 60%–70% of patients on TPM in the MGR trials lost weight, and no change in bodyweight occurred in 20%. The mean overall reductions in baseline bodyweight in MIGR-001, MIGR-002, and MIGR-003 (Brandes et al 2004; Diener 2004; Silberstein et al 2004) were 3.8%, 3.3%, and 2.7% respectively.
Analysis of pooled data from these studies (Bussone et al 2005; Silberstein et al 2005) showed that in patients receiving 100 mg/day TPM, mean weight change was − 2.5 kg, compared with virtually no change on placebo (+0.1 kg). In patients for whom data on body mass index (BMI) were available (n = 378), weight reduction was − 1.9 kg in the patients with normal BMI, − 3.1 kg in overweight patients, and − 3.0 kg in obese patients.
Effect on quality of life
Improvement in HRQOL has been recently reported in an analysis of data pooled from the three MGR trials (Diamond et al 2005). The Migraine-Specific Questionnaire (MSQ, version 2.1) (Jhingran et al 1998) was used to assess the effect of 100 mg/day TPM on HRQOL. This tool evaluates quality of life in three domains: role restriction (degree to which performance of daily activities is limited by migraine), role prevention (degree to which performance of daily activities is interrupted by migraine), and emotional function (examines feelings of frustration and helplessness due to migraine). All these domains improved significantly more in TPM-treated patients than in those on placebo.
Contraindications
The available drugs for migraine prevention are characterized by diverse side-effect profiles and contraindications. On the basis of its lack of major contraindications, TPM can be used in the presence of various conditions that contraindicate the use of other migraine prophylactics: excess weight (amitriptyline, flunarizine, pizotifen, valproate contraindicated); asthma (β-blockers absolutely contraindicated); depression (can be enhanced by flunarizine and β-blockers); heart block, epilepsy, and urinary retention (amitriptyline contraindicated); and liver disease and bleeding disorders (valproate contraindicated).
Concluding remarks
Migraine is a common chronic recurrent neurological disorder, associated with significant morbidity, important impairment in daily functioning, and poor quality of life. Migraine patients with high attack frequency, severe and disabling attacks, poor response to acute treatment, or who overuse acute medications are candidates for prevention treatments.
Several compounds are currently used in migraine prophylaxis; in many of which efficacy has not been determined in studies conducted according to the criteria of the International Headache Society (International Headache Society Clinical Trials Subcommittee 2000), or the recently published guidelines for assessing the clinical benefits of preventive agents in migraine patients (Silberstein 2000). By contrast, TPM satisfies these criteria, having being validated by large, double-blind, multicenter, cross-border, placebo-controlled trials that analyzed patients on an intent-to-treat basis, and which lasted sufficiently long (26 weeks) to assess adequately improvement and adverse events (Brandes et al 2004; Diener et al 2004, Silberstein et al 2004, 2005; Bussone et al 2005).
These trials showed that TPM has sustained efficacy associated with satisfactory safety and tolerability, particularly at the 100 mg/day dose. About half the patients receiving the target dose (100 mg/day) achieved at least a 50% reduction in migraine frequency; a subgroup experienced even greater clinical benefit and about 6% became migraine free during the treatment period.
With regard to tolerability and safety, paresthesia is the most common adverse event, reported by about 50% of patients receiving 100 mg/day TPM. Analysis of the time course of this symptom in patients receiving TPM for migraine prevention showed that most paresthesias were transient, and about half had resolved by the end of the trial (Silberstein et al 2005). Nevertheless about 25% of patients on the 100 mg/day dose discontinued treatment for various reasons during the clinical trials.
We suggest that patients should be warned in advance of possible side-effects of TPM, and should be reassured about their benign and self-limited nature, and also because our clinical experience indicates that many patients will better tolerate them.
Other important characteristics of TPM are that efficacy onset is usually rapid, progresses over time, and is independent of gender. Patient acceptability may be enhanced by lack of weight gain and lack of major contraindications, both of which are common concerns in patients receiving other preventive treatments for migraine. The effect on weight can be considered a favorable outcome in overweight or obese patients or in those on concomitant medications known to increase weight, such as antidepressants or atypical antipsychotics. TPM also has positive effects on quality of life. These positive attributes indicate that TPM can satisfy many migraine patients’ requirements as a prophylactic and so may contribute to reducing the negative impact of migraine on individuals and on society.
Experience with TPM in the treatment of epilepsy and migraine has shown that low initial dosing and slow titration increase tolerability. Our clinical experience is that by starting at 25 mg/day and increasing at 25 mg/week, it is possible to reach a maintenance dose of 50 mg bid in most patients, although slower titration may be necessary in some. It is also usually possible to increase the daily dose to 150 or 200 mg, if necessary.
TPM is already extensively used to treat migraine in clinical practice, both in the US and Europe. In a questionnaire survey of 30 headache specialists from various countries (Tepper et al 2004) conducted to determine practice with migraine prophylaxis, TPM was considered as first-line or second-line treatment by many. We feel that the favorable clinical profile of TPM, together with lack of major contraindications, suggest that the compound should be regarded as a first-line treatment for the prevention of migraine.
References
- Bigal ME, Rapoport AM, Lipton RB, et al. Assessment of migraine disability using the Migraine Disability Assessment (MIDAS) Questionnaire: a comparison of chronic migraine with episodic migraine. Headache. 2003;43:336–42. doi: 10.1046/j.1526-4610.2003.03068.x. [DOI] [PubMed] [Google Scholar]
- Brandes JL. Global trends in migraine care: results from the MAZE survey. CNS Drugs. 2002;16(Suppl 1):13–8. doi: 10.2165/00023210-200216001-00003. [DOI] [PubMed] [Google Scholar]
- Brandes JL, Saper JR, Diamond M, et al. Topiramate for migraine prevention: a randomized controlled trial. JAMA. 2004;291:965–73. doi: 10.1001/jama.291.8.965. [DOI] [PubMed] [Google Scholar]
- Brandes JL. Practical use of topiramate for migraine prevention. Headache. 2005;45(Suppl 1):S66–73. doi: 10.1111/j.1526-4610.2005.4501007.x. [DOI] [PubMed] [Google Scholar]
- Bussone G, Diener HC, Pfeil J, et al. Topiramate 100mg/day in migraine prevention: a pooled analysis of double-blind randomised controlled trials. Int J Clin Pract. 2005;59:961–8. doi: 10.1111/j.1368-5031.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- Dahlöf CHG, Dimenäis E. Migraine patients experience: poor subjective well-being/quality of life even between attacks. Cephalalgia. 1995;15:31–6. doi: 10.1046/j.1468-2982.1995.1501031.x. [DOI] [PubMed] [Google Scholar]
- D’Amico D, Genco S, Perini F. Workplace disability in migraine: an Italian experience. Neurol Sci. 2004;25(Suppl 3):S251–2. doi: 10.1007/s10072-004-0299-z. [DOI] [PubMed] [Google Scholar]
- D’Amico D, Usai S, Grazzi L, et al. The impact of primary headaches on patients’ lives: Italian experience with the MIDAS and the SF-36 questionnaires. Headache Care. 2004;1:123–8. [Google Scholar]
- Diamond M, Dahlof C, Papadopoulos G, et al. Topiramate improves health-related quality of life when used to prevent migraine. Headache. 2005;45:1023–30. doi: 10.1111/j.1526-4610.2005.05183.x. [DOI] [PubMed] [Google Scholar]
- Diener HC, Tfelt-Hansen P, Dahlof C, et al. Topiramate in migraine prophylaxis. Results from a placebo-controlled trial with propranolol as an active control. J Neurol. 2004;251:943–50. doi: 10.1007/s00415-004-0464-6. [DOI] [PubMed] [Google Scholar]
- Dowson AJ, Lipscombe S, Sender J, et al. Migraine in primary care advisors. New guidelines for the management of migraine in primary care. Curr Med Res Opin. 2002;18:414–39. doi: 10.1185/030079902125001164. [DOI] [PubMed] [Google Scholar]
- Dueland AN, Leira R, Burke TA, et al. The impact of migraine on work, family, and leisure among young women – a multinational study. Curr Med Res Opin. 2004;20:1595–604. doi: 10.1185/030079904X3357. [DOI] [PubMed] [Google Scholar]
- Dueland AN, Leira R, Cabelli ST. The impact of migraine on psychological well-being of young women and their communication with physicians about migraine: a multinational study. Curr Med Res Opin. 2005;21:1297–305. doi: 10.1185/030079905X56394. [DOI] [PubMed] [Google Scholar]
- Edmeads J, Findlay H, Tugwell P, et al. Impact of migraine and tension-type headache on life-style, consulting behaviour, and medication use: a Canadian population survey. Can J Neurol Sci. 1993;20:131–7. doi: 10.1017/s0317167100047697. [DOI] [PubMed] [Google Scholar]
- Fraunfelder FW, Fraunfelder FT, Keates EU. Topiramate-associated acute, bilateral, secondary angle-closure glaucoma. Ophthalmology. 2004;111:109–11. doi: 10.1016/j.ophtha.2003.04.004. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. Migraine pathophysiology. Headache. 2005;45(Suppl 1):S14–24. doi: 10.1111/j.1526-4610.2005.4501003.x. [DOI] [PubMed] [Google Scholar]
- Gray RN, Goslin RE, McCrory DC, et al. Evidence Report: Drug Treatments for the Prevention of Migraine Technical Review 23, February (Prepared for the Agency for Health Care Policy and Research under Contract No. 290-94-2025. 1999 Available from the National Technical Information Service; NTIS Accession No. 127953) [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd ed. Cephalalgia. 2004;24(S1):8–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Henry P, Auray JP, Gaudin AF, et al. Prevalence and clinical characteristics of migraine in France. Neurology. 2002;59:232–7. doi: 10.1212/wnl.59.2.232. [DOI] [PubMed] [Google Scholar]
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia. 2000;20:765–86. doi: 10.1046/j.1468-2982.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- Jhingran P, Davis SM, LaVange LM, et al. MSQ: Migraine-Specific Quality-of-Life Questionnaire. Further investigation of the factor structure. Pharmacoeconomics. 1998;13:707–17. doi: 10.2165/00019053-199813060-00007. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–85. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53:537–42. doi: 10.1212/wnl.53.3.537. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Goadsby PJ, Sawyer JPC, et al. Migraine: diagnosis and assessment of disability. Rev Contemp Pharmacother. 2000;11:63–73. [Google Scholar]
- Lipton RB, Hamelsky SW, Stewart WF. Epidemiology and impact of migraine. In: Silberstein SD, Lipton RB, Dalessio DJ, editors. Wolff’s headache and other head pain. New York: Oxford University Press; 2001. pp. 85–107. [Google Scholar]
- Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–57. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Liberman JN, Kolodner KB, et al. Migraine headache disability and health-related quality-of-life: a population-based case-control study from England. Cephalalgia. 2003;23:441–50. doi: 10.1046/j.1468-2982.2003.00546.x. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Arnold LM, Shapira NA, et al. Topiramate in the treatment of binge eating disorder associated with obesity: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160:255–61. doi: 10.1176/appi.ajp.160.2.255. [DOI] [PubMed] [Google Scholar]
- MacGregor EA, Brandes J, Eikermann A, et al. Impact of migraine on patients and their families: the Migraine And Zolmitriptan Evaluation (MAZE) survey – Phase III. Curr Med Res Opin. 2004;20:1143–50. doi: 10.1185/030079904125004178. [DOI] [PubMed] [Google Scholar]
- Osterhaus JT, Gutterman DL, Plachetka JR. Healthcare resource and labor costs of migraine in the US. Pharmacoeconomics. 1992;2:67–76. doi: 10.2165/00019053-199202010-00008. [DOI] [PubMed] [Google Scholar]
- Roncolato M, Fabbri L, Recchia G, et al. An epidemiological study to assess migraine prevalence in a sample of Italian population presenting to their GPs. Eur Neurol. 2000;43:102–6. doi: 10.1159/000008143. [DOI] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Streeter AJ, et al. An overview of the preclinical aspects of TPM: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41:S3–9. [PubMed] [Google Scholar]
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754–62. doi: 10.1212/wnl.55.6.754. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Saper JR, Freitag FG. Migraine: diagnosis and treatment. In: Silberstein SD, Lipton RB, Dalessio DJ, editors. Wolff’s headache and other head pain. New York: Oxford University Press; 2001. pp. 121–237. [Google Scholar]
- Silberstein SD, Neto W, Schmitt J, et al. Topiramate in migraine prevention: results of a large controlled trial. Arch Neurol. 2004;61:490–5. doi: 10.1001/archneur.61.4.490. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Ben-Menachem E, Shank RP, et al. Topiramate monotherapy in epilepsy and migraine prevention. Clin Ther. 2005;27:154–65. doi: 10.1016/j.clinthera.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Solomon GD, Santanello N. Impact of migraine and migraine therapy on productivity and quality of life. Neurology. 2000;55(Suppl 2):S29–36. [PubMed] [Google Scholar]
- Steiner TJ, Scher AI, Stewart WF, et al. The prevalence and disability burden of adult migraine in England and their relationships to age, gender and ethnicity. Cephalalgia. 2003;2:519–27. doi: 10.1046/j.1468-2982.2003.00568.x. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Shechter A, Lipton RB. Migraine heterogeneity. Disability, pain intensity, and attack frequency and duration. Neurology. 1994;44(Suppl 4):24–39. [PubMed] [Google Scholar]
- Tepper SJ, D’Amico D, Baos V, et al. Guidelines for prescribing prophylactic medications for migraine: a survey among headache specialist physicians in different countries. Headache Care. 2004;1:267–72. [Google Scholar]
- Terwindt GM, Ferrari MD, Tijhuis M, et al. The impact of migraine on quality of life in the general population: the GEM study. Neurology. 2000;55:624–29. doi: 10.1212/wnl.55.5.624. [DOI] [PubMed] [Google Scholar]
- Von Korff MR, Stewart WF, Simon DJ, et al. Migraine and reduced work performance. A population-based diary study. Neurology. 1998;50:1741–45. doi: 10.1212/wnl.50.6.1741. [DOI] [PubMed] [Google Scholar]
- Welch KM. Brain hyperexcitability: the basis for antiepileptic drugs in migraine prevention. Headache. 2005;45(Suppl 1):S25–32. doi: 10.1111/j.1526-4610.2005.4501008.x. [DOI] [PubMed] [Google Scholar]