Abstract
The management of facial paralysis is one of the most complex areas of reconstructive surgery. Given the wide variety of functional and cosmetic deficits in the facial paralysis patient, the reconstructive surgeon requires a thorough understanding of the surgical techniques available to treat this condition. This review article will focus on surgical management of facial paralysis and the treatment options available for acute facial paralysis (<3 weeks duration), intermediate duration facial paralysis (3 weeks to 2 yr) and chronic facial paralysis (>2 yr). For acute facial paralysis, the main surgical therapies are facial nerve decompression and facial nerve repair. For facial paralysis of intermediate duration, nerve transfer procedures are appropriate. For chronic facial paralysis, treatment typically requires regional or free muscle transfer. Static techniques of facial reanimation can be used for acute, intermediate, or chronic facial paralysis as these techniques are often important adjuncts to the overall management strategy.
Keywords: Facial paralysis, Surgical management
INTRODUCTION
Facial paralysis can result from a wide variety of etiologies including infectious, neurologic, congenital, neoplastic, traumatic, systemic, and iatrogenic causes (1). Regardless of cause, the management of facial paralysis is complex and often requires multidisciplinary intervention. The evaluation and treatment of facial paralysis is especially intricate because of the wide variation in the potential for regeneration and lack of reliable prognostic indicators for spontaneous recovery (2). Current management of facial paralysis consists of a combination of pharmacologic therapy, physical therapy for facial neuromuscular retraining, and surgical intervention via dynamic and static techniques for facial reanimation. This review article will focus on the wide variety of surgical therapies available to the reconstructive surgeon for successful facial reanimation.
SURGICAL MANAGEMENT OF ACUTE FACIAL PARALYSIS (<3 WEEKS)
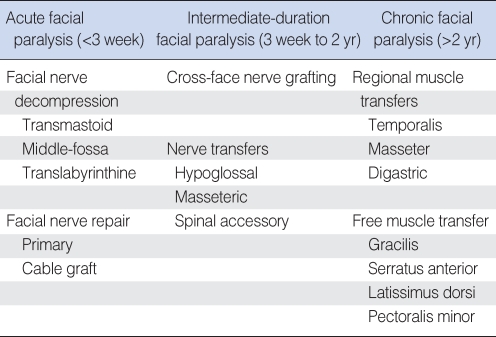
Any surgical intervention for facial paralysis must carefully take into account the patient's age, medical history, residual hearing, segment of nerve injured, and the patient's expectations and risk tolerance (1). Management of acute facial paralysis may involve facial nerve decompression surgery in cases of virally-induced facial paralysis (Bell's palsy, Ramsay-Hunt syndrome) or primary facial nerve repair/grafting in cases of resection or transection of the facial nerve (Table 1).
Table 1.
Surgical treatment options for acute, intermediate, and chronic facial paralysis
Facial nerve decompression (3)
Transmastoid approach
The transmastoid approach for facial nerve decompression can be utilized when the trauma is clearly localized to the tympanic or mastoid segments of the facial nerve. The nerve should be decompressed for 180 degrees of its circumference. Important landmarks for this approach include the lateral semicircular canal, fossa incudis, and digastric ridge. The incus can be removed and then replaced as an interposition graft to achieve decompression of the tympanic segment of the facial nerve all the way to the geniculate ganglion.
Middle fossa approach
The middle fossa approach allows decompression of the facial nerve when the injury extends to the labyrinthine segment. It is sometimes used in combination with the transmastoid approach in cases of temporal bone trauma. Critical landmarks for this approach include the superior semicircular canal, the greater superficial petrosal nerve, and "Bill's bar" or the vertical crest separating the facial nerve from the superior vestibular nerve.
Translabyrinthine approach
The translabyrinthine approach can be utilized for decompression of the entire intratemporal course of the facial nerve in cases where cochleovestibular function is absent or has been destroyed by the trauma.
Facial nerve repair
Primary nerve repair
Primary neurroraphy provides the best return of facial nerve function. However, the primary repair should be tension free. This sometimes necessitates rerouting or mobilization of the adjacent facial nerve segments in order to provide a tension-free anastomosis. It is important to note that the distal nerve segments can be identified intraoperatively by electrical stimulation for up to 72 hr after nerve transection or injury, making early repair critical. Most authors today recommend epineurial repair of the facial nerve as suture placement with fascicular or perineurial repair is difficult and may injure the axons (4).
Cable grafting
Cable nerve grafts are utilized when a tension-free primary nerve repair is not possible. Popular choices for donor nerve grafts include: great auricular nerve, sural nerve, and the medial and lateral antebrachial cutaneous nerves. The ansa cervicalis has been used as a donor nerve as well as there may be some evidence that motor nerve grafts are better than sensory nerve grafts (5). With either primary nerve repair or cable grafting, it is generally accepted that the best possible outcome is House-Brackmann Grade III facial function.
SURGICAL TREATMENT OF INTERMEDIATE DURATION FACIAL PARALYSIS (3 WEEKS to 2 YR)
The treatment of intermediate duration facial paralysis typically occurs in the setting of an anatomically intact facial nerve that has not recovered well. For example, facial paralysis after acoustic neuroma surgery in which the nerve is often intact but can have poor recovery due to stretch injury. Nerve transfers and nerve crossover procedures are typically the treatment of choice in this period as the native facial musculature is still viable (Table 1).
Nerve transfers and cross-facial nerve grafting
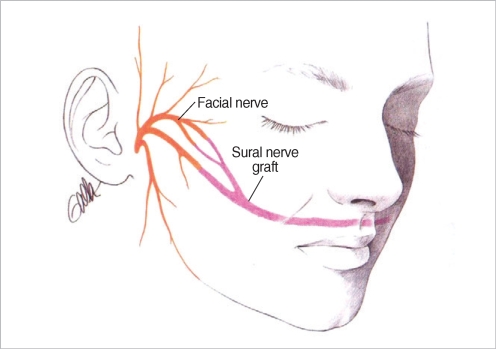
Cross-facial nerve grafting can be utilized if the contralateral facial nerve is intact and functional. Terzis et al. believe that the best outcomes from cross facial nerve grafting are if the period of denervation is less than 6 months (6). The surgical technique is a 2-stage procedure. In the first stage, a modified pre-auricular face-lift incision on the normal, functional side of the face. After elevation of a skin flap anteriorly to the level of the lateral canthus, the superficial muscular aponeurotic system (SMAS) layer is penetrated anterior to the parotid gland and the branches of the facial nerve are identified using a nerve stimulator. Nerve branches are carefully selected for sacrifice depending on the desired innervations function and mapping of the innervations targets of each branch. A long sural nerve graft is tunneled to the contralateral face and the donor facial nerve branches are then sacrificed. Under magnification, the proximal end of the sural nerve graft is then coapted to the donor facial nerve branches (Fig. 1). After a waiting period of 9 to 12 months, the second stage can be undertaken. In the second stage, secondary neurorraphies are performed between selected facial nerve branches and the cross face nerve grafts (6, 7). If the period of denervation is longer than 2 yr, cross facial nerve grafting can be utilized in conjunction with free muscle transfer for smile reanimation (discussed below).
Fig. 1.
Coaptation of the sural nerve graft to the donor facial nerve branches anterior to the parotid gland followed by tunneling of the sural nerve graft to the contralateral paralyzed side of the face.
Nerve transfer procedures have been described using a variety of donor nerves: hypoglossal, spinal accessory, masseteric branch of the trigeminal nerve and motor branches of the cervical plexus. The most commonly used procedure is the hypoglossal-facial transfer. The classic XII-VII transfer involves transection of the entire hypoglossal nerve distal to the ansa cervicalis and coaptation to the main trunk of the facial nerve. Several modifications have been described (8):
"Split" XII-VII transfer: approximately 30-40% of the hypoglossal nerve is divided longitudinally for several centimeters and approximated to the lower division of the facial nerve.
XII-VII jump graft: end-to-side neurorrhaphy between hypoglossal nerve and a donor cable nerve graft (e.g. great auricular nerve) which serves as a jump graft to the main trunk of the facial nerve.
Mobilization of mastoid segment of facial nerve: the facial nerve can be mobilized in its mastoid segment from the 2nd genu distally and rotated inferiorly to allow direct coaptation to the hypoglossal nerve. This typically requires removal of the mastoid tip.
SURGICAL TREATMENT OF CHRONIC FACIAL PARALYSIS (>2 YR)
In most cases of chronic facial paralysis of greater than 2 yr duration, the native facial musculature has atrophied and requires the use of alternative muscles for facial reanimation. Muscle transfer techniques, including regional and free muscle transfer, are the mainstay of dynamic facial reanimation for chronic facial paralysis (Table 1).
Static techniques for facial reanimation (such as oculoplastic procedures, eyelid weights, static facial suspension, etc) can be utilized for facial paralysis of any duration and will be discussed in facial reanimation part below.
Regional muscle transfer
The temporalis muscle transfer is the most commonly utilized regional muscle transfer for dynamic facial reanimation. Preoperatively, it is important to ensure that the patient has normal trigeminal nerve function and that the muscle is not atrophic. In the classic temporalis muscle transposition, a 1.5-2.0 cm wide strip of temporalis muscle is elevated from the cranium and rotated inferiorly over the zygoma to reach the oral commissure. The vector of this rotation is favorable because it is typically in the smile vector. A variety of techniques have been described for filling in the depression in the temple created by the muscle transfer including alloplastic implants, fat grafting, and use of the temporoparietal fascial flap for obliteration of the defect. A number of modifications of the temporalis transfer have been described:
Fascial extensions to the upper and lower lip: Sherris et al. have described the use of split fascia graft extensions to the upper and lower lip in order to allow the temporalis muscle transfer to pull the philtrum and lower lip back to the midline (9).
Temporalis tendon transfer: in this technique, the temporalis tendon at the coronoid process is disarticulated and pulled down to the oral commissure. This technique avoids the midfacial bulkiness over the zygoma as well as eliminating the depression in the temporal fossa from classic temporalis muscle transposition. This technique can be performed via an open preauricular transzygomatic approach or a minimally invasive transbuccal approach through a nasolabial fold incision (10).
Other regional muscle transfers that have been described include the masseter muscle transfer for smile reanimation and the digastric muscle transfer for marginal mandibular nerve injuries. The masseteric muscle transfer is considered inferior to the temporalis muscle transfer because of its more lateral vector of pull (10).
Free muscle transfer
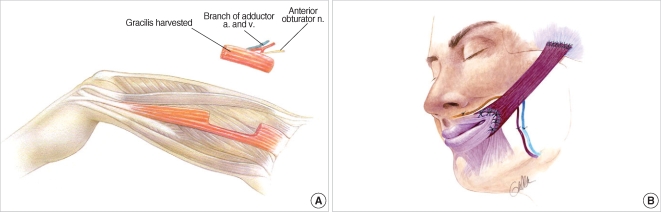
The field of facial reanimation has made a dramatic advance with the advent of microvascular free tissue transfer. Free muscle transfer can be utilized if the native facial musculature has been resected, in cases where there is concurrent trigeminal nerve dysfunction precluding use of regional muscle transfer, and as the only way of achieving involuntary, mimetic smile reanimation when used in conjunction with cross-face nerve grafting. A wide variety of muscles have been described for use in the treatment of facial paralysis including the gracilis, pectoralis minor, serratus anterior, latissimus dorsi, and others (11). The workhorse of free muscle transfer for facial reanimation remains the gracilis muscle. The gracilis muscle is a long, thin muscle located in the medial thigh (Fig. 2A). It is easily harvested and provides an excellent neurovascular pedicle. Its location in the medial thigh permits the use of a two-team approach with one team for flap harvest and one team for preparation of the recipient site. For unilateral facial paralysis, the gracilis muscle transfer is typically done in two stages. In the first stage, a cross-face nerve graft is performed using a sural nerve graft as described above. After 6-12 months, the second stage is performed wherein the gracilis muscle is harvested and transferred to the paralyzed side of the face. Vascular anastomoses are performed to the facial artery and vein or to the superficial temporal vessels. The obturator nerve to the gracilis muscle is coapted to the distal end of the sural nerve graft placed at the first stage (Fig. 2B). Typically, movement of the muscle is detected by six months but may take up to one year or longer.
Fig. 2.
(A) Harvest of gracilis muscle from the medial thigh. (B) Inset of gracilis muscle in the paralyzed side of the face with vascular anastomosis to the facial artery and vein and neurorrhaphy of the obturator nerve to the cross-face nerve graft.
In cases of bilateral facial paralysis (e.g. Mobius syndrome), the gracilis free muscle transfer can be performed as a single stage. In these cases, where there no cross-facial nerve grafting available, the masseteric branch of the trigeminal nerve is used as the donor nerve to drive the gracilis muscle. For bilateral cases, gracilis muscle transfer can be performed sequentially or simultaneously for both sides.
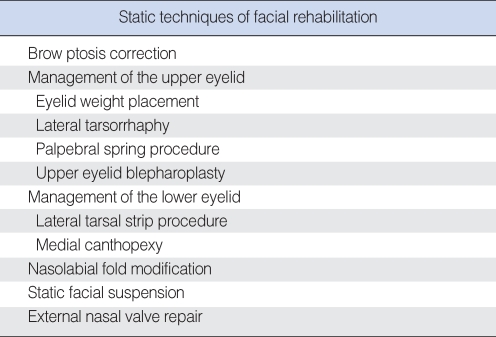
STATIC TECHNIQUES for FACIAL REANIMATION (CAN BE UTILIZED AT ANY DURATION OF FACIAL PARALYSIS)
There are significant benefits to static techniques of facial reanimation that can provide an alternative to or enhance the results of dynamic facial reanimation. Static techniques can be utilized in chronic facial paralysis or also for temporary facial paralysis when nerve recovery is expected (9). Static techniques for facial reanimation will be described for the upper and lower zones of the face (Table 2).
Table 2.
Listing of static facial reanimation techniques. These can be performed for acute, intermediate, or chronic facial paralysis as needed
Brow ptosis correction
Brow ptosis correction is an important part of management of the facial paralysis patient. A variety of treatment approaches have been described: direct brow lift (coronal, mid-forehead, or brow incision), endoscopic brow lift, or minimally invasive temporal brow lift using a biodegradable stabilization device (ENDOTINE, Coapt Systems Inc, Palo Alto, CA, USA) (2, 12).
Management of the eye
Oculoplastic management of the paralyzed eye is of paramount importance as exposure keratitis can lead to permanent visual loss. The upper eyelid can be managed with the following procedures as needed:
Eyelid weight placement: lid loading via placement of a gold or platinum weight is a very effective technique for correction of lagopthalmos. Thin profile platinum weights are becoming increasingly popular as they offer a better cosmetic outcome and decreased incidence of allergy as compared with gold implants.
Palpebral spring procedure: the palpebral spring procedure is a technically difficult procedure that can be employed in lieu of an eyelid weight for lagopthalmos correction. The spring spans between the superior orbital rim periosteum and a pocket at the superior aspect of the tarsus.
Upper eyelid blepharoplasty: in patients with significant dermatochalasis, conservative upper lid blepharoplasty can be performed to remove the excess skin.
Lateral tarsorrhaphy: a permanent "reversible" lateral tarsorrhaphy can be performed using mattress sutures placed to coapt the lateral aspects of the upper and lower lid tarsal plates. Tarsorrhaphies are typically used in cases of exposure keratitis or in cases where there is loss of the corneal sensation in addition to lagopthalmos.
The lower eyelid is managed with the following procedures as needed:
Lateral tarsal strip procedure: the lateral tarsal strip procedure is a powerful technique that can be used to address paralytic lower lid ectropion. In this technique, a lateral canthotomy is performed followed by inferior crus cantholysis. The lower tarsus is trimmed and sutured directly to the lateral orbital rim periosteum.
Medial canthopexy: medial paralytic ectropion of the lower eyelid is treated using a precaruncular medial canthopexy technique in which the medial tarsus is sutured to the periosteum of the lamina papyracea (13-15).
Nasolabial fold modification
Patients with effacement of the nasolabial fold or patients with overprominent nasolabial folds can be treated with a simple suture technique to create or efface the nasolabial fold crease (2).
Static facial suspension
Static facial slings for facial support are typically placed from the zygomatic arch/temporalis fascia to the oral commissure and nasolabial fold. A number of materials have been described for use as the sling material including fascia lata, Gore-Tex, and AlloDerm. In addition, multivector suture techniques have also been described for facial suspension (9).
External nasal valve repair
An often overlooked aspect of the patient with facial paralysis is external nasal valve collapse. This can be treated with a fascia lata sling from the alar base to the zygoma/temporalis fascia to stent open the external nasal valve (8).
CONCLUSION
The reconstructive surgeon has a wide array of surgical treatment options for management of the patient with facial paralysis. An organized, thoughtful approach is necessary when evaluating patients with facial paralysis to ensure that no obvious treatment choices are overlooked. For acute facial paralysis, the main surgical therapies are facial nerve decompression and facial nerve repair. For facial paralysis of intermediate duration, nerve transfer procedures are appropriate. For chronic facial paralysis, treatment typically requires regional or free muscle transfer. It is important to remember that static techniques of facial reanimation can be used for acute, intermediate, or chronic facial paralysis as these techniques are often important adjuncts to the overall management strategy.
References
- 1.Melvin TA, Limb CJ. Overview of facial paralysis: current concepts. Facial Plast Surg. 2008 May;24(2):155–163. doi: 10.1055/s-2008-1075830. [DOI] [PubMed] [Google Scholar]
- 2.Hadlock TA, Greenfield LJ, Wernick-Robinson M, Cheney ML. Multimodality approach to management of the paralyzed face. Laryngoscope. 2006 Aug;116(8):1385–1389. doi: 10.1097/01.mlg.0000225980.38147.c6. [DOI] [PubMed] [Google Scholar]
- 3.Sofferman RA. Facial nerve injury and decompression. In: Nadol JB Jr, Mckenna MJ, editors. Surgery of the ear and temporal bone. Philadelphia (PA): Lippincott Williams and Wilkins; 2005. pp. 435–450. [Google Scholar]
- 4.Humphrey CD, Kriet JD. Nerve repair and cable grafting for facial paralysis. Facial Plast Surg. 2008 May;24(2):170–176. doi: 10.1055/s-2008-1075832. [DOI] [PubMed] [Google Scholar]
- 5.Chu TH, Du Y, Wu W. Motor nerve graft is better than sensory nerve graft for survival and regeneration of motoneurons after spinal root avulsion in adult rats. Exp Neurol. 2008 Aug;212(2):562–565. doi: 10.1016/j.expneurol.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Terzis JK, Konofaos P. Nerve transfers in facial palsy. Facial Plast Surg. 2008 May;24(2):177–193. doi: 10.1055/s-2008-1075833. [DOI] [PubMed] [Google Scholar]
- 7.Tai CY, Mackinnon SE. Surgical options for facial reanimation. Mo Med. 2006 May–Jun;103(3):270–274. [PubMed] [Google Scholar]
- 8.Hadlock TA, Cheney ML, McKenna MJ. Facial reanimation surgery. In: Nadol JB Jr, Mckenna MJ, editors. Surgery of the ear and temporal bone. Philadelphia (PA): Lippincott Williams and Wilkins; 2005. pp. 461–472. [Google Scholar]
- 9.Liu YM, Sherris DA. Static procedures for the management of the midface and lower face. Facial Plast Surg. 2008 May;24(2):211–215. doi: 10.1055/s-2008-1075836. [DOI] [PubMed] [Google Scholar]
- 10.Boahene KD. Dynamic muscle transfer in facial reanimation. Facial Plast Surg. 2008 May;24(2):204–210. doi: 10.1055/s-2008-1075835. [DOI] [PubMed] [Google Scholar]
- 11.Chuang DC. Free tissue transfer for the treatment of facial paralysis. Facial Plast Surg. 2008 May;24(2):194–203. doi: 10.1055/s-2008-1075834. [DOI] [PubMed] [Google Scholar]
- 12.Meltzer NE, Byrne PJ. Management of the brow in facial paralysis. Facial Plast Surg. 2008 May;24(2):216–219. doi: 10.1055/s-2008-1075837. [DOI] [PubMed] [Google Scholar]
- 13.Bergeron CM, Moe KS. The evaluation and treatment of upper eyelid paralysis. Facial Plast Surg. 2008 May;24(2):220–230. doi: 10.1055/s-2008-1075838. [DOI] [PubMed] [Google Scholar]
- 14.Bergeron CM, Moe KS. The evaluation and treatment of lower eyelid paralysis. Facial Plast Surg. 2008 May;24(2):231–241. doi: 10.1055/s-2008-1075839. [DOI] [PubMed] [Google Scholar]
- 15.Fay A, Rubin PA. Oculoplastic considerations and management of facial paralysis. In: Nadol JB Jr, Mckenna MJ, editors. Surgery of the ear and temporal bone. Philadelphia (PA): Lippincott Williams and Wilkins; 2005. pp. 473–484. [Google Scholar]