Abstract
The α6 integrin is essential for early nervous system development in Xenopus laevis. We have previously reported a uPA cleaved form of integrin α6 (α6p), in invasive human prostate cancer tissue, whose presence correlates with increased migration and invasive capacity. We now report that α6 is cleaved during the normal development of Xenopus in a spatially and temporally controlled manner. In addition, unlike normal mammalian tissues, which lack α6p, the major form of the α6 integrin present in adult Xenopus is α6p. The protease responsible for the cleavage in mammals, uPA, is not involved in the cleavage of Xenopus α6. Finally, overexpression of a mammalian α6 mutant which cannot be cleaved leads to developmental abnormalities suggesting a potential role for the cleavage in development.
Keywords: Integrin, Xenopus laevis, Development
Our previous work has shown that a structural variant of the human α6 integrin called α6p exists in a variety of human epithelial cell lines and in human cancer tissues [1,2]. This variant is missing the extracellular domain associated with ligand binding and is produced by proteolytic cleavage of α6 by Urokinase-type Plasminogen Activator (uPA) [1], a serine protease important for glandular development. Using site-directed mutagenesis we have shown that residues R594 and R595 are essential for cleavage and that the cleavage of the α6 extracellular domain promotes tumor cell invasion and migration on laminin [3]
In Xenopus, there is considerable integrin diversity during early development and integrins α2, α3, α4, α5, and α6 are expressed by the end of gastrulation [4]. Although the spatial and temporal expression of a6 mRNA and protein has been described in detail there is no information on the expression of α6 in the adult frog. The earliest stage at which α6 mRNA is detected is the mid-gastrula stage (stage 10) and the expression levels increase up to the tadpole stage (stage 45) which was the last stage analyzed [4]. α6 protein first appears at stage 13 and the levels increase until the last stage analyzed (stage 40) [5]. In addition, it has been demonstrated that integrin α6 is required for early nervous system development in Xenopus [5] a notion supported by results in the mouse where evidence has been provided suggesting an essential role of integrin–laminin interactions for the proper development of the nervous system.
Given the common identity of molecules involved in cancer and development and the parallels and strategic similarities between them we wanted to examine if α6 cleavage is a regulatory mechanism utilized during development.
Materials and methods
Embryos
Sexually mature adult wild-type and laboratory bred Xenopus laevis were obtained from Xenopus Express (France). Induction of females to ovulate was done by injection of 750 U of human gonadotropin (Sigma–Aldrich, MO, USA). Eggs were artificially fertilized and the produced embryos were degelified in 2% cysteine in 0.33× MMR. Embryos were cultured in 0.1× MMR (0.1M NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM HEPES, pH 7.8, 0.1 mM EDTA) and staged according to Nieuwkoop and Faber (1967) [6].
Cells
The DU145 cell line was incubated at 37 °C in a humidified atmosphere of 95% air and 5% CO2, in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL: Gaithersburg, MD, USA) plus 10% fetal bovine serum (FBS). The X. laevis primary cultures were grown in 0.1×MMR plus 5% fetal calf serum at room temperature. The Xenopus cell lines A6 and XL177 were grown in L-15 medium Leibovitz plus 10% fetal calf serum at room temperature.
Antibodies and chemicals used in this study
The anti-α6 integrin rabbit polyclonal antibody α6cytA was generously provided by Dr. Ivan de Curtis (Milano, Italy) and was described previously [7]. The AA6A rabbit polyclonal antibody was raised against the last 16 amino acids in the cytoplasmic domain of the human α6 integrin [2]. The rabbit polyclonal antibody against the N-terminal domain of the α6 integrin was raised against the first 500 amino acids, excluding the signal peptide, of the human α6 integrin. Amiloride and aminobenzamidine were purchased from Sigma–Aldrich (MO, USA). The human wild-type and uncleavable mutant α6 cDNAs were described previously [3].
Whole-mount immunohistochemistry
Immunohistochemistry was carried out on whole-mount tadpoles using a standard protocol [8]. Primary antibodies were followed by HRP-conjugated or Alexa633 secondary antibody incubation and washes. Detection of the HRP-conjugated antibodies was achieved using tyramide signal amplification, following manufacturer’s instructions (Alexa 647 tyramide, Molecular Probes, Invitrogen). After re-fixation, embryos were cleared in 1:2 benzyl alcohol/benzyl benzoate and were then imaged on a Zeiss Axioimager equipped for structured illumination (Apotome) for the creation of optical sections. MosaiX Images obtained using Alexa633 secondary where processed using the Zeiss Widefield Multichannel Unmixing module utilizing the Extraction function to remove autofluorescence from the Alexa633 channel. The Zeiss Inside4D module was used for the creation of the 3D reconstructions.
Human α6 expression studies
The wild-type and uncleavable human α6 integrin cDNAs were cloned into the HindIII and NotI sites of the pcDNA3.1+ vector (Invitrogen Corp). In vitro transcription was performed using the mMessage mMachine T7 kit (Ambion) and the resulting mRNAs were purified using the Mega Clear kit (Ambion). Embryos were degellied with cysteine as described above and equilibrated in 4% Ficol in 0.33× MMR prior to microinjection. Purified human α6 mRNA (1 ng) was injected into 2-cell Xenopus embryos. After injection, the embryos were cultured in 4% Ficol 0.33× MMR at room temperature until stage 9 and then washed and cultured in 0.1× MMR.
Immunoprecipitation
Xenopus laevis tadpoles were lysed in RIPA buffer and then sonicated briefly and immunoprecipitation was performed as described previously [1] using 1 mg of whole cell lysate in a 1 ml reaction with 50 µ1 protein G sepharose beads and 5 µ1of anti-α6 integrin antibody in an eppendorf tube. After analysis on a 7.5% SDS–PAGE gel, the gel was stained using SYPRO RUBY protein stain (Invitrogen) overnight and the bands were visualized and isolated under UV light.
Tandem mass spectrometry coupled to liquid chromatography (LC–MS/MS)
Excised SYPRO RUBY-stained protein bands following SDS– PAGE were digested in trypsin (10 µg/mL) at 37 °C overnight. LC–MS/MS analyses of in-gel trypsin digested [9] protein bands were carried out using a linear quadrupole ion trap ThermoFinnigan LTQ mass spectrometer (San Jose, CA) equipped with a Michrom Paradigm MS4 HPLC, a SpectraSystems AS3000 autosampler, and a nanoelectrospray source, as described previously [10,11]. Tandem MS spectra of peptides were analyzed with TurboSEQUEST™ v 3.1, a program that allows the correlation of experimental tandem MS data with theoretical spectra generated from known protein sequences [12]. The peak list (dta files) for the search were generated by Bioworks 3.1. Parent peptide mass error tolerance, fragment ion mass tolerance, and criteria used for preliminary positive peptide identification are the same as previously described [13,14]. All matched peptides were confirmed by visual examination of the spectra. All spectra were searched against a Xenopus database created from the latest version of the non-redundant protein database downloaded July 7, 2006, from NCBI. At the time of the search the Xenopus protein database from NCBI contained 19,238 entries. The results were also validated using XTandem, another search engine [15], and with Scaffold, a program that relies on various search engine results (i.e., Sequest, XTandem, MASCOT) and which uses Bayesian statistics to reliably identify more spectra [16,17].
Western blotting
Protein samples (50 µg for tissues or 20 µg for cell lines) were analyzed by Western blotting as previously described [1].
Results and discussion
α6 is cleaved during Xenopus development
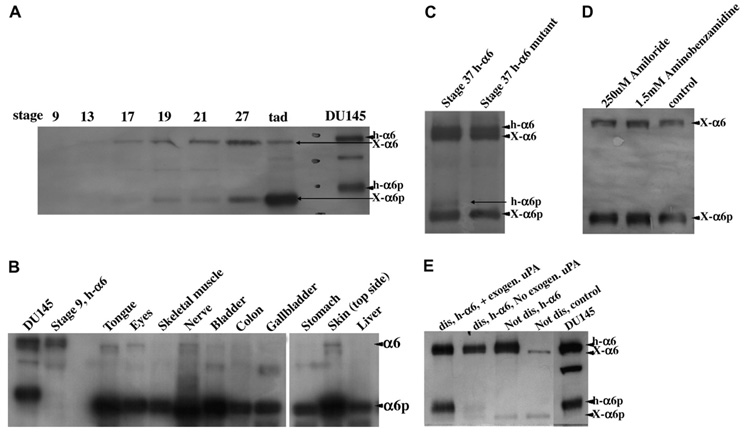
First we wanted to investigate whether α6p is produced during Xenopus development. Analysis of various stages for α6 and α6p integrin expression was performed by Western blot analysis. The results inFig. 1A show that α6 protein becomes detectable at stage 13. A second band corresponding to the human α6p starts to appear at stage 17 and at late tadpole stages (45+) this becomes the predominant band. A second antibody against the α6 C-terminus gave the same results (data not shown). Additional time points were added to get a better overall view of the temporal changes in the ratio of α6 and α6p (Supplementary data 1). The expression of both α6 and α6p increases gradually but at late tadpole stages (45+) the levels of α6 start to decline whereas the levels of α6p reach maximum levels. These data indicate that α6 is cleaved during Xenopus development and that α6p becomes the prevailing form in free swimming tadpoles.
Fig. 1.
α6p is produced during Xenopus development by a protease other than uPA. (A) Whole Xenopus embryos at indicated stages were analyzed for α6 and α6p integrin expression by Western blotting. Note that both Xenopus α6 and α6p run at a lower molecular weight than their human counterparts. (B) α6p is the predominant form of α6 in adult Xenopus as shown by Western blotting. (C) An endogenous Xenopus protease is capable of cleaving the microinjected human wild-type but not the mutated α6. (D) uPA inhibitors do not block α6p production in Xenopus primary cultures. (E) Exogenous uPA is capable of cleaving the human α6 that was microinjected in Xenopus embryos.
We went on to examine if the cleavage of α6 persisted in the adult frogs and examine potential differences in the ratio of α6/α6p between different tissues and organs. The results in(Fig. 1B (and Supplementary data 2) indicate that α6p is present in all organs analyzed and that the levels of α6p are higher than the levels of α6. In addition, analysis of lysates from a whole froglet indicates that α6p is overall the predominant form of α6. Interestingly several organ samples contained complete conversion of the α6 integrin to α6p. This is surprising considering that all mammalian tissues and cultured cell lines to date have always contained full length α6 [1–3,18]. The tissues with the highest full length α6 level were the skin and the testes. The differences in the ratio of α6/α6p observed in different tissues leads to the conclusion that α6 cleavage is spatially controlled.
Previous studies have shown that in humans and mice α6 is only cleaved in cancer tissues [1] and unpublished data). Unlike Xenopus [1], the human α6 is proteolytically cleaved during biosynthesis into a heavy and a light chain and these chains are disulphide linked to each other [19]. It has been shown that mammalian α6 mutants that cannot be cleaved into two chain molecules are capable of ligand binding but not of inside-out signaling [19]. In addition, α6 is a major component of the hemidesmosome and Xenopus hemidesmosomes have been shown to be different than those of other species [20]. These differences may explain the presence of α6p in normal Xenopus tissues.
Verification of the presence of α6p and α6N in Xenopus by tandem mass spectrometry coupled to liquid chromatography (LC–MS/MS)
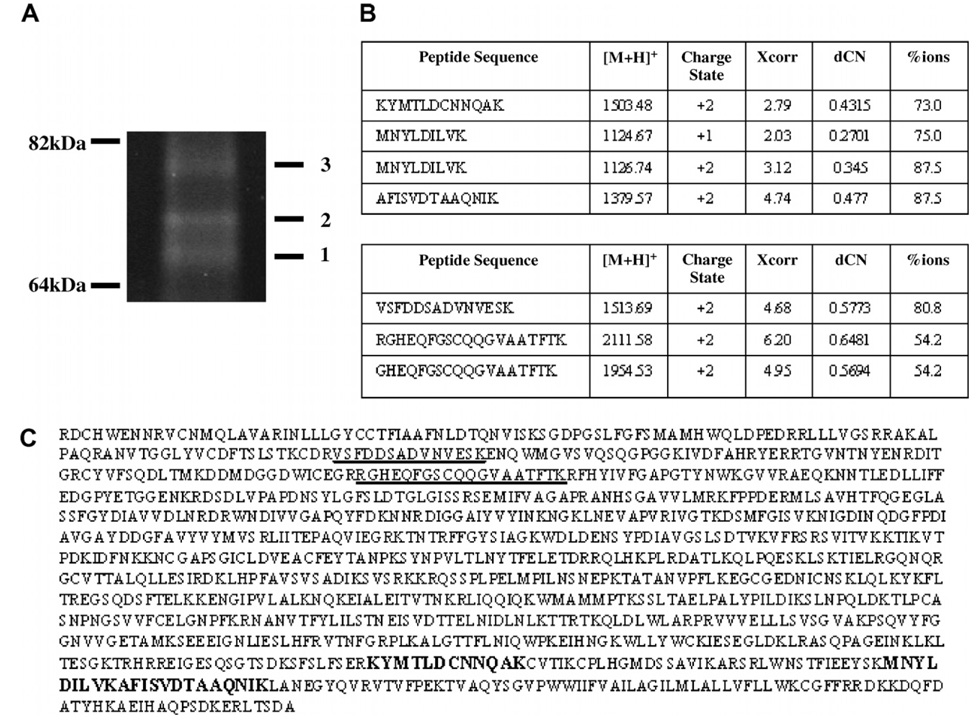
Our next goal was to confirm that the low molecular band we were observing using the Western blot technique was indeed the product of proteolytic cleavage of the full length α6. α6 cleavage in mammalian tissues gives rise to two fragments α6p and α6N (the N-terminal fragment of α6) [18]. To verify that the low molecular weight band was indeed the Xenopus equivalent of α6p we performed a large scale immunoprecipitation reaction using tadpole protein lysate and an anti-α6 integrin antibody. The immunocomplex was analyzed by SDS–PAGE analysis and the resulting gel was stained using SYPRO RUBY protein stain. The bands were visualized under UV light and the results are shown inFig. 2A. The bands of the expected molecular weight were isolated and then analyzed by tandem mass spectrometry coupled to liquid chromatography. The results inFig. 2 show that band #2 is α6p and band #3 is α6N. A total of 4 high-scoring peptides (panel B top) were identified from band #2 analysis which cover 3.1% (bold) of the primary sequence of integrin α6 (panel C). All four peptides spanned the carboxy terminus of the α6 integrin and no peptides spanning the amino-terminus of the α6 integrin were obtained from band #2. These results verified that band #2 was as expected α6p. Also, a total of 3 high-scoring peptides (panel B bottom) were identified from band #3 which cover 3.0% (underlined) of the primary sequence of integrin α6 (panel C). All 3 peptides spanned the amino-terminus of the α6 integrin and no peptides spanning the carboxy-terminus of α6 were identified verifying that band #3 is α6N. These data taken together verify the presence of α6p and confirm that α6p is a product of the proteolytic cleavage of α6.
Fig. 2.
Verification of the presence of α6p and α6N in Xenopus by tandem mass spectrometry coupled to liquid chromatography (LC–MS/MS). (A) Isolation of the fragments of the cleaved α6 integrin by immunoprecipitation. (B) Band #2 produced a total of 4 high-scoring peptides covering 3.1% (bold) of the primary sequence of integrin α6 (C), whereas band #3 produced a total of three high-scoring peptides covering 3.0% (underlined) of the primary sequence of integrin α6 (C).
uPA is not responsible for α6p production in Xenopus
uPA has been identified as the protease responsible for the cleavage of α6 in humans. However, the cleavage site is not conserved in Xenopus (data not shown).To test the potential involvement of a related protease in the cleavage of α6 in Xenopus, we used two inhibitors of uPA, amiloride and aminobenzamidine, to treat primary cell cultures and two established Xenopus epithelial cell lines (A6 and XL177). Although these inhibitors effectively reduced α6p levels in the mammalian cell line DU145 ([1] and data not shown) they failed to produce a significant reduction of the levels of α6p in Xenopus (Fig. 1D and data not shown). These data suggest that uPA is not responsible for the observed α6 cleavage in Xenopus. We went on to test if a protease in Xenopus is capable of cleaving the human α6 integrin. The human α6 integrin, introduced through transcript microinjection, was cleaved in the frog in a similar manner as in human malignancies (Fig. 1C and E). The cleavage resulted in the production of α6p protein which was the exact molecular weight as α6p in the human prostate cancer cell line (DU145). However the Xenopus protease appears to have reduced affinity for the human protein. A very small amount of the total human α6 appears to be cleaved and the cleaved product appears at much later stages (stage 37) than the Xenopus α6p (stage 17). Dissociation of α6 injected embryos coupled with the addition of exogenous uPA leads to a drastic increase of human α6p supporting the above notion (Fig. 1E).
In an effort to address a potential function of the α6 cleavage we overexpressed a mutated human α6 integrin (α6RR) in Xenopus embryos. Unlike the wild-type human α6, the mutated α6 integrin was not cleaved in Xenopus (Fig. 1C). Expression of α6RR in human cells acts as a dominant negative and prevents the cleavage of endogenous α6 [3]. This was not the case in Xenopus, where overexpression of α6RR by injection of transcripts did not reduce the levels of the endogenous Xenopus α6p (Fig. 1C). Despite this, overexpression of the mutant led to a mild phenotype of reduced eye size, axial defects and/or reduced head size (Supplementary data 1). The α6RR mRNA was co injected with GFP mRNA as a linage tracer in the dorsal marginal zone leading to high expression in the head, the notochord and the neural tube. No phenotype was observed when the mRNAs were injected laterally leading to ectopic expression primarily in the somites suggesting that the phenotype is specific. The fact that the presence of full length α6 despite its failure to block the cleavage of the endogenous protein leads to developmental abnormalities suggests a need for complete α6 conversion to α6p in certain tissues and may be an indication that cleavage is a mechanism for α6 deactivation.
α6 and α6p integrin expression and localization in Xenopus embryos
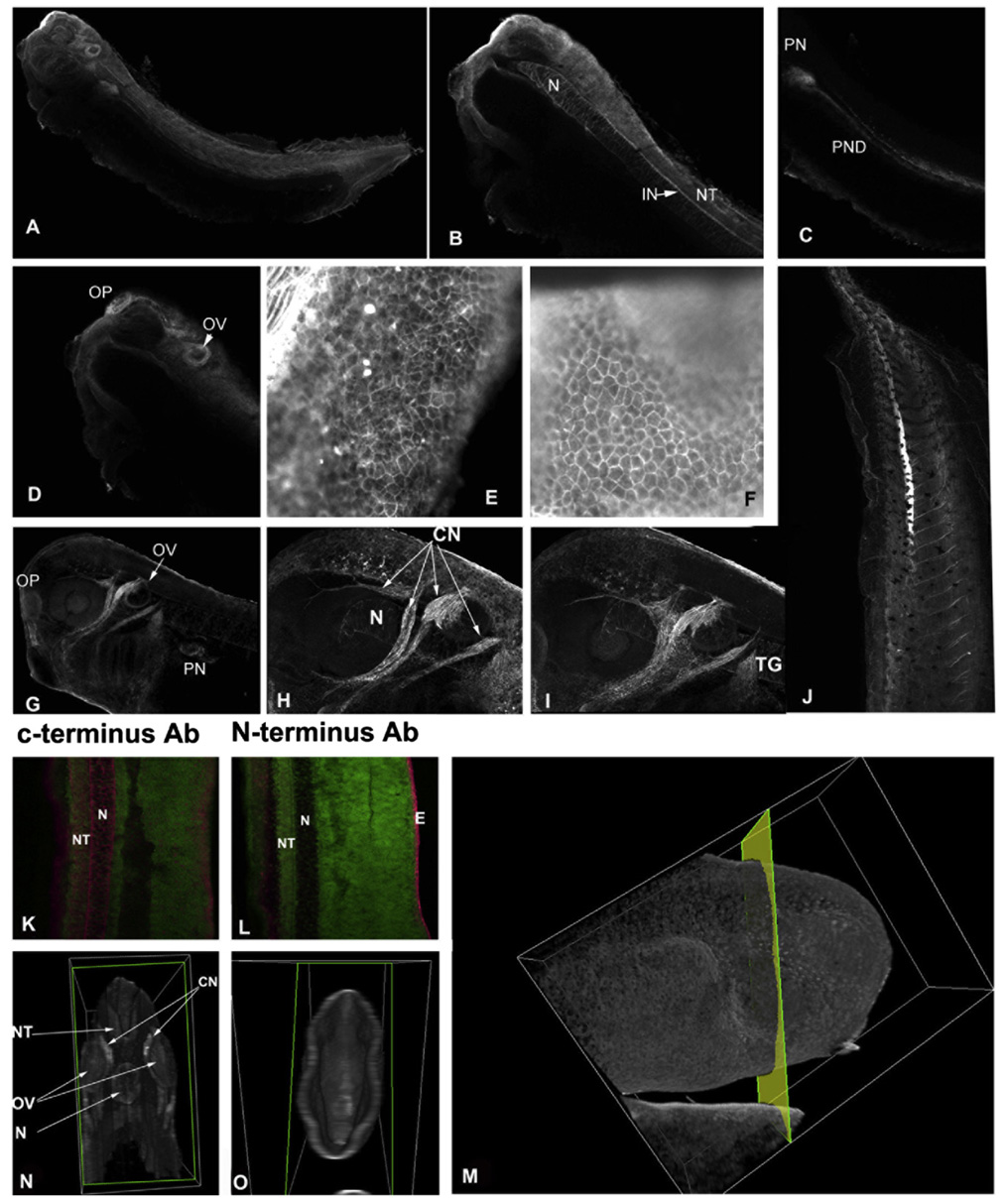
Xenopus embryos (stages 33–34) were used for whole mount immunostaining using a polyclonal anti-α6 antibody against the C-terminus of α6A. This antibody recognizes both α6 and α6p.The staining pattern was confirmed with a second anti-α6 antibody.Fig. 3A is a 2D projection (Maximum Intensity Projection-MIP) of a series of optical sections from a cleared embryo. B, C, and D are individual optical sections where the principal expression domains are shown more clearly. α6 is found throughout the CNS and the notochord (N) with higher expression in the neural tube (NT), the olfactory placode (OP), interneurons (IN), the pronephros (PN), and the pronephric duct (PND). Very strong staining was also observed in a subset of cranial nerves (Fig. 3G–I). The localization of the α6 protein is in agreement with previously published data regarding the localization of the α6 mRNA with some distinctions. Despite the absence of any mRNA in the notochord at stage 32, our results show strong antibody staining in this tissue indicating that translated protein remains abundant at later stages (35). It is interesting to note that a polyclonal antibody against the N-terminal portion of α6 fails to detect protein in the notochord suggesting that α6 in this tissue is almost completely cleaved (Fig. 3K and L). Furthermore 3D reconstruction of embryos stained with the N-terminal antibody shows that no staining is present in internal tissues stained by the C-terminal antibodies (Fig. 3M–O). There is however strong staining of the epidermis indicating, in agreement with the western blot data, that α6 is almost completely cleaved in all tissues with the exception of the epidermis suggesting spatial regulation of cleavage. In the epidermis both antibodies stain the cell–cell boundaries very strongly and colocalize with the actin cytoskeleton (Fig. 3E and F). The fact that in some tissues α6 protein persists long after the mRNA stops being expressed coupled with the fact that in such tissues α6 is completely cleaved supports the hypothesis that the cleavage may be a deactivation mechanism.
Fig. 3.
Integrin α6 and α6p expression and localization in Xenopus embryos. Whole mount immunostaining of a Xenopus embryo (stages 33 and 34) using a polyclonal anti-α6 antibody against the C-terminus of α6A (A). Panel A shows a 2D projection of a series of optical sections from a cleared embryo. B–D are individual optical sections where the principal expression domains are shown more clearly. Panels E and F show the expression of actin and α6, respectively, in the Xenopus tadpole epidermis. The α6 protein is found throughout the CNS and the notochord (N) with higher expression in the neural tube (NT), the olfactory placode (OP), interneurons (IN), the pronephros (PN), and the pronephric duct (PND). (G–I) Both antibodies tested also gave strong signal at a subset of cranial nerves (CN)and the trigerminal gaglia (TG) as seen in panels H and I. At late tadpole stages (stage 42) α6 is expressed in the vascular system with prominence in the newly formed vessels (J). (K and L) Full length a6 is only found in the epidermis and is completely absent from the notochord and other internal tissues. Optical sections of embryos stained with a C-terminal α6 antibody (K) and an N-terminal antibody (L). (N and O) 3D reconstruction of embryos, which were optically sectioned as indicated in M (tissues on the left side of the plane shown were removed leaving only the anterior structures) reveals that α6 is only found in the epidermis of stage 35 embryos with α6p present in the notochord (N), the neural tube (NT), the otic vesicle (OV) and cranial nerves (CN).
A previous study showed that the endothelial cell marker flk-1 can be detected at neurula stages (stage 15) and in the future heart region at stage 18. α6 cleavage coincides with the establishment of the first endothelial cells. The fact that endothelial cells express high levels of the α6 integrin, and that the most closely related protease to uPA in Xenopus is tissue plasminogen activator (tPA), a protease found in the circulatory system, raised the possibility that the α6 cleavage is mediated by tPA in Xenopus. Use of a tPA inhibitor (tPA stop), however failed to block α6p production indicating that tPA is not involved in this process (data not shown).
Overall we have shown that integrin a6 is cleaved during the normal development of X. leavis and that α6p is the major form of the α6 integrin in the adult frog. We have also provided data suggesting that cleavage of α6 may be a rapid deactivation mechanism required for normal development. The fact that little or no α6p is present in normal human or mouse tissues could reflect differences in the hemidesmosome structures between these species and Xenopus or differences in post-translational modifications [20]. At the same time however, the fact that α6p is present in different species suggests that α6 cleavage is a conserved mechanism for the regulation of the α6 integrin function. Future work will be aimed at elucidating the precise role and function of α6 cleavage during development.
Acknowledgment
We thank Dr. Ivan de Curtis (Milan, Italy) for providing us with the α6 integrin antibody. This work was supported by Marie Curie IRGs 036567 and 016613, and the Research Promotion Foundation Grant APONE050502, and CA 56666, CA 23074. Mass spectral proteomic analyses were performed by the Arizona Proteomics Consortium supported by Grants from NIEHS ES06694 and NCI CA023074 and the BIO5 Institute.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc. 2007.12.040.
References
- 1.Demetriou MC, Pennington ME, Nagle RB, et al. Extracellular alpha 6 integrin cleavage by urokinase-type plasminogen activator in human prostate cancer, Exp. Cell Res. 2004;294:550–558. doi: 10.1016/j.yexcr.2003.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis TL, Rabinovitz I, Futscher BW, et al. Identification of a novel structural variant of the alpha 6 integrin. J. Biol. Chem. 2001;276:26099–26106. doi: 10.1074/jbc.M102811200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawar SC, Demetriou MC, Nagle RB, et al. Integrin alpha6 cleavage: a novel modification to modulate cell migration. Exp Cell Res. 2007;313:1080–1089. doi: 10.1016/j.yexcr.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whittaker CA, DeSimone DW. Integrin alpha subunit mRNAs are differentially expressed in early Xenopus embryos. Development. 1993;117:1239–1249. doi: 10.1242/dev.117.4.1239. [DOI] [PubMed] [Google Scholar]
- 5.Lallier TE, Whittaker CA, DeSimone DW. Integrin alpha 6 expression is required for early nervous system development in Xenopus laevis. Development. 1996;122:2539–2554. doi: 10.1242/dev.122.8.2539. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) second ed. North Holland: Amsterdam; 1967. [Google Scholar]
- 7.de Curtis I, Reichardt LF. Function and spatial distribution in developing chick retina of the laminin receptor alpha 6 beta 1 and its isoforms. Development. 1993;118:377–388. doi: 10.1242/dev.118.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber AM, Das B, Huang H, et al. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc. Natl. Acad. Sci. USA. 2001;98:10739–10744. doi: 10.1073/pnas.191361698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shevchenko A, Wilm M, Vorm O, et al. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 10.Andon NL, Hollingworth S, Koller A, et al. Proteomic characterization of wheat amyloplasts using identification of proteins by tandem mass spectrometry. Proteomics. 2002;2:1156–1168. doi: 10.1002/1615-9861(200209)2:9<1156::AID-PROT1156>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Lantz RC, Lynch BJ, Boitano S, et al. Pulmonary biomarkers based on alterations in protein expression after exposure to arsenic, Environ. Health Perspect. 2007;115:586–591. doi: 10.1289/ehp.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eng JK, McCormack AL, Yates III JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 13.Qian WJ, Liu T, Monroe ME, et al. Probability-based evaluation of peptide and protein identifications from tandem mass spectrometry and SEQUEST analysis: the human proteome. J. Proteome. Res. 2005;4:53–62. doi: 10.1021/pr0498638. [DOI] [PubMed] [Google Scholar]
- 14.Cooper B, Eckert D, Andon NL, et al. Investigative proteomics: identification of an unknown plant virus from infected plants using mass spectrometry. J. Am. Soc. Mass Spectrom. 2003;14:736–741. doi: 10.1016/S1044-0305(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 15.Craig R, Beavis RC. TANDEM: matching proteins with tandem mass spectra. Bioinformatics. 2004;20:1466–1467. doi: 10.1093/bioinformatics/bth092. [DOI] [PubMed] [Google Scholar]
- 16.Keller A, Nesvizhskii AI, Kolker E, et al. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 17.Nesvizhskii AI, Keller A, Kolker E, et al. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 18.Demetriou MC, Cress AE. Integrin clipping: a novel adhesion switch? J. Cell Biochem. 2004;91:26–35. doi: 10.1002/jcb.10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delwel GO, Kuikman I, van der Schors RC, et al. Identification of he cleavage sites in the alpha6A integrin subunit: structural requirements for cleavage and functional analysis of the uncleaved alpha6Abeta1 integrin. Biochem. J. 1997;324(Pt 1):263–272. doi: 10.1042/bj3240263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buck RC. Ultrastructural characteristics associated with the anchoring of corneal epithelium in several classes of vertebrates. J. Anat. 1983;137(Pt 4):743–756. [PMC free article] [PubMed] [Google Scholar]