Abstract
GnRH receptor activation elicits release of intracellular Ca2+, which leads to secretion and also activates Ca2+-activated ion channels underlying membrane voltage changes. The predominant Ca2+-activated ion channels in rat and mouse gonadotrophs are Ca2+-activated K+ channels. To establish the temporal relationship between GnRH-induced changes in intracellular [Ca2+] ([Ca2+]i) and membrane current (Im), and to identify specific Ca2+-activated K+ channels linking GnRH-induced increase in [Ca2+]i to changes in plasma membrane electrical activity, we used single female mouse gonadotrophs in the perforated patch configuration of the patch-clamp technique, which preserves signaling pathways. Simultaneous measurement of [Ca2+]i and Im in voltage-clamped gonadotrophs revealed that GnRH stimulates an increase in [Ca2+]i that precedes outward Im, and that activates two kinetically distinct currents identified, using specific toxin inhibitors, as small conductance Ca2+-activated K+ (SK) current (ISK) and large (big) conductance voltage- and Ca2+-activated K+ (BK) current (IBK). We show that the apamin-sensitive current has an IC50 of 69 pM, consistent with the SK2 channel subtype and confirmed by immunocytochemistry. The magnitude of the SK current response to GnRH was attenuated by 17β-estradiol (E2) pretreatment. Iberiotoxin, an inhibitor of BK channels, completely blocked the residual apamin-insensitive outward Im, substantiating that IBK is a component of the GnRH-induced outward Im. In contrast to its suppression of ISK, E2 pretreatment augmented peak IBK. SK or BK channel inhibition modulated GnRH-stimulated LH secretion, implicating a role for these channels in gonadotroph function. In summary, in mouse gonadotrophs the GnRH-stimulated increase in [Ca2+]i activates ISK and IBK, which are differentially regulated by E2 and which may be targets for E2 positive feedback in LH secretion.
The GnRH-stimulated rise in intracellular [Ca2+] activates both small and large conductance Ca2+-activated K+ currents, which are differentially regulated by estradiol-17β (E2) and which may be targets for E2 positive feedback in luteinizing hormone secretion.
In the anterior pituitary gland, the hypothalamic-releasing hormone GnRH has pleiotropic actions on gonadotrophs that span a time scale of minutes to hours. On the shortest time scale, GnRH elicits gonadotrophin secretion and characteristic changes in gonadotroph plasma membrane voltage (Vm). The link between gonadotroph GnRH receptor activation and electrical activity is the release of Ca2+ from intracellular stores that leads to hormone secretion and stimulation of plasma membrane Ca2+-activated ion channels that underlie the Vm changes (reviewed in Ref. 1). The predominant Ca2+-activated ion channels in rat and mouse gonadotrophs are Ca2+-activated K+ channels (1,2).
Activation of K+ channels hyperpolarizes the Vm. In rat gonadotrophs, hyperpolarizing oscillations in Vm track GnRH-induced oscillations in intracellular [Ca2+] ([Ca2+]i) (3). Vm hyperpolarization removes voltage-dependent channel inactivation and, on subsequent depolarization, leads to the entry of extracellular (EC) Ca2+ through voltage-activated Ca2+ channels, which is necessary for Ca2+ homeostasis, including the maintenance of intracellular Ca2+ stores (1).
Several Ca2+-activated ion channels are present in the gonadotroph plasma membrane. Most studied are small conductance Ca2+-activated K+ (SK) channels, long known to be present in sheep (4) and rat gonadotrophs (5,6). In addition, large (big) conductance voltage- and Ca2+-activated K+ (BK) channels (7) and a Ca2+-activated nonspecific cation current (8) have been identified. Not as extensively studied as SK channels, the role of these channels in GnRH action involving Ca2+-dependent secretion and modulation of gonadotroph membrane excitability is not known.
SK channels, which are solely dependent on Ca2+ for activation, are fundamental components of cell excitability in, for example, neurons, smooth muscle cells, and secretory cells (9). Three genes encode the SK channel subunits (SK1, SK2, and SK3), and functional SK channels are complexes of four pore-forming subunits plus constitutively bound calmodulin that mediates Ca2+ gating of the channel (10,11). BK channels, which have dual dependence on Vm and Ca2+, are abundant, e.g. in brain, pancreas, and smooth muscle (12,13). Functional BK channels are complexes of tetrameric pore-forming α-subunits and auxiliary β-subunits, which can modify the Ca2+ sensitivity, inactivation, and pharmacological properties of the channels. Four genes encode for the β subunits (β1-β4), and the tissue-specific expression of these subunits is responsible for much of the functional diversity of BK channels.
Ca2+-activated ion channel regulation as a target for modulation of gonadotroph function has received relatively little attention. However, recently, we showed in female mouse gonadotrophs that 17β-estradiol (E2) pretreatment of pituitary cell cultures decreases peak GnRH-induced membrane current (Im) (2). This indicates an additional layer of functional complexity in the regulation of gonadotroph membrane activity.
The objectives were to establish the temporal relationship between GnRH-induced changes in [Ca2+]i and Im, and to identify specific Ca2+-activated K+ channel types in mouse gonadotrophs that link the GnRH-induced increase in [Ca2+]i to changes in plasma membrane electrical activity and their modulation by E2. The model was single gonadotrophs from adult female mice, and the electrophysiological approach was the perforated-patch configuration of the patch-clamp technique, which preserves signaling pathways, including GnRH-induced Ca2+ oscillations.
Materials and Methods
Materials
Media, trypsin, and sera were from Invitrogen-GIBCO (Carlsbad, CA). Kanamycin sulfate, BSA fraction V, amphotericin B, apamin, iberiotoxin, E2, and GnRH were from Sigma-Aldrich Corp. (St. Louis, MO). Dow Corning R6101 was from K.R. Anderson (Morgan Hill, CA). Fura-2/AM and pluronic F-127 were from Invitrogen-Molecular Probes (Carlsbad, CA). For immunofluorescence, normal goat serum and mounting medium were from Vector Laboratories (Burlingame, CA), and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat antimouse IgG and fluorescein isothiocyanate (FITC)-labeled goat antirabbit IgG were from Sigma-Aldrich. Monoclonal antibody 518B7 to bovine LH was provided by Dr. Jan Roser (University California, Davis, Davis, CA). SK2 polyclonal antibody to rat 542–559 antigen was from Alomone Labs (Jerusalem, Israel).
Animals and cell culture
The animal protocol was approved by the University of California Davis Institutional Animal Care and Use Committee. Adult female wild-type mice [C57/BL/6, Charles River (Hollister, CA)], in 12-h light, 12-h dark, were ovariectomized and maintained for 2 wk after surgery before use (2). Pituitary glands were removed after CO2 narcosis and decapitation. Anterior pituitary tissue was enzymatically dispersed (trypsin) and prepared for cell culture as described (14).
Electrophysiology
Cells were plated in 35-mm dishes incorporating a 10 or 22-mm glass-bottomed well (World Precision Instruments, Sarasota, FL, or Bioscience Tools, San Diego, CA) at low density (∼15,000 cells per well) and flooded with MEM supplemented with 200 μm kanamycin sulfate, 10% fetal bovine serum that had been charcoal treated to remove endogenous steroids (15), and without (no steroid pretreatment) or with (E2 pretreatment) 0.2 nm E2. Cells were maintained in a humidified atmosphere (37 C) of 5% CO2 in air and used on d 2–5 (day of plating = d 0).
Immunocytochemistry
Cells were plated on 12-mm glass coverslips (20,000 cells per coverslip) and cultured as described previously. On d 2, medium was replenished, and on d 3, cells were fixed for immunocytochemistry.
LH secretion
Cells were plated in 22-mm multi-well plates (2 × 105 cells per well) and cultured as described previously with or without 0.2 nm E2. Medium was replenished on d 2, and secretion experiments were performed on d 3.
Experimental procedures
Electrophysiology
Culture medium was replaced with an EC medium, without added E2, consisting of (in mm): 135 NaCl, 5 KCl, 5 CaCl2, 1.3 MgCl2, 4 NaHCO3, 5 glucose, 10 HEPES 1, mg ml−1 BSA, and was adjusted to pH 7.4 with 5 n NaOH. Culture dishes were transferred to an inverted microscope with a stage maintained at 22 C and superfused with EC medium at approximately 1 ml min−1. Gonadotrophs were tentatively identified based on morphological criteria as described (2). Putative gonadotrophs were voltage clamped at the indicated potential. Solution flow was switched between control EC medium and experimental media using a valve placed close to the microscope stage; GnRH, 1 nm, was applied for 1 min or as indicated. For toxin treatment, exposure was started 2 min before GnRH and was continued until the end of the GnRH treatment (or end of the acquisition). A Im response to GnRH in voltage-clamped cells was taken as confirmation that the cell was a gonadotroph. Of 253 cells identified as putative gonadotrophs, 156 (62%) subsequently responded to GnRH, similar to our previously reported success rate, 58% (2). In all cases only data from GnRH-responsive cells are reported.
Measurements were performed using the perforated-patch configuration of the tight-seal patch-clamp recording technique (16). The pipette-filling solution consisted of (in mm): 130 KAspartate, 20 KCl, 10 NaCl, 5 MgSO4, 10 HEPES, and was adjusted to pH 7.2 with 5 n KOH. Pipette fabrication and coating were as described (2). Perforation of the patch was achieved by inclusion of the pore-forming antibiotic amphotericin B. Series resistance compensation was not used; mean series resistance was 24 ± 0.5 MΩ (n = 144), and maximum uncorrected voltage error was less than 4 mV. Pipette potentials were corrected for junction and Donnan potentials, −16 mV, calculated as described (2). Recordings were made with a MultiClamp 700B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA) via a DigiData 1320A computer interface (Molecular Devices) using Clampex (pClamp software suite; Molecular Devices). Data were filtered at 600 Hz and sampled at 50 kHz. GnRH-induced currents were recorded from cells voltage clamped at −50 or −66 mV. Reported currents were normalized to membrane capacitance (pA/pF) to correct for differences in cell size; there was no difference in membrane capacitance between control and E2-pretreated cells [7.26 ± 0.13 pF (n = 73) vs. 7.09 ± 0.13 pF (n = 71); P = 0.341].
Simultaneous [Ca2+]i and electrophysiology
[Ca2+]i was determined ratiometrically with the fluorescent probe fura-2 as described (17,18) using hardware and software from Photon Technology International (Birmingham, NJ). Briefly, cells were loaded with the fluorophore by incubation with 2 μm fura-2/AM in serum-free MEM containing 1 mg ml−1 BSA and without added steroids for 30 min at 37 C. The dishes were rinsed with EC medium and transferred to the microscope stage. Fluorescence, alternately excited at 340 and 380 nm, was collected using a 40 × CF Fluor oil-immersion lens and passed through a bandpass filter to a photomultiplier. [Ca2+]i was calculated every 100 msec. Simultaneous recordings of [Ca2+]i and Im were obtained under the conditions for electrophysiology recording as described previously. For synchronization of simultaneously acquired data, Im data acquisition was triggered by the initiation of [Ca2+]i data acquisition.
Immunocytochemistry
On d 3, cells were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, treated with −20 C methanol, and blocked in 5% normal goat serum/PBS. Cells were exposed to 1:100 anti-SK2 and 1:500 anti-LH overnight at 4 C. Immunofluorescence staining with FITC- and TRITC-conjugated second antibodies was accomplished in successive incubations. As negative controls, either or both primary antibodies were omitted. To establish absence of overlap between the detection of FITC and TRITC, singly labeled cells were imaged under identical conditions as those for dual-labeled cells to confirm proper signal isolation. As specificity control, anti-SK2 was preincubated with SK2 peptide (1:1); when used under identical conditions, preabsorbed antibody showed no positive staining (data not shown).
LH secretion
On d 3, successive-timed incubations were collected before, during, and after three 15-min pulses of 1 nm GnRH. For the toxin-treated groups, either apamin or iberiotoxin was included in the medium beginning 15 min before the first GnRH pulse and continuing for 45 min. Samples were assayed for LH by RIA as described (14,19). The integrated secretory response to GnRH was calculated as the total amount of LH secreted during a 15-min exposure to GnRH plus that secreted in the subsequent 15 min.
Statistical analyses
Differences between two groups were analyzed with the t test using SigmaStat (Systat Software, San Jose, CA) or with the Welch modified two-sample t test using S-Plus (Insightful Corp., Seattle, WA). One-way ANOVA followed by Student-Newman-Keuls for multiple comparisons was used to examine toxin effects on LH secretion (SigmaStat). Two-way ANOVA was used to compare control and E2 groups and the concentration-dependence of apamin inhibition of SK channel current (OriginPro; OriginLab Corp., Northampton, MA).
Results
Simultaneous determination [Ca2+]i and Im
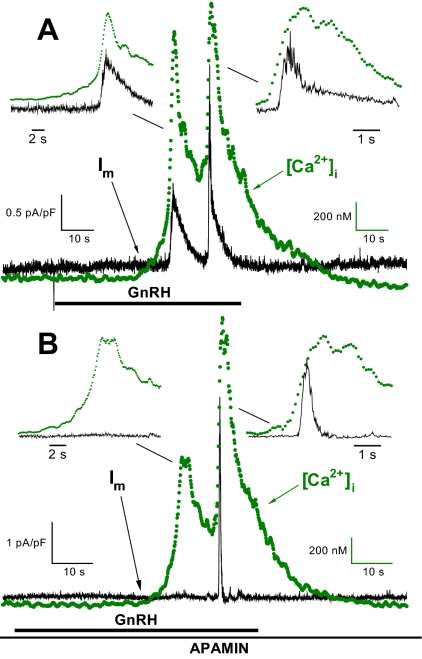
In mouse gonadotrophs (20), as in other species (1), GnRH stimulates an increase in [Ca2+]i. As shown in Fig. 1A, under voltage clamp the GnRH-induced increase in [Ca2+]i precedes the increase in Im; the [Ca2+]i oscillations elicit synchronous changes in Im. The Im oscillations were of two general types and are demonstrated in the expanded time-scale insets in Fig. 1A. The distinguishing characteristics are in the kinetics of the decay to baseline of the Im oscillations. In the first instance (Fig. 1A, left inset) the decay is relatively slow and follows the decline in [Ca2+]i; in the second (Fig. 1A, right inset), the decay is biphasic with an initially fast component followed by a slower one. In all of the five voltage-clamped cells in which [Ca2+]i and Im were recorded simultaneously, one or more of the GnRH-induced Im oscillations (eight of a total of 29 oscillations in the five cells) exhibited the initially rapid and then slower biphasic decay seen in Fig. 1A, right inset (also see Fig. 3A).
Figure 1.
Simultaneous determination of [Ca2+]i and Im in single mouse gonadotrophs. Representative [Ca2+]i and Im responses under voltage clamp (Vm = −50 mV) for control cells (no steroid pretreatment) to 1 nm GnRH (1-min exposure). A, Response to an initial GnRH pulse. The insets show the [Ca2+]i and Im relationships on an expanded time scale. The ordinates have been compressed to show temporal relationships; for both oscillations the increase in [Ca2+]i precedes the increase in Im. B, The same cell response to a second GnRH pulse (17-min interpulse interval) in the presence of 100 nm apamin. There is no Im response to the initial [Ca2+]i oscillation; however, the second [Ca2+]i oscillation elicits a kinetically distinct fast, transient Im response. The insets show the ordinate-adjusted oscillations on an expanded time scale; the increase in [Ca2+]i precedes the increase in the apamin-insensitive Im. s, sec.
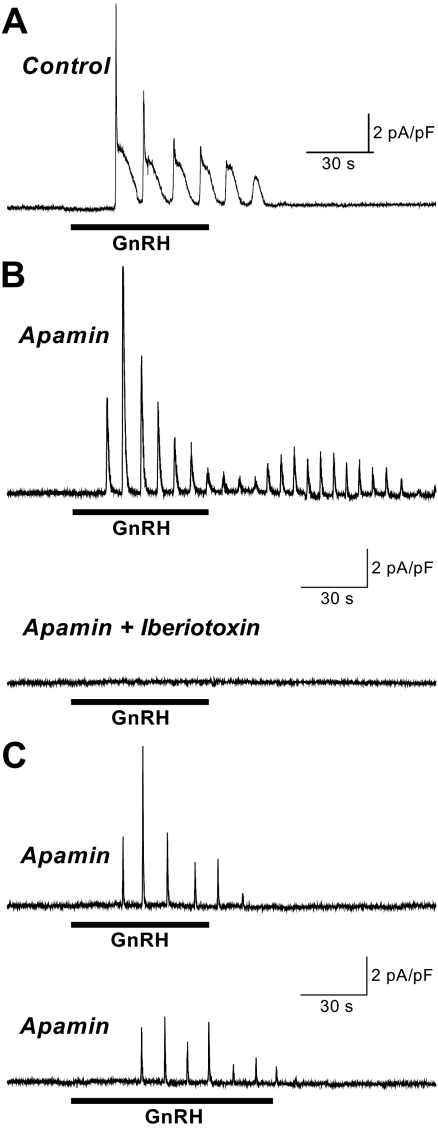
Figure 3.
Iberiotoxin inhibition of apamin-insensitive Im. For all records Vm was voltage clamped at −50 mV. Representative Im records from individual gonadotrophs. A, Control response to GnRH (E2 pretreatment) demonstrating the biphasic decay of Im when the magnitude of peak IBK exceeds that of ISK. B, Response to GnRH (no steroid pretreatment) in the presence of 100 nm apamin reveals IBK (upper panel). Following the same cell with GnRH in the presence of 100 nm apamin and 100 nm iberiotoxin shows IBK inhibition (lower panel). C, Representative control records showing the IBK response to GnRH (no steroid pretreatment) in the presence of 100 nm apamin (upper panel), followed by second response to GnRH in the presence of 100 nm apamin alone (no iberiotoxin, lower panel), showing that the absence of a response to GnRH in the presence of apamin plus iberiotoxin is due to IBK inhibition and not simply a failure to respond to two successive GnRH pulses in the presence of apamin. The horizontal line below each trace indicates the time of 1 nm GnRH application. In B and C, the scale bars apply to both traces in the panel. s, sec.
We next determined the sensitivity of the GnRH-induced Im to the toxin apamin, a specific blocker of Ca2+-activated SK channels (21,22,23). Figure 1B shows the response to a GnRH pulse in the presence of 100 nm apamin. In the five cells in which apamin was applied, the toxin did not alter the [Ca2+]i response to GnRH but partially inhibited Im in all five cells. Note that the residual, apamin-insensitive Im has a distinct fast, transient kinetic profile as shown on an expanded time scale (Fig. 1B, right inset). Furthermore, the change in the shape of the decay of the second GnRH-induced Im response in the presence of apamin (compare insets on right in Fig. 1, A and B) suggests that the more complex decay in the Fig. 1A inset (right) results from the presence of both apamin-sensitive SK channels and apamin-insensitive channel activity.
Apamin inhibition of GnRH-induced SK current (ISK)
To further examine apamin inhibition of GnRH-induced current, we determined the concentration dependence of peak Im inhibition by the SK channel-specific toxin. We have determined that the apamin-insensitive current shown in Fig. 1B is sensitive to the BK channel blocker iberiotoxin (see below); therefore, for this series the voltage-clamp potential was lowered to −66 mV to reduce the probability of activation of voltage- and Ca2+-sensitive BK channels. In addition, any Im responses that exhibited the fast transient illustrated in Fig. 1 were omitted. Each cell served as its own control, receiving a control GnRH treatment followed by a second GnRH treatment in the presence of 0–100 nm apamin; intertreatment interval was no less than 15 min. Data for each cell were normalized (peak ISK during second GnRH exposure/peak ISK during first) to give the fraction of remaining current. Apamin inhibition of the normalized, fractional GnRH-induced ISK was not different between control and E2-pretreated cells (P = 0.64), indicating that the ability of apamin to inhibit ISK is independent of E2 pretreatment, and, therefore, data were pooled.
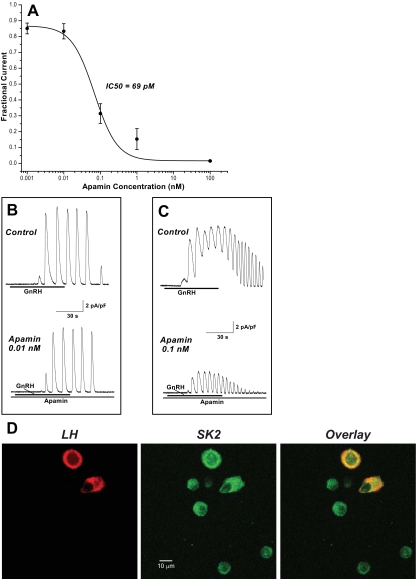
Figure 2A shows the concentration dependence of apamin inhibition. Half-maximal concentration for inhibition estimated from the fitted curve is 69 pm. Figure 2, B and C, shows representative records of concentration-dependent apamin inhibition of GnRH-induced ISK for data with no contaminating BK current (IBK). The control panels in Fig. 2, B and C, illustrate the variability in Im response to GnRH between gonadotrophs as shown previously (2); however, for an individual gonadotroph, the GnRH response pattern is relatively consistent.
Figure 2.
Apamin inhibition of GnRH-induced peak ISK and localization of SK2 to gonadotrophs. A, Concentration dependence of apamin inhibition of normalized, fractional ISK under voltage clamp (Vm = −66 mV) and no contaminating IBK (mean ± sem; n = 8–17; absence of an error bar indicates that the sem is smaller than the diameter of the symbol). For apamin-treated and control (zero apamin) cells, mean normalized, fractional remaining current data were fit to a logistic equation (weighted by the inverse of the variance) to obtain an estimate of the apamin concentration at half inhibition (IC50). B and C, Representative voltage-clamp ISK records from individual gonadotrophs showing the response to GnRH alone (upper panel), followed by GnRH in the presence of the indicated apamin concentration in the same cell (lower panel). The horizontal line below each trace indicates the time of 1 nm GnRH application. The scale bars apply to all traces. D, Colocalization of LH (red) and SK2 (green) to mouse anterior pituitary cells fixed and immunofluorescently stained with anti-LH and anti-SK2 (putative epitope location at intracellular C terminal) and analyzed by confocal laser scanning microscopy (Zeiss LSM 510, 63 × oil-immersion lens; Carl Zeiss MicroImaging, Inc., Thornwood, NY) with excitation as described (47). The same field is shown in the three panels. Far right, Overlay of both labels. Yellow is the result of colocalization of red and green labels, and demonstrates presence of SK2 in gonadotrophs. Note SK2 presence in other anterior pituitary cells. For each channel the pinhole was set to give an optical slice of 2 μm; field was optically sectioned with 20 overlapping slices to ensure acquisition of an image from the middle of each cell in the field. During the 4-d culture, pituitary cells retain rounded shape; therefore, depending on cell volume, images are captured at different depths for cells in this field. Representative of five separate experiments, 10 fields examined per experiment. s, sec.
In other systems, SK2 is the most sensitive SK isoform to the blocking action of apamin, reported in the 30- to 90-pm range, and next is SK3 at 500 pm or more (21,22,23,24). The results in Fig. 2 support the conclusion that SK2 is the predominant SK channel subtype responsible for the apamin-sensitive component of GnRH-induced Im in mouse gonadotrophs; a significant presence of the other SK subtypes would shift the concentration dependence of inhibition to the right.
To examine for localization of SK2 channels to cultured gonadotrophs, dual-label immunofluorescent staining was used. As shown in Fig. 2D, cells that express LH, a gonadotroph marker, also express SK2. In five separate experiments, all cells staining positive for LH (n = 68) were positive for SK2. Also shown is SK2 presence in cells not containing LH, indicating that its expression in anterior pituitary cells is not gonadotroph exclusive.
Iberiotoxin inhibition of apamin-insensitive Im
For these experiments, cells were voltage clamped at a more depolarized Vm, −50 mV, to increase the probability of activation of the voltage and Ca2+-sensitive BK channel in response to the GnRH-induced increase in [Ca2+]i. Figure 3A shows the response to GnRH in an E2-pretreated control cell (no toxin treatment) displaying biphasic oscillations with a decreasing fast component as the response proceeds from the first through the fifth oscillation. For toxin treatment each cell served as its own control, receiving an initial GnRH treatment in the presence of 100 nm apamin, followed by a second GnRH treatment in the presence of apamin only or apamin and 100 nm iberiotoxin, a selective, high-affinity inhibitor of BK channels (25). Figure 3, B and C, upper panels, shows Im recordings from mouse gonadotrophs in response to GnRH (both cells, no steroid pretreatment) in the presence of apamin. The residual apamin-insensitive outward current is seen to activate and inactivate rapidly. As shown in Fig. 3B, lower panel, the apamin-insensitive outward Im is inhibited when a second GnRH exposure includes apamin and iberiotoxin. The inhibition of the peak apamin-insensitive current at this iberiotoxin concentration was essentially complete [fractional current (second GnRH pulse/first): apamin + iberiotoxin/apamin, 0.10 ± 0.02, n = 7; apamin/apamin control, 0.81 ± 0.21, n = 7; P < 0.001; four E2 and three no steroid-pretreated cells per group]. Figure 3C, lower panel, illustrates the apamin-insensitive Im control response to a second GnRH pulse in the presence of apamin. These results support the conclusion that the residual apamin-insensitive outward Im in mouse gonadotrophs is IBK.
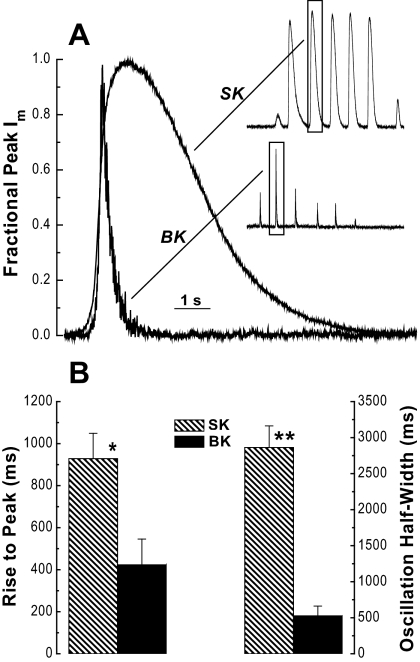
Figure 4 compares the kinetic behavior of the mouse gonadotroph currents identified as IBK and ISK. When individual oscillations for these two currents are normalized to peak current height and to the time when they reach one half of the normalized peak, it is evident that mouse gonadotroph apamin-sensitive ISK and iberiotoxin-sensitive IBK have distinct, easily recognized kinetic profiles (Fig. 4A). Quantitative analysis of two characteristics is presented in Fig. 4B. In the absence of toxin(s), the coexistence of ISK and IBK in Im records is not always evident, at least in part, because IBK is transient and can be obscured by the longer duration ISK. However, when Vm was voltage clamped at −50 mV to increase the probability of IBK activation and 100 nm apamin was present, IBK was found in 65% (17/26) of no-steroid pretreated cells and 67% (14/21) of E2-pretreated cells selected as presumptive gonadotrophs that subsequently responded to a GnRH challenge. This is similar to our overall success rate in this study (62%) for selecting mouse gonadotrophs from mixed cell populations of dispersed mouse anterior pituitary cells. These data indicate that IBK is consistently expressed and readily separated from ISK in mouse gonadotrophs.
Figure 4.
Kinetics of ISK and IBK. A, Single ISK and IBK oscillations (SK inset from Fig. 2B, upper panel, and BK inset from Fig. 3C, upper panel) on an expanded time scale and normalized to maximum peak height and the time at which one half of the peak height was attained. B, Quantitative analysis of kinetic variables for IBK and ISK oscillations shows significant differences in the time required to increase to peak value (*, P = 0.013; n = 10 and 7) (left) and the width in ms (msec) of the oscillation when it is has increased to one half of the peak value (**, P < 0.001; n = 10 and 7) (right). A single Im oscillation was analyzed for each cell. Records that had been baseline corrected and filtered at 100 Hz post-acquisition were analyzed for the rise time to peak as an estimate of the speed of activation and for half-width (oscillation width in ms at one half of the peak height) as an estimate of oscillation duration. s, sec.
E2 modulation of ISK and IBK
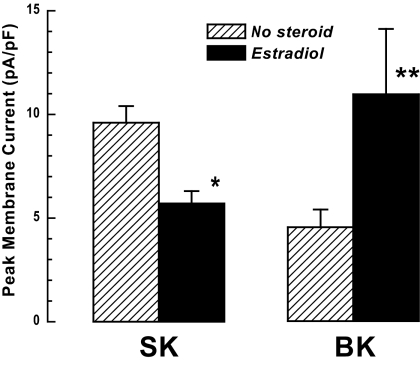
Our previous study showing that E2 pretreatment reduces the GnRH-induced peak outward Im in voltage-clamped mouse gonadotrophs (2) was expanded to analyze effects specific to the two components, ISK and IBK. For ISK we analyzed records obtained at Vm = −66 mV, in which the transient IBK component was not present; E2 pretreatment significantly attenuates GnRH-induced peak ISK (Fig. 5), as well as decreasing the mean current during the response and the current area per oscillation (supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo. endojournals.org). In marked contrast, analysis of peak IBK in voltage-clamped (−50 mV) mouse gonadotrophs in the presence of apamin to inhibit ISK showed that E2 pretreatment significantly augments peak IBK in mouse gonadotrophs (Fig. 5).
Figure 5.
E2 effect on ISK and IBK. SK: control (no steroid) and E2 (0.2 nm) pretreated gonadotrophs voltage clamped at −66 mV were challenged with 1 nm GnRH. Peak ISK was significantly reduced by E2 pretreatment (*, P < 0.008; control, n = 41; E2, n = 34). The Cm (membrane capacítance), baseline Im, the number of oscillations, and their frequency were not affected by E2 pretreatment (supplemental Table 1). BK: control (no steroid) and E2-pretreated (0.2 nm) gonadotrophs voltage clamped at −50 mV were challenged with 1 nm GnRH in the presence of 100 nm apamin to inhibit ISK. Peak IBK was significantly augmented by E2 pretreatment (**, P < 0.04; control, n = 16; E2, n = 12).
SK and BK channels and LH secretion
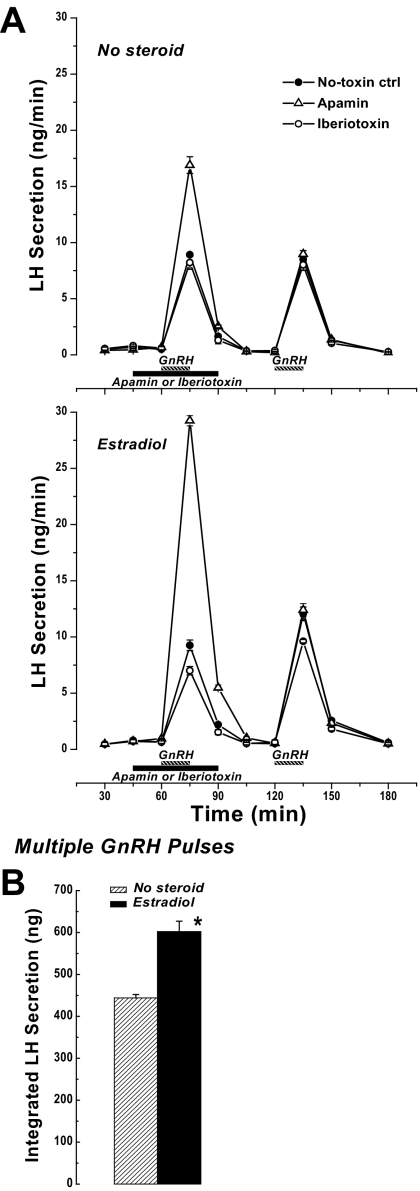
We next asked whether pharmacological blockade of SK or BK channels, and a presumptive change in membrane excitability, would affect the ability of GnRH to stimulate LH secretion. In addition, because of the differential action of E2 on peak ISK and IBK induced by GnRH (Fig. 5), the LH secretory responses were determined for cells cultured with or without E2 pretreatment. The context for this series is the demonstrated ability of preovulatory E2 levels to increase the gonadotroph secretory response to GnRH in vivo and in vitro (e.g. Refs. 14 and 26,27,28). Consistent with E2-positive action on LH secretion, mouse pituitary cells cultured in a background of 0.2 nm E2 respond to three hourly pulses of GnRH with LH secretion that is significantly greater (P < 0.001) than that found in the absence of added steroid (Fig. 6B).
Figure 6.
LH secretion. A, Effect of SK or BK channel blockade on GnRH-stimulated LH secretion. After 3 d culture without (upper graph) or with (lower graph) 0.2 nm E2, LH secretion from mouse anterior cells was monitored in response to 15-min pulses of 1 nm GnRH. For the toxin-treated groups, either 100 nm apamin or 100 nm iberiotoxin was included in the medium beginning at 15 min before the first GnRH pulse and continuing a total of 45 min. Results are expressed as mean ± sem from three to six independent experiments per treatment group. Where not shown, the error bar is smaller than the symbol representing the mean. B, Effect of E2 (0.2 nm, 3 d) on LH secretion in response to three 15-min pulses of 1 nm GnRH with 1-h interpulse intervals. Secretion rates for pulses 1 and 2 are shown in A [no-toxin ctrl (control)]. Mean ± sem from six independent experiments per treatment group. *, Significantly different from no steroid (P < 0.001).
Addition of the SK blocker apamin to pituitary cultures 15 min before the first GnRH pulse resulted in a significantly enhanced LH secretory response that was 2-fold for no-steroid treated cells (Fig. 6A, upper graph; P < 0.001) and 3-fold for cells with an E2 background (lower graph; P < 0.001) compared with no-toxin control. Regardless of the steroid background, apamin addition had no significant effect on baseline secretion, and the effect on GnRH-stimulated secretion was reversible as evidenced by the temporal profile of stimulated-LH secretion and the response to a subsequent GnRH pulse, which was indistinguishable from control.
Addition of iberiotoxin to block BK channel activity had no significant effect on GnRH-stimulated LH secretion for cells cultured without added steroids (Fig. 6A, upper graph). However, for cells pretreated with E2, BK channel blockade with iberiotoxin resulted in a significant reduction in GnRH-stimulated LH secretion (P = 0.011) (Fig. 6A, bottom graph).
Discussion
We previously reported that GnRH induces a large amplitude outward Im in voltage-clamped mouse gonadotrophs that is characteristic of current flow through Ca2+-activated K+ channels (2). In addition, we found that the magnitude of this current is suppressed by E2 pretreatment, suggesting that a decrease in Ca2+-activated K+ channel activity to increase membrane excitability is one mode of action of E2 in modulating gonadotroph function. An objective of the present study was to identify the specific Ca2+-activated K+ channel types in mouse gonadotrophs that could link the GnRH-induced increase in [Ca2+]i to changes in plasma membrane electrical activity. Using simultaneous measurement of [Ca2+]i and Im in voltage-clamped mouse gonadotrophs, we show that GnRH stimulates an increase in [Ca2+]i that precedes the outward Im. For this outward Im, we found that the GnRH-stimulated increase in [Ca2+]i activates two kinetically distinct outward currents that were identified as ISK and IBK.
Ca2+-activated K+ channel identification
Single-channel conductances distinguish members of the Ca2+-activated K+ channel family, and subfamily members are further differentiated by distinct kinetic and pharmacological properties. Work defining SK members largely has been possible because of the toxin apamin, for which the only known receptors are SK channels (23). The three SK channel subtypes have different sensitivity to the blocking action of apamin, with SK2 > SK3 ≫ SK1. We show that the apamin-sensitive current in mouse gonadotrophs has an IC50 consistent with SK2 channel data in other systems (21,22,23,24), and SK2 presence is confirmed by immunocytochemistry. The unique toxin sensitivities of BK as well as SK2 channels allowed us to establish that the two are jointly expressed in mouse gonadotrophs and are components of GnRH-induced outward Im.
ISK and IBK kinetics
A striking observation in this study is the difference in the kinetic profiles of the current oscillations for IBK (fast transient) and ISK (longer duration). The basis for the marked differences in ISK and IBK kinetic behavior is an intriguing unknown, but BK and SK channel characteristics suggest some interesting possibilities. Because these experiments were done under voltage clamp, voltage-activated events are eliminated, voltage-gated Ca2+ entry across the plasma membrane does not occur, and the activation of voltage- and Ca2+-sensitive BK channels is limited to Ca2+ at a fixed voltage. Under these conditions SK and BK channels are responding only to the GnRH-stimulated release of Ca2+ from intracellular stores. This mimics gonadotroph function in that secretion is initiated by release of Ca2+ from intracellular stores, and is independent of and relatively insensitive to EC Ca2+ entry through voltage-gated Ca2+ channels (1). SK channels are sensitive to [Ca2+] in the submicromolar range consistent with the observation that ISK tracks global [Ca2+]i in the rat (3) and with the ISK profiles in the mouse as shown here. For IBK, we show that the transient current is too fast to reflect fully the time course of the change in global [Ca2+]i. There are at least two, not necessarily exclusive, explanations. First, BK channel activation generally requires higher [Ca2+] than that for SK channels. If BK channels and intracellular Ca2+ release sites are colocalized, the rapid closure of BK channels may reflect the rise and fall of [Ca2+] in the microdomain near the channels (29). Although microdomain relationships are not known for BK channels, SK channels and intracellular Ca2+ release sites colocalize in the rat gonadotroph (1). Second, BK channels may close despite the continued presence of elevated [Ca2+]i. Regarding this possibility, inactivation of BK channels can occur as a result of coexpression of the pore-forming α-subunits with either the β2 or a β3 splice variant (13,30). Expression of specific BK-β subunits has not been established for gonadotrophs.
SK channel function
A general function of SK and BK channels is integration of changes in [Ca2+]i with changes in membrane potential. The specific functional consequences of this interplay depend on cell type and association with other channels. The consequence of SK channel activation generally is Vm hyperpolarization and a decrease in membrane excitability. For example, SK channels in myometrial cells are involved in regulating uterine contractility (31), in vascular endothelial cells are reported to regulate agonist-induced nitric oxide synthesis (32), and in neurons have a role in regulating firing frequency (11). For endocrine cells a decrease in SK channel activity and, thus, increased electrical excitability would be expected to increase agonist-stimulated secretion. As we report here for gonadotrophs, one outcome of the presumptive increase in excitability when SK channel activity is reduced by apamin is marked enhancement in GnRH-stimulated LH secretion. Similar outcomes have been reported for adrenal chromaffin cells; apamin inhibition of SK channels augments catecholamine secretion stimulated by muscarinic or nicotinic agonists or pituitary adenylate cyclase-activating polypeptide (33,34,35). To our knowledge the present study is the first to demonstrate the consequences of SK channel interference for LH secretion. However, studies with rat lactotrophs and the GH3 rat cell line report that apamin blockade can further increase the elevated secretion of prolactin found in the absence of dopamine but does not augment secretion induced by TRH (36,37).
BK channel function
The contribution of BK channel activation to membrane excitability is more complex, depending on cell type and expression of auxiliary β-subunits. The overall function of BK channels is the integration of [Ca2+]i signals with changes in membrane potential leading to negative feedback for limiting depolarization-driven Ca2+ influx. However, differential expression of β-subunits can confer striking differences in gating and kinetics of the pore-forming α-subunits, contributing to a variety of physiological roles. For example, in neurons BK channels with auxiliary β4-subunits are thought to down-regulate neurotransmitter release by shortening the duration of depolarization associated with Ca2+ entry (38); in vascular smooth muscle cells, which predominantly express the β1-subunit, IBK-induced extended hyperpolarization leads to relaxation critical for control of vascular tone (39). In contrast, BK channels in cells that predominantly express the β2 auxiliary subunit exhibit rapid inactivation, allowing for repetitive firing with a presumptive positive consequence to secretion in, for example, adrenal chromaffin cells (40).
For somatotrophs, the role of BK channels in shaping the action potential waveform has been examined (1), but there is no information on channel kinetics implicating the association with a specific β-subunit. As shown here for mouse gonadotrophs, BK channels exhibit rapid inactivation, and the kinetic profile is consistent with that found when β2 is the predominate subunit in other cell types. Also in line with that possibility, BK channel blockade decreased GnRH-stimulated LH secretion but notably only when the gonadotrophs had been treated with E2.
E2 modulation of SK and BK channels
Additional regulators of SK and BK channel function are cell-specific factors modulating channel expression or activity. Previously, we showed for mouse gonadotrophs that E2 attenuates GnRH-induced, here identified as ISK, as well as voltage-activated Im (2). Conversely, for BK channels, E2 pretreatment augments peak IBK. A potential physiological consequence of this differential action of E2 is evident in the LH secretory response after selective removal of either SK or BK. The augmentation of GnRH-stimulated LH secretion occurring when SK channels are blocked can be interpreted as reflecting increased membrane excitability (decreased ISK) and prominence of IBK with its rapid inactivation that can promote abbreviated hyperpolarization; in E2-treated cells under the same SK channel blockade, the even greater augmentation of LH secretion could be a reflection of the E2-induced increase in functional BK channel density. Consistent with this interpretation, when the compensating effect of BK channel kinetics is pharmacologically blocked in E2-treated cells, the consequence is a decrease in stimulated LH secretion.
Not addressed are possible mechanisms for E2 modulation of SK and BK channels. In other systems the focus primarily has been on regulation of SK or BK expression (e.g. Refs. 31 and 41,42,43). However, for SK channels, evidence suggests that SK2 binding components are targets for modulation. SK2-associated calmodulin can be phosphorylated by another member of the multiprotein channel assembly, casein kinase 2 (CDK2), resulting in a change in channel activity (44). The challenge is identification of potential modifiers of CDK2 and protein phosphatase 2A, another assembly component. No studies have tested the possibility that E2 can affect SK2 channel activity through modulation of SK2-associated CDK2 activity; however, there is suggestive evidence for estrogen receptor α/ERK/MAPK-mediated increase in CDK2 activity in endocervical cells (45). For BK channels a subnanomolar E2 background as used here has been shown to selectively increase expression of α- and β4-subunits in a GnRH-secreting neuronal cell line, GT1–7 (43). In addition, a number of splice variants of BK-α and the β-subunits have been identified to be under hormonal regulation and associated with distinct channel properties (12); e.g. an alternatively spliced BK-α, which reverses phosphorylation regulation, is expressed in pregnant myometrium under E2 control (46). Mechanisms underlying the action of E2 on ISK and IBK in gonadotrophs remain to be determined.
In summary, we find that the GnRH-induced, Ca2+-activated outward Im in mouse gonadotrophs is carried by both BK and SK Ca2+-activated K+ channels. Furthermore, these currents are differentially modulated by E2 pretreatment. Inhibition of SK or BK channels affects GnRH-stimulated LH secretion, implicating more directly a physiological role for these channels in gonadotroph function, including as targets for E2 positive feedback on LH secretion. The effect on membrane electrical excitability in gonadotrophs of a GnRH-stimulated increase in [Ca2+]i includes the interplay of ISK and IBK activation. Contributions of these currents during GnRH-induced [Ca2+]i oscillations will reflect their individual expression level and modulation by E2, their activation timing as reflecting their [Ca2+] sensitivity, their inactivation kinetics, and their spatial relationship to Ca2+ release sites.
Supplementary Material
Acknowledgments
We thank Allison Barrie for her excellent technical support.
Footnotes
This work was supported by National Institutes of Health Grant HD12137.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 23, 2008
Abbreviations: BK, Large (big) conductance voltage- and Ca2+-activated K+; [Ca2+]i, intracellular [Ca2+]; CDK2, casein kinase 2; E2, 17β-estradiol; EC, extracellular; FITC, fluorescein isothiocyanate; IBK, large (big) conductance voltage- and Ca2+-activated K+ current; Im, membrane current; ISK, small conductance Ca2+-activated K+ current; SK, small conductance Ca2+-activated K+; TRITC, tetramethyl rhodamine isothiocyanate; Vm, membrane voltage.
References
- Stojilkovic SS, Zemkova H, Van Goor F 2005 Biophysical basis of pituitary cell type-specific Ca2+ signaling-secretion coupling. Trends Endocrinol Metab 16:152–159 [DOI] [PubMed] [Google Scholar]
- Waring DW, Turgeon JL 2006 Estradiol inhibition of voltage-activated and gonadotropin-releasing hormone-induced currents in mouse gonadotrophs. Endocrinology [Erratum (2007) 148:231] 147:5798–5805 [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Kukuljan M, Iida T, Rojas E, Catt KJ 1992 Integration of cytoplasmic calcium and membrane potential oscillations maintains calcium signaling in pituitary gonadotrophs. Proc Natl Acad Sci USA 89:4081–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyward PM, Chen C, Clarke IJ 1995 Inward membrane currents and electrophysiological responses to GnRH in ovine gonadotropes. Neuroendocrinology 61:609–621 [DOI] [PubMed] [Google Scholar]
- Tse A, Hille B 1992 GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science 255:462–464 [DOI] [PubMed] [Google Scholar]
- Kukuljan M, Stojilkovic SS, Rojas E, Catt KJ 1992 Apamin-sensitive potassium channels mediate agonist-induced oscillations of membrane potential in pituitary gonadotrophs. FEBS Lett 301:19–22 [DOI] [PubMed] [Google Scholar]
- Van Goor F, Zivadinovic D, Stojilkovic SS 2001 Differential expression of ionic channels in rat anterior pituitary cells. Mol Endocrinol 15:1222–1236 [DOI] [PubMed] [Google Scholar]
- Vergara L, Rojas E, Stojilkovic SS 1997 A novel calcium-activated apamin-insensitive potassium current in pituitary gonadotrophs. Endocrinology 138:2658–2664 [DOI] [PubMed] [Google Scholar]
- Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H 2005 International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev 57:463–472 [DOI] [PubMed] [Google Scholar]
- Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP 2004 Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol 554(Pt 2):255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M 2004 Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5:758–770 [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A 2006 High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7:921–931 [DOI] [PubMed] [Google Scholar]
- Torres YP, Morera FJ, Carvacho I, Latorre R 2007 A marriage of convenience: β-subunits and voltage-dependent K+ channels. J Biol Chem 282:24485–24489 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Waring DW 1990 Rapid augmentation by progesterone of agonist-stimulated luteinizing hormone secretion by cultured pituitary cells. Endocrinology 127:773–780 [DOI] [PubMed] [Google Scholar]
- Horwitz KB, McGuire WL 1978 Nuclear mechanisms of estrogen action. Effects of estradiol and anti-estrogens on estrogen receptors and nuclear receptor processing. J Biol Chem 253:8185–8191 [PubMed] [Google Scholar]
- Marty A, Neher E 1995 Tight-seal whole-cell recording. In: Sakmann B, Neher E, eds. Single-channel recording. 2nd ed. New York: Plenum Press; 31–52 [Google Scholar]
- Thomas P, Mellon PL, Turgeon JL, Waring DW 1996 The LβT2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology 137:2979–2989 [DOI] [PubMed] [Google Scholar]
- Thomas P, Waring DW 1997 Modulation of stimulus-secretion coupling in single rat gonadotrophs. J Physiol 504(Pt 3):705–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon JL, Waring DW 2001 Luteinizing hormone secretion from wild-type and progesterone receptor knockout mouse anterior pituitary cells. Endocrinology 142:3108–3115 [DOI] [PubMed] [Google Scholar]
- Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U 2008 Functional characterization of genetically labeled gonadotrophs. Endocrinology 149:2701–2711 [DOI] [PubMed] [Google Scholar]
- Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP 1996 Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273:1709–1714 [DOI] [PubMed] [Google Scholar]
- Benton DC, Monaghan AS, Hosseini R, Bahia PK, Haylett DG, Moss GW 2003 Small conductance Ca2+-activated K+ channels formed by the expression of rat SK1 and SK2 genes in HEK 293 cells. J Physiol 553(Pt 1):13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolting A, Ferraro T, D'hoedt D, Stocker M 2007 An amino acid outside the pore region influences apamin sensitivity in small conductance Ca2+-activated K+ channels. J Biol Chem 282:3478–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, Angelo K, Frøkjær-Jensen C, Klærke DA, Olesen SP, Jensen BS 2001 Pharmacological modulation of SK3 channels. Neuropharmacology 40:879–887 [DOI] [PubMed] [Google Scholar]
- Garcia ML, Knaus HG, Munujos P, Slaughter RS, Kaczorowski GJ 1995 Charybdotoxin and its effects on potassium channels. Am J Physiol 269(1 Pt 1):C1–C10 [DOI] [PubMed] [Google Scholar]
- Frawley LS, Neill JD 1984 Biphasic effects of estrogen on gonadotropin-releasing hormone-induced luteinizing hormone release in monolayer cultures of rat and monkey pituitary cells. Endocrinology 114:659–663 [DOI] [PubMed] [Google Scholar]
- Naik SI, Young LS, Charlton HM, Clayton RN 1984 Pituitary gonadotropin-releasing hormone receptor regulation in mice. II: females. Endocrinology 115:114–120 [DOI] [PubMed] [Google Scholar]
- Fink G 1988 Oestrogen and progesterone interactions in the control of gonadotrophin and prolactin secretion. J Steroid Biochem 30:169–178 [DOI] [PubMed] [Google Scholar]
- Fakler B, Adelman JP 2008 Control of KCa channels by calcium nano/microdomains. Neuron 59:873–881 [DOI] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ 2003 Inactivation of BK channels by the NH2 terminus of the β2 auxiliary subunit: an essential role of a terminal peptide segment of three hydrophobic residues. J Gen Physiol 121:125–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Cornwell T, Korniyenko I, Solodushko V, Bond CT, Adelman JP, Taylor MS 2007 Myometrial expression of small conductance Ca2+-activated K+ channels depresses phasic uterine contraction. Am J Physiol Cell Physiol 292:C832–C840 [DOI] [PubMed] [Google Scholar]
- Sheng JZ, Braun AP 2007 Small- and intermediate-conductance Ca2+-activated K+ channels directly control agonist-evoked nitric oxide synthesis in human vascular endothelial cells. Am J Physiol Cell Physiol 293:C458–C467 [DOI] [PubMed] [Google Scholar]
- Uceda G, Artalejo AR, López MG, Abad F, Neher E, García AG 1992 Ca2+-activated K+ channels modulate muscarinic secretion in cat chromaffin cells. J Physiol 454:213–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A, Urabe M, Yuhi T, Yamamoto R, Yanagita T, Niina H, Kobayashi H 1995 Large-and small conductance Ca2+-activated K+ channels: their role in the nicotinic receptor mediated catecholamine secretion in bovine adrenal medulla. Naunyn Schmiedebergs Arch Pharmacol 352:545–549 [DOI] [PubMed] [Google Scholar]
- Fukushima Y, Nagayama T, Hikichi H, Mizukami K, Yoshida M, Suzuki-Kusaba M, Hisa H, Kimura T, Satoh S 2002 Role of K+ channels in the PACAP-induced catecholamine secretion from the rat adrenal gland. Eur J Pharmacol 437:69–72 [DOI] [PubMed] [Google Scholar]
- Wang X, Inukai T, Greer MA, Greer SE 1994 Evidence that Ca2+-activated K+ channels participate in the regulation of pituitary prolactin secretion. Brain Res 662:83–87 [DOI] [PubMed] [Google Scholar]
- Vacher P, Vacher AM, Mollard P 1998 Tubocurarine blocks a calcium-dependent potassium current in rat tumoral pituitary cells. Mol Cell Endocrinol 139:131–142 [DOI] [PubMed] [Google Scholar]
- Wang ZW 2008 Regulation of synaptic transmission by presynaptic CaMKII and BK channels. Mol Neurobiol 38:153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT 2000 Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 278:C235–C256 [DOI] [PubMed] [Google Scholar]
- Solaro CR, Prakriya M, Ding JP, Lingle CJ 1995 Inactivating and noninactivating Ca2+- and voltage-dependent K+ current in rat adrenal chromaffin cells. J Neurosci 15:6110–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MA, Kelly MJ, Rønnekleiv OK 2002 Distribution, neuronal colocalization, and 17β-E2 modulation of small conductance calcium-activated K+ channel (SK3) mRNA in the guinea pig brain. Endocrinology 143:1097–1107 [DOI] [PubMed] [Google Scholar]
- Palmer ML, Schiller KR, O'Grady SM 2008 Apical SK potassium channels and Ca2+-dependent anion secretion in endometrial epithelial cells. J Physiol 586:717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura I, Ui-Tei K, Saigo K, Ishii H, Sakuma Y, Kato M 2008 17β-Estradiol at physiological concentrations augments Ca2+-activated K+ currents via estrogen receptor β in the gonadotropin-releasing hormone neuronal cell line GT1–7. Endocrinology 149:774–782 [DOI] [PubMed] [Google Scholar]
- Allen D, Fakler B, Maylie J, Adelman JP 2007 Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci 27:2369–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodeski GI 2007 Estrogen modulation of MgATPase activity of nonmuscle myosin-II-B filaments. Endocrinology 148:279–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Eghbali M, Helguera G, Song M, Stefani E, Toro L 2005 Alternative splicing of Slo channel gene programmed by estrogen, progesterone and pregnancy. FEBS Lett 579:4856–4860 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Shyamala G, Waring DW 2001 PR localization and anterior pituitary cell populations in vitro in ovariectomized wild-type and PR-knockout mice. Endocrinology [Erratum (2002) 143:1065] 142:4479–4485 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.