Abstract
Objectives
Hearing loss is the most common sensory disorder in humans and genetic causes are estimated to cause more than 50% of all incidents of congenital hearing loss. To develop an efficient method for a genetic diagnosis of hearing loss, we have developed and validated a genetic hearing loss DNA chip that allows the simultaneous analysis of 7 different mutations in the GJB2, SLC26A4, and the mtDNA 12S rRNA genes in Koreans.
Methods
A genotyping microarray, based on the allele-specific primer extension (ASPE) method, was used and preliminary validation was examined from the five patients and five controls that were already known their genotypes by DNA sequencing analysis.
Results
The cutoff Genotyping index (GI) of genotyping for each mutation was set up and validated to discriminate among the genotypes. The result of the DNA chip assay was identical to those of previous results.
Conclusion
We successfully designed the genetic hearing loss DNA chip for the first time in Korea and it would be useful for a clinical genetic diagnosis of hearing loss. Further consideration will be needed in order to examine the accuracy of this DNA chip with much larger patient sample numbers.
Keywords: Hearing loss, DNA chip, Gene
INTRODUCTION
Hearing impairment is the most common sensory disorder in humans. It occurs in one in 1,000 newborns and genetic causes are estimated to cause more than 50% of all incidents of congenital hearing loss in children. Genetic hearing impairment is a highly heterogeneous disorder, in which about 70% of cases are non-syndromic. It can follow a pattern of autosomal dominant, autosomal or X-linked recessive transmission, or mitochondrial inheritance (1).
To date, more than 120 loci for nonsyndromic hearing loss (NSHL) have been mapped in the human genome and approximately 47 genes have been identified as causative genes by means of the positional cloning (Hereditary Hearing Loss Homepage; http://webh01.ua.ac.be/hhh/). Among these genes, GJB2, SLC26A4 and mitochondrial 12S rRNA genes have been extensively studied in many studies and the spectrum and frequencies of mutations vary in different ethnic populations (2-4). Despite the fact that large number of known rare mutations in these genes has been reported, mutation hot spots were also found to be race-specific. For example, 235delC for GJB2 and H723R for SLC26A4 are the most frequent mutations in East Asians (5-7). Also, the frequency of mtDNA A1555G mutation is dependent on racial or geographic origins and the frequency estimate is 1.4% for Koreans (8).
DNA microarrays, sometimes called DNA chips, are designed to allow many hybridization experiments to be performed at a time. A large number of DNA probes, each one with a different sequence, are immobilized at defined positions on a solid surface. The probes can be synthetic oligonucleotides or other short DNA molecules such as cDNAs. The array is incubated with labeled target DNA to allow hybridization to take place. Which oligonucleotides have hybridized to the target DNA is determined by scanning the surface of the array and recording the positions at which the signal emitted by the label is detectable and software tools are used for data extraction and analysis. Conventional gel-based or direct sequencing analysis has been used to screen known deafness genes, but these methods are time-consuming and expensive. Because specific mutations have showed the pattern of high frequency mutations in diverse populations, the demand for rapid and cheap genetic analysis has risen sharply. For this purpose, the DNA microarray is a method that offers simultaneous screening for a large number of specific mutations in known genes and makes it meaningful to initially select hotspot mutations for neonatal screening or the genetic testing of a genetically heterogeneous condition such as deafness. In this study, we have developed a genetic hearing loss DNA chip that allows the simultaneous analysis of 7 different mutations in regards to the GJB2, SLC26A4, and the mtDNA 12S rRNA genes.
MATERIALS AND METHODS
DNA samples
Various previously genotyped five patients and five control DNA samples were provided by the Department of Otorhinolaryngology-Head and Neck Surgery, Kyungpook National University Hospital. All participants provided written informed consent according to the protocol approved by the Ethics Committee of Kyungpook National University Hospital prior to the study.
Polymerase chain reaction (PCR) amplification
Genomic DNA was extracted from peripheral blood using Flexi-Gene DNA extraction kit (QIAGEN, Hilden, Germany), or from buccal swab specimens by using Puregene Buccal Cell Core Kit A (QIAGEN).
All PCR reactions were performed in a 25 µL volume containing 1× PCR buffer (Solgent, Daejeon, Korea), 10 mM deoxynucleotide triphosphate (dNTP), 10 pmol each of forward and reverse oligonucleotide primers, 1U/µL of Taq DNA polymerase (Solgent), and 25 ng genomic DNA. The parameters for PCR consisted of a denaturation cycle at 95℃ for 2 min, followed by 30 cycles at 94℃ for 30 sec, 57℃ for 30 sec, 72℃ for 30 sec, a final extension cycle at 72℃ for 5 min, and a stabilization step at 4℃. PCR was performed using a PTC-200 thermal cycler and an iCycler™, Thermal cycler (BIO-RAD, Hercules, CA, USA). For purification of the PCR products, an ExoSAP IT Kit (USB Corp., Cleveland, OH, USA) was used.
Genotyping and data analysis
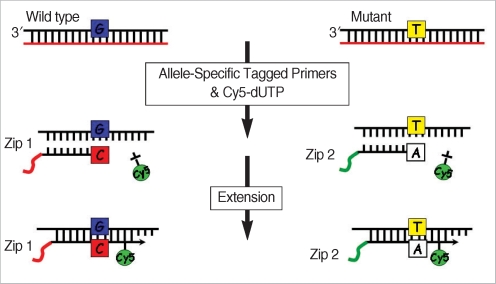
The principle of allele specific primer extension (ASPE) reaction is described in Fig. 1. In brief, an ASPE reaction consisted of 5 mL of pooled PCR products, specific normal and mutant primer mixes, cy5-dUTP, i-starTaq DNA polymerase (iNtRON Biothnology, Seongnam, Korea). A total of 20 µL of pooled ASPE reaction was suspended in 50 µL hybridization buffer (5× SSC, 0.1% SDS, 25 mg/mL Cot-1 DNA, 25% formamide, 0.5 mg/mL polyA, 10% dextran sulfate). After being heated for 5 min at 95℃, the hybridization mixture was added to the DNA chip. The slide was incubated at 45℃ for 30 min and washed once at 50℃ in 2× SSC, 0.1% SDS for 10 min and four times at room temperature in 0.1× SSC for 1 min. The DNA chip was scanned in an GenePix 4000B scanner (Axon, Union City, CA, USA) and the data was incorporated into the Microsoft Excel program (Microsoft Corp., Redmond, WA, USA).
Fig. 1.
The principle of allele-specific primer extension (ASPE). ASPE is a solution based, sequence specific enzymatic reaction technology that can be used to assay multiple SNPs in a single tube. A primer anneals and is expended if there is a perfect match at the SNP site. The matched reaction is detected by fluorescence (Cy5) using scanner.
RESULTS
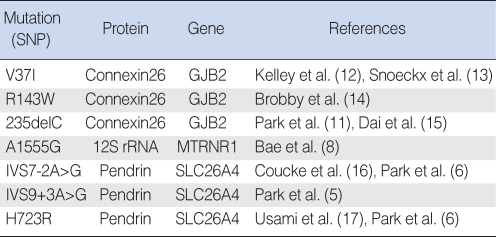
Based on the previous studies in Korea, a genetic hearing loss DNA chip was designed in order to detect the following changes: the GJB2 V37I, R143W, and 235delC; the SLC26A4 IVS7-2A>G, IVS9+3A>G, and H723R; the mtDNA A1555G mutation (Table 1) (5, 8-11). Thirty five mer of cZipCode sequences with 10 nucleotides spacer (CAGGCCAAGTCT) adjacent to the 5'-end were printed as the probes on the DNA chip as illustrated in Fig. 2. All Zipcode sequences were selected from the database and bioinformatics' tools and designed to hybridize with the complementary ASPE reaction product on the microarray. Allele-specific oligonucleotides to the normal and mutant sequences linked to the 25 mer of ZipCode at the 5' end were synthesized to enable an accurate discrimination of a nucleotide substitution in the designated position.
Table 1.
The contents of genetic hearing loss chip used in this study
SNP: single nudeotide polymorphism.
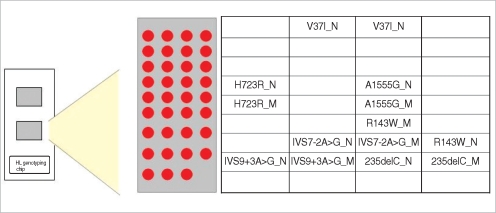
Fig. 2.
Layout of the genetic hearing loss DNA chip. Oligonucleotides to wild-type sequences are indicated by a-N, and to mutant sequences by a-M.
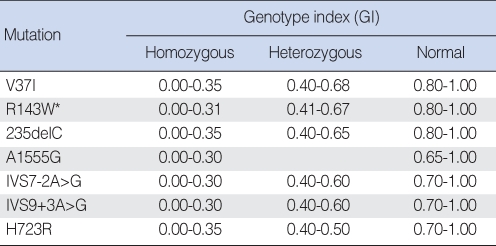
We have validated the genetic hearing loss DNA chip from five patients with a total of six sequence mutations and five controls, with the exception of the R143W mutation, which were previously confirmed as the genotypes of each mutation by DNA sequencing analysis. Since no subject with R143W mutation was identified in our samples, only normal controls were used for DNA chip validation purposes. Of the five sequence mutations, two were in the GJB2 gene, two in SLC26A4 gene, and one in mitochondrial 12S rRNA genes. Genotype index (GI) was quantitated from the spot intensities of 10 µm pixel size in the scanner using the red Cy5 channel (635 nm). Using the background subtracted median pixel intensity as the spot value (SV), the GI for each normal and mutant spot pair was calculated by the following program, GI=SVnormal/(SVnormal+SVmutant) (18). Cutoff GI values for genotypes for each mutation were justified, as shown in Table 2, and applied for the genotyping on the DNA chip.
Table 2.
Genotype index values used to determine genotype for the 7 mutations
*GI value of R143W mutation from Siemering et al. (18).
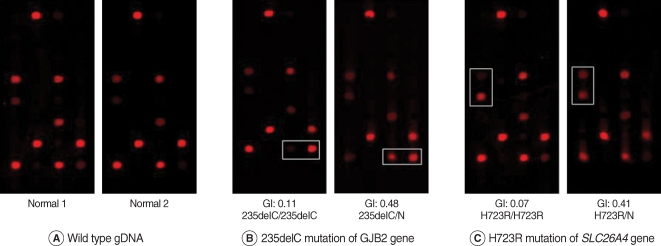
Representative results of the current DNA chip are presented in Fig. 3. Fig. 3A shows the normal control genotypes that were homozygous for wild-type. In contrast, the DNA chip assay showed the homozygote and heterozygote for 235delC mutation of GJB2 gene and the homozygote and heterozygote for H723R mutation for SLC26A4 gene (Fig. 3B, C). The result of DNA chip assay was identical to those of DNA sequencing analysis demonstrating on a clear distinction between the homozygous and heterozygous mutation.
Fig. 3.
Examples of hybridization results. The genotype indexes calculated from the hybridization signals of mutant spots are also shown.
DISCUSSION
Genetic hearing loss is a highly heterogeneous disorder, in which a number of genes cause the same phenotype. Over the last few decades, mutations within the prevalent three genes, GJB2, SLC26A4, and mitochondrial 12S rRNA genes have been extensively reported across many populations and are currently recognized as being the most common deafness genes. Moreover, the mutation spectra and frequencies in these genes have been demonstrated to be dependent on each population. Recently, Han et al. (9) has reported that the GJB2 pathogenic mutations in a Korean population were identified in 3% of the newborns with normal hearing and V37I mutation was the most common, followed by 235delC mutation (9, 13). SLC26A4 gene associated with enlarged vestibular aquaduct (EVA) also accounts for as much as 10% of hereditary hearing loss in diverse populations. In our population, 5 different mutation alleles are detected in most of patients with the clinical symptom of EVA (5). Especially, the haplotype analysis has shown that 235delC in the GJB2 gene and H723R in the SLC26A4 gene are common founder mutations, indicating that the ethnic background should be considered when performing genetic testing (6, 7, 17). In addition, the mtDNA A1555G mutation has been identified as the most prevalent mitochondrial mutation (19). Although the prevalence of this mutation in Koreans was lower than those of other Asians, it is still considered as the most frequent mutation out of the mtDNA genes (8).
Early diagnosis of the genetic etiology for congenital hearing loss expedites early intervention and aids in management decisions and genetic counseling. However, routine mutation analysis of patients suffering from hearing loss has been hampered by the high costs associated with application of conventional analysis techniques. Recently, microarray approach has been extensively used for analyzing the expression levels of thousands of genes and for doing multiplex genotyping of SNPs and large-scale mutation screening. For the HL, several studies using microarray have proposed for high-throughput mutation screening of deafness genes and demonstrated accurate and reliable results (18, 20, 21). Since some mutations on the mutation panels have only identified in Caucasians and vice versa, genetic screening based on the mutation spectrum of a corresponding population may be an appropriate and effective strategy for detecting mutations in causative genes.
In this study, we first designed a chip-based genotyping strategy according to the principle of single nucleotide ASPE reaction and were successfully able to simultaneously screen 7 mutations in three causative genes for hearing loss in Korea. This DNA chip-based assay will provides a relatively rapid and cost-effective for mutation detection in clinical genetic diagnosis. Further consideration will need to examine the accuracy of this DNA chip with much larger numbers of patient samples.
Footnotes
This work was supported by grants from Korean Society of Otorhinolaryngology-Head and Neck Surgery.
References
- 1.Morton NE. Genetic epidemiology of hearing impairment. Ann N Y Acad Sci. 1991;630:16–31. doi: 10.1111/j.1749-6632.1991.tb19572.x. [DOI] [PubMed] [Google Scholar]
- 2.Kokotas H, Petersen MB, Willems PJ. Mitochondrial deafness. Clin Genet. 2007 May;71(5):379–391. doi: 10.1111/j.1399-0004.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- 3.Kopp P, Pesce L, Solis-S JC. Pendred syndrome and iodide transport in the thyroid. Trends Endocrinol Metab. 2008 Sep;19(7):260–268. doi: 10.1016/j.tem.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Martinez AD, Acuna R, Figueroa V, Maripillan J, Nicholson B. Gap-junction channels dysfunction in deafness and hearing loss. Antioxid Redox Signal. 2009 Feb;11(2):309–322. doi: 10.1089/ars.2008.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HJ, Lee SJ, Jin HS, Lee JO, Go SH, Jang HS, et al. Genetic basis of hearing loss associated with enlarged vestibular aqueducts in Koreans. Clin Genet. 2005 Feb;67(2):160–165. doi: 10.1111/j.1399-0004.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 6.Park HJ, Shaukat S, Liu XZ, Hahn SH, Naz S, Ghosh M, et al. Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians: global implications for the epidemiology of deafness. J Med Genet. 2003 Apr;40(4):242–248. doi: 10.1136/jmg.40.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan D, Park HJ, Ouyang XM, Pandya A, Doi K, Erdenetungalag R, et al. Evidence of a founder effect for the 235delC mutation of GJB2 (connexin 26) in east Asians. Hum Genet. 2003 Dec;114(1):44–50. doi: 10.1007/s00439-003-1018-1. [DOI] [PubMed] [Google Scholar]
- 8.Bae JW, Lee KY, Choi SY, Lee SH, Park HJ, Kim UK. Molecular analysis of mitochondrial gene mutations in Korean patients with nonsyndromic hearing loss. Int J Mol Med. 2008 Aug;22(2):175–180. [PubMed] [Google Scholar]
- 9.Han SH, Park HJ, Kang EJ, Ryu JS, Lee A, Yang YH, et al. Carrier frequency of GJB2 (connexin-26) mutations causing inherited deafness in the Korean population. J Hum Genet. 2008;53(11-12):1022–1028. doi: 10.1007/s10038-008-0342-7. [DOI] [PubMed] [Google Scholar]
- 10.Lee KY, Choi SY, Bae JW, Kim S, Chung KW, Drayna D, et al. Molecular analysis of the GJB2, GJB6 and SLC26A4 genes in Korean deafness patients. Int J Pediatr Otorhinolaryngol. 2008 Sep;72(9):1301–1309. doi: 10.1016/j.ijporl.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park HJ, Hahn SH, Chun YM, Park K, Kim HN. Connexin26 mutations associated with nonsyndromic hearing loss. Laryngoscope. 2000 Sep;110(9):1535–1538. doi: 10.1097/00005537-200009000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Kelley PM, Harris DJ, Comer BC, Askew JW, Fowler T, Smith SD, et al. Novel mutations in the connexin 26 gene (GJB2) that cause autosomal recessive (DFNB1) hearing loss. Am J Hum Genet. 1998 Apr;62(4):792–799. doi: 10.1086/301807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snoeckx RL, Huygen PL, Feldmann D, Marlin S, Denoyelle F, Waligora J, et al. GJB2 mutations and degree of hearing loss: a multicenter study. Am J Hum Genet. 2005 Dec;77(6):945–957. doi: 10.1086/497996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brobby GW, Müller-Myhsok B, Horstmann RD. Connexin 26 R143W mutation associated with recessive nonsyndromic sensorineural deafness in Africa. N Engl J Med. 1998 Feb 19;338(8):548–550. doi: 10.1056/NEJM199802193380813. [DOI] [PubMed] [Google Scholar]
- 15.Dai P, Yu F, Han B, Wu H, Yuan YY, Li Q, et al. Features of nationwide distribution and frequency of a common gap junction beta-2 gene mutation in China. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007 Nov;42(11):804–808. [PubMed] [Google Scholar]
- 16.Coucke PJ, Van Hauwe P, Everett LA, Demirhan O, Kabakkaya Y, Dietrich NL, et al. Identification of two different mutations in the PDS gene in an inbred family with Pendred syndrome. J Med Genet. 1999 Jun;36(6):475–477. [PMC free article] [PubMed] [Google Scholar]
- 17.Usami S, Abe S, Weston MD, Shinkawa H, Van Camp G, Kimberling WJ. Non-syndromic hearing loss associated with enlarged vestibular aqueduct is caused by PDS mutations. Hum Genet. 1999 Feb;104(2):188–192. doi: 10.1007/s004390050933. [DOI] [PubMed] [Google Scholar]
- 18.Siemering K, Manji SS, Hutchison WM, Du Sart D, Phelan D, Dahl HH. Detection of mutations in genes associated with hearing loss using a microarray-based approach. J Mol Diagn. 2006 Sep;8(4):483–489. doi: 10.2353/jmoldx.2006.050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usami S, Abe S, Akita J, Namba A, Shinkawa H, Ishii M, et al. Prevalence of mitochondrial gene mutations among hearing impaired patients. J Med Genet. 2000 Jan;37(1):38–40. doi: 10.1136/jmg.37.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cremers FP, Kimberling WJ, Kulm M, de Brouwer AP, van Wijk E, te Brinke H, et al. Development of a genotyping microarray for Usher syndrome. J Med Genet. 2007 Feb;44(2):153–160. doi: 10.1136/jmg.2006.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardner P, Oitmaa E, Messner A, Hoefsloot L, Metspalu A, Schrijver I. Simultaneous multigene mutation detection in patients with sensorineural hearing loss through a novel diagnostic microarray: a new approach for newborn screening follow-up. Pediatrics. 2006 Sep;118(3):985–994. doi: 10.1542/peds.2005-2519. [DOI] [PubMed] [Google Scholar]