Abstract
We report here the desensitization and internalization of the relaxin receptor (RXFP1) after agonist activation in both primary human decidual cells and HEK293 cells stably transfected with RXFP1. The importance of β-arrestin 2 in these processes has also been demonstrated. Thus, in HEK-RXFP1 cells the desensitization of RXFP1 was significantly increased when β-arrestin 2 was overexpressed. After relaxin activation, β-arrestin 2 was translocated to the cell membrane and RXFP1 underwent rapid internalization. We have previously shown that RXFP1 forms dimers/oligomers during its biosynthesis and trafficking to the plasma membrane, we now show that internalization of RXFP1 occurs through this dimerization/oligomerization. In nonagonist stimulated cells, it is known that the majority of the RXFP1 is located intracellularly and was confirmed in the cells used here. Constitutive internalization of RXFP1 could account for this and indeed, slow but robust constitutive internalization, which was increased after agonist stimulation was demonstrated. A carboxyl-terminal deleted RXFP1 variant had a similar level of constitutive agonist-independent internalization as the wild-type RXFP1 but lost sensitivity to agonist stimulation. This demonstrated the importance of the carboxyl terminus in agonist-stimulated receptor internalization. These data suggest that the autocrine/paracrine actions of relaxin in the decidua are under additional controls at the level of expression of its receptor on the surface of its target cells.
Both desensitization and relaxin-induced and constitutive internalization of relaxin’s receptor (RXFP1) occurs through its dimerization/oligomerization and the influence of β-arrestin-2.
Relaxin is structurally related to insulin (1), and its main systemic source in pregnant women is the corpus luteum (2). Recent work has expanded its role to several important nonreproductive tissues: cardiovascular, renal, and lung tissues and in prostate and thyroid cancers (3,4,5,6,7). We have shown that human relaxin is produced by the maternal decidua during pregnancy and acts locally within the decidua and the apposed chorion (8,9) by binding to these cells (10).
The relaxin receptor, relaxin family peptide receptor (RXFP)-1, belongs to the G protein-coupled receptor (GPCR) superfamily and was originally named the leucine-rich repeat-containing G protein-coupled receptor (LGR)-7 (11) and most recently RXFP1. RXFP1 and the closely related RXFP2 are mosaic proteins containing an extracellular region with 10 leucine-rich repeats and seven-transmembrane helix domains. RXFP1 and RXFP2 are activated by their endogenous ligands, relaxin, and insulin-like peptide 3 (INSL3), respectively. Among the members of the LGR subfamily are the glycoprotein hormone receptors such as LH receptor (LHR), FSH receptor (FSHR), and TSH receptor (TSHR) (12). RXFP1 and RXFP2 belong to the LGR C subfamily distinguished by a unique low-density lipoprotein class A module (LDL-A) at their N termini. We demonstrated that the LDL-A module of RXFP1 has a role in receptor maturation, cell surface delivery, and ligand-induced receptor signaling (13). Mutations in the LDL-A module of RXFP2 affects the proper cell surface delivery of this receptor (14). After ligand binding, both receptors couple to Gs and activate adenylate cyclase to increase intracellular cAMP levels and stimulate protein kinase A (15).
After ligand activation, GPCR signaling is strictly regulated by different mechanisms, depending on the cell type. Agonist-mediated activation of GPCRs is generally followed by initialization of the mechanisms leading to rapid signal attenuation, termed desensitization (16). GPCR signaling is regulated by a well-characterized cascade of events involving G protein coupling and activation, receptor phosphorylation by GPCR kinases, and subsequent β-arrestin recruitment (17). Activated receptors interact with intracellularly localized β-arrestin proteins, which sterically uncouples them from the G proteins and terminates signaling. This also initiates receptor internalization into endocytic vesicles that can contribute to receptor desensitization (17,18). The arrestin proteins act as scaffold proteins; thus, GPCR internalization is predominantly mediated via recruitment of β-arrestin 1 and/or 2 to agonist-activated receptor. Two different classes of GPCRs have been identified based on their binding affinities for β-arrestin 1 and 2 (19). The class A receptors have a higher affinity for β-arrestin 2 than β-arrestin 1. The class B receptors have a similar affinity for both proteins and do not discriminate between β-arrestin 1 and 2 (19).
LHR, FSHR and TSHR, subclass A receptors, with homology to LGR7/RXFP1, undergo β-arrestin-dependent desensitization and show enhanced internalization via β-arrestin 2 (20,21,22). An understanding of the down-regulation of RXFP1 signaling is of interest because overexpression of either relaxin or RXFP1 in the human fetal membranes correlates with preterm delivery (8,23,24). We therefore investigated the desensitization and internalization of RXFP1 after agonist activation in both primary human decidual cells and RXFP1 transfected human embryonic kidney 293 (HEK293) cells and shown that RXFP1 desensitizes after relaxin treatment and that β-arrestin 2 increases this desensitization. After relaxin activation, RXFP1 undergoes rapid β-arrestin 2-dependent internalization and is then translocated to the membrane. RXFP1 forms dimers during its biosynthesis and trafficking to the plasma membrane (25), and we now report here that agonist-induced internalization/endocytosis of RXFP1 occurs through this dimerization/oligomerization. Our results also show that RXFP1 undergoes slow but robust constitutive internalization, which increases in the presence of relaxin. In contrast, RXFP1 missing its carboxyl terminal tail displays constitutive agonist-independent internalization, but relaxin fails to cause agonist-dependent internalization of this receptor variant.
Materials and Methods
Hormones and cell lines
Recombinant human relaxin was a generous gift from BAS Medical Inc. (Palo Alto, CA) and human INSL3 was purchased from Phoenix Pharmaceuticals (Belmont, CA). Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and HEK293 cells were obtained from American Type Culture Collection (Manassas, VA; no. CRL-1573).
DNA constructs and plasmids
The hemagglutinin (HA)-tagged wild-type RXFP1 and LDL-A module-deleted RXFP1 were generated as previously described (13). The carboxyl terminal-deleted RXFP1 (RXFP1-687, stop codon after Pro687) was generated by overlap extension PCR. β-Arrestin 1 and 2 were generous gifts from Dr. J. L. Benovic (Thomas Jefferson University, Philadelphia, PA) and FLAG tagged RXFP2 was a gift from Dr. A. I. Agoulnik (Baylor Collage of Medicine, Houston, TX). We generated a β-arrestin 2-yellow fluorescence protein (YFP) construct by subcloning the YFP sequence as previously described (25).
Cell culture and transfection
Isolated decidual cells and HEK293 cells were maintained in RPMI 1640 medium and DMEM medium, respectively, supplemented with 10% fetal bovine serum and 100 μg/ml penicillin/streptomycin in a humidified atmosphere at 37C and 95% air-5% CO2. Transient transfections of HEK293 cells were performed using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Stably transfected HEK-RXFP1 were previously developed (25) and stably transfected HEK-delLDLRXFP1 cells were created by the same method.
Primary decidual cell isolation
Isolation of primary decidual cells from human fetal membranes was performed by Percoll gradient methodology (26,27) with modifications. Briefly, fetal membranes from women undergoing elective cesarean section before labor were collected. Fetal membranes were dissected from the placenta and transported to the laboratory in sterile PBS. The decidua was separated from the chorion by gentle scraping with a glass slide and digested with 0.02% collagenase A and 20 U/ml deoxyribonuclease 1 at 37 C for 60 min. The cells were collected by filtration and centrifugation, overlaid onto a discontinuous Percoll gradient (5–60% Percoll) and centrifuged at 400 × g for 20 min. Decidual cells migrating between 1.017 and 1.045 g/ml were collected, washed, and plated onto 2% gelatin-coated plates in RPMI 1640 containing 10% fetal bovine serum and antibiotics. Cultures were grown to confluence and passaged three times before use to eliminate nonadherent cells.
Membrane preparations, β-arrestin 2-YFP translocation, Western blot analysis, and immunocytochemistry
Membrane preparations of primary decidual cells and HEK-RXFP1 cells and Western blot analysis were performed as described previously (13). Blotted samples were incubated with a monoclonal RXFP1 antibody [LGR7 monoclonal antibody (M01), clone 3E3 at 1:500; Abnova, Taipei, Taiwan] followed by a secondary horseradish peroxidase-conjugated mouse antibody (Bio-Rad, Hercules, CA; 1:3000). For RXFP1 detection in decidual cells, we exposed the film for a longer time (20–30 min) before visualization.
To test translocation of β-arrestin 2 to the membrane of HEK-RXFP1 and HEK-delLDLRXFP1 cells, these were transfected with the β-arrestin 2-YFP construct. After 48 h, the cells were collected and incubated with or without 10 nm relaxin for 10 min. Cells were then cross-linked with disuccinimidyl suberate (Pierce, Rockford, IL) 2 mm in PBS for 30 min at room temperature followed by quenching of the reaction with 50 mm Tris (pH 7.5) for 15 min at room temperature, and membrane preparations were isolated as described previously (13). YFP fluorescence was measured of 1 μg membrane using a Victor 2 fluorometer (PerkinElmer Life Sciences, Wellesley, MA). Three independent experiments with different membrane preparations were performed and expressed as means ± sem.
Immunocytochemistry of decidual cells and HEK-RXFP1 cells were performed as described previously (25). For RXFP1 visualization, cells were incubated with antibody to RXFP1 [LGR7 monoclonal antibody (M01), Abnova, at 1:500 dilution] for 1 h at room temperature. In colocalization studies, cells were permabilized and the endoplasmic reticulum (ER) was immunostained with calreticulin (SPA-600 at 1:200 dilution; StressGen, Ann Arbor, MI). Appropriate fluorescently labeled AlexaFluor antibody (1:1000 dilution; Molecular Probes, Eugene, OR) were applied and laser confocal scanning microscopy was performed using a LSM-5 system (Zeiss, Thornwood, NY).
Fluorescence-activated cell sorting (FACS) analysis
Isolated decidual cells were seeded onto 6-well plates coated with 2% gelatin. After 2 d of incubation, cells were treated with 10 nm relaxin for appropriate times and FACS was performed. The cells were washed with PBS, fixed with 4% paraformaldehyde for 10 min at room temperature, and blocked with 1% BSA in PBS and antibody to RXFP1 (Abnova; 1:500) overnight at 4C. The next day, cells were washed three times with PBS, incubated with AlexaFluor 488 antibody (Molecular Probes; 1:1000) for 1 h at room temperature, washed three times with PBS, and the cells collected. Fluorescence was monitored by using a Epics XL cytometer (Beckman Coulter, Miami, FL) and 5000 cells/sample. Three independent experiments were performed and expressed as means ± sem. The mean fluorescence of stained cells with primary and secondary antibody, minus the mean fluorescence of stained cells with only the secondary antibody, was used to calculate the percent internalization.
Quantitative RT-PCR (qRT-PCR) of arrestins
Total RNA was extracted by Rneasy minikit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. Primers for β-arrestin 1 and 2, and the housekeeping gene (18S) were purchased from Applied Biosystems (Taqman Assay on Demand, Foster City, CA). The cDNAs were prepared using GeneAmp reagents (Applied Biosystems). Reverse transcription and qRT-PCR were performed as described previously (25). Each reaction was performed in triplicate, the results were normalized to the expression of 18S, and data were represented as relative gene expression as mean values ± sem.
Intracellular cAMP determination and desensitization
Intracellular cAMP was determined with the cAMP Biotrak enzyme immunoassay EIA system (GE Healthcare, Piscataway, NJ) as described previously (13).
In desensitization studies, decidual cells, HEK-RXFP1 or HEK-RXFP1 cotransfected with β-arrestin 1 or 2 were pretreated for various times (0–120 min) with 0.1 nm relaxin, then briefly washed, and further stimulated with 0.1 nm relaxin for 20 min. Intracellular cAMP levels were determined with the cAMP Biotrak enzyme immunoassay system (GE Healthcare) (13). Each experiment was performed in duplicate and expressed as means ± sem for three to six observations.
Receptor internalization and the constitutive internalization assay
We used a cell surface ELISA described previously (13). Internalization was measured as a decrease in cell surface receptor levels, and agonist-treated cells were compared with nontreated cells. They were stimulated with 10 nm relaxin or INSL3 and incubated for 1–60 min. Each experiment was performed in duplicate and expressed as means ± sem for three observations.
Constitutive internalization was measured as described by Pawson et al. (28). The value from the cells transfected with the empty vector was subtracted from the values obtained from the cells transfected with the receptor variants. Receptors remaining at the cell surface after warming to 37 C, in either the presence or absence of ligand were expressed as the percentage of receptors at the cell surface at time zero. Each experiment was performed in duplicate and expressed as means ± sem for three observations.
Statistical analysis
Results were analyzed by one-way ANOVA with Student-Newman-Keuls multiple comparison methods, using GraphPad Instat software (San Diego, CA).
Results
Characterization of RXFP1 expression in primary decidual cells and HEK293 cells stably transfected with RXFP1 (HEK-RXFP1)
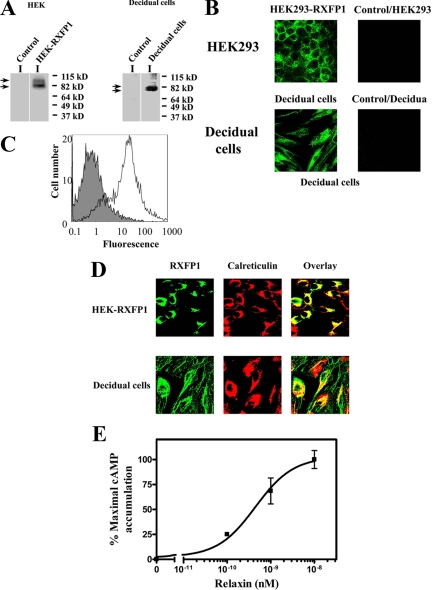
It has been previously shown that the RXFP1 gene and protein are expressed in the decidua and chorionic cytotrophoblast of the fetal membranes (23), agreeing with binding studies (10). To further characterize RXFP1 protein expression, we isolated primary decidual cells and compared them with HEK-RXFP1. RXFP1 protein was detected in total membrane preparations by Western blot using the monoclonal antibody (3E3) against the extracellular N terminus of the protein (Fig. 1A). HEK293 cells do not endogenously express RXFP1 (29); therefore, untransfected HEK293 cells were used as controls, and RXFP1 protein was undetectable (Fig. 1A). This also confirmed the specificity of the antibody. In HEK-RXFP1 cells, two RXFP1 protein forms were detected with the same molecular masses (80 and 95 kDa) as previously detected using an HA antibody (13). Decidual cells expressed lower molecular mass forms (80 and 75 kDa) compared with HEK-RXFP1.
Figure 1.
A, RXFP1 protein expression in HEK-RXFP1 and primary decidual cells. Membrane preparations from HEK-RXFP1 cells, decidual cells, and untransfected HEK293 cells (control) were analyzed by Western blotting. RXFP1 expression was detected in HEK-RXFP1 and decidual cells, indicated by arrows, and not in untransfected HEK293 cells (control). B, Immunocytochemistry of RXFP1 in HEK-RXFP1 cells and decidual cells. Nonpermeabilized cells were stained with a monoclonal antibody to RXFP1 followed by a mouse AlexaFluor 488-conjugated secondary antibody, showing membrane localization of RXFP1. No staining was detected in untransfected HEK293 cells (control). C, FACS analysis of isolated decidual cells. Cell surface expression of RXFP1 was measured by FACS in nonpermeabilized decidual cells. The fluorescence of the control cells (no primary antibody to RXFP1) is shown by the solid histogram compared with the cell population stained with primary antibody to RXFP1 and mouse AlexaFluor 488 secondary antibody in the open histogram. D, The subcellular localization of RXFP1 in HEK-RXFP1 (upper row) and decidual cells (bottom row). Cells were fixed, permeabilized and double labeled for confocal microscopy. The ER resident proteins were labeled with antibody to calreticulin (middle panels), and RXFP1 proteins were labeled in parallel using a monoclonal antibody to RXFP1 (left panels). Secondary antibodies were AlexaFluor 488 or AlexaFluor 546. The overlay (right panels) shows colocalization of RXFP1 with the ER marker calreticulin. E, Functional characterization of RXFP1 in isolated decidual cells. Relaxin caused a dose-dependent stimulation of cAMP production from decidual cells. cAMP accumulation is expressed as percentage of the maximal relaxin response. The results shown are mean of ± sem of four independent decidual cell isolation experiments performed in duplicate.
RXFP1 expression was confirmed by immunostaining (Fig. 1B). Both nonpermeabilized HEK-RXFP1 cells and decidual cells were labeled, demonstrating the cell surface expression of RXFP1. Previous studies have shown that RXFP1 is delivered to the cell surface in transfected HEK293 cells (13,25). The plasma membrane localization of RXFP1 was further studied in decidual cells by flow cytometry (FACS). Nonpermeabilized cells were identified as a broad peak of fluorescent cells, which was shifted when labeled with the antibody against the receptor N terminus and Alexa Fluor 488 antimouse IgG, compared with controls (no primary antibody) (Fig. 1C). Thus, the results of flow cytometry and immunocytochemistry confirmed the decidual cell surface delivery of RXFP1. RXFP1 was previously localized in HEK293 cells in the ER as a preprotein (13). Therefore, we used immunocytochemistry to identify this in decidual cells. Indeed, RXFP1 was colocalized with the ER marker calreticulin in the decidual cells and HEK-RXFP1 cells (Fig. 1D), showing that RXFP1 protein expression, processing, and trafficking in decidual cells was similar to that in HEK-RXFP1 cells. Furthermore, we functionally characterized the decidual cells and obtained a dose-dependent increase in cAMP levels after relaxin treatment (Fig. 1E). The calculated EC50 (0.4 ± 0.2 nm) was similar to that shown previously in HEK-RXFP1 cells (13). Therefore, our results showed that RXFP1 is expressed in decidual cells and was delivered to the plasma membrane and responded to relaxin treatment.
Desensitization of primary decidual cells and HEK-RXFP1 after relaxin treatment and the effects of arrestins
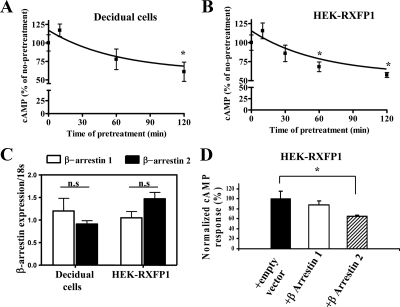
Functional desensitization of GPCRs is an important down-regulation process affecting further cell signaling after prolonged agonist exposure and prevents the cells from overstimulation. We characterized this after relaxin treatment in both decidual and HEK-RXFP1 cells. Pretreatment with 0.1 nm relaxin for 10–120 min caused a time-dependent attenuation of intracellular cAMP levels after a second treatment with 0.1 nm relaxin (Fig. 2, A and B), showing that RXFP1 undergoes desensitization in response to relaxin. In both decidual and HEK-RXFP1 cells, in the desensitization assay, there was a slight increase in the first 10 min, which was not significant compared with 0 min. After 120 min pretreatment, the capacity for intracellular production of cAMP was reduced to 60% of the initial level in decidual cells (Fig. 2A) and 57% in HEK-RXFP1 (Fig. 2B) cells. These results showed that signaling of RXFP1 by relaxin was down-regulated by relaxin pretreatment.
Figure 2.
Desensitization of RXFP1 and the effect of β-arrestins. Isolated decidual cells (A) or HEK-RXFP1 (B) were exposed to 0.1 nm relaxin for the times indicated. Cells were rapidly washed and restimulated with 0.1 nm relaxin. The intracellular cAMP levels were measured and its accumulation expressed as percentage of the maximal relaxin response. Pretreatment with relaxin caused a time-dependent attenuation of cAMP levels after the second treatment with relaxin. The results shown are mean of ± sem of three independent experiments performed in duplicate. *, P < 0.05 compared with 0 min. C, qRT-PCR analysis of mRNA expression of β-arrestins 1 and 2 in decidual cells and HEK-RXFP1 cells. Total RNA was obtained from decidual cells isolated from different patients (n = 4) and HEK-RXFP1cells (n = 4). After RT qRT-PCR was performed using Applied Biosystems primers for β-arrestins 1 and 2. β-Arrestin expression was normalized to the expression of 18S in each sample and shown as relative gene expression ± sem. Both arrestins were expressed in both cell types, and there were no significant (ns) differences in their levels of expression. D, Effects of β-arrestin 1 and 2 on desensitization of RXFP1 in HEK-RXFP1 cells. HEK-RXFP1 cells were cotransfected with either β-arrestin 1 or 2 or cotransfected with an empty vector (control). These cells were pretreated with 0.1 nm relaxin for 60 min, quickly washed, and restimulated with 0.1 nm relaxin. The cAMP responses (percent) were normalized to the control cells. Overexpression of β-arrestin 2 significantly increased attenuation of the RXFP1 response after relaxin treatment. Data are means ± sem of three independent experiments performed in duplicate. *, P < 0.05 compared with controls (HEK-RXFP1 transfected with an empty vector).
It has been shown that β-arrestins mediate the desensitization of GPCRs by the uncoupling of the activated receptors from the heteromeric G proteins after agonist treatment (30,31,32). Cells endogenously express two β-arrestin forms, β-arrestin 1 and β-arrestin 2 (16,33). To investigate their role in RXFP1 desensitization, we first measured the levels of expression of β-arrestin 1 and 2 (Fig. 2C). The results showed that both β-arrestin genes were detectable in decidual cells and HEK-RXFP1 cells, with no significant differences in their levels in either cell type (Fig. 2C). Furthermore, the contribution of these β-arrestins to desensitization of RXFP1 was shown by additionally transfecting HEK-RXFP1 cells with either β-arrestin 1 or β-arrestin 2 constructs (Fig. 2D). Controls were HEK-RXFP1 transfected with an empty vector. Overexpression of β-arrestin 2 led to significantly increased attenuation of the RXFP1 response after relaxin treatment (cAMP inhibited by 35.4 ± 4.9%, P < 0.05 compared with control; Fig. 2D). Overexpression of β-arrestin 1 caused no significant changes (Fig. 2D). These results suggest that β-arrestin 2 plays a role in relaxin-mediated desensitization of RXFP1.
Relaxin-induced internalization of RXFP1 in decidual cells and HEK293 cells
Internalization of the receptor into intracellular vesicles after agonist treatment can contribute to functional desensitization (17,18); we investigated this for RXFP1. Cell surface expression of RXFP1 in decidual cells was quantified by FACS. Their treatment with 10 nm relaxin resulted in RXFP1 sequestration from the cell surface, shown by the rapid loss of measurable receptors (Fig. 3A). Relaxin caused rapid internalization of 30% of RXFP1 initially present on the cell surface (P < 0.05), evident at 5 min and remaining constant for 60 min (Fig. 3A).
Figure 3.
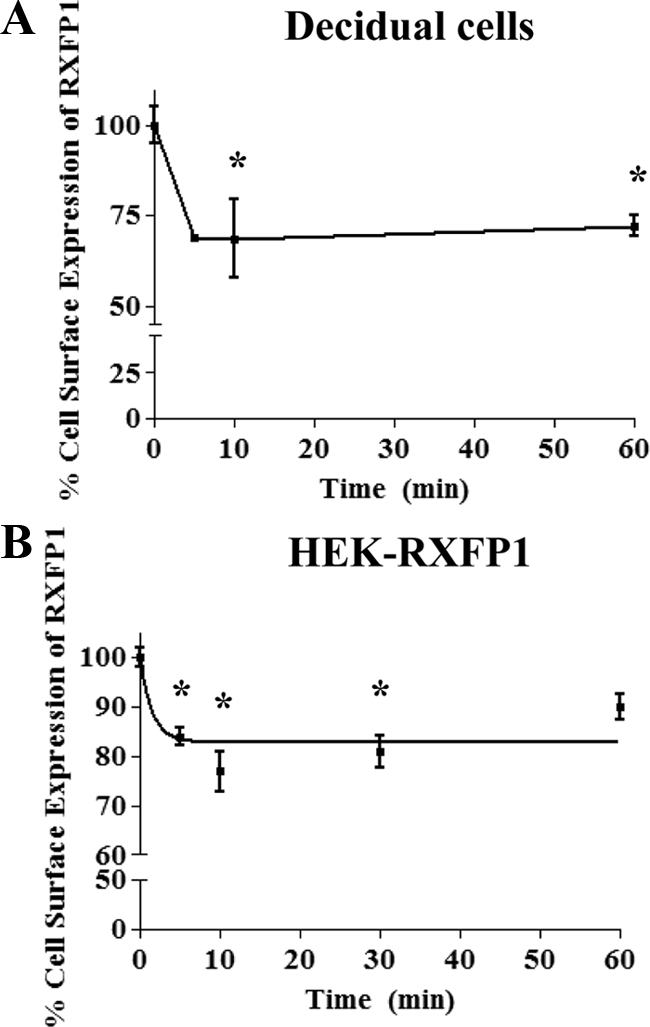
Internalization of RXFP1 after relaxin treatment. A, Relaxin induced cell surface expression of RXFP1 measured by FACS analysis in decidual cells. Decidual cells were treated with 10 nm relaxin for different times (5–60 min) and FACS performed. Fluorescence was normalized to the basal RXFP1 cell surface expression at 0 min (untreated). Relaxin treatment resulted in the rapid loss of RXFP1 cell surface expression by 5 min, remaining constant for up to 60 min. *, P < 0.05 compared with unstimulated cells. B, Relaxin induced cell surface expression of RXFP1 measured by cell surface ELISA in HEK-RXFP1 cells. Cells were treated with 10 nm relaxin for different times (5–60 min) and the cell surface ELISA performed. Data were normalized to the basal RXFP1 cell surface expression for untreated cells at 0 min. Relaxin treatment caused the rapid loss of RXFP1 cell surface expression by 5 min. Data are means ± sem of three independent experiments performed in duplicate. *, P < 0.05 compared with unstimulated cells.
The cell surface expression of RXFP1 in stably transfected HEK cells was performed by cell surface ELISA using an HA antibody. Treatment of cells with 10 nm relaxin induced rapid sequestration of receptors and reached maximum levels of 20–23% in the first 30 min of treatment (Fig. 3B). Therefore, RXFP1 exhibited a similar agonist-dependent internalization but slightly lower than that of decidual cells.
Effect of β-arrestins on relaxin-induced internalization of RXFP1
To show whether RXFP1 internalization was β-arrestin dependent, HEK-RXFP1 cells were cotransfected with β-arrestin constructs (Fig. 4A, upper panel). Overexpression of β-arrestin 2 significantly increased (P < 0.05) loss of cell surface expression at 30 and 60 min after 10 nm relaxin by 30 ± 2 and 28 ± 3% compared with controls, 15 ± 3 and 7 ± 2%, respectively (Fig. 4A, upper panel). β-Arrestin 1 had no effect on ligand-induced internalization of RXFP1 (Fig. 4A, upper panel). This agrees with the effect of β-arrestin 2 on desensitization of RXFP1 (Fig. 2D). Effects of internalization and β-arrestin 2 using a LDL-A module-deleted RXFP1 variant (delLDL-RXFP1) was investigated. We previously showed the LDL-A module of RXFP1 is important in receptor activation, the delLDL-RXFP1 variant had no cAMP response after relaxin activation (13). Others have shown that this variant retains relaxin binding, demonstrating its involvement in signaling but not ligand binding (34). The internalization assay was performed with HEK293 stably transfected with this variant. No internalization occurred after relaxin treatment and cotransfection of β-arrestin 2 had no effect on its internalization (Fig. 4A, lower panel).
Figure 4.
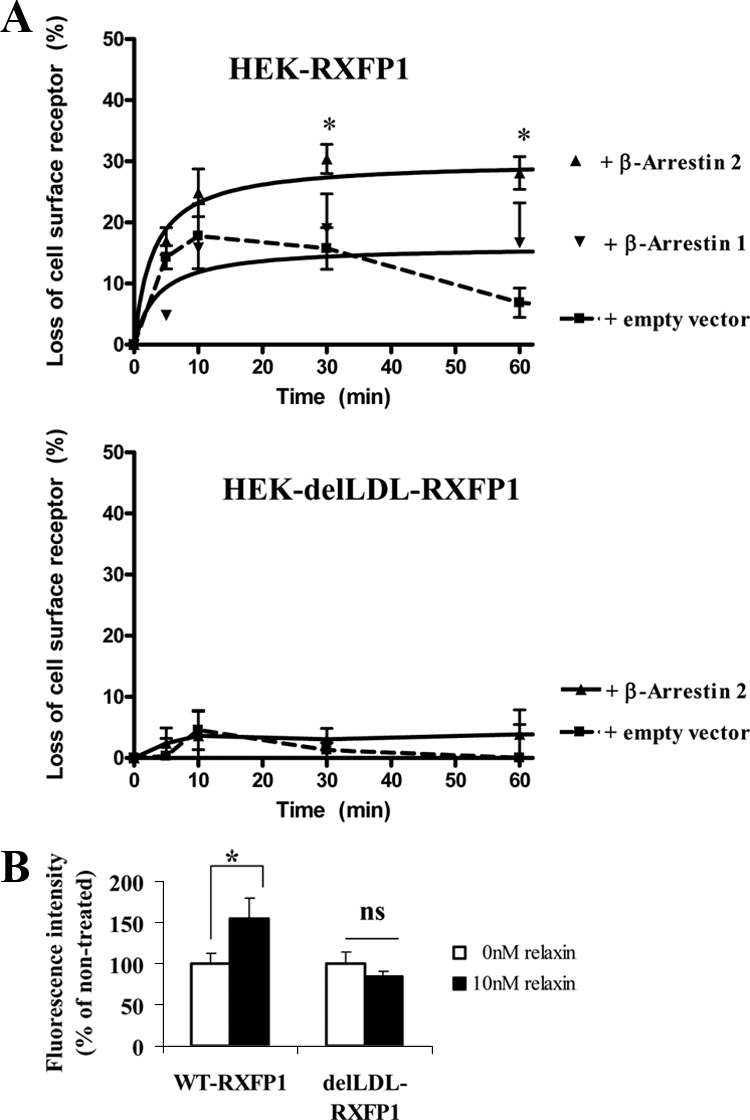
A. Effects of β-arrestins 1 and 2 on the internalization of RXFP1 and delLDL-RXFP1 in stably transfected HEK293 cells. HEK-RXFP1 cells (upper panel) or HEK-delLDL-RXFP1 cells (lower panel) were transfected with either of the β-arrestins or an empty vector. Cells were stimulated with 10 nm relaxin for the time indicated (5–60 min) and the cell surface expression of RXFP1 measured by ELISA. Overexpression of β-arrestin 2 caused the significantly increased loss of cell surface expression in HEK-RXFP1 cells, whereas overexpression of β-arrestin 1 had no significant effect (upper panel). There was no internalization of the delLDL-RXFP1 variant with or without cotransfection with β-arrestin 2 and treatment with relaxin up to 60 min. Data are means ± sem of three independent experiments performed in duplicate and expressed as the percentage loss of cell surface expression compared with unstimulated cells (0 min). *, P < 0.05 compared with cells transfected with an empty vector. B, Translocation of β-arrestin 2-YFP to the membrane after relaxin treatment. HEK-RXFP1 cells and HEK-delLDL-RXFP1 cells were cotransfected with β-arrestin 2-YFP and stimulated with 10 nm relaxin for 10 min. Membranes were isolated and β-arrestin 2-YFP monitored by fluorescence measurement of YFP. A significant increase in the fluorescence of the membrane preparation from the relaxin-treated HEK-RXFP1 cells but no significant (ns) increase in the delLDL-RXFP1 variant after relaxin treatment. β-Arrestin 2 therefore has an important role in RXFP1 internalization. Data are means ± sem of three independent experiments performed in duplicate and expressed as percentage of unstimulated cells (0 min, 100%). *, P < 0.05 compared with unstimulated cells.
Agonist-induced activation of GPCRs causes translocation of arrestin proteins to the plasma membrane (35). We also investigated whether activated β-arrestin 2 is translocated to the membrane fraction in agonist-induced HEK-RXFP1 cells. Cotransfected cells using a β-arrestin 2-YFP were treated with 10 nm relaxin for 10 min and fluorescence of membrane fractions were compared from treated and untreated cells. Western blot confirmed the membrane fraction using Na/K ATPase as a marker (data not shown). A significant increase (P < 0.05) was measured in relaxin-treated HEK-RXFP1 cells (150 ± 14%) compared with controls (Fig. 4B). In contrast, β-arrestin 2 translocation to the cell membrane in HEK-delLDL-RXFP1 cells after relaxin treatment showed no significant changes compared with controls (Fig. 4B), confirming our previous results (Fig. 4A, lower panel). Thus, β-arrestin 2 plays an important role in RXFP1 internalization and activated β-arrestin 2 is translocated to the membrane after relaxin treatment.
Agonist-induced internalization of RXFP1 occurs through dimerization
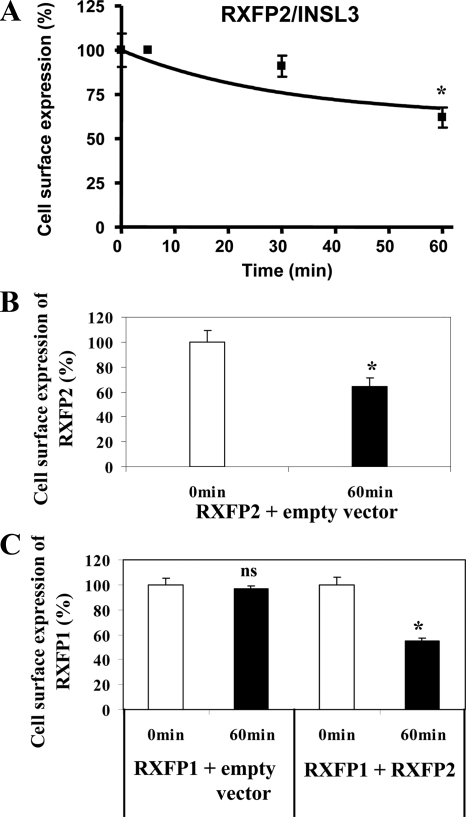
Recent studies have shown that RXFP1 forms dimers (25,36). This occurs early in the ER and is maintained through its trafficking to the plasma membrane (25). We used RXFP1/RXFP2 heterodimerization to study the role of RXFP1 dimers in internalization. We exploited two facts: first, that RXFP1 and RXFP2 form constitutive heterodimers (37) and second, that INSL3 only binds and activates RXFP2 (38). HEK293 cells transfected with RXFP2 were treated with 10 nm INSL3 and significant (P < 0.05) internalization of RXFP2 occurred after 60 min of treatment (Fig. 5A). Thus, INSL3 induced internalization of RXFP2 (64 ± 7%) compared with controls (Fig. 5B). In contrast, cells transfected with RXFP1 showed no INSL3-induced internalization (Fig. 5C), confirming that INSL3 does not activate RXFP1. However, in HEK293 cells cotransfected with RXFP1 and RXFP2, RXFP1 internalization was then detected by 60 min with 10 nm INSL3 (Fig. 5C). Thus, cell surface expression of RXFP1 was significantly decreased (55 ± 3%) compared with controls (Fig. 5C), confirming RXFP1/RXFP2 dimerization. These results show that internalization of RXFP1 occurs through dimer formation and suggests that INSL3 ligand, which does not normally affect signaling of RXFP1, could affect the function of RXFP1 if RXFP1/RXFP2 constitutive heterodimers are present in the plasma membrane.
Figure 5.
RXFP1 internalization occurs through dimerization. A, INSL3 induced loss of cell surface expression of RXFP2 measured by cell surface ELISA in RXFP2 transiently transfected HEK293 cells. Cells were treated with 10 nm INSL3 for the times shown (5–60 min) and the cell surface ELISA performed. *, P < 0.05 compared with unstimulated cells. B and C, RXFP1 internalization was caused through RXFP1/RXFP2 heterodimerization. HEK293 cells transiently cotransfected with RXFP2 and an empty vector were treated with 10 nm INSL3 for 60 min and the cell surface ELISA performed (B). HEK293 cells transiently cotransfected with either RXFP1 and an empty vector or with RXFP1 and RXFP2, were treated with 10 nm INSL3 for 60 min, and the cell surface ELISA performed (C). Cell surface expression was normalized to the basal RXFP2 (A and B) or RXFP1 (C) cell surface expression for the untreated cells at 0 min. Cell surface expression of RXFP1 was significantly decreased in the cells cotransfected with RXFP1 and RXFP2, confirming that dimerization had occurred. Data are means ± sem of three independent experiments performed in duplicate. *, P < 0.05 compared with unstimulated cells. ns, Not significant.
Constitutive internalization of RXFP1 and the role of its C-terminal tail
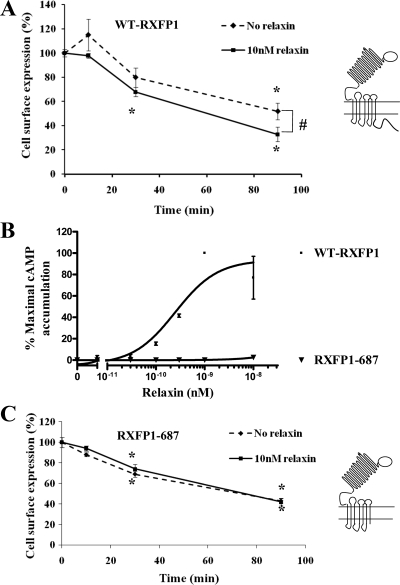
It has been reported that some GPCRs internalize constitutively without agonist induction (28,39,40,41); this was investigated for RXFP1. Internalization of RXFP1 was measured in HEK-RXFP1 cells by showing its disappearance from the cell surface in the absence of relaxin. We showed that RXFP1 undergoes constitutive internalization to intracellular sites (48% loss of cell surface expression after 90 min; Fig. 6A). Agonist-dependent constitutive internalization of RXFP1 over the same time-frame was shown after prelabeling with the HA antibody at 4 C in the presence of 10 nm relaxin. Constitutive internalization of RXFP1 was significantly (P < 0.05) enhanced by 90 min in the presence of relaxin (Fig. 6A).
Figure 6.
Constitutive internalization of RXFP1. A, HEK-RXFP1 cells were preincubated with an HA antibody, washed, and incubated at 37 C for the times indicated, in the absence (♦) or presence (▪) of 10 nm relaxin. Receptors remained at the cell surface after incubation at 37 C either with or without relaxin were expressed as percentage of receptors at the cell surface at 0 min. Relaxin significantly enhanced the constitutive internalization of RXFP1. Data are means ± sem of three independent experiments performed in duplicate. *, P < 0.05 compared with 0 min; #, P < 0.05 compared with nonstimulated cells. WT-RXFP1, Wild-type RXFP1 . B, Functional characterization of variant RXFP1-687 in HEK293 cells. HEK293 cells were transiently transfected with either wild-type RXFP1 or variant RXFP1-687 with the carboxyl terminus deleted. Relaxin caused a dose-dependent stimulation of cAMP production in cells transfected with WT-RXFP1 (▪), whereas it had no effect on the cells transfected with RXFP1-687 (▾). This showed the importance of the carboxyl terminus of RXFP1 in signal transduction. Accumulation of cAMP was expressed as percentage of the maximal relaxin response of the WT-RXFP1. Data are mean ± sem of two independent experiments performed in duplicate. C, Constitutive internalization of RXFP1-687 in HEK293 cells. HEK293 cells transiently transfected with RXFP1-687 were preincubated with an HA antibody, washed, and warmed to 37 C for the times indicated in the absence (♦) or presence (▪) of 10 nm relaxin. Receptors remained at the cell surface after warming to 37 C in either the absence or presence of relaxin and were expressed as a percentage of receptors at the cell surface at 0 min. The RXFP1-687 variant underwent constitutive internalization to the similar extent as WT-RXFP1. However, there was no significant change in the presence of relaxin, showing that the carboxyl terminus of RXFP1 is important in agonist-induced internalization of RXFP1. Data are means ± sem of three independent experiments performed in duplicate. *, P < 0.05 compared with 0 min.
Constitutive internalization of GPCRs is dependent on their carboxyl-terminal region (28). This was examined for RXFP1 by production of a carboxyl terminal deleted variant (RXFP1-687). As seen in Fig. 6B, the RXFP1-687 variant lost receptor function after relaxin treatment, showing the importance of its carboxyl terminus in signal transduction. The constitutive internalization of RXFP1-687 in the absence of ligand was shown after prelabeling the cell surface receptors with HA antibody. There was constitutive internalization to an intracellular site to a similar extent as wild-type RXFP1 (55% loss of cell surface expression of RXFP1-687 after 90 min incubation; Fig. 6C), showing that constitutive internalization of RXFP1 did not appear to be dependent on its intracellular carboxyl terminus. Furthermore, measurement of the agonist-dependent internalization of RXFP1-687 showed no increase in the amount of internalization above the constitutive level in the presence of 10 nm relaxin (Fig. 6C), suggesting that the intracellular carboxyl terminal of RXFP1 is important in agonist-induced internalization of this receptor.
Discussion
We report here that the relaxin receptor RXFP1 exhibits desensitization and internalization after agonist activation and β-arrestin 2 is important in these processes in both primary human decidual cells and HEK293 cells stably transfected with RXFP1 used as a control.
The primary relaxin binding sites in the human fetal membranes are decidua and chorionic cytotrophoblast (10), confirmed by RXFP1 mRNA expression (23). Overexpression of relaxin or RXFP1 correlates with the preterm premature rupture of the membranes and preterm birth, suggesting their importance in this pathology (8,23). Therefore, an understanding of the regulation of signaling and trafficking of RXFP1 is important for the potential development of pharmacotherapeutics. Primary decidual cells were used as physiologically relevant cells together with HEK293 cells transfected with RXFP1 as controls. RXFP1protein in HEK-RXFP1 cells is expressed as a mature form delivered to the cell surface and an immature form accumulated in the ER (13). In decidual cells, RXFP1 expression was studied by Western blot, immunocytochemistry, and flow cytometry. Lower-molecular-weight forms compared with the RXFP1 protein expressed in HEK-293 cells were detected. RXFP1 is highly glycosylated in HEK293 cells (13), and these differences suggest that the two cell types glycosylate RXFP1 differently. Immunofluorescence of decidual cells showed the RXFP1 protein localized in the ER, confirming similarity of its biosynthesis and expression in decidual cells and HEK293 cells. Further studies are needed to explore any role of the intracellularly retained RXFP1. Several other GPCRs are inefficiently expressed at the plasma membrane and accumulate intracellularly (42). A new paradigm is emerging in which it appears that there can be a pool of functional receptors retained inside the cell, which can be rapidly mobilized to the plasma membrane as needed, maintaining the cells at a high level of physiological responsiveness (42).
Relaxin causes increased cAMP accumulation in human endometrial glandular epithelial cells (43). Here we detected a relaxin dose-dependent increase in cAMP with an EC50 similar to that of HEK293 cells transfected with RXFP1. Thus, our results show the RXFP1 protein expressed by primary decidual cells, delivery to the cell surface and their response to relaxin with a similar efficacy as HEK293 transfected with RXFP1.
Receptor desensitization is an important physiological process resulting in attenuation of receptor responsiveness (16). This mechanism protects the cells from acute and chronic overstimulation after agonist activation. However, receptor desensitization can also significantly limit the therapeutic usefulness of many receptor agonists. Different mechanisms can contribute to receptor desensitization. GPCR kinases and arrestin proteins can contribute to receptor desensitization, which also initiate agonist-stimulated GPCR endocytosis and intracellular trafficking. Desensitization, internalization, and functional importance of the arrestin proteins have been demonstrated for other LGR GPCRs: TSHR, LHR, and FSHR. These receptors undergo β-arrestin 1 and 2-dependent desensitization with enhanced internalization by β-arrestin 2 (20,21,22), TSHR has a higher affinity to β-arrestin 2 than β-arrestin 1 (22). We investigated the desensitization and down-regulation of signaling of RXFP1 after relaxin treatment. Interestingly, in these desensitization experiments, both decidual cells, and HEK-RXFP1 cells showed slight increases in the first 10 min. This can be interpreted in context of a previous report showing that relaxin activation induced a rapid cAMP response also through recruitment of protein kinase Cζ to the membrane and activation of adenylyl cyclase (44). Another possibility is that relaxin induces a rapid but brief increase in the cell surface delivery of RXFP1, although we did not detect this with the cell surface ELISA (Figs. 3, 4, and 6), probably because the measurement of cAMP is more sensitive than the cell surface ELISA; therefore, further studies are needed to clarify this. Decidual cells desensitized to a similar level as HEK-RXFP1 after relaxin treatment, suggesting similar desensitization, also validating HEK-RXFP1 cells as a model. Therefore, we investigated which β-arrestin would affect this process using HEK-RXFP1 cells. The decidual cells and HEK-RXFP1 cells endogenously expressed the arrestin proteins at similar levels. However, cAMP accumulation after relaxin treatment was significantly decreased in HEK-RXFP1 cells cotransfected with β-arrestin 2 compared with the controls, showing that β-arrestin 2 is predominant in the cAMP-dependent regulation of RXFP1 desensitization.
Internalization or endocytosis of GPCRs after agonist induction removes receptors from the cell surface and may contribute to desensitization and allow the fine-tuning of the resulting signal (17,18). Internalization after relaxin-treatment was shown here in both decidual cells and HEK-RXFP1. Rapid internalization of RXFP1 occurred with a 20–30% loss of the cell surface receptor after 5 min and was maintained for 60 min. The time courses of desensitization and internalization were slightly different, with rapid internalization compared with slower desensitization. Relaxin treatment of THP1 cells induces activation and recruitment of protein kinase Cζ to the plasma membrane and activation of adenylyl cyclase, inducing the second wave of cAMP production and might contribute to the slower desensitization after relaxin activation shown here (44). Receptor activation causes G proteins to localize to the endosomes in which they stimulate effector proteins (45,46), suggesting a possible mechanism of cointernalization of agonist-activated receptor with G protein and signaling from endosomes, which might contribute to a slower desensitization process. Further studies need to be performed to dissect the mechanism underlying this slow desensitization of RXFP1.
The arrestin proteins are central players in desensitization and GPCR internalization, intracellular trafficking, and signaling (17,18). Our results demonstrate that β-arrestin 2 significantly increased internalization of RXFP1. Thus, RXFP1 belongs to class A receptors, which internalize through a β-arrestin 2-dependent mechanism (19). Furthermore, β-arrestin 2 activation was shown here after relaxin treatment. The delLDL-RXFP1 variant retains relaxin binding (34) but is unable transduce signal (13), and only marginal internalization was shown after relaxin exposure. In addition, β-arrestin 2 had no effect on the internalization of delLDL-RXFP1 or its translocation to the membrane, showing that arrestin is normally activated by relaxin and translocated to the membrane.
GPCRs often internalize as oligomers (47). Some receptors cointernalize in a heterodimer pair, even when the agonist is present for only one of the receptors (48). Dimerization appears to be required for such cointernalization (49). Therefore, we investigated whether internalization of RXFP1 occurs as dimers/oligomers by using the known heterodimerization of RXFP1 and RXFP2 (37). Relaxin is a ligand and activates both RXFP1 and RXFP2 (50); we therefore used INSL3 as ligand, which binds and activates only RXFP2 and not RXFP1 (50). We have shown that INSL3 promoted internalization of RXFP2 but not RXFP1. However, when RXFP1 and RXFP2 were coexpressed, INSL3 treatment decreased cell surface expression of the RXFP1, showing that RXFP2 internalization also promoted internalization of RXFP1, suggesting that RXFP1 internalization occurs through the dimerization of the receptor.
In nonstimulated cells, the majority of the RXFP1 is localized intracellularly [∼60–70% of total receptors (13)]. We confirmed this here, suggesting the existence of an intracellular pool of receptors under basal conditions. Constitutive internalization occurs for some GPCRs, and we investigated this for RXFP1. Using HEK-RXFP1 cells, we detected slow, agonist-independent constitutive internalization of RXFP1. Although our carboxyl-terminal deleted (RXFP1-687) variant showed no signal transduction after relaxin treatment, it showed a similar level of constitutive agonist-independent internalization as the wild-type receptor, suggesting that the carboxyl-terminal is uninvolved in its constitutive internalization. This agrees with a recent study of GnRH receptor, in which constitutive agonist-independent internalization was not dependent on its cytoplasmic carboxyl-terminal tail (28). Constitutive internalization has been associated with constitutive activation states of GPCRs, fitting well with a canonical model of activation-dependent trafficking of GPCRs. Constitutive internalization influences receptor function in vivo, as shown for the vasopressin receptor (51). Our data show that in addition to the rapid agonist-induced internalization of RXFP1, it undergoes slower constitutive agonist-independent internalization and that the activation state of the receptor is not involved in this process.
In summary, we demonstrate the desensitization and internalization of RXFP1 after relaxin treatment. Other GPCRs have been shown to desensitize and internalize more rapidly and completely than shown here for RXFP1. However, RXFP1 is expressed only at very low levels on any cell type studied to date, and we believe that the amounts of desensitization and internalization shown here may be physiologically important for the action of relaxin. We have also shown that β-arrestin 2 was predominant compared with β-arrestin 1, suggesting classification of RXFP1 as a member of class A GPCRs. Dimerization/oligomerization of RXFP1 was shown to be necessary for receptor internalization. This occurred constitutively and was significantly increased in the presence of ligand. Relaxin treatment of human endometrial (52) and decidual cells (23) increases RXFP1 mRNA, but the data presented here suggest that relaxin action in the fetal membranes is also controlled by the level of its receptors expressed on the cell surface of its target cells.
Acknowledgments
We thank the nurses and staff of the labor and delivery unit of Kapiolani Medical Center for Women and Children for their help with tissue collection. Special thanks go to S. Yamamoto for collection of tissues and preparing primary cells. We also acknowledge BAS Medical for the generous gift of human relaxin and Dr. J. Benovic (Jefferson Medical College, Philadelphia, PA) for β-arrestin 1 and 2 cDNA and Dr. A. I. Agoulnik (Baylor Collage of Medicine, Houston, TX) for the RXFP2 cDNA.
Footnotes
This work was supported by Grant HD24314 from the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 30, 2008
Abbreviations: ER, Endoplasmic reticulum; FACS, fluorescence-activated cell sorting; FSHR, FSH receptor; GPCR, G protein-coupled receptor; HA, hemagglutinin; HEK293, human embryonic kidney 293; HEK-RXFP1, HEK293 cells stably transfected with RXFP1; INSL3, insulin-like peptide 3; LDL-A, low-density lipoprotein class A; LGR, leucine-rich repeat containing G-protein coupled receptor; LHR, LH receptor; qRT-PCR, quantitative RT-PCR; RXFP, relaxin family peptide receptor; TSHR, TSH receptor; WT, wild type; YFP, yellow fluorescent protein.
References
- James R, Niall H, Kwok S, Bryant-Greenwood GD 1977 Primary structure of porcine relaxin: homology with insulin and related growth factors. Nature 267:544–546 [DOI] [PubMed] [Google Scholar]
- Sherwood OD 2004 Relaxin’s physiological roles and other diverse actions. Endocr Rev 25:205–234 [DOI] [PubMed] [Google Scholar]
- Hombach-Klonisch S, Bialek J, Trojanowicz B, Weber E, Holzhausen HJ, Silvertown JD, Summerlee AJ, Dralle H, Hoang-Vu C, Klonisch T 2006 Relaxin enhances the oncogenic potential of human thyroid carcinoma cells. Am J Pathol 169:617–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CS, Hewitson TD 2006 Relaxin in cardiovascular and renal disease. Kidney Int 69:1498–1502 [DOI] [PubMed] [Google Scholar]
- Mookerjee I, Solly NR, Royce SG, Tregear GW, Samuel CS, ML. T 2006 Endogenous relaxin regulates collagen deposition in an animal model of allergic airway disease. Endocrinology 147:754–761 [DOI] [PubMed] [Google Scholar]
- Feng S, Agoulnik IU, Bogatcheva NV, Kamat AA, Kwabi-Addo B, Li R, Ayala G, Ittmann MM, Agoulnik AI 2007 Relaxin promotes prostate cancer progression. Clin Cancer Res 13:1695–1702 [DOI] [PubMed] [Google Scholar]
- Smith MC, Danielson LA, Conrad KP, Davison JM 2006 Influence of recombinant human relaxin on renal hemodynamics in healthy volunteers. J Am Soc Nephrol 17:3192–3197 [DOI] [PubMed] [Google Scholar]
- Bogic LV, Yamamoto SY, Millar LK, Bryant-Greenwood GD 1997 Developmental regulation of the human relaxin genes in the decidua and placenta: overexpression in the preterm premature rupture of the fetal membranes. Biol Reprod 57:908–920 [DOI] [PubMed] [Google Scholar]
- Koay ES, Bryant-Greenwood GD, Yamamoto SY, Greenwood FC 1986 The human fetal membranes: a target tissue for relaxin. J Clin Endocrinol Metab 62:513–521 [DOI] [PubMed] [Google Scholar]
- Garibay-Tupas JL, A. Maaskant R, Greenwood FC, Bryant-Greenwood GD 1995 Characteristics of the binding of 32P-labelled human relaxins to the human fetal membranes. J Endocrinol 145:441–448 [DOI] [PubMed] [Google Scholar]
- Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ 2002 Activation of orphan receptors by the hormone relaxin. Science 295:671–674 [DOI] [PubMed] [Google Scholar]
- Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, van Duin M, Hsueh AJ 2000 The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol 14:1257–1271 [DOI] [PubMed] [Google Scholar]
- Kern A, Agoulnik AI, Bryant-Greenwood GD 2007 The low-density lipoprotein class A module of the relaxin receptor (leucine-rich repeat containing G-protein coupled receptor 7): its role in signaling and trafficking to the cell membrane. Endocrinology 148:1181–1194 [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Ferlin A, Feng S, Truong A, Gianesello L, Foresta C, Agoulnik AI 2007 T222P mutation of the insulin-like 3 hormone receptor LGR8 is associated with testicular maldescent and hinders receptor expression on the cell surface membrane. Am J Physiol Endocrinol Metab 292:E138–E144 [DOI] [PubMed] [Google Scholar]
- Hsu SY 2003 New insights into the evolution of the relaxin-LGR signaling system. Trends Endocrinol Metab 14:303–309 [DOI] [PubMed] [Google Scholar]
- Ferguson SS 2001 Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53:1–24 [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL 2007 Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol 69:451–482 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK 2005 Transduction of receptor signals by β-arrestins. Science 308:512–517 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS 2000 Differential affinities of visual arrestin, β arrestin1, and β arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275:17210–17210 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Lazari MF, Li S, Korgaonkar C, Ascoli M 1999 Role of the rate of internalization of the agonist-receptor complex on the agonist-induced down-regulation of the lutropin/choriogonadotropin receptor. Mol Endocrinol 13:1295–1304 [DOI] [PubMed] [Google Scholar]
- Lazari MF, Liu X, Nakamura K, Benovic JL, Ascoli M 1999 Role of G protein-coupled receptor kinases on the agonist-induced phosphorylation and internalization of the follitropin receptor. Mol Endocrinol 13:866–876 [DOI] [PubMed] [Google Scholar]
- Frenzel R, Voigt C, Paschke R 2006 The human thyrotropin receptor is predominantly internalized by β-arrestin 2. Endocrinology 147:3114–3122 [DOI] [PubMed] [Google Scholar]
- Lowndes K, Amano A, Yamamoto S, Bryant-Greenwood GD 2006 The human relaxin receptor (LGR7): expression in the fetal membranes and placenta. Placenta 27:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashima LS, Yamamoto SY, Yasuda M, Millar LK, Bryant-Greenwood GD 2002 Decidual relaxins: gene and protein up-regulation in preterm premature rupture of the membranes by complementary DNA arrays and quantitative immunocytochemistry. Am J Obstet Gynecol 187:785–797 [DOI] [PubMed] [Google Scholar]
- Kern A, Hubbard D, Amano A, Bryant-Greenwood GD 2008 Cloning, expression, and functional characterization of relaxin receptor (leucine-rich repeat-containing G protein-coupled receptor 7) splice variants from human fetal membranes. Endocrinology 149:1277–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simhan HN, Chura JC, Rauk PN 2004 The effect of the anti-inflammatory cytokines interleukin-4 and interleukin-10 on lipopolysaccharide-stimulated production of prostaglandin E2 by cultured human decidual cells. J Reprod Immunol 64:1–7 [DOI] [PubMed] [Google Scholar]
- Cakmak H, Schatz F, Huang ST, Buchwalder L, Rahman M, Arici A, Lockwood CJ 2005 Progestin suppresses thrombin- and interleukin-1β-induced interleukin-11 production in term decidual cells: implications for preterm delivery. J Clin Endocrinol Metab 90:5279–5286 [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Faccenda E, Maudsley S, Lu ZL, Naor Z, Millar RP 2008 Mammalian type I gonadotropin-releasing hormone receptors undergo slow, constitutive, agonist-independent internalization. Endocrinology 149:1415–1422 [DOI] [PubMed] [Google Scholar]
- Ivell R, Balvers M, Pohnke Y, Telgmann R, Bartsch O, Milde-Langosch K, Bamberger AM, Einspanier 2003 A Immunoexpression of the relaxin receptor LGR7 in breast and uterine tissues of humans and primates. Reprod Biol Endocrinol 1:114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippig S, Andexinger S, Daniel K, Puzicha M, Caron MG, Lefkowitz RJ, Lohse MJ 1993 Overexpression of β-arrestin and β-adrenergic receptor kinase augment desensitization of β2-adrenergic receptors. J Biol Chem 268:3201–3208 [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV 2006 The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther 110:465–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick JG, Gurevich VV, Benovic JL 1997 Mechanism of quenching of phototransduction. Binding competition between arrestin and transducin for phosphorhodopsin. J Biol Chem 272:18125–18131 [DOI] [PubMed] [Google Scholar]
- Ménard L, Ferguson SS, Zhang J, Lin FT, Lefkowitz RJ, Caron MG, Barak LS 1997 Synergistic regulation of β2-adrenergic receptor sequestration: intracellular complement of β-adrenergic receptor kinase and beta-arrestin determine kinetics of internalization. Mol Pharmacol 51:800–808 [PubMed] [Google Scholar]
- Scott DJ, Layfield S, Yan Y, Sudo S, Hsueh AJ, Tregear GW, Bathgate RA 2006 Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique low density lipoprotein class A modules. J Biol Chem 281:34942–34954 [DOI] [PubMed] [Google Scholar]
- Zhang J, Barak LS, Anborgh PH, Laporte SA, Caron MG, Ferguson SS 1999 Cellular trafficking of G protein-coupled receptor/β-arrestin endocytic complexes. J Biol Chem 274:10999–11006 [DOI] [PubMed] [Google Scholar]
- Svendsen AM, Zalesko A, Kønig J, Vrecl M, Heding A, Kristensen JB, Wade JD, Bathgate RA, De Meyts P, Nøhr J 2008 Negative cooperativity in H2 relaxin binding to a dimeric relaxin family peptide receptor 1. Mol Cell Endocrinol 296:10–17 [DOI] [PubMed] [Google Scholar]
- Svendsen AM, Vrecl M, Ellis TM, Heding A, Kristensen JB, Wade JD, Bathgate RA, De Meyts P, Nøhr J 2008 Cooperative binding of insulin-like peptide 3 to a dimeric relaxin family peptide receptor 2. Endocrinology 149:1113 [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Truong A, Feng S, Engel W, Adham IM, Agoulnik AI 2003 GREAT/LGR8 is the only receptor for insulin-like 3 peptide. Mol Endocrinol 17:2639–2646 [DOI] [PubMed] [Google Scholar]
- Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J 2002 β-Arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem 277:1292–1300 [DOI] [PubMed] [Google Scholar]
- Mohammad S, Baldini G, Granell S, Narducci P, Martelli AM, Baldini G 2007 Constitutive traffic of melanocortin-4 receptor in neuro2A cells and immortalized hypothalamic neurons. J Biol Chem 282:4963–4974 [DOI] [PubMed] [Google Scholar]
- McDonald NA, Henstridge CM, Connolly CN, Irving AJ 2007 An essential role for constitutive endocytosis, but not activity, in the axonal targeting of the CB1 cannabinoid receptor. Mol Pharmacol 71:976–984 [DOI] [PubMed] [Google Scholar]
- Achour L, Labbé-Jullié C, Scott MG, Marullo S 2008 An escort for GPCRs: implications for regulation of receptor density at the cell surface. Trends Pharmacol Sci 29:528–535 [DOI] [PubMed] [Google Scholar]
- Chen GA, Huang JR, Tseng L 1988 The effect of relaxin on cyclic adenosine 3′,5′-monophosphate concentrations in human endometrial glandular epithelial cells. Hum Reprod Update 4:355–358 [DOI] [PubMed] [Google Scholar]
- Nguyen BT, Dessauer CW 2005 Relaxin stimulates protein kinase Cζ translocation: requirement for cyclic adenosine 3′,5′-monophosphate production. Mol Endocrinol 19:1012–1023 [DOI] [PubMed] [Google Scholar]
- Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG 2006 Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein α subunit at the endosome. Cell 126:191–203 [DOI] [PubMed] [Google Scholar]
- García-Regalado A, Guzmán-Hernández ML, Ramírez-Rangel I, Robles-Molina E, Balla T, Vázquez-Prado J, Reyes-Cruz G 2008 GPCR-promoted trafficking of Gβ1γ2 leads to AKT activation at endosomes via a mechanism mediated by Gβ1γ2-Rab11a interaction. Mol Biol Cell 19:4188–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttenrauch F, Pollok-Kopp B, Oppermann M 2005 G protein-coupled receptor kinases promote phosphorylation and β-arrestin-mediated internalization of CCR5 homo- and hetero-oligomers. J Biol Chem 280:37503–37515 [DOI] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferre S, Fuxe K 2002 Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem 277:18091–18097 [DOI] [PubMed] [Google Scholar]
- Stanasila L, Perez J-B, Vogel H, Cotecchia S 2003 Oligomerization of the α1a- and α1b-adrenergic receptor subtypes. J Biol Chem 278:40239–40251 [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ 2006 International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharm Rev 58:7–31 [DOI] [PubMed] [Google Scholar]
- Barak LS, Oakley RH, Laporte SA, Caron MG 2001 Constitutive arrestinmediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc Natl Acad Sci USA 98:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazella J, Tang M, Tseng L 2004 Disparate effects of relaxin and TGFβ1: relaxin increases, but TGFβ1 inhibits, the relaxin receptor and the production of IGFBP-1 in human endometrial stromal/decidual cells. Hum Reprod 19:1513–1518 [DOI] [PubMed] [Google Scholar]