Abstract
Past studies have shown that melanoma cells have largely adapted to endoplasmic reticulum (ER) stress. In this study, we report that melanoma cells under ER stress are more resistant to apoptosis induced by the microtubule-targeting chemotherapeutic drugs, docetaxel and vincristine, and this is, at least in part, due to activation of the phosphoinositide 3-kinase (PI3K)/Akt pathway mediated by the X-box-binding protein 1 (XBP-1) axis of the unfolded protein response. Treatment with the ER stress-inducer tunicamycin (TM) or thapsigargin before the addition of docetaxel or vincristine reduced the levels of apoptosis induced by the drugs. This was associated with inhibition of mitochondrial release of apoptogenic proteins and activation of Bax and Bak. Induction of ER stress resulted in the rapid activation of the PI3K/Akt pathway that seemed to be important in antagonizing docetaxel and vincristine, in that inhibition of Akt blocked the effect of pretreatment with TM on apoptosis induced by the drugs. Neither docetaxel nor vincristine triggered ER stress in melanoma cells, but the basal activity of XBP-1 signaling seemed to play a role in the protection against the drugs because small interfering RNA knockdown of XBP-1 enhanced docetaxel- and vincristine-induced apoptosis. In addition, inhibition of XBP-1 decreased the constitutive levels of activation of Akt and blocked the activation of Akt induced by TM. Taken together, these results identify activation of the PI3K/Akt pathway by XBP-1-mediated signaling of the unfolded protein response as a resistance mechanism against docetaxel and vincristine in melanoma cells under ER stress.
Introduction
Melanoma continues to increase in incidence in many parts of the world, but there is currently no curative treatment once the disease has spread beyond the primary site because of the absence of effective systemic therapies. This is believed to be largely due to the resistance of melanoma cells to induction of apoptosis by available chemotherapeutic drugs and biological reagents [1,2]. Inappropriate activation of survival signaling pathways such as those mediated by mitogen-activated protein kinase kinase (MEK)/extracellular-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/Akt, either as consequences of genetic alterations or resulting from environmental stimulations, is known to play a central role in the resistance of melanoma to apoptosis [1,2].
A number of cellular stress conditions, such as nutrient deprivation, hypoxia, and alterations in glycosylation status, lead to the accumulation of unfolded and/or misfolded proteins in the endoplasmic reticulum (ER) lumen and cause so-called ER stress [3–5]. The ER responds to stress conditions by activating a range of signaling pathways that couples the ER protein folding load with the ER protein folding capacity and is termed the unfolded protein response (UPR) [3–5]. The UPR of mammalian cells is initiated by three ER transmembrane proteins, namely, activating transcription factor 6 (ATF6), inositol-requiring enzyme 1 (IRE1), and double-stranded RNA-activated protein kinase-like ER kinase (PERK), which act as proximal sensors of ER stress. Under unstressed conditions, the luminal domains of these sensors are occupied by the ER chaperon glucose-regulated protein 78 (GRP78) [3–5]. Upon ER stress, sequestration of GRP78 by unfolded proteins activates these sensors by inducing phosphorylation and homodimerization of IRE1 and PERK and relocalization of ATF6 to the Golgi where it is cleaved by Sites 1 and 2 proteases leading to its activation as a transcriptional factor [3–5].
There is increasing evidence that the UPR is activated in various solid tumors, e.g., elevated expression of GRP78 has been reported in a number of cancers [6,7]. Our previous studies have shown that GRP78 is also expressed at higher levels in most melanoma cell lines and that the levels of GRP78 expression on melanoma tissue sections increase with melanoma progression [8–10]. Similarly, another effector of UPR activation, the spliced X-box-binding protein 1 (XBP-1) messenger RNA (mRNA), is frequently expressed inmelanoma cell lines and freshmelanoma isolates [10].Given the highly malignant nature of melanoma, it is conceivable that the rapid growth rate and perhaps inadequate vascularization would create a microenvironment with hypoxia and glucose deprivation, which in turn results in ER stress [8,11]. Recently, it was shown that the UPR can be activated at early stages of melanoma initiation by the ongogenic form of HRAS (HRASG12V) [12].
Although the UPR is fundamentally a cytoprotective response, excessive or prolonged UPR can result in apoptosis by activation of many of the same molecules that have important roles in other apoptotic cascades [13–16]. Nevertheless, most human melanoma cell lines are not sensitive to apoptosis induced by ER stress [8,10]. Multiple mechanisms, either constitutively activated, or induced by the UPR, play roles in the protection of melanoma cells against ER stress-induced apoptosis. For example, up-regulation of the antiapoptotic Bcl-2 family protein Mcl-1 is critical for neutralizing the BH3-only proteins PUMA and Noxa that are also upregulated by ER stress in melanoma cells [17]. Moreover, activation of the UPR is known to protect against apoptosis induced by various chemotherapeutic drugs in many cancer types [6,7,11]. We have found that up-regulation of GRP78 plays a role in antagonizing the DNA-damaging drugs cisplatin and adriamycin in melanoma [10]. Activation of X-box-binding protein 1 (XBP-1) has also been shown to inhibit apoptosis induced by a number of chemotherapeutic drugs [18].
In this study, we have examined the role of activation of the UPR in regulating the sensitivity of melanoma cells to apoptosis induced by two microtubule-targeting chemotherapeutic drugs, docetaxel and vincristine. We show in this report that induction of ER stress leads to increased resistance of melanoma cells to apoptosis induced by the drugs. This is, at least in part, due to the activation of the PI3K/Akt pathway mediated by the XBP-1 axis of theUPR.We demonstrate that neither docetaxel nor vincristine triggers ERstress inmelanoma cells, but the basal activity of the UPR plays a role in protection ofmelanoma cells against the drugs.
Materials and Methods
Cell Lines
Human melanoma cell lines Mel-RM, MM200, Mel-CV, ME4405, Sk-Mel-28, and Mel-FH have been described previously [19]. They were cultured in Dulbecco's modified Eagle's medium containing 5% fetal calf serum (Commonwealth Serum Laboratories, Melbourne, Australia).
Antibodies, Recombinant Proteins, and Other Reagents
Tunicamycin (TM) and thapsigargin (TG) were purchased from Sigma Chemical Co. (Castle Hill, Australia). They were dissolved in dimethyl sulfoxide and made up in stock solutions of 1 mM. Docetaxel (Taxotere) was kindly provided by Aventis Pharma S.A. (Antony, France) and was stored as a 100-mM solution in absolute ethanol at -80°C and diluted with the medium before use. Vincristine was provided by Baxter Healthcare Pty., Ltd (Newcastle, Australia) that is in 0.9% sodium chloride solution at 1 mg/25 ml. The PI3K inhibitor, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002), was purchased from Calbiochem (Kilsyth, Victoria, Australia). The rabbit polyclonal antibodies against Smac, GRP78, XBP-1, IRE1α, ATF6, and PERK were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse monoclonal antibody (mAb) against cytochrome c was from Pharmingen (Bioclone, Marrickville, Australia). The rabbit polyclonal anti-Bax against amino acids 1 through 20 was purchased from Upstate Biotechnology (Lake Placid, NY). The mouse mAb against Bak (Ab-1) was purchased from Calbiochem (La Jolla, CA). Isotype control antibodies used were the ID4.5 (mouse immunoglobulin G 2a [IgG2a]) mAb against Salmonella typhi supplied by Dr. L. Ashman (Institute for Medical and Veterinary Science, Adelaide, Australia), the 107.3 mouse IgG1 mAb purchased from PharMingen (San Diego, CA), and rabbit IgG from Sigma Chemical Co.
Apoptosis
Quantitation of apoptotic cells by measurement of sub-G1 DNA content using the propidium iodide method was carried out as described elsewhere [19,20].
Flow Cytometry
Immunostaining on intact and permeabilized cells was carried out as described previously [19,20]. Analysis was carried out using a Becton Dickinson (Mountain View, CA) FACScan flow cytometer.
Mitochondrial Membrane Potential (ΔΨm)
Changes in ΔΨm were studied by staining the cells with the cationic dye, JC-1, according to the manufacturer's instructions (Molecular Probes, Eugene, OR) as described previously [20].
Western Blot Analysis
Western blot analysis was carried out as described previously [10,17]. Labeled bands were detected by Immun-Star HRP Chemiluminescent Kit, and images were captured and the intensity of the bands was quantitated with the Bio-Rad VersaDoc image system (Bio-Rad, Regents Park, NSW, Australia).
Detection of XBP-1 mRNA Splicing
The method used for the detection of unspliced and spliced XBP1 mRNA was as described previously [11,21].
Small Interference RNA
Melanoma cells were seeded at 4 x 104 cells per well in 24-well plates and allowed to reach approximately 50% confluence on the day of transfection [10,17]. The small interference RNA (siRNA) constructs used were obtained as the siGENOME SMARTpool reagents (Dharmacon, Lafayette, CO), the siGENOME SMARTpool GRP78 (M-008198-0010), the siGENOME SMARTpool IRE1α (M-004951-01-0010), the siGENOME SMARTpool ATF6 (M-009917-00-0010), the siGENOME SMARTpool PERK (M-004883-01-0010), the siGENOME SMARTpool Akt3 (M-003002-01-0010), and the siGENOME SMARTpool XBP-1 (M-009552-02). The nontargeting siRNA control, SiConTRolNon-targeting SiRNA pool (D-001206-13-20), was also obtained from Dharmacon. Cells were transfected with 50 to 100 nM siRNA in Opti-MEM medium (Invitrogen, Carlsbad, CA) with 5% fetal calf serum using Lipofectamine reagent (Invitrogen) according to themanufacturer's transfection protocol. Twenty-four hours after transfection, the cells were switched into a medium containing 5% fetal calf serum and were treated as designed.
Results
Induction of ER Stress Protects Melanoma Cells from Docetaxel- and Vincristine-Induced Apoptosis
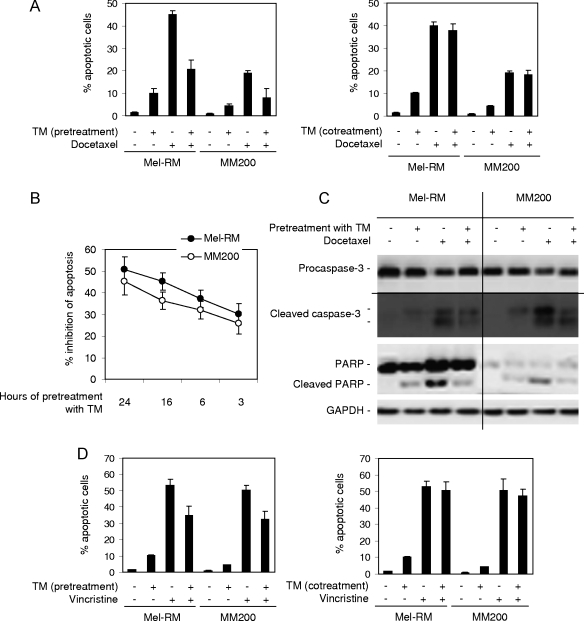
Human melanoma cells have largely adapted to ER stress [8,17]. To study the potential effect of this adaptation on the sensitivity of melanoma cells to microtubule-targeting chemotherapeutic drugs, we treated Mel-RM and MM200 cells with TM, a naturally occurring antibiotic that induces ER stress by inhibiting glycosylation [22], for 24 hours before the addition of docetaxel for a further 48 hours. As reported before [16,17,21], TM alone induced only moderate levels of apoptosis (<10% apoptotic cells), whereas docetaxel induced apoptotic cell death in approximately 45% and 20% of Mel-RM and MM200 cells, respectively (Figure 1A). Pretreatment with TM for 24 hours markedly reduced the levels of apoptosis induced by docetaxel (P < .01, 2-tailed Student's t test), but coadministration of TM and docetaxel resulted in similar levels of apoptosis to those induced by docetaxel alone (Figure 1A). These results indicate that the inhibitory mechanism(s) activated by TM needs to be present before exposing melanoma cells to docetaxel.
Figure 1.
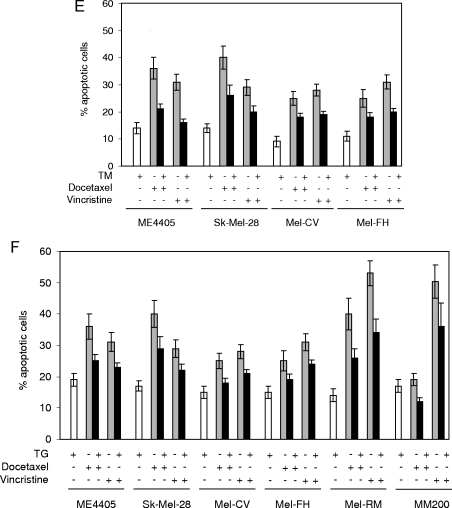
Induction of ER stress protects melanoma cells against apoptosis induced by docetaxel and vincristine. (A) Mel-RM and MM200 cells were pretreated with TM (3 µM) for 24 hours before the addition of docetaxel (20 nM) for a further 48 hours (left panel) or were cotreated with TM (3 µM) and docetaxel (20 nM) for 48 hours (right panel). Apoptosis was measured by the propidium iodide method using flow cytometry. (B) Mel-RM and MM200 cells were pretreated with TM (3 µM) for indicated periods before the addition of docetaxel (20 nM) for another 48 hours. Apoptosis was measured by the propidium iodide method using flow cytometry. The data shown (y-axis) are percentages of inhibition of apoptosis. (C) Mel-RM and MM200 cells were pretreated with TM (3 µM) for 6 hours before the addition of docetaxel (20 nM) for a further 36 hours. Whole cell lysates were subjected to Western blot analysis. (D) Mel-RM and MM200 cells were pretreated with TM (3 µM) for 6 hours before the addition of vincristine (50 ng/ml) for another 48 hours (left panel) or were cotreated with TM (3 µM) and vincristine (50 ng/ml) for 48 hours (right panel). Apoptosis was measured by the propidium iodide method using flow cytometry. (E) Melanoma cells were pretreated with TM (3 µM) for 24 hours before the addition of vincristine (50 ng/ml) for a further 48 hours. Apoptosis was measured by the propidium iodide method using flow cytometry. (F) Melanoma cells were pretreated with TG (1 µM) for 24 hours before the addition of docetaxel (20 nM) or vincristine (50 ng/ml) for a further 48 hours. Apoptosis was measured by the propidium iodide method using flow cytometry. The data shown are either the mean ± SE (A, B, D, E, and F) or are a representative (C) of three individual experiments.
To study the time frame required for the activation of the TM-mediated protective mechanism(s), we reduced the periods of treatment with TM before the addition of docetaxel. As shown in Figure 1B, although pretreatment with TM for as short as 3 hours reduced the levels of docetaxel-induced apoptosis by around 30% in both Mel-RM and MM200 cells, the degrees of inhibition seemed to be progressively attenuated with shorter pretreatment periods. Figure 1C shows that pretreatment with TM partially inhibited docetaxel-induced activation of caspase-3 and cleavage of its substrate poly (ADP-ribose) polymerase.
As shown in Figure 1D, pretreatment with TM also significantly blocked apoptosis induced by another microtubule-targeting drug, namely, vincristine, in Mel-RM and MM200 cells (P < .01, 2-tailed Student's t test). In contrast, coadministration of TM and vincristine did not have any notable effect on apoptosis induced by vincristine (Figure 1D). Protection of melanoma cells from docetaxel- and vincristine-induced apoptosis by preexposure to TM was confirmed in four other melanoma cell lines (Figure 1E). Similarly, pretreatment with TG that induces ER stress by inhibiting ER Ca2+ ATPases [23] also protected against apoptosis induced by the drugs in a panel of melanoma cell lines (Figure 1F).
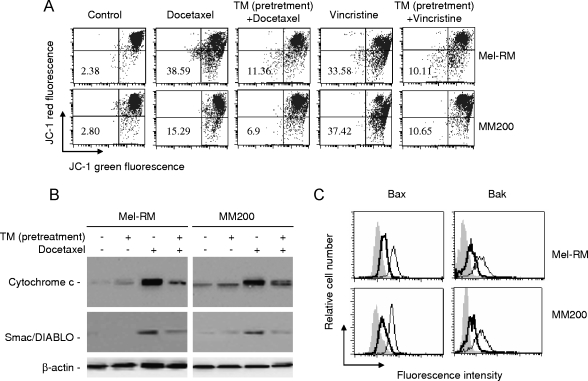
Induction of ER Stress Inhibits Mitochondrial Apoptotic Events Induced by Docetaxel and Vincristine
To elucidate the stage at which apoptotic signaling induced by docetaxel and vincristine is inhibited by pretreatment with TM, we examined the effect of pretreatment with TM on changes in ΔΨm induced by the drugs. As shown in Figure 2A, both docetaxel and vincristine induced reduction in ΔΨm as reported before [24,25], but the reduction was attenuated by pretreatment with TM. Figure 2B shows that pretreatment with TM reduced the cytosolic levels of cytochrome c and Smac/DIABLO in comparison with those in cells treated with docetaxel alone, indicating inhibition of mitochondrial release of these apoptogenic proteins. Similarly, pretreatment with TM blocked the activation of Bax and Bak induced by the docetaxel as detected with the antibodies that specifically recognize the activated forms of Bax and Bak, respectively (Figure 2C) [20,26].
Figure 2.
Pretreatment with TM inhibits mitochondrial apoptotic events induced by docetaxel and vincristine. (A) Mel-RM and MM200 cells were pretreated with TM (3 µM) for 6 hours before the addition of docetaxel (20 nM) or vincristine (50 ng/ml) for a further 36 hours. Cells were subjected to measurement of ΔΨm by JC-1 staining in flow cytometry. The number in each left-bottom quadrant represents the percentage of cells with reduction in ΔΨm. (B) Mel-RM and MM200 cells with or without pretreatment with TM (3 µM) for 6 hours were treated with docetaxel (20 nM) for a further 36 hours. Cytosolic fractions were isolated and were subjected to Western blot analysis of cytochrome c and Smac/DIABLO expression. (C) Mel-RM and MM200 cells with or without pretreatment with TM (3 µM) for 6 hours were treated with docetaxel (20 nM) for a further 36 hours. Cells were permeabilized and subjected to measurement of activation of Bax and Bak by flow cytometry using antibodies that specifically recognize activated Bax and Bak, respectively. The filled histograms were generated from cells treated with TM alone, the thin open histograms were generated from cells treated with docetaxel alone, and the thick open histograms were generated from cells pretreated with TM followed by the addition of docetaxel. The data shown are representative of three individual experiments.
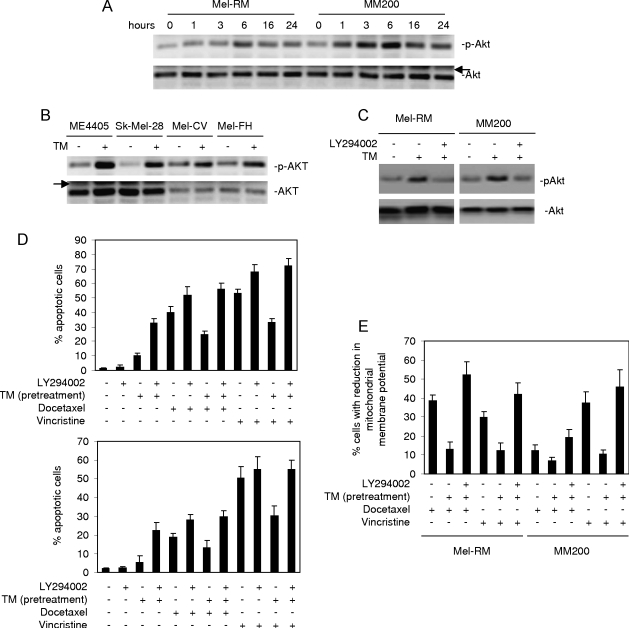
Protection of Melanoma Cells against Docetaxel- and Vincristine-Induced Apoptosis by Pretreatment with TM Is Associated with Activation of Akt
Our previous studies have shown that induction of apoptosis of melanoma cells by docetaxel and vincristine is inhibited by activation of the MEK/ERK pathway [24,25]. However, treatment with TM does not induce further activation of MEK/ERK that is constitutively activated in melanoma cells [21]. This argues against a major role of this survival pathway in the protection induced by pretreatment with TM. To elucidate the protective mechanism(s) activated by TM, we monitored the activation status of the PI3K/Akt survival pathway in Mel-RM and MM200 cells before and after treatment with TM. Figure 3A shows that exposure to TM induced increases in activation (phosphorylation) of Akt that were detectable as soon as 1 to 3 hours. The elevated levels of activation of Akt sustained for up to 16 to 24 hours after exposure to TM (Figure 3A). Activation of Akt by TM was observed in four other melanoma cell lines (Figure 3B).
Figure 3.
Activation of the PI3K/Akt pathway contributes to protection of melanoma cells against docetaxel and vincristine by pretreatment with TM. (A) Whole cell lysates from Mel-RM and MM200 cells treated with TM (3 µM) for indicated periods were subjected to Western blot analysis. The arrowhead points to nonspecific bands. (B) Whole cell lysates from melanoma cells treated with TM (3 µM) for 6 hours were subjected to Western blot analysis. The arrowhead points to nonspecific bands. (C) Mel-RM and MM200 cells were treated with LY294002 (20 µM) for 1 hour before the addition of TM (3 µM) for another 6 hours. Whole cell lysates were subjected to Western blot analysis. (D) Mel-RM (upper panel) and MM200 (lower panel) cells were treated with LY294002 (20 µM) for 1 hour before the addition of TM (3 µM) for 6 hours followed by docetaxel (20 nM) or vincristine (50 ng/ml) for a further 48 hours. Apoptosis was measured by the propidium iodide method using flow cytometry. (E) Mel-RM and MM200 cells were treated with LY294002 (20 µM) for 1 hour before the addition of TM (3 µM) for 6 hours followed by docetaxel (20 nM) or vincristine (50 ng/ml) for a further 48 hours. Mitochondrial membrane potential was measured using JC-1 staining by flow cytometry. The data shown are either representative (A, B, and C) or are the mean ± SE (D and E) of three individual experiments.
To study if the activation of Akt by ER stress plays a role in the protection of melanoma cells against docetaxel and vincristine, we treated Mel-RM and MM200 cells with the PI3K inhibitor LY294002 before the addition of TM followed by docetaxel and vincristine. Whereas LY294002 inhibited TM-induced activation of Akt (Figure 3C), it reversed the resistance of melanoma cells to apoptosis induced by TM and enhanced the apoptosis induced by docetaxel or vincristine alone (Figure 3D). In the presence of LY294002, protection against docetaxel- and vincristine-induced apoptosis and reduction in ΔΨm by pretreatment with TM were abrogated (Figure 3, D and E).
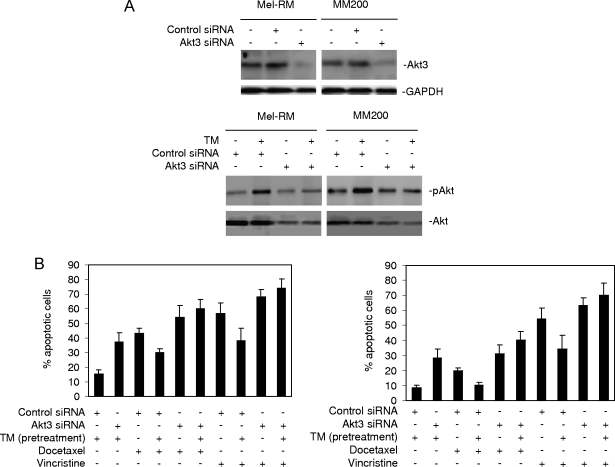
To confirm that the effect of LY294002 was due to the inhibition of the activation of Akt, we transfected an siRNA pool for Akt3, the major isoform of Akt in melanoma [27], into Mel-RM and MM200 cells. siRNA knockdown of Akt3 markedly reduced its expression levels and activation of Akt induced by TM (Figure 4A). Similar to LY294002, knockdown of Akt3 by siRNA inhibited the effect of pretreatment with TM on apoptosis induced by docetaxel and vincristine (Figure 4B).
Figure 4.
siRNA knockdown of Akt3-enhanced apoptosis induction abolishes protection against apoptosis induced by docetaxel and vincristine by pretreatment with TM. (A) Upper panel: Mel-RM and MM200 cells were transfected with the control or Akt3 siRNA. Twenty-four hours later, whole cell lysates were subjected to Western blot analysis. Lower panel: Mel-RM and MM200 cells were transfected with the control or Akt3 siRNA. Twenty-four hours later, cells were treated with TM (3 µM) for 6 hours. Whole cell lysates were subjected to Western blot analysis. (B) Mel-RM (left panel) and MM200 (right panel) cells were transfected with the control or Akt3 siRNA. Twenty-four hours later, cells were treated with TM (3 µM) for 6 hours followed by the addition of docetaxel (20 nM) or vincristine (50 ng/ml) for a further 48 hours. Apoptosis was measured by the propidium iodide method using flow cytometry. The data shown are either representative (A) or are the mean ± SE (B) of three individual experiments.
ER Stress-Induced Activation of Akt Is Associated with the IRE1α and ATF6 Pathways of the UPR
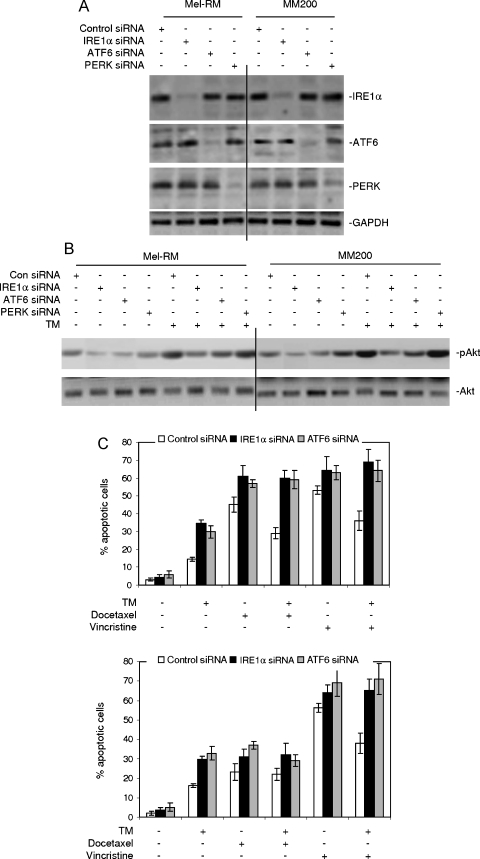
We investigated the signaling pathways of the UPR responsible for the activation of the PI3K/Akt pathway in melanoma cells under ER stress by transfecting siRNA pools for IRE1α, ATF6, and PERK into Mel-RM and MM200 cells, respectively. Figure 5A shows that siRNA knockdown of each of these UPR transducers inhibited its expression by at least 70% and had no notable effects on the expression of the others. As shown in Figure 5B, the levels of activation of Akt induced by TM were reduced in cells transfected with the siRNA for IRE1α and, to a lesser extent, in cells transfected with the siRNA for ATF6. Notably, the basal levels of activation of Akt were also decreased in cells with IRE1α or ATF6 knocked down. Inhibition of PERK by siRNA did not have any notable effect on activation of Akt before and after treatment with TM.
Figure 5.
siRNA knockdown of IRE1α or ATF6 blocks activation of Akt by TM. (A) Mel-RM and MM200 cells were transfected with the control, IRE1α, ATF6, or PERK siRNA. Twenty-four hours later, whole cell lysates were subjected to Western blot analysis. (B) Mel-RM and MM200 cells were transfected with the control, IRE1α, ATF6, or PERK siRNA as shown in panel A. Twenty-four hours later, cells were treated with TM (3 µM) for 6 hours. Whole cell lysates were subjected to Western blot analysis. (C) Mel-RM (upper panel) and MM200 (lower panel) cells were transfected with the control, IRE1α, or ATF6 siRNA. Twenty-four hours later, cells were treated with TM (3 µM) for 6 hours followed by the addition of docetaxel (20 nM) or vincristine (50 ng/ml) for a further 48 hours. Apoptosis was measured by the propidium iodide method using flow cytometry. The data shown are either representative (A and B) or are the mean ± SE (C) of three individual experiments.
We studied if the basal activity of each arm of the UPR pathways plays a role in regulating sensitivity of melanoma cells to docetaxel and vincristine. As shown in Figure 5C, siRNA inhibition of IRE1α or ATF6 enhanced apoptosis induced by docetaxel and vincristine and reversed resistance to TM-induced apoptosis in Mel-RM and MM200 cells. As expected, induction of apoptosis by docetaxel or vincristine in melanoma cells pretreated with TM was also enhanced by siRNA knockdown of IRE1α or ATF6 (Figure 5C). Inhibition of PERK by siRNA did not result in any notable effect on apoptosis induced by TM, docetaxel, or vincristine alone and in the protection against docetaxel or vincristine by pretreatment with TM (data not shown).
ER Stress-Induced Activation of Akt Is Mediated by XBP-1
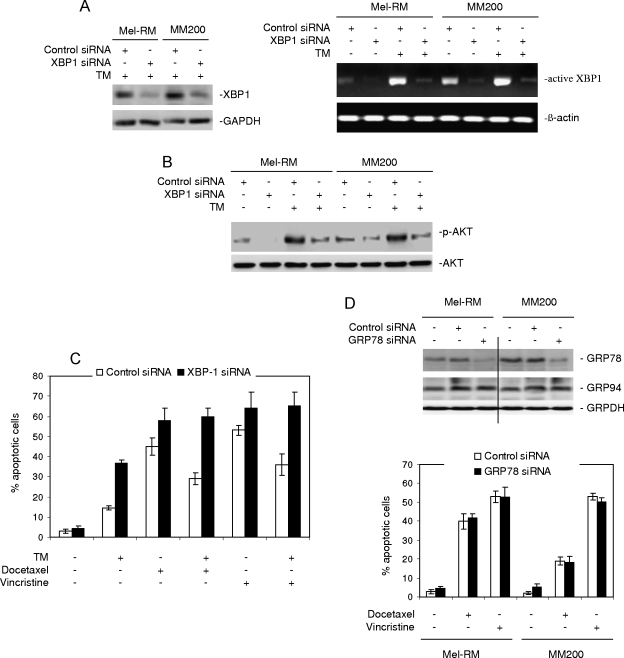
The IRE1α and ATF6 signaling pathways of the UPR converge on the UPR effector XBP-1, as XBP-1 is transcriptionally regulated by ATF6, but its activation is mediated by IRE1α [3–5]. We therefore envisage that XBP-1 plays a role in the activation of Akt induced by the UPR. To test this, we transfected an siRNA pool for XBP-1 into Mel-RM and MM200 cells. Figure 6A shows that the XBP-1 siRNA inhibited the XBP-1 levels by more than 70% in both cell lines. Activation of XBP-1, as indicated with XBP-1 mRNA splicing, by TM was also markedly reduced in cells transfected with the XBP-1 siRNA (Figure 6A). As shown in Figure 6B, inhibition of XBP-1 by siRNA reduced the basal levels of activation of Akt and blocked TM-induced activation of Akt in both cell lines, indicating that XBP-1 plays a role in the activation of Akt in melanoma cells when submitted to ER stress. Figure 6C shows that siRNA knockdown of XBP-1 sensitized melanoma cells to apoptosis induced by TM, docetaxel, and vincristine. Protection of melanoma cells against docetaxel and vincristine by pretreatment with TM was abolished in melanoma cells with XBP-1 knocked down by siRNA (Figure 6C).
Figure 6.
XBP-1 mediates the activation of Akt induced by TM in melanoma cells. (A) Left panel: Mel-RM and MM200 cells were transfected with the control or XBP-1 siRNA. Twenty-four hours later, cells were treated with TM for 16 hours. Whole cell lysates were subjected to Western blot analysis. Right panel: Mel-RM and MM200 cells were transfected with the control or XBP-1 siRNA. Twenty-four hours later, cells were treated with TM for 16 hours. Reverse transcription-polymerase chain reaction products of XBP1 mRNA were digested with Apa-LI for 90 minutes followed by electrophoresis. (B) Mel-RM and MM200 cells were transfected with the control or GRP78 siRNA. Twenty-four hours later, cells were treated with TM for another 6 hours. Whole cell lysates were subjected to Western blot analysis. (C) Mel-RM and MM200 cells were transfected with the control or GRP78 siRNA. Twenty-four hours later, cells were treated with TM for another 6 hours, followed by the addition of docetaxel (20 nM) or vincristine (50 ng/ml) for a further 48 hours. Apoptosis was measured by the propidium iodide method using flow cytometry. (D) Upper panel: Mel-RM and MM200 cells were transfected with the control or GRP78 siRNA. Twenty-four hours later, whole cell lysates were subjected to Western blot analysis of GRP78 and GRP94. Lower panel: Mel-RM and MM200 cells were transfected with the control or GRP78 siRNA. Twenty-four hours later, cells were treated with docetaxel (20 nM) or vincristine (50 ng/ml) for a further 48 hours. Apoptosis was measured by the propidium iodide method using flow cytometry. The data shown are either representative (A, B, and D) or are the mean ± SE of three individual experiments.
We also examined if GRP78, which is known to protect against various chemotherapeutic drugs in melanoma cells [6,7,11], is involved in regulating the sensitivity of melanoma cells to docetaxel and vincristine. Figure 6D shows that transfection of an siRNA pool for GRP78 markedly inhibited its expression, but had no effect on the expression of GRP94, in Mel-RM and MM200 cells. siRNA knockdown of GRP78 did not have any notable effect on the levels of apoptosis induced by docetaxel or vincristine (Figure 6D).
Docetaxel and Vincristine Do Not Trigger ER Stress in Melanoma Cells
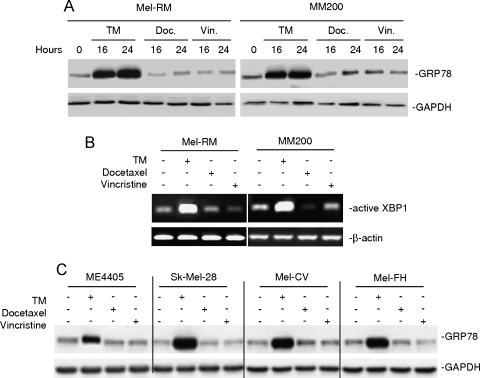
A number of clinically available chemotherapeutic drugs and those in development for clinical use can induce ER stress in melanoma [16,28]. To test if docetaxel and vincristine can similarly induce ER stress and activate the UPR in melanoma cells, we monitored the levels of the GRP78 protein and the spliced (active) XBP-1 mRNA in melanoma cells treated with docetaxel or vincristine. Cells treated with TM were included as controls. Whereas TM induced marked up-regulation of the GRP78 protein and spliced XBP-1 mRNA in Mel-RM and MM200 cells, neither docetaxel nor vincristine caused notable changes in the expression of the indicators of activation of the UPR (Figure 7, A and B). The inability of the drugs in the induction of GRP78 was also confirmed in four other melanoma cell lines (Figure 7C).
Figure 7.
Neither docetaxel nor vincristine triggers activation of the UPR in melanoma cells. (A) Mel-RM and MM200 cells with or without treatment with TM (3 µM), docetaxel (20 nM), or vincristine (50 ng/ml) for indicated periods were subjected to Western blot analysis. (B) Mel-RM and MM200 cells with or without treatment with TM (3 µM), docetaxel (20 nM), or vincristine (50 ng/ml) for 16 hours. Reverse transcription-polymerase chain reaction products of XBP1 mRNA were digested with Apa-LI for 90 minutes followed by electrophoresis. (C) Melanoma cells were treated with TM (3 µM), docetaxel (20 nM), or vincristine (50 ng/ml) for 16 hours. Whole cell lysates were subjected to Western blot analysis. The data shown are representative of three individual experiments.
Discussion
Resistance of melanoma cells to chemotherapeutics is a major obstacle to successful treatment of melanoma once it has spread beyond locoregional sites [1,2,29]. In the present study, we show that induction of ER stress renders melanoma cells even more resistant to two microtubule-targeting chemotherapeutic drugs, docetaxel and vincristine. Our results suggest that adaptation of melanoma cells to ER stress may be an important mechanism of resistance of melanoma cells to the drugs.
In the search for the protective mechanism(s) against docetaxel and vincristine resulting from preloaded ER stress, we found that exposure to TM induced activation of the PI3K/Akt pathway. Kinetically, TM-induced activation of Akt occurred as early as 1 to 3 hours, and the elevated activation levels sustained till 16 to 24 hours. This conformed to the time frame needed by TM to activate the protective mechanism(s) against the drugs.Moreover, inhibition of Akt activation by either the PI3K inhibitor LY294002 or the siRNA knockdown of Akt3 reversed the protection against vincristine- or docetaxel-induced apoptosis by pretreatment with TM. When LY294002 was implemented after initiating the activation of Akt, its effect on TM-mediated protection against the drugs was attenuated. This further confirms the importance of preexisting Akt activity in antagonizing apoptotic signaling induced by docetaxel and vincristine, neither of which induces activation of the PI3K/Akt pathway in melanoma cells [24,25]. Taken together, these results identify activation of Akt as an important protective mechanism against docetaxel and vincristine in melanoma cells undergoing ER stress.
ER stress-induced activation of Akt has been speculated to be due to the increases in intracellular calcium [30], as disruption of calcium homeostasis is one of the characteristics of ER stress and calcium plays critical roles in the activation of PI3K [1–3,31,32]. However, activation of Akt by glucosamine through induction of ER stress was shown not to be associated with increased release of calcium into the cytosol [33]. We found that TM-induced activation of Akt was partially inhibited by siRNA knockdown of IRE1α or ATF6, indicating that these pathways of the UPR are involved in the activation of Akt in melanoma cells under ER stress. Because XBP-1 is transcriptionally regulated by ATF6 and is activated by IRE1α [3–5], it seemed that XBP-1 may play a part in the activation of Akt mediated by the UPR. In this study, inhibition of XBP-1 by siRNA reduced the levels of constitutively activated Akt and blocked TM-induced Akt activation, verifying a role of XBP-1 in UPR-mediated activation of Akt. It was recently shown that ER stress activated Akt in a zebrafish embryonic cell line through XBP-1-mediated up-regulation of insulin growth factor-1 (IGF-1) [34]. However, IGF-1/IGF-1 receptor signaling in melanoma cells originates mainly from exogenous IGF-1 because melanoma cells express no, or minimal, IGF-1 [35,36]. Nevertheless, it is possible that XBP-1 will activate a factor or factors similar to IGF-1 that in turn causes activation of the PI3K/Akt pathway in melanoma cells under ER stress. IRE1α is known to be responsible for ER stress-induced activation of Jun N-terminal kinase and p38 by forming complexes with TNF receptor-associated factor 2 and apoptosis signal-regulating kinase 1 [37,38]. Whether this is also involved in the activation of Akt by TM in melanoma cells remains to be studied.
Activation of Akt is known to protect against apoptosis induced by a number of stimuli [39–41]. Several proapoptotic proteins, such as the BH3-only protein Bad, forkhead transcription factors, and caspase-9, have been identified as direct targets of Akt [39,40]. In addition, induction of the inhibitor of apoptosis protein (IAP) family members XIAP and cIAP2 by Akt has also been reported to be responsible for the inhibition of ER stress-induced apoptosis downstream of mitochondria [30]. However, inhibition of docetaxel- and vincristine-induced apoptotic signaling in melanoma cells submitted to ER stress seemed to take place upstream of mitochondrial apoptotic events, suggesting that activation of Akt by TM may act on one or more Bcl-2 family proteins to protect melanoma cells from docetaxel- and vincristine-induced apoptosis.
A Bcl-2 family protein that may play a role in antagonizing docetaxel and vincristine in melanoma cells submitted to ER stress isMcl-1, which can be regulated by Akt [42–44]. Mcl-1 is upregulated by ER stress and plays a critical role in the protection of melanoma cells from ER stress-induced apoptosis by neutralizing the BH3-only proteins PUMA and Noxa [17]. The latter are also upregulated by ER stress [17]. However, neither PUMA nor Noxa plays a part in apoptosis induced by docetaxel and vincristine [24,25]. Nevertheless, Mcl-1 can also bind, albeit loosely, to another BH3-only protein Bad, which is known to be involved in docetaxel-induced apoptosis of melanoma cells [45].
A number of clinically available chemotherapeutic drugs and those in development for clinical use can induce ER stress in various types of cancer cells [16,28,46]. We have shown previously that the DNA-damaging drugs cisplatin and adriamycin induce ER stress in melanoma cell lines and that induction of GRP78 by the UPR plays an important role in antagonizing these drugs [16]. Unlike cisplatin and adriamycin, docetaxel and vincristine did not cause ER stress in the melanoma cells. In this study, the sensitivity of melanoma cells to docetaxel- and vincristine-induced apoptosis could not be enhanced by the inhibition of GRP78. These results are in contrast to a recent report in which another microtubule-targeting drug, namely, paclitaxel, was shown to induce apoptosis of human lymphoma cells by triggering ER stress [47]. Melanoma cells are biologically different from other cell types in many aspects [2,8]. The difference in response to microtubule-targeting drugs between the present study and others may therefore be due to inherent characteristics of different cell types.
An important finding of this study is that the constitutive activation of the UPR is involved in the resistance of melanoma cells to apoptosis induced by docetaxel and vincristine. Significantly, inhibition of IRE1α and ATF6 reduced the constitutive levels of Akt activation in melanoma cells, suggesting that activation of the UPR by chronic ER stress contributes to the constitutive activation of the PI3K/Akt pathway. The observation that inhibition of Akt partially reversed the resistance of melanoma cells to TM-induced apoptosis indicates that activation of Akt in turn plays a role in the adaptation of melanoma cells to ER stress, as does the MEK/ERK pathway [21].
In summary, we demonstrate in this report that induction of ER stress protects melanoma cells against the microtubule-targeting chemotherapeutic drugs docetaxel and vincristine. This is mediated, at least in part, by the activation of PI3K/Akt through the XBP-1 axis of the UPR. In addition, we show that XBP-1 contributes to the basal levels of Akt activation in melanoma cells, which in turn plays a role in the resistance of melanoma cells to apoptosis induced by ER stress and by docetaxel and vincristine. The results from this study suggest that targeting XBP-1 may be useful in improving the therapeutic effects of microtubule-targeting drugs in melanoma. Moreover, therapeutics that can induce ER stress in melanoma cells such as cisplatin and fenretinide may cause an antagonistic effect when combined with docetaxel or vincristine [16,28].
Abbreviations
- ATF6
activating transcription factor 6
- ER
endoplasmic reticulum
- GRP78
glucose-regulated protein 78
- IRE1α
inositol-requiring transmembrane kinase and endonuclease 1α
- mAb
monoclonal antibody
- PERK
protein kinase-like ER kinase
- TG
thapsigargin
- TM
tunicamycin
- UPR
unfolded protein response
- XBP-1
X-box-binding protein 1
Footnotes
This work was supported by the NSW State Cancer Council, the Melanoma and Skin Cancer Research Institute Sydney, the Hunter Melanoma Foundation, NSW, and the National Health and Medical Research Council, Australia. X.D. Zhang is a Cancer Institute NSW Fellow.
References
- 1.Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 2.Hersey P, Zhuang L, Zhang XD. Current strategies in overcoming resistance of cancer cells to apoptosis melanoma as a model. Int Rev Cytol. 2006;251:131–158. doi: 10.1016/S0074-7696(06)51004-6. [DOI] [PubMed] [Google Scholar]
- 3.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 4.Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279:25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- 5.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 6.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 8.Hersey P, Zhang XD. Adaptation to ER stress as a driver of malignancy and resistance to therapy in human melanoma. Pigment Cell Melanoma Res. 2008;21:358–367. doi: 10.1111/j.1755-148X.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang L, Lee CS, Scolyer RA, McCarthy SW, Zhang XD, Thompson JF, Hersey P. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology. 2009 doi: 10.1111/j.1365-2559.2009.03242.x. DOI: 10:1111/J.1365-2559. [DOI] [PubMed] [Google Scholar]
- 10.Jiang CC, Mao ZG, Avery-Kiejda KA, Wade M, Hersey P, Zhang XD. Glucose-regulated protein 78 antagonizes cisplatin and adriamycin in human melanoma cells. Carcinogenesis. 2009;30:197–204. doi: 10.1093/carcin/bgn220. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 12.Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR, Pointer JN, Gruber SB, Su LD, Nikiforov MA, et al. Antioncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol. 2006;8:1053–1063. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- 13.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 14.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 15.Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang CC, Lucas K, Avery-Kiejda KA, Wade M, deBock CE, Thorne RF, Allen J, Hersey P, Zhang XD. Up-regulation of Mcl-1 is critical for survival of human melanoma cells upon endoplasmic reticulum stress. Cancer Res. 2008;68:6708–6717. doi: 10.1158/0008-5472.CAN-08-0349. [DOI] [PubMed] [Google Scholar]
- 18.Koong AC, Chauhan V, Romero-Ramirez L. Targeting XBP-1 as a novel anti-cancer strategy. Cancer Biol Ther. 2006;5:756–759. doi: 10.4161/cbt.5.7.2973. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999;59:2747–2753. [PubMed] [Google Scholar]
- 20.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspaseindependent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934–4942. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- 21.Jiang CC, Chen LH, Gillespie S, Wang YF, Kiejda KA, Zhang XD, Hersey P. Inhibition of MEK/ERK sensitizes human melanoma cells to endoplasmic reticulum stress-induced apoptosis by enhancing caspase-4 activation. Cancer Res. 2007;67:9750–9761. doi: 10.1158/0008-5472.CAN-07-2047. [DOI] [PubMed] [Google Scholar]
- 22.Elbein AD. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- 23.Sagara Y, Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J Biol Chem. 1991;266:13503–13506. [PubMed] [Google Scholar]
- 24.Mhaidat NM, Zhang XD, Jiang CC, Hersey P. Docetaxel-induced apoptosis of human melanoma is mediated by activation of c-Jun NH2-terminal kinase and inhibited by the mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway. Clin Cancer Res. 2007;13:1308–1314. doi: 10.1158/1078-0432.CCR-06-2216. [DOI] [PubMed] [Google Scholar]
- 25.Zhu BK, Wang P, Zhang XD, Jiang CC, Chen LH, Avery-Kiejda KA, Watts R, Hersey P. Activation of Jun N-terminal kinase is a mediator of vincristine-induced apoptosis of melanoma cells. Anticancer Drugs. 2008;19:189–200. doi: 10.1097/CAD.0b013e3282f3138a. [DOI] [PubMed] [Google Scholar]
- 26.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, Kester M, Sandirasegarane L, Robertson GP. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–7010. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 28.Lovat PE, Corazzari M, Armstrong JL, Martin S, Pagliarini V, Hill D, Brown AM, Piacentini M, Birch-Machin MA, Redfern CP. Increasing melanoma cell death using inhibitors of protein disulfide isomerases to abrogate survival responses to endoplasmic reticulum stress. Cancer Res. 2008;68:5363–5369. doi: 10.1158/0008-5472.CAN-08-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallagher SJ, Thompson JF, Indsto J, Scurr LL, Lett M, Gao BF, Dunleavey R, Mann GJ, Kefford RF, Rizos H. p16INK4a expression and absence of activated B-RAF are independent predictors of chemosensitivity in melanoma tumors. Neoplasia. 2008;10:1231–1239. doi: 10.1593/neo.08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu P, Han Z, Couvillon AD, Exton JH. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem. 2004;279:49420–49429. doi: 10.1074/jbc.M407700200. [DOI] [PubMed] [Google Scholar]
- 31.Bonilla M, Nastase KK, Cunningham KW. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 2002;21:2343–2353. doi: 10.1093/emboj/21.10.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pérez-García MJ, Ceña V, de Pablo Y, Llovera M, Comella JX, Soler RM. Glial cell line-derived neurotrophic factor increases intracellular calcium concentration. Role of calcium/calmodulin in the activation of the phosphatidylinositol 3-kinase pathway. J Biol Chem. 2004;279:6132–6142. doi: 10.1074/jbc.M308367200. [DOI] [PubMed] [Google Scholar]
- 33.Matthews JA, Belof JL, Acevedo-Duncan M, Potter RL. Glucosamine-induced increase in Akt phosphorylation corresponds to increased endoplasmic reticulum stress in astroglial cells. Mol Cell Biochem. 2007;298:109–123. doi: 10.1007/s11010-006-9358-5. [DOI] [PubMed] [Google Scholar]
- 34.Hu MC, Gong HY, Lin GH, Hu SY, Chen MH, Huang SJ, Liao CF, Wu JL. A key regulator of unfolded protein response, activates transcription of IGF1 and Akt phosphorylation in zebrafish embryonic cell line. Biochem Biophys Res Commun. 2007;359:778–783. doi: 10.1016/j.bbrc.2007.05.183. [DOI] [PubMed] [Google Scholar]
- 35.Lee JT, Brafford P, Herlyn M. Unraveling the mysteries of IGF-1 signaling in melanoma. J Invest Dermatol. 2008;128:1358–1360. doi: 10.1038/jid.2008.124. [DOI] [PubMed] [Google Scholar]
- 36.Rodeck U, Melber K, Kath R, Menssen HD, Varello M, Atkinson B, Herlyn M. Constitutive expression of multiple growth factor genes by melanoma cells but not normal melanocytes. J Invest Dermatol. 1991;97:20–26. doi: 10.1111/1523-1747.ep12477822. [DOI] [PubMed] [Google Scholar]
- 37.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuura H, Nishitoh H, Takeda K, Matsuzawa A, Amagasa T, Ito M, Yoshioka K, Ichijo H. Phosphorylation-dependent scaffolding role of JSAP1/JIP3 in the ASK1-JNK signaling pathway. A new mode of regulation of the MAP kinase cascade. J Biol Chem. 2002;277:40703–40709. doi: 10.1074/jbc.M202004200. [DOI] [PubMed] [Google Scholar]
- 39.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 40.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 41.Hollander MC, Balogh AR, Liwanag J, Han W, Linnoila RI, Anver MR, Dennis PA. Strain-specific spontaneous and NNK-mediated tumorigenesis in Pten+/- mice. Neoplasia. 2008;10:866–872. doi: 10.1593/neo.08406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, Yang-Yen HF. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–6206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo ML, Chuang SE, Lin MT, Yang SY. The involvement of PI 3-K/Akt-dependent up-regulation of Mcl-1 in the prevention of apoptosis of Hep3B cells by interleukin-6. Oncogene. 2001;20:677–685. doi: 10.1038/sj.onc.1204140. [DOI] [PubMed] [Google Scholar]
- 44.Mei Y, Xie C, Xie W, Tian X, Li M, Wu M. Noxa/Mcl-1 balance regulates susceptibility of cells to camptothecin-induced apoptosis. Neoplasia. 2007;9:871–881. doi: 10.1593/neo.07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 46.Joo JH, Liao G, Collins JB, Grissom SF, Jetten AM. Farnesol-induced apoptosis in human lung carcinoma cells is coupled to the endoplasmic reticulum stress response. Cancer Res. 2007;67:7929–7936. doi: 10.1158/0008-5472.CAN-07-0931. [DOI] [PubMed] [Google Scholar]
- 47.Liao PC, Tan SK, Lieu CH, Jung HK. Involvement of endoplasmic reticulum in paclitaxel-induced apoptosis. J Cell Biochem. 2008;104:1509–1523. doi: 10.1002/jcb.21730. [DOI] [PubMed] [Google Scholar]