Abstract
A 6-year-old male, blue Doberman pinscher crossbreed was presented with coat abnormalities; in particular, flank alopecia and pruritus. Based on medical the history, clinical evidence, and histopathological examination, color dilution alopecia was diagnosed. The dog was with oral melatonin treated for 3 months without success.
Résumé
Alopécie à dilution de couleur chez une race croisée de Doberman Pinscher bleu. Un Doberman Pinscher âgé de 6 ans est présenté avec des anomalies du pelage; en particulier, une alopécie du flanc et du prurit. En fonction de l’anamnèse, des signes cliniques et de l’examen histopathologique, l’alopécie à dilution de couleur a été diagnostiquée. Le chien a été traité sans succès à la mélatonine pendant 3 mois.
(Traduit par Isabelle Vallières)
A 6-year-old, 38 kg, intact male, blue Doberman pinscher crossbreed was presented to the Milan University Medical Teaching Hospital because of progressive coat abnormalities, in particular, flank alopecia and pruritus. The dog had not been vaccinated regularly or exposed to antiparasitic prophylaxis. The dog had accompanied his owners as they traveled throughout Italy (Emilia-Romagna and Toscana) and abroad (southern coast of France and Spain). Until the appearance of the first dermatological clinical signs, the dog had had no previous history of illness. The changes in the dog’s skin, in particular a bilaterally symmetrical loss of hair with pruritus on the flanks, had been seen from about 18 mo of age. Oral antibiotic therapy with amoxicillin/clavulanate (Synulox; Pfizer Italia Srl., Latina, Italy), 25 mg/kg bodyweight (BW), PO, q12h for 3 wk had resulted in a partial resolution of the pruritus, without improvement in the density of the coat.
Case description
No significant clinical abnormalities were seen on physical examination. Dermatological examination revealed a poor, dry, and brittle coat with pruritic, diffuse, bilaterally symmetrical alopecia of the thorax, flanks, and abdomen; it extended from the back of the neck to the lumbar area and was associated with a diffuse papulopustular dermatitis with excessive scaling and crusting concentrated mainly on the dorsal and the lateral trunk. Hematological and complete biochemical profiles, serum protein electrophoresis, an adrenocorticotropic hormone (ACTH) stimulation test with IM injection of 0.25 mg of synthetic ACTH (Synacten; Novartis Farma S.p.a., Origgio, Varese, Italy), indirect immunofluorescence assay for Leishmania infantum, and total thyroxine (T4) and thyroid stimulating hormone (TSH) serum concentration tests were performed; results from them all were within the reference limits. Ultrasonography of the testicles was also conducted to determine it there was a Sertoli cell tumor; the results were negative.
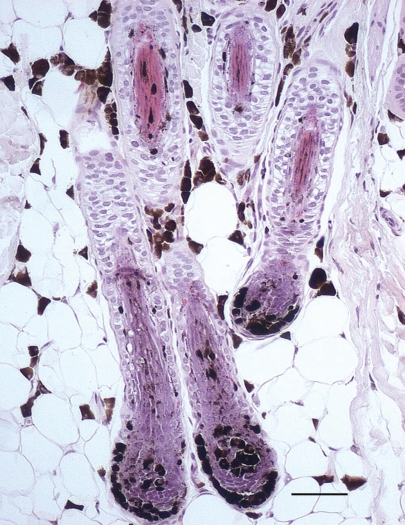
Cytological examination of follicle pustular contents (Quick Color PKL; Pokler Italia Srl, Genova, Italy) revealed many neutrophils phagocytosing cocci, suggesting a bacterial folliculitis. A bacterial culture of the exudate showed the presence of Staphylococcus intermedius, which was determined to be susceptible to the antimicrobial drugs amoxicillin clavulanate, cephalexin, enrofloxacin, marbofloxacin, clindamycin, doxycycline, and trimethoprim-sulfonamide. Results from the examination of skin scrapings and culturing for fungi were negative for ectoparasites and dermatophytes, respectively. Direct microscopic examination of plucked hairs revealed hair shaft distortion and the presence of clumped melanin (macromelanosomes) variably distributed along the whole length of the hair shaft, causing deformation of the medulla and the cortex. These clumps were numerous and associated with cuticular deformities and fractures (Figure 1). Color dilution alopecia (CDA) or congenital black hair follicular dysplasia were suspected and a therapeutic plan was established with the administration of cephalexin (Rilexine; Virbac S.A., Corras Cedex, France), 30 mg/kg BW, PO, q12h for 3 wk; fipronil and (S)-methoprene combination spot-on application (Frontline Combo; Merial, Tolose, France) 2 times/mo; and shampoo therapy (with salicylic acid, zinc gluconate, vitamin B6, piroctone olamine, and linoleic and linolenic acids) 2 times/wk. Skin biopsies from affected areas (right and left flank, back, and abdomen) and a normal zone (neck) were submitted for histopathological examination. On microscopic examination, the appearance of the skin biopsies was very similar among the various lesions: the epidermis was characterized by minimal hyperplasia and by melanin aggregates in the basal layers. Major changes were centered in and around the hair follicles. Infundibula were keratin-plugged and dilated by an accumulation of lamellar keratin (follicular keratosis) and hair shafts containing large clumps of melanin. The majority of hair shafts, follicles, and bulbs were characterized by abnormal and massive melanin clumping. Variable distortion of the hair follicles was also present. Approximately 50% of the hair follicles were in telogen arrest and 50% were in anagen. Pigment aggregates and melanomacrophages were present around the hair follicles, especially in the deep dermis and panniculus surrounding the bulbs. Lesions were more severe on the left flank, with the follicular isthmus often being completely filled by pigment aggregates and frequently distorted (witch’s foot), than on right flank and dorsum (Figure 2). Microscopically, the lesions were consistent with hair follicle pigmentary dysplasia. These histological features were considered to be characteristic of either CDA or congenital black hair follicular dysplasia. Based on the signalement (dog crossbred with a blue Doberman pinscher), clinical signs (skin disease limited to the color diluted areas), laboratory data, and results of histopathological examination, CDA was diagnosed. The antibiotic therapy previously prescribed was continued for 1 wk beyond the resolution of the bacterial infection. To increase hair regrowth, melatonin (Melatonin pura; ESI SpA, Albissola Marina, Italy), 5 mg, PO, q12h for 3 mo, was prescribed in association with shampoo therapy q15d, as described in previous studies on dogs with alopecia X, recurrent flank alopecia, and pattern baldness (1,2). Administration of melatonin was suspended after 3 mo, when no improvement in overall hair growth was noted. However, the secondary bacterial infections and pruritus were effectively controlled with the biweekly shampoo therapy.
Figure 1.

Large melanin clump (macromelanosome) in the hair cortex and medulla (Bar = 60 μm).
Figure 2.
Inferior segment of 4 hair follicles characterized by abnormal melanin clumping in one hair shaft, in the outer root sheaths, and in the hair bulbs. Several melanomacrophages are present in the septae of the adjacent panniculus. Isthmus of hair follicles is misshapen and completely filled by abundant melanin clumping. (Hematoxylin and eosin stain. Bar = 50 μm)
Discussion
Color dilution alopecia, or color mutant alopecia, is an uncommon genetic-based skin disease with delayed onset (3); it has been described in many canine breeds and is more prevalent in color dilute individuals, especially those with a fawn (a dilution of a normally red or brown coat) or blue (a dilution of the normal black and tan color) coat (3). According to Miller (4), it is prevalent in the blue and fawn Doberman pinscher with an incidence rate of 57.9% and 89.5%, respectively. The disorder, however, has also been reported in color dilution variants of other breeds, including the Yorkshire terrier, dachshund, schnauzer, Irish setter, chow chow, Italian greyhound, standard poodle, whippet, Chihuahua, saluki, and Bernese mountain dog (3,5,6) but, rarely, in crossbreed dogs (5,7). The pathogenetic mechanisms of alopecia in CDA are not clearly understood (6). Color dilution alopecia is based on autosomal recessive gene transmission. The dilution gene -d, especially the allele called -d1, may play an important role in the genetic transmission of color mutant alopecia (3), but other alleles and other factors should be taken into consideration, such as disorders of calcium ion conduction and protein kinase C activation, which play an important role in the keratinization process (8). In fact, not all dogs with color dilution develop coat abnormalities (8). Review of most of the literature indicates that there are multiple levels of possible anomalies: abnormal keratinization, abnormalities in pigment transfer, defects in melanization, abnormal storage of undegraded melanosomes, and defects of hair follicle function (4,8,9). The disease is not manifested at birth (6). The onset of hair loss usually begins between 4 and 18 mo of age, although in some cases, the disease will be latent until the animal is 3 to 6 y old (9). Sex predilections have not been noted (9). The condition is progressive with a gradual onset of dry, dull, and poor hair coat (6,9). Hair shafts are brittle and broken, especially along the dorsal midline (6). After several years, hypotrichosis is evident and can progress until a complete alopecia extends from the trunk to the flanks, usually sparing the head, tail, and limbs and all nondiluted coat areas (6,9). The skin in the affected areas is usually scaly, and follicular papules may develop and progress to frank comedones and, potentially, to recurrent bacterial folliculitis (6,9). Although pruritus is usually absent in CDA (6), it may occur to varying degrees due to the presence of bacterial folliculitis (6), as in the patient in this report. Except for the dermatologic lesions, color-mutant dogs seem to be in good general health. Diagnosis of CDA is based on ruling out any inflammatory causes of alopecia (demodicosis, bacterial folliculitis, and dermatophytosis) and the common noninflammatory causes of alopecia, such as hypothyroidism and hyperadrenocorticism (9). The primary differential diagnosis for CDA is black hair follicular dysplasia (6,9), which is virtually identical histopathologically with CDA, but tan areas are spared in CDA, whereas only black areas are affected in black hair follicular dysplasia (6). Currently, most literature supports the theory that the 2 lesions are manifestations of the same disease (3,9). Signalement, clinical history, the characteristic presence of diluted pigment, and the skin microscopic lesions were determinants in establishing a definitive diagnosis in this study (6). Hair examination of dogs with CDA will typically show numerous large melanin aggregates (macromelanosomes) in the hair cortex and medulla, distortion and breakage of the hair cortex, and fractures of the hair cuticle (3), as was detected in this case. Histological analysis indicates epidermal and follicular hyperkeratosis with most of the hair follicles dilated and filled with large melanin clumps. Abnormal clumping of melanin is also observed in the epidermis, dermis, and epithelia of hair follicles and around hair bulbs (6). Free melanin deposits are present at every level of the hair follicle and shaft, often with numerous perifollicular and peribulbar melanophages and pigmentary incontinence (9), as was observed on our histopathological examinations. It has been suggested that matrix hair cells are damaged by the cytotoxic effects of melanin precursor, which initiates the hair follicle dysplasia seen in the microscopic lesions and contributes to the development of the alopecia (3,4). Presence of subcuticular and intramedullary vacuoles that deform hair structure and contain melanosomes, as seen by transmission electron microscopy, may also explain hair fragility and ease of rupture (3) and might be responsible for liberation of the melanin clumps that are released into hair follicles. There is no specific or curative therapy for color dilution alopecia (6). The vital prognosis is good, but it is preferable to prevent affected animals from breeding. Frequent baths, given once or twice/wk, with keratomodulating/antiseborrhoeic agents (benzoyl peroxide, salicylic acid, or ethyl lactate) can control the recurrence of bacterial infections, encourage restoration of normal keratinocyte multiplication, and inhibit or reduce the formation of comedones and sebum production (6). We administered oral melatonin supplementation to encourage hair regrowth, but it did not have a beneficial effect. To the authors’ knowledge, there are no reports of melatonin administration in dogs with color mutant alopecia, but oral or topical melatonin has been used to treat various other forms of canine alopecia with variable success (1,2,10). The mechanism by which melatonin may result in hair regrowth is not known. Melatonin is thought to play either a direct (on hair follicles) or an indirect role (within the central nervous system to alter secretion of melanocyte-stimulating hormone or prolactin secretion, or both) in the control of moulting and hair growth in mammals (2,10), but knowledge of this process is still very incomplete. It was originally hypothesized that melatonin exerted its effect on hair growth through modulating sex hormone and adrenal steroid hormone concentrations; however, decreases in hormone concentrations were not associated with hair regrowth in neutered dogs with alopecia X (1). In a recent study, it was theorized that melatonin may cause hair regrowth in dogs with hair cycle arrest by down-regulating the expression of estrogen receptors, which seem to have a role in the regulation of the anagen and telogen phases of hair (1). There are many potential reasons for failure of the treatment of the dog in our study. First, there are reports about the possible mechanisms of action, bioavailability, dosages, and seasonal/daily timing of melatonin administration, which made it difficult to determine a correct method of administration. We chose to follow the general directions given by Paradis (2). Additionally, because melatonin is classified as a nutraceutical, there is lack of standardization of the product, which may result in an inconsistent drug content in the final preparation. Finally, the real mechanism for development of color mutant alopecia is unknown; therefore, treatment with melatonin simply may not be effective.
To the authors’ knowledge, only 2 cases of CDA in crossbreed dogs have been reported (5,7).
Footnotes
Authors’ contributions
Dr. Perego was involved with the clinical aspects of the case, performed the dermatological procedures and examinations, and wrote the manuscript. Dr. Proverbio assisted in the interpretation of the clinical findings, the diagnostic imaging, and the hematological and biochemical evaluations. She also assisted with the preparation of the manuscript. Dr. Roccabianca performed the histopathological studies on the skin. Dr. Spada was involved with the clinical aspects of the case and cared for the dog. She also monitored the therapeutic treatments. All authors read and approved the manuscript. CVJ
References
- 1.Frank LA, Donnell RL, Kania SA. Oestrogen receptor evaluation in Pomeranian dogs with hair cycle arrest (alopecia X) on melatonin supplementation. Vet Dermatol. 2006;16:252–258. doi: 10.1111/j.1365-3164.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 2.Paradis M. Melatonin therapy for canine alopecia. In: Bonagura JD, editor. Kirk’s Current Veterinary Medicine. 13th ed . Philadelphia: WB Saunders; 2000. pp. 546–549. [Google Scholar]
- 3.Beco L, Fontaine J, Gross TL, Charlier G. Colour dilution alopecia in seven Dachshunds. A clinical study and the hereditary, microscopical and ultrastructural aspect of the disease. Vet Dermatol. 1996;7:91–97. doi: 10.1111/j.1365-3164.1996.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller WH. Colour dilution alopecia in Doberman Pinscher with blue or fawn coat colours: A study on the incidence and histopathology of this disorder. Vet Dermatol. 1990;1:113–121. doi: 10.1111/j.1365-3164.1990.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller WH. Alopecia associated with coat colour dilution in two Yorshire Terriers, one Saluki and one mix–breed. J Am Anim Hosp Assoc. 1991;27:39–43. [Google Scholar]
- 6.Kim J, Kang K, Sohn H, Woo G, Jean Y, Hwang E. Color — dilution alopecia in dogs. J Vet Sci. 2005;6:259–261. [PubMed] [Google Scholar]
- 7.Finnie JW, Tham VL. Colour mutant alopecia in a Kelpie x Border Collie dog. Aust Vet J. 1993;70:388–389. doi: 10.1111/j.1751-0813.1993.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 8.Roperto F, Cerundolo R, Restucci B, et al. Colour dilution alopecia (CDA) in ten Yorkshire Terriers. Vet Dermatol. 1995;6:171–178. doi: 10.1111/j.1365-3164.1995.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 9.Gross TL, Ihrke PJ, Walder EJ. Veterinary Dermatopathology: A Macroscopic and Microscopic Evaluation of Canine and Feline Skin Disease . St Louis: Mosby Year Book; 1992. pp. 298–302. [Google Scholar]
- 10.Diaz SF, Torres SM, Nogueira SAF, Gilbert S, Jessen CR. The impact of body site, topical melatonin and brushing on hair regrowth after clipping normal Siberian Husky dogs. Vet Dermatol. 2006;17:45–50. doi: 10.1111/j.1365-3164.2005.00497.x. [DOI] [PubMed] [Google Scholar]