Abstract
The last few decades have seen the hippocampal formation at front and center in the field of synaptic transmission. However, much of what we know about hippocampal short- and long-term plasticity has been obtained from research at one particular synapse; the Schaffer collateral input onto principal cells of the CA1 subfield. A number of recent studies, however, have demonstrated that there is much to be learned about target-specific mechanisms of synaptic transmission by study of the lesser known synapse made between the granule cells of the dentate gyrus; the so-called mossy fiber synapse, and its targets both within the hilar region and the CA3 hippocampus proper. Indeed investigation of this synapse has provided an embarrassment of riches concerning mechanisms of transmission associated with feedforward excitatory and inhibitory control of the CA3 hippocampus. Importantly, work from a number of labs has revealed that mossy fiber synapses possess unique properties at both the level of their anatomy and physiology, and serve as an outstanding example of a synapse designed for target-specific compartmentalization of synaptic transmission. The purpose of the present review is to highlight several aspects of this synapse as they pertain to a novel mechanism of bidirectional control of synaptic plasticity at mossy fiber synapses made onto hippocampal stratum lucidum interneurons. It is not my intention to pour over all that is known regarding the mossy fiber synapse since many have explored this topic exhaustively in the past and interested readers are directed to other fine reviews (Henze et al., 2000; Urban et al., 2001; Lawrence et al., 2003; Bischofberger et al., 2006; Nicoll and Schmitz, 2005).
Keywords: hippocampus, local circuit inhibitory interneuron, long-term depression, glutamate receptors, plasticity, mossy fiber, mGluR7
Anatomy of the granule cell mossy fiber axon
Before discussing the physiological properties of the mossy fiber synapse, a short description of the granule cell anatomy and in particular the mossy fiber axon is warranted. The granule cell is the principal cell of the dentate gyrus and releases the neurotransmitter glutamate (Spruston and McBain, 2006). Granule cells typically possess small, ovoid cell bodies with a single apical, and conical dendritic tree, which extends into the molecular layer and terminates close to the hippocampal fissure. Compared to other principal cells of the hippocampal formation the granule cell is relatively small, possessing a somata approximately 10 μm in diameter and about 18 μm along its long axis. The number of dendritic branches is highly variable but the total dendritic length is significantly shorter than their CA1 and CA3 pyramidal neuron counterparts. The axon of the granule cell emerges close to the basal pole of the somata where it passes unmyelinated into the hilus and the CA3 hippocampus.
Based solely on their anatomy these axons are unique among hippocampal and cortical neurons (and indeed throughout the mammalian central nervous system) in that they form anatomically specialized synapses depending on the nature of their postsynaptic targets. Indeed the anatomy of the granule cell axons was first noted as peculiar by the anatomist Sala and then subsequently described in greater detail by Ramon y Cajal. In his landmark original work Textura del sistema del hombre y los vertebrados (1899 and 1904) Cajal remarked: “In well impregnated material from the 8-day old rabbit or the newborn Guinea-pig, one can easily see that these varicosities are triangular or stellate masses of cytoplasm with angles that give rise either to short, thick, diverging processes or to thin, rather long filaments ending in a varicosity. Although perhaps less striking this appearance is definitely reminiscent of certain fibers in the cerebellum that we referred to as mossy fibers”. Cajal's early appreciation of the anatomy of the granule cell axon is evident in his Figure 479 from his original text and reproduced here as Fig. 1A.
Fig. 1.
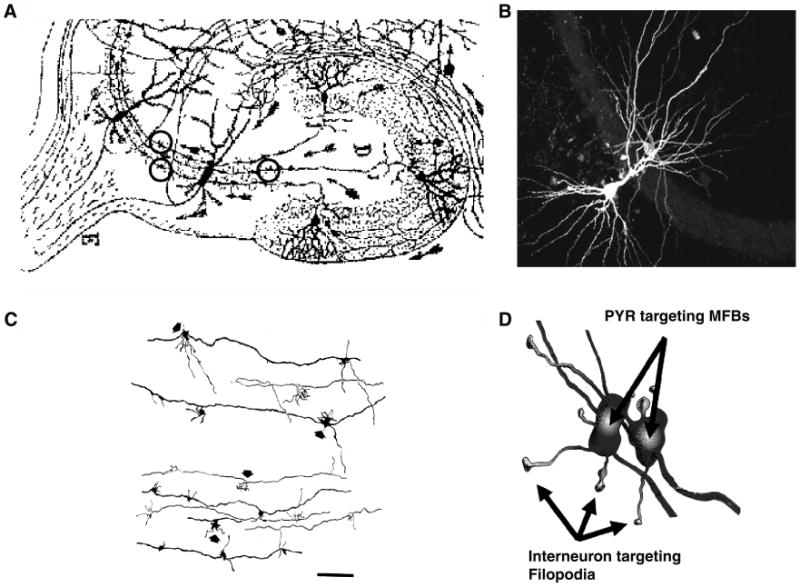
Anatomy of the mossy fiber–CA3 synapse. (A) Enlargement of Ramon y Cajal's classic drawing of the hippocampal formation to illustrate the mossy fiber pathway as visualized by his Golgi impregnation technique. Three individual mossy fiber boutons are circled to illustrate that even at this level of resolution Cajal could appreciate the anatomical structure of the mossy fiber bouton as being something more than a typical swelling or en passant synapse. (B) Triple fluorescence image shows the juxtaposition of a CA3 pyramidal cell, a stratum lucidum interneuron, and the MF pathway (stained for calbindin immunoreactivity). (C) Camera lucida reconstruction of a Golgi-impregnated mossy fiber axon illustrating individual boutons and their associated filopodial extensions (indicated by arrows) C. A cartoon rendering of reconstructed mossy fiber boutons, which innervate pyramidal cells and their filopdial extensions which typically innervate the smooth dendrites of local circuit inhibitory interneurons. Figures have been modified and reproduced with permission from: Panel A, Ramon y Cajal (1904, 1995); Panel C, Amaral and Dent (1981); Panel D, Acsády et al. (1998); Panel B, from K Pelkey and C.J. McBain, unpublished observation.
Typically, each granule cell gives rise to a single unmyelinated axonal fiber approximately 0.2 μm in diameter, with a total length (including collaterals) of more than 3 mm (Claiborne et al., 1986; Frotscher et al., 1991; Acsády et al., 1998). These axons form a number of local collaterals, which innervate the numerous cell types present within the hilar subfield. After leaving the hilar region, the mossy fiber axon contains few, if any further branch points and projects to the apical and in some cases basal dendrites of CA3 pyramidal neurons (Claiborne et al., 1986). The mossy-fiber projection to CA3 forms a tight bundle running parallel to the CA3 pyramidal cell body layer in a region corresponding to approximately the first 100 μm of the CA3 pyramidal neuron apical dendrite, the stratum lucidum, so-called because of its translucent appearance under the light microscope (Fig. 1B).
Three basic types of mossy fiber presynaptic terminal exist along the entire length of the main axon: large mossy terminals (4–10 μm in diameter), filopodial extensions (0.5–2.0 μm) that project from the large mossy boutons, and small en passant varicosities (0.5–2.0 μm) (Amaral, 1979; Claiborne et al., 1986; Frotscher et al., 1991; Acsády et al., 1998) (Fig. 1C, D). Both of these latter, small terminals are somewhat larger than the typical varicosities found in axons of CA3 pyramidal neurons (Acsády et al., 1998). The total number of large mossy terminals along a single axon is about 10 in the hilar region and about 12 (range 10–18) in the CA3 region, with the terminals being distributed somewhat evenly spaced (approximately every 150 μm) along the mossy fiber axon (Acsády et al., 1998). The large mossy fiber terminals, which contain up to 35 individual release sites envelop the postsynaptic thorny excrescences of CA3 pyramidal neuron dendrites. In contrast the mossy cells constitute the principal target of the large mossy terminals in the hilus. Although large mossy fiber boutons do not typically innervate GABAergic interneurons, they have on occasion been observed to directly contact the dendrites of stratum lucidum basket cell interneurons in Guinea pig (Frotscher, 1985). More typical however, the small filopodial extensions, and en passant terminals emerging from the parent axon, predominantly innervate only inhibitory interneuron targets in the stratum lucidum (Acsády et al., 1998). These terminals form single, often perforated, asymmetric synapses on the cell bodies, dendrites, and spines of GABAergic inhibitory interneurons.
In 1998, Lazlo Acsády, then in Gyorgyi Buzsaki's Lab, made the important observation that the total number of filopodial extensions and small, en passant synapses outnumbered the large mossy fiber terminals by approximately 10-fold (Acsády et al., 1998). Specifically, a single granule cell typically gives rise to 7–12 large mossy boutons in the hilus and 11–18 boutons in the CA3 region. In contrast, the number of small terminals (en passant and filopodial) was considerably higher both in the hilus (120–150) and the CA3 region (40–50). This circuit arrangement is such that each large mossy fiber bouton in the CA3 subfield gives rise to approximately 2.5 small bulbous ending filopodial extensions, indicating that an average granule cell mossy fiber axon in the CA3 subfield has 25–35 filopodial extensions associated with ∼10 large boutons (Fig. 1D). Since the larger mossy fiber boutons primarily target CA3 pyramidal cells and filopodial extensions target the smooth dendrites of local circuit inhibitory interneurons this would suggest that, if based solely on the total number of actual synapses, the primary targets of dentate gyrus granule cells are inhibitory interneurons (Acsády et al., 1998). This anatomical segregation of terminal types is unprecedented throughout the mammalian central nervous systems and suggests that it may serve to provide a functional specialization of synaptic output.
Basic properties of mossy fiber-inhibitory interneuron transmission
Without prior electrophysiological knowledge, based on these anatomical properties alone, it is possible to speculate that the physiological properties of each synapse type within this synaptic arrangement would differ, since the large bouton alone has 20–35 release sites compared to the single release site of each filopodial extension. In fact, electrophysiological experiments have largely confirmed the hypothesis that the large and small synaptic specializations of the mossy fibers are functionally distinct. Individual mossy fiber release sites onto principal cells have a low initial release probability (Pr = 0.01–0.05) (Jonas et al., 1993). However, because of the large number of release sites per bouton this endows these presynaptic terminals with a high degree of short-term- and frequency-dependent facilitation of transmission (Salin et al., 1996; Toth et al., 2000; Lawrence et al., 2004). In contrast, mossy fiber synapses onto interneurons in stratum lucidum possess a greater than one order of magnitude higher initial release probability (0.1–0.5) (Lawrence et al., 2004). This relatively high release probability endows these filopodial synapses with either mild facilitation or depression in response to brief stimulus trains (Toth et al., 2000).
Consideration of these different initial release probabilities at the mossy fiber bouton and filopodia synapse highlights an important feature of this synapse, namely that dentate gyrus granule cells firing at different frequencies will differentially impact the CA3 network function. The higher probability of release and higher potency of each release site at the filopodial synapse onto inhibitory interneurons (Lawrence et al., 2004), coupled to their interconnectivity, will tend to provide a robust feedforward inhibitory drive to all downstream targets at low (<0.5 Hz) in vivo discharge rates of dentate granule cells (Jung and McNaughton, 1993). This would suggest that at low frequencies at least, the output of the mossy fiber pathway largely drives a net inhibition onto the CA3 network. This is a hypothesis that has been well appreciated from in vivo recordings where synchronous activation of dentate granule cells in vivo during dentate spikes results in a net inhibition of CA3 pyramidal cell firing (Bragin et al., 1995a, b; Penttonen et al., 1997). However, the relative release dynamics change at higher dentate granule-cell firing frequencies (Salin et al., 1996; Toth et al., 2000; Henze et al., 2002). Data from in vivo recordings demonstrate that when the animal moves into the dentate granule cell's place field, cells fire in short, high-frequency bursts during the in-field discharge (Henze et al., 2002). This high frequency granule cell discharge transiently alters the network dynamics of mossy fiber transmission such that pyramidal cells are now robustly excited due to the massive facilitation of mossy fiber transmission at events late in the epoch. In contrast, since the mossy fiber–interneuron synapse can only weakly facilitate or often depresses in response to such high-frequency afferent drive, this results in an erosion of mossy fiber drive of inhibitory interneuron targets. The net effect is to provide an expansion of the CA3 recurrent excitability and the triggering of action potential discharge within the CA3 pyramidal cell population (Henze et al., 2002; Lawrence et al., 2003; Mori et al., 2004). Thus, the dynamics of excitation and inhibition within the CA3 network will shift depending on the firing frequency of the dentate granule cells.
Two types of AMPA/NMDAR populate mossy fiber–interneuron synapses
One important aspect of mossy fiber synapses onto stratum lucidum interneurons is the nature of the AMPA and NMDA receptors that populated the postsynaptic sites. We have demonstrated that in rat hippocampus a continuum of AMPA receptor types exist at these synapses (Toth et al., 2000; Lei et al., 2002) (Fig. 2). Of particular interest, at one end of the continuum there are MF–interneuron synapses that comprise GluR2-lacking, Ca2+-permeable (CP-) AMPA receptors. These CP AMPA receptors lack the GluR2 subunit, show inward rectifying current–voltage relationships and are blocked by exogenous polyamine containing toxins (Fig. 3). These CP-AMPARs typically occur at synapses that also possess NMDA receptors containing the NR2B subunit (Fig. 2). At the other end of the continuum, GluR2-lacking, Ca2+-impermeable (CI-) AMPARs are typically found associated with NR2B lacking NMDARs (Lei et al., 2002) (Fig. 2). These CI-AMPARs possess linear current voltage relationships and are largely resistant to exogenous polyamine toxins (Fig. 3). The ratios of these two synapse types show some developmental regulation, with CP-AMPA receptor containing synapses modestly predominating (62% of measured synapses) early in development. However, it is important to appreciate that there is not a complete conversion of one synapse type to another on developmental maturation, at least at time points up to postnatal day 40 (Lei et al., 2002). At postnatal day 40, approximately 45% of mossy fiber–interneuron synapses are comprised of CI-AMPARs, whereas only 37% are comprised CP-AMPARs. The remaining ∼20% of synapses are a mixed population that possess intermediate rectification ratios, AMPARs with low sensitivity to polyamine block and NMDARs with intermediate sensitivity to the NR2B preferring antagonist ifenprodil (Figs. 2 and 3). At this time it is unclear what rules dictate the expression of each type of AMPA and NMDA receptor and how these synapse types map onto their presynaptic granule cell afferents. Although not as rigorously determined, the ratio of mossy fiber–CP-AMPAR: CI-AMPAR synapses in the mouse is significantly higher across all ages tested than that observed in the rat.
Fig. 2.
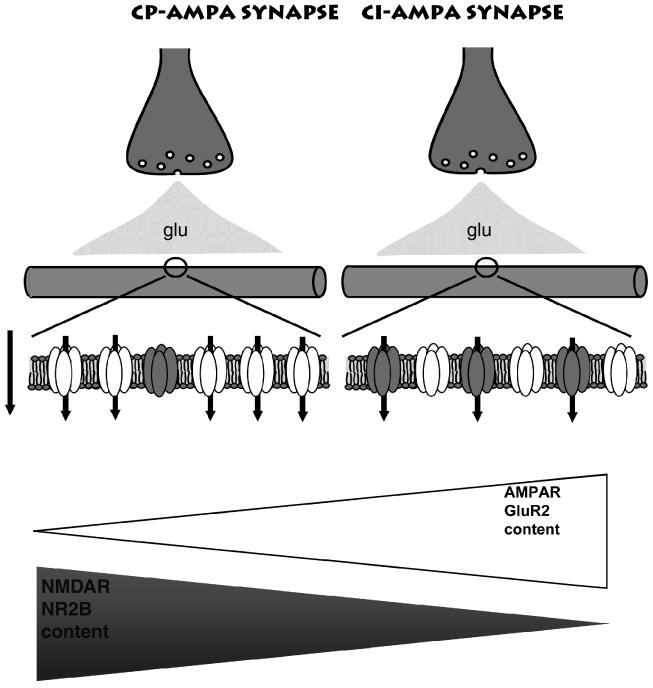
A continuum of glutamate receptors exist at mossy fiber–interneuron synapses. A schematic illustrating the typical synapses that make up the population of mossy fiber–interneuron connections. Typically, GluR2-lacking Ca2+-permeable (CP) AMPA receptors are found at synapses also populated with NR2B-containing NMDA receptors. At the other end of the continuum is a population of synapses that contain GluR2-containign, CP-AMPA receptors together with NR2B-lacking NMDA receptors. Figure modified and reproduced with permission from Bischofberger and Jonas (2002).
Fig. 3.
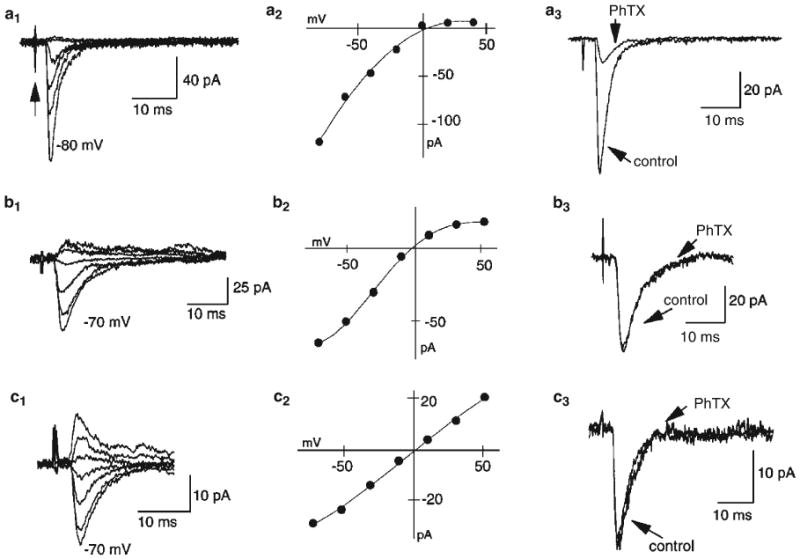
Rectification properties and PhTx sensitivity of EPSCs on stratum lucidum interneurons. Examples of mossy-fiber-evoked EPSCs onto three stratum lucidum interneurons (left panels). Low-intensity stimulation of mossy fibers evokes EPSCs that show a continuum of I–V relationships (center panels) from either strongly rectifying to essentially linear. Five EPSCs were evoked at each potential (20-mV increments) and averaged. The stimulus artifact is indicated by the arrow. (a, b) Two cells where EPSCs demonstrated strong and moderate inward rectification (center), respectively. The rectification indices of these two cells were 0.01 (a) and 0.12 (b). Of the two sets of evoked EPSCs, only the one with strong inward rectification (a) was blocked by the polyamine toxin PhTx-433 (10 mM, right panels). (c) In contrast EPSCs with linear current–voltage relationships (rectification index of 0.88) are insensitive to PhTx (right). Figure reproduced from Toth and McBain (1998).
Some differences in the basic features of transmission at CP-AMPAR and CI-AMPAR synapses exist (Toth et al., 2000; Walker et al., 2002). One of two important features worthy of further consideration is the presence of an entirely postsynaptic mechanism of short-term plasticity at CP-AMPARs, which results from the use-dependent unblock of intracellular polyamines from the intracellular pore of the channel (Rozov et al., 1998; Rozov and Burnashev, 1999; Toth et al., 2000). Traditionally most mechanisms of short-term synaptic plasticity are considered to be presynaptic in origin and typically arise from changes in the release probability due to accumulation of residual calcium loads, or exhaustion of the readily releasable pool of neurotransmitter. However, CP-AMPA receptors lacking the GluR2 subunit posses an unexpected and entirely postsynaptic form of short-term plasticity due to their susceptibility to block by intracellular polymines (Rozov et al., 1998; Rozov and Burnashev, 1999).
Within the pore of AMPA receptors a single amino acid, the so-called Q/R site not only dictates their Ca permeability but also influences their sensitivity to block by both endogenous intracellular polyamines such as spermine and spermidine as well as externally applied polyamine toxins such as philanthotoxin (Bowie and Mayer, 1995; Kamboj et al., 1995; Koh et al., 1995; Isaac et al., in press). The interaction with intracellular polyamines is in part voltage-dependent, with block being greatest at depolarized potentials. It is this polyamine block that is responsible for the highly rectifying current voltage relationship of GluR2-lacking AMPA receptors. The affinity of polyamines for their intracellular binding site within the AMPA receptor is such that it provides a tonic block of native Ca-permeable AMPA receptors. Relief of this block is use- and voltage-dependent (Bowie and Mayer, 1995) and typically requires multiple receptor activations (i.e. multiple synaptic events) to force the polyamine from the inner vestibule of the channel. This use-dependence results in an apparent increased current flow due to the progressive unblock of the pore and imparts a novel mechanism of short-term facilitation to CP-AMPA receptors. Because of the voltage-dependence of the polyamine block, facilitation is greatest when the cell is depolarized and is almost completely absent at resting or hyperpolarized membrane potentials. Such a mechanism is observed at mossy fiber-CP AMPARs interneuron synapses (Toth et al., 2000). Indeed short trains (five events) of synaptically evoked currents were observed to induced facilitation when measured at −20 mV but not at resting potential. This facilitation is absent when polyamines are omitted from the intracellular recording solution or when endogenous polyamines are chelated with ATP.
A second important feature that differentiates activity through CP- versus CI-AMPAR synapses is the magnitude of temporal summation observed at either synapse when measured under current clamp conditions (Lei et al., 2002). At CI-AMPAR containing synapses the presence of NR2B-lacking NMDARs endows synapses with a distinct temporal feature when compared with CP-AMPAR containing synapses (which contain NR2B-containing NMDARs). The presence of the larger amplitude NR2B-lacking NMDA receptor-mediated excitatory postsynaptic potentials (EPSPs) at CI-AMPAR synapses endows these synapses with a broad depolarizing envelope and significant temporal summation during repetitive stimulation when measured at resting potentials (Lei et al., 2002). This long-lasting depolarization results in repeated action potential firing and a reduced action potential precision. In contrast, temporal summation at CP-AMPAR/NR2B-containing NMDARs is modest. Synaptic events triggered by NR2B-containing NMDARs possess a slower time course, but their small amplitude renders them unable to significantly influence transmission at these synapses at resting membrane potentials (Bischofberger et al., 2002; Lei et al., 2002). Consequently, repetitive activation of CP-AMPAR synapses results in rapid and brief synaptic potentials (Lei et al., 2002; Walker et al., 2002) that trigger single action potentials at their peak with little jitter. Consistent with a modest role for NMDA receptors at this synapse type, addition of an NMDA receptor antagonist has little impact on the temporal envelope underlying repetitive activation and the temporal precision of action potential initiation is largely unchanged (Lei et al., 2002). This scenario suggests that CP-AMPAR containing synapses are designed for precision timing and rapid synaptic transmission (Lawrence and McBain, 2003; Jonas et al., 2004) whereas robust depolarization and multiple action potentials with no requirement for precision timing is a feature of mossy fiber–CI-AMPAR interneuron synapses.
Mossy fiber-inhibitory interneuron plasticity
In the 1990s, Maccaferri and McBain published several papers that suggested that excitatory synapses onto inhibitory interneurons lacked the NMDAR-dependent forms of long-term potentiation (LTP) that were observed at excitatory synapses onto principal cells (Maccaferri and McBain, 1995, 1996; for reviews, see McBain and Maccaferri, 1997; McBain et al., 1999). Our failure to observe the most widely studied form of postsynaptic expressed LTP at synapses onto interneurons drove us to consider whether presynaptically expressed forms of long-lasting plasticity were similarly absent at excitatory synapses onto inhibitory interneurons. To this end, we turned our attention to the mossy fiber synapse where a well-established form of presynaptically expressed NMDAR-independent form of LTP had been described (Nicoll and Schmitz, 2005) (Figs. 4 and 5). Our hypothesis was that if this form of LTP was expressed as a change in presynaptic transmitter release probability, then these changes should distribute to all presynaptic terminals, regardless of the postsynaptic target type. It was also at this time that we became aware of the mossy fiber bouton versus filopodial arrangements described by Acsády et al. (1998) (Fig. 1); indeed this circuit arrangement fueled the experiments that were to follow. Given that the filopodial extensions originate from the large MF terminal, it might be assumed that any change in presynaptic release probability arising from LTP in the large MF bouton would distribute evenly to all synapses, resulting in a potentiation of transmission at both MF-pyramidal cell and interneuron synapses.
Fig. 4.
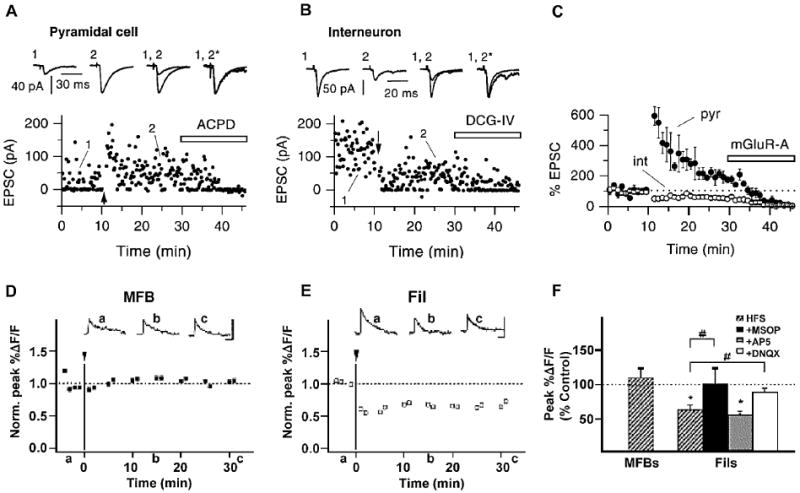
Target-cell specific presynaptic LTD in the hippocampal MF pathway. A–C Representative single recording examples (A, B) and group data (C) illustrating divergent forms of NMDA receptor independent presynaptic plasticity at MF-PYR and MF-SLIN synapses following HFS (arrows). MF-PYR synapses undergo LTP while mossy fiber filopodial–interneuron synapses undergo LTD. Traces above are averaged EPSCs obtained at the times indicated, and are also shown scaled to each other (*). Application of an mGluR agonist (ACPD or DCG-IV) at the end of each recording confirms the MF origin of evoked synaptic events. Arrows in each panel reflect the time point for delivery of the induction protocol. D–F: Presynaptic two-photon Ca2+ imaging reveals compartmentalized VGCC regulation at MFB and Fil release sites. HFS (arrow and line) does not alter MFB CaTs that are undergoing LTP (D, F) but produces LTD of filopodial CaTs (E, F). The HFS-induced LTD of filopodial–interneuron synaptic CaTs is prevented by antagonizing presynaptic mGluR7 with MSOP and also by blocking postsynaptic CP-AMPARs with DNQX but is not affected by NMDAR inhibition with AP5 (F). Figures have been modified and reproduced Panels A–C from Maccaferri et al. (1998), Panels D–F from Pelkey et al. (2006).
Fig. 5.
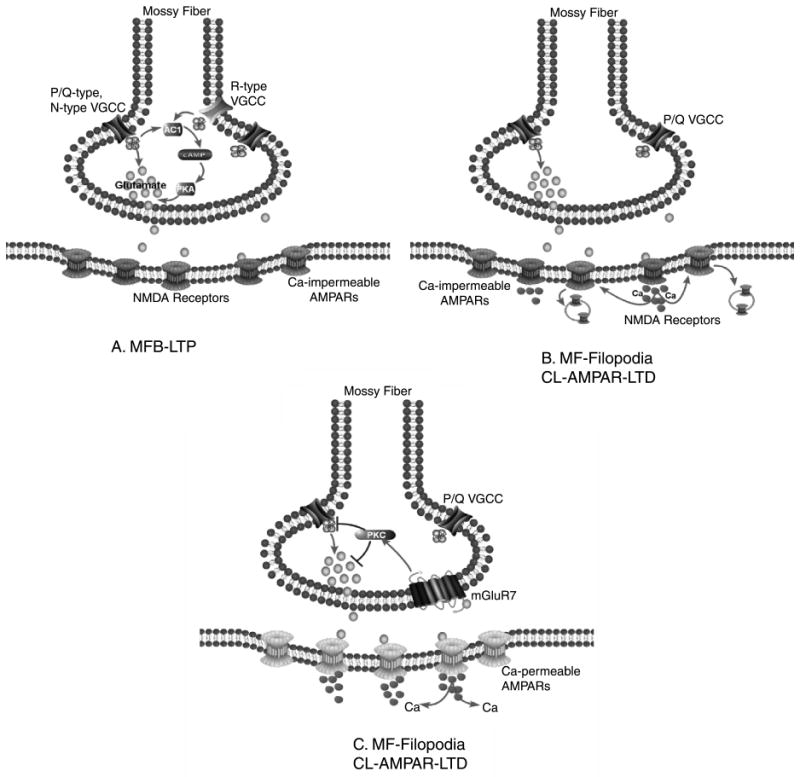
Target-specific plasticity at mossy fiber synapses. Three schematics to illustrate the prevailing mechanisms underlying the three forms of plasticity at mossy fiber synapses onto pyramidal cells (Panel A) and inhibitory interneurons (Panels B and C). (A) A high-frequency non-associative induction paradigm produces LTP at mossy fiber bouton-pyramidal cell synapses that is NMDAR-independent and has a presynaptic locus of expression. Expression is thought to involve a Ca2+-dependent increase in neurotransmitter release via a mechanism involving adenylyl cyclase 1 (AC1), cAMP, and protein kinase A (PKA) modulation of the release machinery. B and C: At filopodial synapses onto mossy fiber-CA3 interneuron a non-associative induction paradigm induces two forms of long-term depression. (B) Long-term depression at mossy fiber–interneuron synapses comprised of Ca2+-impermeable (CI-) AMPA receptor synapses has a postsynaptic locus of induction and expression, is NMDA receptor dependent, and involves an endocytosis of surface AMPA receptors reminiscent of NMDAR-dependent LTD observed at principal cell synapses. (C) Long-term depression at mossy fiber–interneuron synapses comprised of CP-AMPA receptors has a requirement for a postsynaptic increase in intracellular Ca2+ but is expressed presynaptically. This form of LTD is NMDA receptor independent, requires activation of presynaptic mGluR7 and downstream PKC-dependent cascades to reduce transmitter release probability via a reduction of the voltage-gated Ca2+ transient generated by P/Q Ca2+ channels.
MF-principal cell LTP arises via an adenylyl cyclase-cAMP-dependent mechanism involving PKA phosphorylation of Rab3/Rim1a to ultimately strengthen synaptic transmitter release (Castillo et al., 1997, 2002; Nicoll and Schmitz, 2005) (Fig. 5). Although there are arguments for and against a requirement for a postsynaptic induction locus (Yeckel et al., 1999; Nicoll and Schmitz, 2005) almost all camps agree that expression of mossy fiber-pyramidal cell LTP proceeds as an increase in initial release probability of transmitter glutamate release. In marked contrast, the same high-frequency induction protocol that causes LTP at principal cell synapses induces two distinct forms of long-term depression (LTD) at mossy fiber–interneuron synapses (Figs. 4 and 5) (Maccaferri et al., 1998; Lei and McBain, 2004; Pelkey et al., 2005). One can immediately appreciate that this differential, strengthening of synapses onto CA3 pyramidal cells combined with a simultaneous weakening of transmission onto stratum lucidum interneurons will have important consequences for the dynamics of the CA3 circuit.
When these results were originally published (Maccaferri and McBain, 1998) we were unaware that two distinct mossy fiber–interneuron synapse types existed; i.e. synapses formed with either CI-AMPARs or CP-AMPARs (Figs. 2 and 3). However once we had characterized more fully the presence of CI- versus CP-AMPARs at mossy fiber–interneuron synapses, Toth et al. (2000) and Lei et al. (2002) revisited these original experiments to establish whether common mechanisms underlay LTD at either synapse type. Indeed high-frequency stimulation of mossy fiber afferents induced LTD at both mossy fiber–CP-AMPAR and CI-AMPAR synapse types. Although the induction locus was postsynaptic in both cases, and blocked by inclusion of BAPTA in the recording pipette, the locus of LTD expression was presynaptic at CP-AMPAR but postsynaptic at CI-AMPARs (Figs. 4 and 5) (Lei and McBain, 2004). Importantly the mechanisms underlying either form of LTD were also distinct. LTD at CI-AMPARs is NMDAR dependent and blocked by the antagonist, AP5, and by inclusion of intracellular BAPTA indicating a role for Ca2+-elevation via NMDA receptor activation. Moreover, parameters consistent with changes in presynaptic transmitter release, paired pulse ratio, coefficient of variance (CV), failure rate, and the magnitude of block by the low-affinity glutamate receptor antagonist γ-DGG were all unchanged after LTD of CI-AMPA receptor-mediated transmission. In addition, this form of LTD relies on an AP-2 dependent internalization of AMPAR by a mechanism similar to that seen at other synapses expressing NMDAR-dependent LTD (Lei et al., 2004).
At mossy fiber CP-AMPAR-interneuron synapses, LTD is NMDAR-independent. Moreover, this form of depression is accompanied by changes in the paired pulse ratio, failure rate, and the CV, all indices consistent with changes in presynaptic transmitter release probability. However, none of these parameters by themselves definitively prove a presynaptic locus. To explore the presynaptic locus of LTD at CP-AMPAR synapses in more detail, Lei and McBain (2004) took advantage of the low-affinity glutamate receptor antagonist γ-DGG. This compound has been used at a number of synapses to probe for changes in the transmitter release profile under a variety of conditions (Liu et al., 1999; Wadiche and Jahr, 2001). At CP-AMPAR synapses, the magnitude of γ-DGG block of the evoked EPSC was increased following LTD expression, indicative of a lower concentration of transmitter release following depression. These data strongly support the hypothesis that LTD at CP-AMPARs proceeds as a change in glutamate release probability either by alterations in multivesicular release or for example by converting full fusion vesicular release events into partial fusion, thus reducing the cleft glutamate concentration. Independent support of this presynaptic locus of expression came from experiments using postsynaptic delivery of peptides (pep2m) designed to interfere with AMPA receptor internalization. In contrast to CI-AMPARs, infusion of pep2m into the postsynaptic compartment had no effect of either basal synaptic transmission or the ability to induce LTD at CP-AMPAR synapses (Lei and McBain, 2004).
Taken together these data highlight two important aspects of mossy fiber transmission onto inhibitory interneurons. First, mossy fiber–interneuron synapses are constructed from two distinct types of postsynaptic glutamate receptor, GluR2-lacking CP-AMPA receptors and GluR2-containing, CI AMPA receptors. Importantly, distinct forms of long-lasting plasticity are generated at these synapses, that appear to be largely dictated by the nature of the AMPA receptor present on the postsynaptic side. Interestingly, in synapses that contain a mixture of either CP-AMPARs and CI-AMPARs, i.e. cells with only mild sensitivity to philanthotoxin or are weakly rectifying, it appears that LTD defaults to the presynaptic mechanism (Lei and McBain, 2004).
Before discussing this presynaptic form of LTD in greater detail, it is worthwhile pausing to emphasize that these data suggest that a common non-associative induction paradigm triggers a presynaptic form of strengthening at mossy fiber bouton synapses onto pyramidal cells yet simultaneously triggers a long-lasting depression at filopodial synapses onto inhibitory interneurons. This observation strengthens the hypothesis that the anatomical arrangement of filopodia emerging from the parent mossy fiber bouton may serve to function as a solution for a compartmentalization of a biochemical mechanism (for further discussion, see Pelkey and McBain, 2007). Importantly, these data suggest that each synapse type can independently regulate activity-induced changes in synaptic strength using distinct signaling cascades. Evidence in support of this hypothesis comes from our original observation that the adenylyl cyclase activator, forskolin, which has been used repeatedly as a exogenous means to trigger LTP at mossy fiber-CA3 synapses, has no impact on synaptic strength at the filopodial–interneuron synapse under naïve conditions (Maccaferri et al., 1998). This indicates that some aspect of the cAMP-dependent cascade present in the mossy fiber bouton may be absent from the filopodial extension or that the target(s) of the adenylyl cyclase-activated cascade may be inactive or sequestered.
mGluR7 functions as a trigger for bidirectional plasticity at the mossy fiber–interneuron synapses
The above discussion highlighted that the filopodial extensions and the parent mossy fiber bouton function independently with regard to their respective transmitter release probabilities, short-term mechanism of plasticity, and their differential sensitivity to high-frequency stimulation. This might suggest that the mechanism underlying transmitter release at each terminal is controlled by different synaptic machinery. At the large mossy fiber bouton, transmitter exocytosis is controlled by the high threshold P/Q (Cav2.1) and N-type (Cav2.2) voltage-gated calcium channels (VGCCs), with some evidence for a minor role for R-type VGCCs (Bischofberger et al., 2006; Nicoll and Schmitz, 2006). We considered it possible that the filopodial extensions may utilize a different compliment of VGCCs to trigger transmitter release. However, both evoked mossy fiber–interneuron synaptic events as well as presynaptic Ca2+ transients were similarly sensitive to the P/Q and N-type Ca2+ channel blockers ω-agatoxin IVa and ω-conotoxin GVIa. These data suggest that at the level of transmitter release, the filopodial extensions and the large parent mossy fiber bouton utilize the same VGCC types (Pelkey et al., 2006).
One important clue regarding the control of transmitter release/plasticity at the filopodial extensions versus the mossy fiber bouton came from the study of Shigemoto et al. (1997). In an elegant study of the distribution of metabotropic glutamate receptors throughout the hippocampal formation, they demonstrated that the Group III mGluR7b isoform was expressed exclusively within the mossy fiber pathway. Surprisingly, mGluR7b was largely restricted to the small filopodial and en passant terminals made onto smooth dendrites of inhibitory interneurons and was largely absent at the larger mossy fiber boutons. Given that many mGluRs function as auto- or heterosynaptic receptors to regulate presynaptic release probability we set out to determine whether mGluR7 performed a similar function at the filopodial synapse onto interneurons (Pelkey et al., 2005). Surprisingly, brief application of the Group III agonist, L-AP4, at a concentration capable of activating mGluR7, induced a long-lasting, chemical depression of excitatory transmission at mossy fiber–CP-AMPAR synapses. This chemical LTD is virtually identical to that induced by high-frequency stimulation at the same synapse; L-AP4 caused an increase in the paired pulse ratio, increased CV, and failure rate suggesting a presynaptic locus of expression for this chemical LTD. To our surprise, application of L-AP4 in the absence of synaptic transmission fails to trigger the chemical LTD and instead only a reversible form of short-term depression is observed. Like high-frequency induced LTD, inclusion of the calcium chelator BAPTA in the intracellular solution prevented chemical LTD induction. This suggests that similar to high-frequency stimulation-induced LTD, postsynaptic activity is also required for induction of the chemical-induced form of LTD. How postsynaptic activation/induction triggers presynaptic LTD expression either driven by L-AP4 or HFS is presently unclear.
LTD induced by either exogenous application of L-AP4, or high-frequency stimulation, results in a persistent depression of the presynaptic voltage-gated calcium channel signal monitored by two-photon microscopy (Fig. 4D–F). This persistent depression of the presynaptic Ca2+ transient arises through a preferential depression of P/Q voltage-gated Ca2+ channels and is blocked by an antagonist of mGluR7 (MSOP) (Pelkey et al., 2006). Importantly, blockade of P/Q channels prevents both the depression of synaptic events and calcium transients by either L-AP4 or high-frequency stimulation indicating that the remaining functional N-type channels play no (or little) role in this form of LTD. A similar application of L-AP4 at the mossy fiber bouton synapses made onto CA3 pyramidal cells failed to result in a persistent depression of the presynaptic Ca2+ transient, consistent with an absence of mGluR7 at these terminals. Moreover, although there likely exist other Group III mGluRs at mossy fiber-pyramidal cell synapses (Shigemoto et al., 1997), their activation triggers only a short-term and reversible depression, suggesting that Group III mediated LTD is not a common phenomenon and so far is limited only to the mossy fiber filopodia– interneuron synapse. In addition, although the Group II mGluR agonist DCG-IV is known to trigger a chemical form of LTD at the mossy fiber-CA3 pyramidal cell synapse, this form of LTD is not accompanied by a persistent depression of the presynaptic Ca2+ transient (Pelkey et al., 2006). These data indicate that synaptic depression at mossy fiber–CP-AMPAR interneuron synapses arises by an mGluR7-dependent persistent reduction in P/Q calcium channel function.
Of particular interest, mGluR7 not only plays a role as a conventional autoreceptor at these synapses but also acts as a metaplastic switch at MF-SLIN synapses. An “occlusion” experiment designed to prove that chemical- and high-frequency-induced LTD share one and the same mechanism, instead revealed that following L-AP4 application, the same HFS induction protocol that resulted in a depression at naïve synapses, now strengthened or de-depressed synaptic transmission (Pelkey et al., 2006) (Fig. 6). Interestingly, HFS induction restored the synaptic strength close to the original “setting” observed during the control epoch. This series of experiments allowed us to determine that mGluR7 activation and cell surface expression governs the direction of plasticity at mossy fiber–interneuron synapses. In naïve slices, mGluR7 activation during high-frequency stimulation generates MF-SLIN LTD by depressing presynaptic transmitter release through a PKC-dependent mechanism that involves the persistent downregulation of the Ca2+ transients arising via P/Q VGCC activation (Fig. 5). However, like many G-protein coupled receptors following agonist exposure, mGluR7 undergoes receptor internalization. Two avenues of investigation allowed us to reach this conclusion. First, using overexpression of a myc-tagged mGluR7 in cultured hippocampal neurons, we demonstrated that the same duration application of L-AP4 resulted in a robust internalization of both isoforms of mGluR7a and b (Pelkey et al., 2005). This observation was then extended to immunoelectron microscopy of mossy fiber filopodial terminals. At the level of the electron microscope, application of L-AP4 was observed to result in both a loss of mGluR7b from mossy fiber filopodia presynaptic grid and its accumulation deep within the presynaptic terminal. This internalization unmasks the ability of MF-SLIN synapses to undergo presynaptic potentiation or de-depression in response to the same HFS that induced LTD in naïve slices (Fig. 6). Thus, the selective mGluR7 accumulation at MF terminals contacting SLINs and not PYRs provides cell-target specific plasticity and bi-directional control of feedforward inhibition (Pelkey et al., 2005, 2006).
Fig. 6.
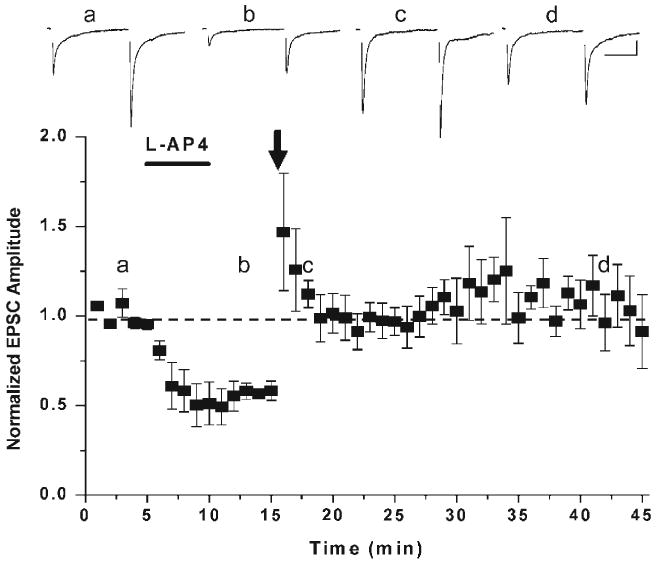
Activation of mGluR7 unmasks presynaptic MF-interneuron LTP. Application of the Group III mGluR agonist, L-AP4, triggers a chemical form of presynaptic mGluR7-dependent long-lasting depression. Subsequent delivery of a non-associative high-frequency stimulation (indicated by arrow) which in naïve tissue would induce LTD, now induces an LTP of MF–interneuron transmission. Synaptic events across the top of the dot plot are averaged paired pulse data taken from the time points indicated by the letters. Figure has been reproduced from Pelkey et al. (2005).
Unfortunately, at this time the underlying mechanism(s) for this novel form of mossy fiber– interneuron LTP is unknown. However, it is possible that it may simply proceed as the molecular inverse of the mGluR7-mediated LTD or alternatively may arise via an entirely distinct sequence of events. Importantly, it is currently also unclear whether the emergence of the de-depression or potentiation of mossy fiber–interneuron transmission provides the signal necessary to allow reinsertion of mGluR7 back onto the presynaptic terminal. An important issue that remains is, what is the duration of the temporal window controlled by the surface expression and internalization of mGluR in vivo? These are important questions that future research will likely uncover the answers. However, it is highly likely that the presence or absence of mGluR7 on the presynaptic mossy fiber–interneuron terminal represents a key trigger for the bidirectional control of this important feedforward inhibitory pathway.
Implications for the mossy fiber-CA3 circuit
In most systems studied so far, it has been difficult to gauge how long-lasting plasticity at one synapse could influence activity in either the feedforward or feedback inhibitory circuit. However, our data reveal that a common induction paradigm strengthens transmission at the mossy fiber inputs onto CA3 pyramidal cells, while simultaneously weakening transmission onto stratum lucidum interneurons. These mossy fiber–interneuron synapses represent the primary feedforward inhibitory drive onto the CA3 pyramidal cells. Consideration of the number of excitatory inputs onto stratum lucidum interneurons, their high initial release probability of synaptic transmission, as well as the large diverging output of these cells onto their CA3 pyramidal cell targets, suggests that plasticity within this circuit could be critically important for the regulation of network excitability. Specifically, a mechanism that simultaneously strengthens excitatory drive of the mossy fiber-CA3 pyramidal cell transmission and weakens the mossy fiber driven di-synaptic feedforward inhibitory input onto the same cells may act to broaden the temporal window for mossy fiber-CA3 pyramidal cell integration.
Typically, in the naïve state, low-frequency mossy fiber synaptic transmission onto CA3 pyramidal cells occurs via a multitude of release sites, each with a low initial release probability. In contrast, mossy fiber inputs onto stratum lucidum interneurons occurs via presynaptic terminals that have a one order of magnitude higher release probability when driven at the same activation frequency. Under these conditions, point to point mossy fiber-pyramidal cell transmission will only sparsely activate the CA3 network. Importantly, this mossy fiber driven activation of CA3 pyramidal cell will arise only in a brief temporal window because the feedforward inhibitory drive to CA3 pyramidal cells will largely constrain the ability of principal cells to fire an action potential in response to its monosynaptic mossy fiber input (Pouille and Scanziani, 2001; Lawrence et al., 2004). Weakening mossy fiber transmission onto inhibitory interneurons will reduce their likelihood of firing an action potential in response to a single mossy fiber input (Toth et al., 2000; Lawrence and McBain, 2003; Lawrence et al., 2004), ultimately contributing to an erosion of the feedforward inhibitory input and the consequent opening of the temporal window for CA3 pyramidal cell action potential initiation. Couple this with the increased probability of transmitter release at potentiated mossy fiber-CA3 pyramidal cell synapses suggests that such a mechanism could exist to increase the net excitation within the CA3 auto-associative network.
Repeated high frequency activation of the mossy fiber pathway will however, likely exacerbate this bifurcation of synaptic drive in the CA3 network to further strengthen transmission onto its pyramidal cell while weakening transmission onto inhibitory interneuron targets. In the absence of a mechanism to re-set or re-establish the balance of synaptic transmission at both mossy fiber targets, the system would be rendered inherently unstable, ultimately resulting in a loss of inhibitory control and the potential for pathological levels of CA3 pyramidal cell network activation. Expression of the Group III mGluR7b at mossy fiber–interneuron synapses may be a mechanism that allows this divergence of signal processing to exist only in a narrow temporal window. Repetitive activation of the mossy fiber–interneuron synapse will trigger mGluR7 activation and its consequent internalization. This internalization is an essential feature that triggers re-strengthening of mossy fiber–interneuron synaptic transmission back to naïve conditions and may exist as a mechanism to reset the inhibitory synaptic tone in the CA3 network.
Clearly, many issues remain to be resolved. However, it is apparent that the mossy fiber-CA3 circuit has designed both an anatomical and physiological template that permits the differential control of basic synaptic transmission as well as endowing this unique synapse with opposing forms of plasticity that depends on the nature of the postsynaptic target. The presence of mGluR7b at the mossy fiber–interneuron filopodial synapse is a key protein in determining the strength and direction of the plasticity induced by a brief non-associative induction paradigm. Typically, metabotropic receptors are considered “autoreceptors” whose function is to decrease presynaptic release probability. At the mossy fiber–interneuron synapse mGluR7 can indeed play this role, however when synaptic activation persists during the epoch of mGluR7 activation this receptor can also trigger a long-lasting form of synaptic depression. mGluR7 is then able to undergo internalization on binding agonist and render the synapse competent for long-lasting potentiation in response to an identical non-associative induction paradigm. It remains to be formally tested as to whether this is a general property of all synapses bearing mGluR7 on their presynaptic terminals, or whether this is a mechanism peculiar only to the mossy fiber filopodial synapse.
Acknowledgments
This work was supported by am NICHD-NIH intramural award to Chris J. McBain.
Footnotes
References
- Acsády L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18(9):3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. Synaptic extensions from the mossy fibers of the fascia dentate. Anat Embryol. 1979;155:241–251. doi: 10.1007/BF00317638. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195(1):51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Frotscher M, Jonas P. Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal network. Eur J Physiol. 2006;453:361–372. doi: 10.1007/s00424-006-0093-2. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Jonas P. TwoB or not twoB: differential transmission at glutamatergic mossy fiber–interneuron synapses in the hippocampus. Trends Neurosci. 2002;25:600–603. doi: 10.1016/s0166-2236(02)02259-2. [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasday Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995a;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasday Z, van Landeghem M, Buzsaki G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J Neurophysiol. 1995b;73:1691–1705. doi: 10.1152/jn.1995.73.4.1691. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388(6642):590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415(6869):327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12(2):261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J Comp Neurol. 1992;325(2):169–182. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246(4):435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Frotscher M. Mossy fibres form synapses with identified pyramidal basket cells in the CA3 region of the guinea-pig hippocampus: a combined Golgi-electron microscope study. J Neurocytol. 1985;14:245–259. doi: 10.1007/BF01258450. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Seress L, Schwerdtfeger WK, Buhl E. The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J Comp Neurol. 1991;312:145–163. doi: 10.1002/cne.903120111. [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98(3):407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Isaac JTR, Ashby M, McBain CJ. The GluR2 AMPA receptor subunit: a critical determinant of AMPA receptor function and target for regulation in synaptic plasticity. Neuron in press. [Google Scholar]
- Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron diversity series: fast in, fast out — temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27(1):30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Jonas P, Major G, Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, McNaughton B. Spatial selectivity of unit activity in the hippocampal granule granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol. 1995;486(Pt 2):297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DS, Burnashev N, Jonas P. Block of native Ca2+-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol. 1995;486(Pt 2):305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, Grinspan ZM, McBain CJ. Quantal transmission at mossy fibre targets in the CA3 region of the hippocampus. J Physiol. 2004;554:175–193. doi: 10.1113/jphysiol.2003.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation — feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26(11):631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Distinct NMDA receptors provide differential modes of transmission at mossy fiber– interneuron synapses. Neuron. 2002;33(6):921–933. doi: 10.1016/s0896-6273(02)00608-6. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Two loci of expression for long-term depression at hippocampal mossy fiber–interneuron synapses. J Neurosci. 2004;24(9):2112–2121. doi: 10.1523/JNEUROSCI.4645-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron. 1995;15(1):137–145. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Long-term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. J Neurosci. 1996;16:5334–5343. doi: 10.1523/JNEUROSCI.16-17-05334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Toth K, McBain CJ. Target-specific expression of presynaptic mossy fiber plasticity. Science. 1998;279:1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Maccaferri G. Synaptic plasticity in hippocampal interneurons? A commentary. Can J Physiol Pharmacol. 1997;75:488–494. [PubMed] [Google Scholar]
- Mori M, Abegg MH, Gahwiler BH, Gerber U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature. 2004;431:453–456. doi: 10.1038/nature02854. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6(11):863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron. 2005;46(1):89–102. doi: 10.1016/j.neuron.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, McBain CJ. Differential regulation at functionally divergent release sites along a common axon. Curr Opin Neurobiol. 2007;17:366–373. doi: 10.1016/j.conb.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Topolnik L, Lacaille JC, McBain CJ. Compartmentalized Ca2+ channel regulation at divergent mossy fiber release sites underlies target-cell dependent plasticity. Neuron. 2006;52:497–510. doi: 10.1016/j.neuron.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Sik A, Acsady L, Buzsaki G. Feed-forward and feed-back activation of the dentate gyrus in vivo during dentate spikes and sharp wave bursts. Hippocampus. 1997;7:437–450. doi: 10.1002/(SICI)1098-1063(1997)7:4<437::AID-HIPO9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feedforward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. In: Histology of the Nervous System. Swanson N, Swanson L, translators. Vol. 2. Oxford University Press; New York: 1904 1995. [Google Scholar]
- Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature. 1999;401(6753):594–598. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- Rozov A, Zilberter Y, Wollmuth LP, Burnashev N. Facilitation of currents through rat Ca2+-permeable AMPA receptor channels by activity-dependent relief from polyamine block. J Physiol. 1998;511(Pt 2):361–377. doi: 10.1111/j.1469-7793.1998.361bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17(19):7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, McBain CJ. Structural and functional properties of hippocampal neurons. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford University Press; 2006. [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20(22):8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NN, Henze DA, Barrionuevo G. Revisiting the role of the mossy fiber synapse. Hippocampus. 2001;11:408–417. doi: 10.1002/hipo.1055. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Multivesicular release at climbing fiber–Purkinje cell synapses. Neuron. 2001;32:301–313. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- Walker HC, Lawrence JJ, McBain CJ. Activation of kinetically distinct synaptic conductances on inhibitory interneurons by electrotonically overlapping afferents. Neuron. 2002;35:161–171. doi: 10.1016/s0896-6273(02)00734-1. [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci. 1999;2(7):625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]


