Abstract
There is an inverse correlation between exposure to sunlight (the major source of vitamin D) and the risk for prostate cancer, the most common noncutaneous cancer and second most common cause of death from cancer in American men. The active metabolite of vitamin D, 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] acting through the vitamin D receptor decreases prostate cancer cell growth and invasiveness. The precise mechanisms by which 1,25(OH)2D3 inhibits growth in prostate cancer have not been fully elucidated. Treatment with 1,25(OH)2D3 causes an accumulation in the G0/G1 phase of the cell cycle in several prostate cancer cell lines. One potential target known to regulate the G0/G1 to S phase transition is c-Myc, a transcription factor whose overexpression is associated with a number of cancers including prostate cancer. We find that 1,25(OH)2D3 reduces c-Myc expression in multiple prostate epithelial cell lines, including C4-2 cells, an androgen-independent prostate cancer cell line. Reducing c-Myc expression to the levels observed after 1,25(OH)2D3 treatment resulted in a comparable decrease in proliferation and G1 accumulation demonstrating that down-regulation of c-Myc is a major component in the growth-inhibitory actions of 1,25(OH)2D3. Treatment with 1,25(OH)2D3 resulted in a 50% decrease in c-Myc mRNA but a much more extensive reduction in c-Myc protein. Treatment with 1,25(OH)2D3 decreased c-Myc stability by increasing the proportion of c-Myc phosphorylated on T58, a glycogen synthase kinase-3β site that serves as a signal for ubiquitin-mediated proteolysis. Thus, 1,25(OH)2D3 reduces both c-Myc mRNA levels and c-Myc protein stability to inhibit growth of prostate cancer cells.
1α,25(OH)2D3 reduces c-Myc expression through decreases in RNA expression and decreased protein stability; equivalent reductions in c-Myc using siRNA are sufficient to inhibit proliferation and cause comparable G1 accumulation.
There is increasing interest in the role of vitamin D metabolites in reducing the risk for a variety of cancers (1,2). Some studies have found inverse correlations between the risk for prostate cancer and exposure to sunlight (our major source of vitamin D) or the serum levels of 25-hydroxyvitamin D3 (3,4,5), whereas others have found no correlation or even an increase in risk (6). 1α,25-Dihydroxyvitamin D3 [1,25(OH)2D3], the biologically active form of vitamin D, is the ligand for the vitamin D receptor (VDR), a member of the ligand activated nuclear receptor family of transcription factors (7). There are a number of reports demonstrating that 1,25(OH)2D3 and less calcemic analogs inhibit growth of prostate cancer cells in vitro (8,9) as well as in mouse xenograft models (10). In addition, there is some evidence from clinical trials of beneficial effects of combination treatments with calcitriol [1,25(OH)2D33] and dexamethasone or docetaxel (11). However, the precise mechanisms by which 1,25(OH)2D3 causes growth inhibition in prostate cancer are still unclear.
The actions of 1,25(OH)2D3 include cell cycle arrest (12) and, less commonly, induction of apoptosis (8,13). In many cells 1,25(OH)2D3 treatment induces G0/G1 accumulation (12,14,15,16). Progression to S phase requires phosphorylation and inactivation of the retinoblastoma (Rb) protein releasing the transcription factor E2F. Several studies have shown that 1,25(OH)2D3 treatment can reduce cyclin-dependent kinase (Cdk)-2 activity and induce the expression of cyclin-dependent kinase inhibitors p21 and p27 in prostate cancer cell lines (8,17,18). Our laboratory has shown that a consequence of 1,25(OH)2D3 treatment in LNCaP cells, an androgen-dependent prostate cancer cell line, is the reduction of c-Myc expression (19). c-Myc is a protooncogene important for progression through the early portion of the cell cycle; c-Myc induces the expression of cyclin D1 and cyclin D2 as well as Cdc25A, a phosphatase that dephosphorylates an inhibitory site in Cdk2, facilitating activation of the kinase and phosphorylation of Rb (20). Thus, 1,25(OH)2D3 may play a role in inhibiting cell cycle progression before the increases in cyclin-dependent kinase inhibitors reported previously.
Overexpression of c-Myc is observed in prostate cancer and many other cancers (21,22,23). Moreover, artificial overexpression of c-Myc is sufficient to induce prostate cancer in rodent models (24,25,26,27). Thus, c-Myc plays an important role in prostate cancer, and treatments that reduce its expression are of great interest. Regulation of c-Myc expression is complex. Transcription of c-Myc is induced both by growth factor signaling (28,29) and the wnt signaling pathway (30). β-Catenin/T-cell factor (TCF) responsive elements have been identified in the c-Myc promoter (31). In addition to transcriptional regulation, regulation of transcriptional elongation of c-Myc mRNA has been reported (32). Finally, c-Myc protein has a relatively short half-life (33). Posttranslational modifications and degradation processes mediated by E3 ubiquitin-ligases also regulate c-Myc protein levels (34).
A number of 1,25(OH)2D3 targets with potential to contribute to the growth-inhibitory actions of 1,25(OH)2D3 have been identified. These include IGF binding protein-3 (18), CCAAT/enhancer binding protein-Δ (35), and inhibition of targets in the prostaglandin pathway (36). Our goal was to determine how effective the reduction in c-Myc is in reproducing the growth inhibition induced by 1,25(OH)2D3 in C4-2 androgen-independent prostate cancer cells and identify the mechanisms by which 1,25(OH)2D3 reduces c-Myc expression. Our data show that the reduction in c-Myc is a major factor in 1,25(OH)2D3-mediated cell cycle accumulation and that 1,25(OH)2D3 reduces c-Myc expression both by reducing mRNA levels and by reducing protein stability.
Materials and Methods
1,25(OH)2D3 was obtained from Solvay Pharmaceuticals (Weesp, The Netherlands). 1,25(OH)2D3 stock solutions were prepared in ethanol and stored in the dark at −20 C. Isoton II and zapoglobulin II were purchased from Beckman Coulter Inc. (Fullerton, CA). Dulbecco’s PBS, TriZOL reagent, and Lipofectamine reagent were obtained from Invitrogen (Carlsbad, CA). Enhanced chemiluminescence (ECL) and ECL+ reagents were purchased from Amersham Pharmacia Biotech Inc. (Piscataway, NJ). SuperSignal West Femto maximum sensitivity substrate was purchased from Thermo Scientific (Rockford, IL). Cycloheximide was purchased from Sigma Chemicals (St. Louis, MO). (2′Z,3′E)-6-Bromoindirubin-3-oxime (BIO) was purchased from EMD Chemicals, Inc. (Gibbstown, NJ). Glycogen synthase kinase (GSK)-3β inhibitor I (I) was purchased from Calbiochem (San Diego, CA). Tissue culture supplies were obtained from Fisher Scientific (Pittsburgh, PA). All other chemicals were reagent grade unless otherwise stated.
Cell culture
The human prostate cancer cell line LNCaP and the normal prostate epithelial cell line RWPE-1 were obtained from American Type Culture Collection (Manassas, VA); the C4-2 prostate cancer cell line was obtained from Urocor Inc. (Oklahoma City, OK). The LNCaP cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). The RWPE-1 cells were grown in keratinocyte serum-free medium (Invitrogen) supplemented with 5 ng/ml human recombinant epithelial growth factor and 0.05 mg/ml bovine pituitary extract (Invitrogen). The C4-2 cells were grown in T medium (37) supplemented with 5% heat-inactivated FBS (56 C for 30 min). All cells were grown in a 37 C humidified incubator with an atmosphere of 5% CO2.
Cell growth assays
C4-2 cells were plated in six-well plates at a density of 100,000 cells/well. The medium was changed and fresh vehicle or 1,25(OH)2D3 added every 3 d. Cells were washed with PBS, harvested by scraping in PBS, and counted using a Coulter Particle Counter Z1 (Hialeah, FL).
Western blot analysis
Cells were washed, scraped into PBS, and pelleted by centrifugation. Cell pellets were resuspended in radioimmunoprecipitation assay buffer (Upstate, Temecula, CA) or buffer (pH 7.4) of 10 mm Tris, 1 mm EDTA, and 12 mm monothioglycerol containing 0.4 m NaCl plus 1× protease inhibitor mix and 1× phosphatase inhibitor mix (Pierce, Rockford, IL). Protein was extracted with three freeze/thaw cycles and the extract clarified by centrifugation. Protein concentrations were determined by Bradford analysis. Equal amounts of proteins were separated on sodium dodecyl sulfate polyacrylamide gels and transferred to nitrocellulose membranes. The nitrocellulose membrane was blocked with 1% milk Tris-buffered saline and Tween 20 [TBST; 10 mm Tris (pH 7.5), 0.15 m NaCl, 1% Tween 20] for at least an hour at room temperature. Primary mouse monoclonal antibody against c-Myc (9E10 clone; Santa Cruz Biotechnology, Santa Cruz, CA) was diluted 1:200 in 1% milk-TBST. Primary rabbit monoclonal antibody (Y69 clone, Abcam, Cambridge, MA) against c-Myc was diluted 1:5000 in 1% milk-TBST. Primary rabbit polyclonal antibody against β-catenin (H-102; Santa Cruz) was diluted 1:400 in 1% milk-TBST. Primary mouse monoclonal antibody against phospho-tyrosine (clone 4G10; Millipore, Temecula, CA) was diluted 1:1000 in 1% milk-TBST. Primary rabbit polyclonal (Thr58/Ser62; Cell Signaling, Danvers, MA) against phospho-c-Myc was diluted 1:200 in 1% BSA-TBST. Primary mouse monoclonal antibody against tubulin (clone AA2; Millipore) was diluted 1:10,000 in 1% milk-TBST. Primary mouse monoclonal antibody against actin (Millipore) was diluted 1:10,000 in 1% milk-TBST.
All primary incubations were performed overnight at 4 C. All membranes were washed three times with 1% milk-TBST at room temperature. Membranes that were incubated with mouse primary antibodies were next incubated with rabbit antimouse IgG (Zymed Laboratories, South San Francisco, CA) diluted to 1:10,000 in 1% milk- or BSA-TBST for 1 h at room temperature. All membranes were washed with 1% milk- or BSA-TBST and incubated in horseradish peroxidase-conjugated donkey antirabbit IgG (Amersham Pharmacia Biotech) diluted 1:30,000 in TBST for 1 h at room temperature. Membranes were washed three times with TBST followed by two Tris-buffered saline [10 mm Tris (pH 7.5), 0.15 m NaCl] washes. Proteins were detected using ECL, ECL+, or Femto reagents according to manufacturer’s recommendations. Films were scanned using the Molecular Dynamics Personal Densitometer SI (Amersham Pharmacia Biotech) or GeneSnap 7.04 (SynGene, Cambridge, UK) and quantified used Image Quant 5.2 (Amersham Pharmacia Biotech) or GeneTools 4.00 (SynGene).
Small interfering RNA (siRNA)
C4-2 cells, plated in six-well plates at a density of 100,000 cells per well, were transfected with the indicated amounts of control or target-specific siRNA using Lipofectamine (Invitrogen) following the manufacturer’s recommendations in serum-free medium (OPTI-MEM; Invitrogen). c-Myc (siRNA no. 1, catalog no. 4250) and silencer negative control siRNA (catalog no. 4611) were purchased from Ambion (Austin, TX). c-Myc (siRNA no. 2; catalog no. M-003282-04-0005) and nontargeting control siRNA (catalog no. D-001210-01-05) were purchased from Dharmacon (Lafayette, CO). β-Catenin (also known as CTNNB1; catalog no. M-003482) siRNA was purchased from Millipore. Six hours after transfection, medium was removed and replaced with fresh full medium (T medium supplemented with 5% heat inactivated FBS) and cells treated with vehicle or 100 nm 1,25(OH)2D3 for 48 h.
Quantitative RT-PCR (qPCR)
RNA was isolated using TriZOL reagent (Invitrogen) following the manufacturer’s recommendations, quantified, and diluted to 100 ng/μl for preparation of cDNA using SuperScript II reverse transcriptase (Invitrogen) as recommended by the manufacturer. Primer and probe sets for 18S (4319413E-040211), c-Myc (Hs99999003_m1; Applied Biosystems, Foster City, CA), cdc25A (5′-TCATTGATTCTAGAGAGACCTGTTTCAG-3′ and 5′-AGTGAAGCCGTGATGGTAAGGA-3′), E2F (5′-TCCAAGAACCACATCCAGTG-3′ and 5′-CTGGGTCAACCCCTCAAG-3′), and c-Myc (5-CGTCTCCACACATCAGCACAA-3′ and 5-CTCTTGGCAGCAGGATAGTCCTT-3′) were used to measure cDNA using the 7500 real-time PCR system (Applied Biosystems).
[3H]thymidine incorporation
Cells were incubated with 2 μCi/ml [3H]thymidine (MP Biomedicals, Solon, OH) for 3 h. Incorporated [3H]thymidine was extracted and counted as previously described (37).
Cell cycle analysis
C4-2 cells, plated in 6-well plates at a density of 100,000 cells/well, were transfected with siRNA as described above and treated with vehicle or 1,25(OH)2D3 for 2 d. Cells were washed with PBS, fixed in ethanol, and prepared for fluorescence activated cell sorting analysis as described previously (38), analyzed using the Coulter EPICS XL-MCL, and quantified by flow cytometry using FlowJo version 8.8.2 (for Mac; Tree Star, Inc. Ashland, OR).
c-Myc stability
C4-2 cells, plated in 10-cm plates at a density of 500,000 cells/well, were pretreated with vehicle or 1,25(OH)2D3 for 3 d and then incubated with 10 μg/ml cycloheximide (CHX) for the indicated times. Cell extracts were prepared and c-Myc detected using the Y69 antibody as described above.
GSK-3β inhibitors
C4-2 cells, plated in six-well plates at a density of 100,000 cells/well, were treated with 2 μm BIO or 10 μm GSK-3β I for 24 h (alone) or 6 h [when cells were pretreated with vehicle or 1,25(OH)2D3]. c-Myc and phospho-c-Myc were detected as described above.
Statistics
The SigmaStat program (Jandel Scientific, San Rafael, CA) was used to perform Student’s unpaired t test to compare the means of two groups and one-way ANOVA was used to compare multiple means to control group or an all pair-wise multiple comparison procedure was used (Holm-Sidak method). Values are indicated as means and the error bars indicate sem. P < 0.05 was considered statistically significant. All experiments were performed a minimum of three times and a representative experiment is shown.
Results
1,25(OH)2D3-mediated growth inhibition is accompanied by down-regulation of c-Myc
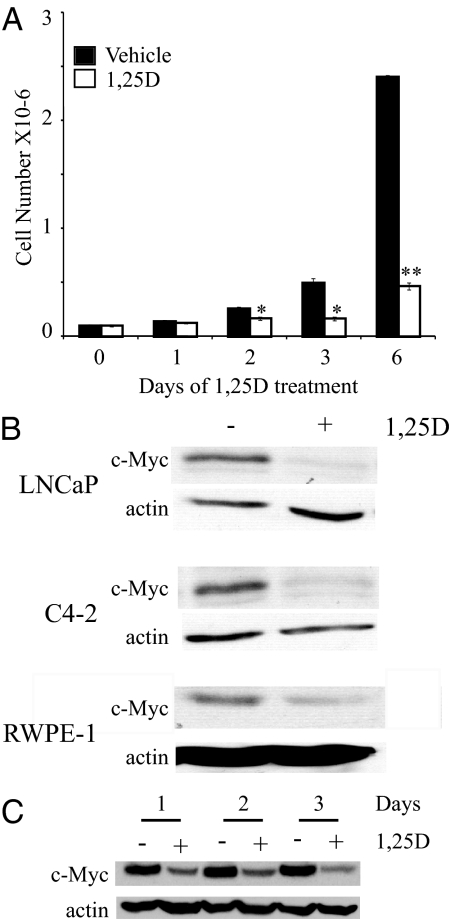
To determine whether down-regulation of c-Myc might be responsible for 1,25(OH)2D3-mediated growth inhibition, we first compared the time course of growth inhibition with the time required to down-regulate c-Myc. Treatment of androgen-independent C4-2 cells grown in medium containing FBS with 100 nm 1,25(OH)2D3 inhibits cell growth with initial differences in cell number detected at 2 d of 1,25(OH)2D3 treatment; the differences in cell number and percent growth inhibition increase with time (Fig. 1A). The treated cells essentially stop growing, although there is no net loss in cell number. Previous publications from our laboratory showed that treatment with growth-inhibitory concentrations of 1,25(OH)2D3 reduces c-Myc protein levels in the LNCaP human androgen-dependent prostate cancer cell line (19,39). 1,25(OH)2D3 treatment also strongly reduces c-Myc expression in C4-2 cells and to a lesser extent in the nontransformed human prostate epithelial cell line RWPE-1 (Fig. 1B). Consistent with the down-regulation contributing to 1,25(OH)2D3 mediated growth inhibition, c-Myc proteins levels begin to decrease before the changes in cell number and expression is strongly inhibited by 1,25(OH)2D3 treatment (Fig. 1C).
Figure 1.
1,25(OH)2D3 treatment reduces cell growth in C4-2 cells and c-Myc expression in normal and malignant prostate epithelial cell lines. A, C4-2 cells were treated with vehicle or 100 nm 1,25(OH)2D3 (1,25D) for the indicated times, harvested, and counted using a Coulter counter. Data are expressed as the mean ± sem. *, Significance at P < 0.05 with respect to vehicle-treated cells within the same time frame; **, significance at P < 0.005 with respect to vehicle-treated cells within the same time frame. B, LNCaP, C4-2, and RWPE-1 cells were treated with vehicle (−) or 100 nm 1,25(OH)2D3 (1,25D; +) for 3 d. Cells were harvested and c-Myc and actin protein levels were detected by Western blotting. C, C4-2 cells were treated with vehicle (−) or 100 nm 1,25(OH)2D3 (1,25D; +) for the indicated times. Cells were harvested, extracts run on an SDS-PAGE gel, and proteins (c-Myc and actin) detected by Western blotting.
Reducing c-Myc expression is sufficient to inhibit proliferation and induce G1 accumulation in C4-2 cells
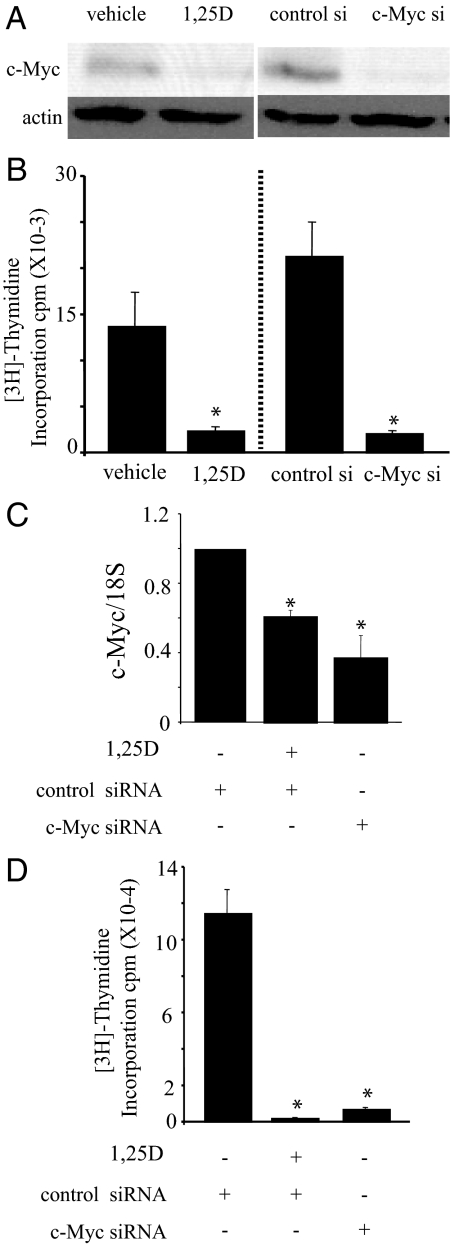
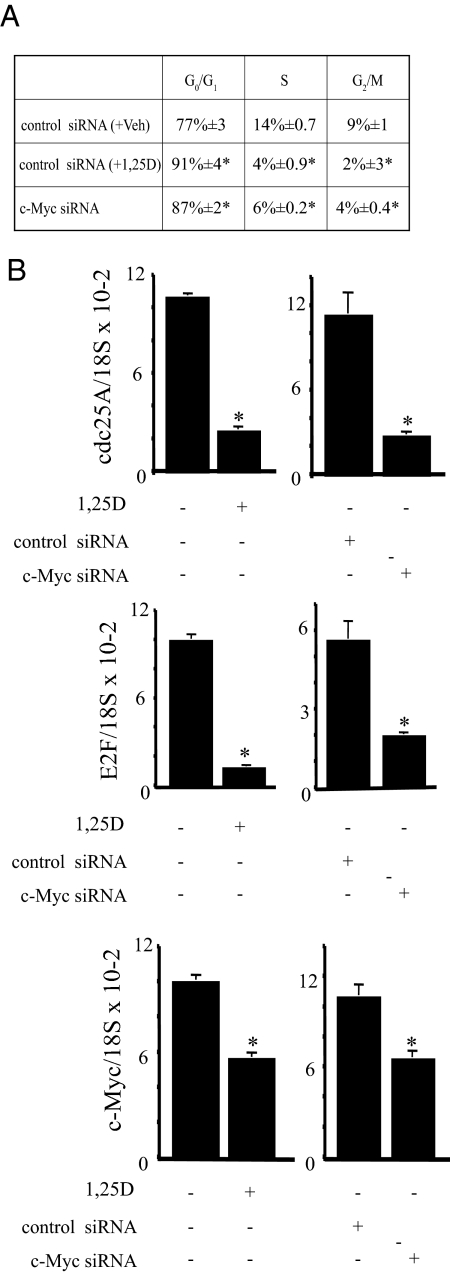
To investigate the role of reduced c-Myc levels in 1,25(OH)2D3-mediated growth inhibition in C4-2 cells, c-Myc expression was reduced using siRNA. The reduction in c-Myc protein by c-Myc targeted siRNA (siRNA no. 1) compared with control siRNA was similar to the reduction in c-Myc achieved by treatment with 1,25(OH)2D3 (Fig. 2A). Reducing c-Myc expression led to a significant reduction in proliferation, as measured by [3H]thymidine incorporation and the reduction was similar to that caused by 1,25(OH)2D3 treatment (Fig. 2B). Similar results were obtained with a second independent c-Myc-targeted siRNA (siRNA no. 2) (Fig. 2, C and D). Because reducing c-Myc expression with siRNA similar to levels achieved by 1,25(OH)2D3 treatment is sufficient to inhibit proliferation, we asked whether reducing c-Myc expression would cause G1 accumulation as has been reported for 1,25(OH)2D3 treatment. Cells were transfected with control or c-Myc siRNA no. 2 and treated with vehicle or 100 nm 1,25(OH)2D3 as indicated for 48 h and the percentages of cells in G0/G1, S, and G2/M phases of the cell cycle were determined by flow cytometry using the FlowJo 8.8.2 (Mac; Tree Star) program (Fig. 3A). The cell cycle distribution of 1,25(OH)2D3-treated and c-Myc siRNA-treated cells show remarkably similar increases in G1 (from 77% in control to ∼87–91% in treated) with corresponding reductions in S and G2/M and are statistically different from the control (Fig. 3A). Thus, reducing c-Myc expression is sufficient to induce G1 accumulation. To determine whether reducing c-Myc or treating with 1,25(OH)2D3 induces changes in gene expression consistent with reduced c-Myc activity, we measured expression of Cdc25A, E2F1, p21, and p27 mRNA. Both Cdc25A and E2F1 expression were reduced when cells were treated with c-Myc siRNA or 1,25(OH)2D3 (Fig. 3B). However, we did not detect changes in p21 or p27 in either case (data not shown).
Figure 2.
Proliferation in the C4-2 prostate cancer cell line is reduced by c-Myc siRNA or 1,25(OH)2D3 treatment. A, C4-2 cells were treated with vehicle or 100 nm 1,25(OH)2D3 (1,25D) or transiently transfected with 75 pm of control or c-Myc siRNA no. 1 for 2 d. Cells were harvested, extracts run on an SDS-PAGE gel, and c-Myc and actin protein levels detected by Western blotting. Representative westerns corresponding to the samples in B are shown. B, C4-2 cells were treated with vehicle or 100 nm 1,25(OH)2D3 (1,25D) or transiently transfected with 75 pm of control or c-Myc siRNA no. 1 for 2 d. Proliferation was measured by [3H]thymidine incorporation. Data are expressed as the mean ± sem. *, Significance at P < 0.005 with respect to vehicle or control siRNA. C, C4-2 cells were transiently transfected with 150 pm control or c-Myc siRNA no. 2. C4-2 cells transfected with control siRNA were further treated with vehicle or 100 nm 1,25(OH)2D3 (1,25D) for 2 d. c-Myc mRNA was measured by qPCR and normalized with 18S RNA. Results from two separate experiments, each performed in triplicate, were pooled and analyzed. Data are expressed as the mean normalized to control ± sem. *, Significance at P ≤ 0.001 with respect to control siRNA plus vehicle. D, C4-2 cells were transiently transfected with 150 pm control or c-Myc siRNA no. 2. C4-2 cells transfected with control siRNA were further treated with vehicle or 100 nm 1,25(OH)2D3 (1,25D) for 2 d. Proliferation was measured by [3H]thymidine incorporation. Data are expressed as the mean ± sem. Results from two separate experiments, each performed in triplicate, were pooled and analyzed. *, Significance at P ≤ 0.001 with respect to control siRNA plus vehicle. 1,25(OH)2D3, 1,25D.
Figure 3.
c-Myc siRNA or 1,25(OH)2D3 induces G0/G1 accumulation. A, C4-2 cells were transiently transfected with 150 pm control or c-Myc siRNA. Control siRNA-transfected cells were treated with vehicle (+Veh) or 100 nm 1,25(OH)2D3 (+1,25D) for 2 d. Cells were then stained with propidium iodide (PI) for total DNA content and analyzed by flow cytometry using FlowJo. G0/G1, S, and G2/M phases of a representative experiment performed in triplicate are indicated. Data are expressed as the mean ± sem for each treatment group. *, Significance at P ≤ 0.05 with respect to vehicle or control siRNA. B, cdc25A, E2F, and c-Myc mRNA were measured by qPCR and normalized with 18S RNA. Results from a representative experiment performed in triplicate are shown. Data are expressed as the mean ± sem. *, Significance at P ≤ 0.05 with respect to vehicle or control siRNA.
Wnt signaling and the regulation of c-Myc in C4-2 cells
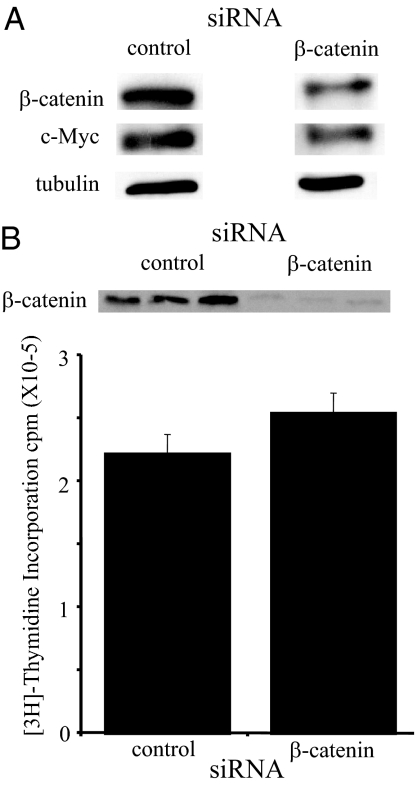
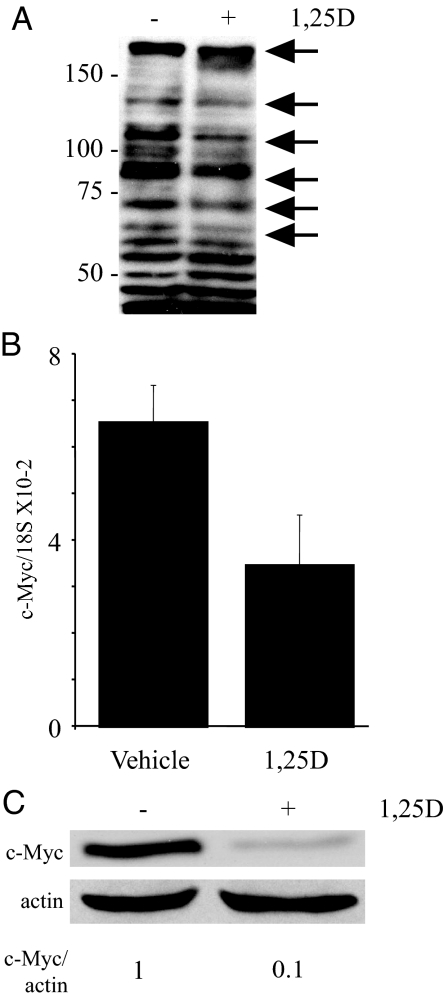
Expression of c-Myc is often induced by signaling pathways such as the canonical wnt signaling pathway and 1,25(OH)2D3 inhibits wnt signaling under some conditions (40). To assess the overall contribution of canonical wnt signaling through β-catenin-dependent transcription to c-Myc expression in C4-2 cells, β-catenin expression was reduced using β-catenin-specific siRNA and expression of β-catenin and c-Myc measured. Expression of c-Myc is diminished when β-catenin expression is reduced verifying that wnt signaling contributes to c-Myc expression in C4-2 cells (Fig. 4A). However, the reduction is not as extensive as that observed with 1,25(OH)2D3 treatment (Fig. 1) nor was reducing β-catenin expression sufficient to inhibit proliferation in C4-2 cells (Fig. 4B). Moreover, we were unable to reproducibly detect a 1,25(OH)2D3-dependent reduction in β-catenin/TCF activity measured using a TOP Flash reporter (Millipore, Billerica, MA; data not shown). Thus, 1,25(OH)2D3 must regulate c-Myc expression through multiple pathways. Tyrosine kinase signaling also has been implicated in inducing expression of c-Myc mRNA (28,41,42,43). Our laboratory has shown previously that 1,25(OH)2D3 reduces the tyrosine phosphorylation of a subset of proteins in LNCaP and C81 LN (an androgen independent derivative of LNCaP) prostate cancer cell lines including HER2 in C81 LN cells (39). To test whether tyrosine kinase signaling was reduced by 1,25(OH)2D3 treatment in C4-2 cells, we performed Western blot analysis using an antibody against phosphotyrosine residues. Similar to our results in other cell lines (39), we found that 1,25(OH)2D3 treatment reduces tyrosine phosphorylation of a subset of proteins in C4-2 cells (Fig. 5A).
Figure 4.
Reducing β-catenin modestly reduces c-Myc protein. A, C4-2 cells were transiently transfected with 80 pm control siRNA or β-catenin siRNA. Two days after transfection, C4-2 cells were harvested for protein analysis and extracts run on an SDS-PAGE gel. β-Catenin, c-Myc, and tubulin protein levels were detected by Western blotting. B, C4-2 cells were transiently transfected with 80 pm control siRNA or β-catenin siRNA. Three days after transfection, C4-2 cells were harvested for protein analysis and extracts run on an SDS-PAGE gel. β-Catenin protein levels were detected by Western blotting. In parallel, proliferation was measured after 3 d of treatment with siRNA by [3H]thymidine incorporation. Data are expressed as the mean ± sem.
Figure 5.
1,25(OH)2D3 reduces c-Myc mRNA and protein. A, C4-2 cells were treated with vehicle or 100 nm 1,25(OH)2D3 (1,25D) for 3 d. Cells were harvested for protein analysis, extracts run on an SDS-PAGE gel, and phosphotyrosine residues in proteins detected by Western blotting. Arrows indicate reduction in phosphotyrosine residues after 1,25D treatment in a subset of proteins. B, C4-2 cells were treated with vehicle or 100 nm 1,25(OH)2D3 (1,25D) for 3 d. c-Myc mRNA was measured by qPCR and normalized with 18S. Data are expressed as the mean ± sem. B, C4-2 cells were treated with vehicle (−) or 100 nm 1,25(OH)2D3 (1,25D; +) for 3 d. Cells were harvested for protein analysis, extracts run on an SDS-PAGE gel, and proteins (c-Myc and actin) detected by Western blotting. Intensity of signals was measured with a densitometer. Densitometric values represent c-Myc/actin normalized to vehicle (−).
1,25(OH)2D3 treatment reduces the stability of c-Myc
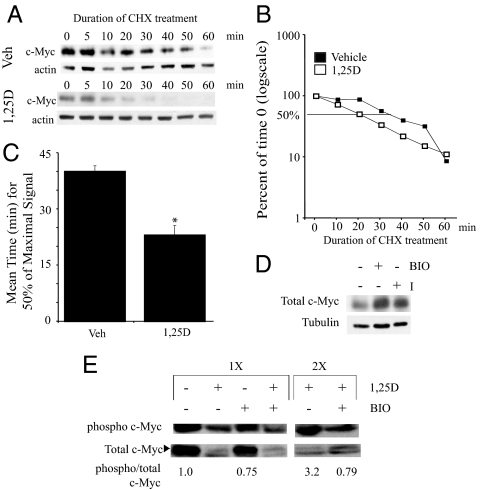
Our initial studies (Figs. 1 and 2) suggested that c-Myc protein levels were reduced more than c-Myc mRNA levels. In a direct comparison within a single experiment, we found that treating C4-2 cells with 1,25(OH)2D3 for 3 d reduces c-Myc protein expression approximately 90% (Fig. 5C) with only an approximately 50% reduction in c-Myc mRNA (Fig. 5B), suggesting an additional posttranscriptional level of control. Densitometric scans of c-Myc protein in Fig. 1C yielded similar reductions in protein (∼80–85%). Because c-Myc expression is also regulated at the level of protein stability (34), we asked whether 1,25(OH)2D3 treatment reduced c-Myc protein stability. To address this, cells were pretreated with vehicle or 1,25(OH)2D3 for 3 d; CHX was then added to inhibit de novo protein synthesis, and cells were harvested at the indicated times. As previously established, 1,25(OH)2D3 decreases the total amount of c-Myc protein. Furthermore, c-Myc expression decreases more quickly in the presence of 1,25(OH)2D3 compared with control-treated cells (Fig. 6A). To determine half-life, expression of c-Myc was normalized to actin and levels of control or 1,25(OH)2D3-treated c-Myc expression at T = 0 for the CHX treatment were set as 100. In this experiment, 50% of maximal signal remains at about 35 min in vehicle-treated cells compared with about 20 min for the 1,25(OH)2D3-treated cells (Fig. 6B). Figure 6C shows the averages and means of the half-lives for c-Myc in vehicle-treated cells and 1,25(OH)2D3-treated cells from five independent experiments. The average reduction in half-life is about 50%.
Figure 6.
1,25(OH)2D3 treatment reduces stability of c-Myc protein in C4-2 cells. A, C4-2 cells were pretreated with vehicle or 100 nm 1,25(OH)2D3 (1,25D) for 3 d. Before harvesting, cells were treated with CHX for 0–60 min. Cells were harvested for protein analysis at the indicated times, extracts run on an SDS-PAGE gel, and c-Myc and actin protein levels detected by Western blotting. A representative western is shown. B, Percent of time 0 was calculated from the Western blot after normalization to actin. Percentage reductions are graphed on a log scale. The line indicates 50% of maximal signal. C, Graph indicating mean values of 50% of maximum signal from five independent experiments for each treatment group. *, Significance at P ≤ 0.005 with respect to vehicle-treated cells. D, C4-2 cells were treated with vehicle, BIO, or GSK-3β I for 24 h. Cells were harvested for protein analysis, extracts run on an SDS-PAGE gel, and c-Myc and tubulin protein levels detected by Western blotting. E, Cells were pretreated with vehicle (−) or 100 nm 1,25(OH)2D3 (1,25D; +) for 3 d. Then cells were treated with vehicle (−) or BIO (+) for 6 h. Twice as much protein extract (2 times) compared with 1 time were run on an SDS-PAGE gel to enhance signal in selected cases. c-Myc and phosphorylated c-Myc (T58/S62) were detected by Western blotting. The signals were measured with a densitometer. Results are presented as ratios of phosphorylated c-Myc to total c-Myc normalized to the ratio for the control sample (arbitrarily set at 1).
1,25(OH)2D3 increases phosphorylation of T58 in c-Myc, a signal for degradation
Phosphorylation of T58 in c-Myc serves as a signal to induce degradation through binding of SCFFbw7 (E3 ubiquitin ligase complexes) (34,44). GSK-3β has been identified as the primary kinase responsible for T58 phosphorylation targeting c-Myc for degradation (34). Thus, we asked whether GSK-3β plays a role in regulating the basal levels of c-Myc protein in C4-2 cells. Treatment with either of two GSK-3β inhibitors for 24 h (I or BIO) increased levels of c-Myc protein (Fig. 6D). Because GSK-3β negatively regulates the expression of c-Myc protein in C4-2 cells, we asked whether 1,25(OH)2D3 increases phosphorylation of T58 facilitating c-Myc degradation. C4-2 cells were pretreated with vehicle or 100 nm 1,25(OH)2D3 for 3 d and then with vehicle or the GSK-3β inhibitor, BIO, for 6 h before extraction and measurement of total c-Myc and P-T58 c-Myc by Western blotting. The ratio of phospho to total c-Myc in 1,25(OH)2D3-treated cells is substantially increased compared with vehicle-treated C4-2 cells (fold increase over multiple experiments ranged from 3 to 6) (Fig. 6E), suggesting that 1,25(OH)2D3 enhances GSK-3β activity, leading to increased phosphorylation and degradation. Furthermore, the reduction in the ratio of phospho to total c-Myc in 1,25(OH)2D3-treated plus BIO cells compared with 1,25(OH)2D3-treated cells is greater than the reduction in control plus BIO-treated compared with control cells, suggesting that the 1,25(OH)2D3 stimulated phosphorylation is GSK-3β dependent (Fig. 6E). Whether the residual phosphorylation is due to incomplete inhibition of GSK-3β by BIO or the presence of another kinase that also phosphorylates T58 is not known, but collectively the data indicate that 1,25(OH)2D3 enhances c-Myc phosphorylation and that this phosphorylation event is reduced by the GSK-3β inhibitor.
Discussion
Our current and previous studies show that 1,25(OH)2D3 down-regulates c-Myc in a variety of prostate cancer cell lines as well as to a lesser extent in nontransformed RWPE-1 prostate epithelial cells. We found that down-regulation of c-Myc is achieved through regulation both at the RNA and protein levels. There is strong evidence that increased c-Myc expression is a major factor in prostate cancer. c-Myc is a protooncogene that is frequently deregulated either through gene translocation, gene amplification, or mutations in many human cancers including prostate cancer (21,23,45) Amplification of chromosome 8q24 (c-Myc locus) is associated with more aggressive prostate cancer (22). The most severe subclass in this analysis included a gain of 8q24 (MYC) combined with a loss at 10q23 (phosphatase and tensin homolog deleted on chromosome 10) among other chromosomal alterations (22). Loss or inactivation of this phosphatase results in higher Akt activity, leading to inactivation of GSK-3β and increased stability and thus expression of c-Myc. LNCaP cells and its androgen-independent derivative C4-2, which were used for this study, both lack functional phosphatase and tensin homolog deleted on chromosome 10 (46,47). Interestingly, recent studies have revealed that a substantial proportion of prostate cancers contain a translocation or deletion that fuses the androgen regulated TMPRSS2 promoter to the coding region of an E26 transformation specific (ETS) factor, most commonly ETS-related gene (ERG;48). Induction of c-Myc by this fusion has been identified as a major factor in regulating growth of VCaP prostate cancer cells (49).
There are numerous studies addressing the mechanisms by which 1,25(OH)2D3 inhibits cell growth and identifying candidates that contribute to the beneficial actions of 1,25(OH)2D3 (9,13,19,39,50,51,52). 1,25(OH)2D3 inhibits growth of both normal (53,54) and malignant (55) prostate cells and at least in some cases tends to induce a more differentiated phenotype. Understanding the mechanisms by which 1,25(OH)2D3 inhibits growth will aid both to identify tumors that are likely to respond to treatment as well as to ascertain which treatments might be enhanced when combined with a VDR agonist. Treatment with 1,25(OH)2D3 causes G0/G1 accumulation in many cell types including some of the prostate cancer cell lines (8,12,19). Transition from G1 to S phase requires the transcription factor E2F, which is sequestered by active Rb (56). Inactivation of Rb is accomplished by phosphorylation by the Cdks, Cdk2, Cdk4, and Cdk6 (57). Cdk activities depend on not only the levels of Cdk but also levels of their partner cyclins whose expression is regulated during cell cycle progression, levels of the Cdk inhibitors (p16, p21, and p27) (58) and the phosphorylation state of the kinases themselves. 1,25(OH)2D3 treatment results in a reduction in Cdk2 activity (8). In some cases, this reduction in activity has been attributed to increases in p21 and/or p27 (8,17,18). Treatment of LNCaP cells with 1,25(OH)2D3 also causes enhanced cytoplasmic localization of Cdk2 (17).
1,25(OH)2D3-induced down-regulation of c-Myc is observed in several types of cancer cells (19,59,60). Although there may be redundant mechanisms by which 1,25(OH)2D3 causes G1 accumulation, our data are the first to show that mimicking the down-regulation of c-Myc by 1,25(OH)2D3 by reducing c-Myc expression with siRNA is sufficient to cause both the growth inhibition and G1 accumulation observed in 1,25(OH)2D3-treated C4-2 cells. A previous report using an antisense oligonucleotide to reduce c-Myc expression in LNCaP cells that were serum starved overnight and then grown in reduced serum (2.5%) also showed growth inhibition (61). The change in growth was accompanied by an increase in a sub-G1 population, suggesting that the cells were becoming apoptotic under these conditions. In C4-2 cells in full serum, reducing c-Myc causes a G1 accumulation similar to 1,25(OH)2D3. Some of the previously reported actions of 1,25(OH)2D3 may be secondary to the down-regulation of c-Myc. For example, c-Myc induces expression of Cdc25A, and we have shown previously that 1,25(OH)2D3 treatment reduces Cdc25A expression in LNCaP cells (39). We have now demonstrated that 1,25(OH)2D3 treatment or depleting c-Myc using siRNA reduces Cdc25A expression in C4-2 cells (Fig. 3B). Cdc25A is required for the removal of an inhibitory phosphate on Cdk2 (62). Thus, reducing c-Myc expression results in reduced Cdk2 activity. Yang and Burnstein (17) reported that 1,25(OH)2D3 treatment of LNCaP cells resulted in increases in the stability of p27 through reduced Cdk2-dependent phosphorylation of p27. 1,25(OH)2D3 treatment reduces E2F activity in LNCaP cells (63). Recently c-Myc has been shown to play a role in inducing expression of E2F, promoting proliferation (64,65). We find that treatment with 1,25(OH)2D3 or depleting c-Myc mRNA reduces E2F1 mRNA (Fig. 3B). Thus, the observed reduction in activity likely is a combination of reduced E2F1 expression due to down-regulation of c-Myc and sequestration of E2F1 by activated Rb.
The necessity for tightly regulating the expression of c-Myc in normal cells has resulted in the evolution of a complex series of regulatory mechanisms. The rates of transcriptional initiation and elongation are both regulated (32,66,67). The protein has a relatively short half-life (<1 h), and its stability is regulated by a complex series of phosphorylation and dephosphorylation (68) followed by proteasome mediated degradation (34). We found that 1,25(OH)2D3 modestly reduces c-Myc mRNA levels. The expression of c-Myc mRNA is often regulated by wnt signaling through β-catenin/TCF/lymphoid enhancer binding factor (31). We found that reducing β-catenin expression modestly reduces c-Myc expression; however, reducing β-catenin expression was insufficient to inhibit proliferation, suggesting that there are additional mechanisms regulating c-Myc expression in C4-2 cells. Consistent with the observation that tyrosine kinase activity has been implicated in the induction of c-Myc mRNA in some cells (41,42,43), we observed decreases in tyrosine phosphorylated proteins after 1,25(OH)2D3 treatment potentially contributing to the reduction of c-Myc mRNA. One of the mechanisms by which growth factor signaling increases c-Myc mRNA is through p42/p44 MAPK-dependent activation (in some cases induced by HER2) of ETS factors, such as ETV1/ER81 (28,48,69), an ETS factor that is overexpressed in the LNCaP lineage (48). In some cells, 1,25(OH)2D3 induces homeobox gene 4, a protein that inhibits transcriptional elongation of c-Myc mRNA (32). However, we did not see evidence of homeobox gene 4 induction on 1,25(OH)2D3 treatment (data not shown).
The discrepancy between the steady-state levels of c-Myc mRNA and steady-state protein levels in cells treated with 1,25(OH)2D3 suggested regulation at the level of protein expression. We found that 1,25(OH)2D3 treatment reduced the half-life of c-Myc protein by about 50%. Phosphorylation of c-Myc at T58 serves as a signal for proteasome-mediated degradation (34). T58 is phosphorylated by GSK-3β, which requires a prior phosphorylation at S62 to phosphorylate T58 (70). S62 is then dephosphorylated and the protein ubiquitinated and degraded (71). Consistent with GSK-3β playing a role in regulating c-Myc levels in C4-2 cells, we showed that treatment with a GSK-3β inhibitor increases c-Myc expression. Moreover, the increased ratio of T58 P c-Myc/total c-Myc is consistent with increased activity of GSK-3β in 1,25(OH)2D3-treated cells. GSK-3β activity can be inhibited through wnt signaling (72,73) and through growth factor-mediated activation of Akt (70,74). Thus, 1,25D may contribute to activation of GSK-3β through either or both of these pathways. The importance of phosphorylation of T58 in regulating the expression of c-Myc is highlighted by the frequency of T58 mutations in human Burkitt’s lymphoma (75,76).
Other VDR target genes have been suggested as contributing to 1,25(OH)2D3-mediated growth inhibition in prostate cancer cells. These include IGF binding protein-3 (18), CCAAT/enhancer binding protein-Δ (35), and inhibition of the prostaglandin pathway (36). Whether these pathways are redundant with down-regulation of c-Myc or cooperate in producing the down-regulation remains to be determined.
Our findings that down-regulation of c-Myc mimics the growth inhibition and G1 accumulation induced by 1,25(OH)2D3 suggests that this is a major factor in the beneficial actions of 1,25(OH)2D3. Moreover, the ability of 1,25(OH)2D3 to reduce both c-Myc mRNA and c-Myc protein stability indicates that 1,25(OH)2D3 treatment can reduce overexpression caused by a variety of genetic alterations.
Acknowledgments
The authors thank William E. Bingman III for technical expertise and Dr. Carolyn Smith’s and Dr. Austin Cooney’s laboratories for reagents.
Footnotes
This work was supported by National Institutes of Health Grant 5R01CA107691 (to N.L.W.), Department of Defense Prostate Cancer Research Program Grant W81-04-1-0192 (to N.L.W.), and Training Grant 5T32HD07165 (to J.N.P.R.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 22, 2009
Abbreviations: BIO, 2′Z,3′E)-6-Bromoindirubin-3-oxime; Cdk, cyclin-dependent kinase; CHX, cycloheximide; ECL, enhanced chemiluminescence; FBS, fetal bovine serum; GSK, glycogen synthase kinase; I, inhibitor I; 1,25(OH)2D3, 1α,25-dihydroxyvitamin D3; qPCR, quantitative RT-PCR; Rb, retinoblastoma; siRNA, small interfering RNA; TBST, Tris-buffered saline and Tween 20; TCF, T-cell factor; VDR, vitamin D receptor.
References
- Masuda S, Jones G 2006 Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther 5:797–808 [DOI] [PubMed] [Google Scholar]
- Barreto AM, Schwartz GG, Woodruff R, Cramer SD 2000 25-Hydroxyvitamin D3, the prohormone of 1,25-dihydroxyvitamin D3, inhibits the proliferation of primary prostatic epithelial cells. Cancer Epidemiol Biomarkers Prev 9:265–270 [PubMed] [Google Scholar]
- Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P 2000 Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control 11:847–852 [DOI] [PubMed] [Google Scholar]
- John EM, Koo J, Schwartz GG 2007 Sun exposure and prostate cancer risk: evidence for a protective effect of early-life exposure. Cancer Epidemiol Biomarkers Prev 16:1283–1286 [DOI] [PubMed] [Google Scholar]
- John EM, Schwartz GG, Koo J, Van Den Berg D, Ingles SA 2005 Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res 65:5470–5479 [DOI] [PubMed] [Google Scholar]
- Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, Horst RL, Hollis BW, Huang WY, Shikany JM, Hayes RB 2008 Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst 100:796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler MR, Mangelsdorf DJ, Komm BS, Terpening CM, Yamaoka K, Allegretto EA, Baker AR, Shine J, McDonnell DP, Hughes M, Weigel NL, O'Malley BW, Pike JW 1988 Molecular biology of the vitamin D hormone. Recent Prog Horm Res 44:263–305 [DOI] [PubMed] [Google Scholar]
- Moffatt KA, Johannes WU, Hedlund TE, Miller GJ 2001 Growth inhibitory effects of 1α, 25-dihydroxyvitamin D(3) are mediated by increased levels of p21 in the prostatic carcinoma cell line ALVA-31. Cancer Res 61:7122–7129 [PubMed] [Google Scholar]
- Skowronski RJ, Peehl DM, Feldman D 1993 Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology 132:1952–1960 [DOI] [PubMed] [Google Scholar]
- Blutt SE, Polek TC, Stewart LV, Kattan MW, Weigel NL 2000 A calcitriol analogue, EB1089, inhibits the growth of LNCaP tumors in nude mice. Cancer Res 60:779–782 [PubMed] [Google Scholar]
- Trump DL, Hershberger PA, Bernardi RJ, Ahmed S, Muindi J, Fakih M, Yu WD, Johnson CS 2004 Anti-tumor activity of calcitriol: pre-clinical and clinical studies. J Steroid Biochem Mol Biol 89–90:519–526 [DOI] [PubMed] [Google Scholar]
- Blutt SE, Allegretto EA, Pike JW, Weigel NL 1997 1,25-Dihydroxyvitamin D3 and 9-cis-retinoic acid act synergistically to inhibit the growth of LNCaP prostate cells and cause accumulation of cells in G1. Endocrinology 138:1491–1497 [DOI] [PubMed] [Google Scholar]
- Blutt SE, McDonnell TJ, Polek TC, Weigel NL 2000 Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology 141:10–17 [DOI] [PubMed] [Google Scholar]
- Elstner E, Linker-Israeli M, Said J, Umiel T, de Vos S, Shintaku IP, Heber D, Binderup L, Uskokovic M, Koeffler HP 1995 20-Epi-vitamin D3 analogues: a novel class of potent inhibitors of proliferation and inducers of differentiation of human breast cancer cell lines. Cancer Res 55:2822–2830 [PubMed] [Google Scholar]
- Simboli-Campbell M, Narvaez CJ, van Weelden K, Tenniswood M, Welsh J 1997 Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res Treat 42:31–41 [DOI] [PubMed] [Google Scholar]
- Studzinski GP, Bhandal AK, Brelvi ZS 1985 Cell cycle sensitivity of HL-60 cells to the differentiation-inducing effects of 1-α,25-dihydroxyvitamin D3. Cancer Res 45:3898–3905 [PubMed] [Google Scholar]
- Yang ES, Burnstein KL 2003 Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J Biol Chem 278:46862–46868 [DOI] [PubMed] [Google Scholar]
- Boyle BJ, Zhao XY, Cohen P, Feldman D 2001 Insulin-like growth factor binding protein-3 mediates 1α,25-dihydroxyvitamin d(3) growth inhibition in the LNCaP prostate cancer cell line through p21/WAF1. J Urol 165:1319–1324 [PubMed] [Google Scholar]
- Polek TC, Stewart LV, Ryu EJ, Cohen MB, Allegretto EA, Weigel NL 2003 p53 is required for 1,25-dihydroxyvitamin D3-induced G0 arrest but is not required for G1 accumulation or apoptosis of LNCaP prostate cancer cells. Endocrinology 144:50–60 [DOI] [PubMed] [Google Scholar]
- Mateyak MK, Obaya AJ, Sedivy JM 1999 c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol 19:4672–4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu NC, Zimonjic DB 2002 Chromosome-mediated alterations of the MYC gene in human cancer. J Cell Mol Med 6:151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J, Li C, Giacomini CP, Salari K, Huang S, Wang P, Ferrari M, Hernandez-Boussard T, Brooks JD, Pollack JR 2007 Genomic profiling reveals alternative genetic pathways of prostate tumorigenesis. Cancer Res 67:8504–8510 [DOI] [PubMed] [Google Scholar]
- Jenkins RB, Qian J, Lieber MM, Bostwick DG 1997 Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res 57:524–531 [PubMed] [Google Scholar]
- Thompson TC, Southgate J, Kitchener G, Land H 1989 Multistage carcinogenesis induced by ras and myc oncogenes in a reconstituted organ. Cell 56:917–930 [DOI] [PubMed] [Google Scholar]
- Williams K, Fernandez S, Stien X, Ishii K, Love HD, Lau YF, Roberts RL, Hayward SW 2005 Unopposed c-MYC expression in benign prostatic epithelium causes a cancer phenotype. Prostate 63:369–384 [DOI] [PubMed] [Google Scholar]
- Thompson TC, Egawa S, Kadmon D, Miller GJ, Timme TL, Scardino PT, Park SH 1992 Androgen sensitivity and gene expression in ras + myc-induced mouse prostate carcinomas. J Steroid Biochem Mol Biol 43:79–85 [DOI] [PubMed] [Google Scholar]
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL 2003 Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 4:223–238 [DOI] [PubMed] [Google Scholar]
- Dwyer J, Li H, Xu D, Liu JP 2007 Transcriptional regulation of telomerase activity: roles of the Ets transcription factor family. Ann NY Acad Sci 1114:36–47 [DOI] [PubMed] [Google Scholar]
- Dean M, Levine RA, Ran W, Kindy MS, Sonenshein GE, Campisi J 1986 Regulation of c-myc transcription and mRNA abundance by serum growth factors and cell contact. J Biol Chem 261:9161–9166 [PubMed] [Google Scholar]
- Sun J, Jin T 2008 Both Wnt and mTOR signaling pathways are involved in insulin-stimulated proto-oncogene expression in intestinal cells. Cell Signal 20:219–229 [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW 1998 Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512 [DOI] [PubMed] [Google Scholar]
- Pan Q, Simpson RU 1999 c-myc intron element-binding proteins are required for 1, 25-dihydroxyvitamin D3 regulation of c-myc during HL-60 cell differentiation and the involvement of HOXB4. J Biol Chem 274: 8437–8444 [DOI] [PubMed] [Google Scholar]
- Hann SR, Eisenman RN 1984 Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol Cell Biol 4:2486–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN, Clurman BE 2004 The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci USA 101:9085–9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezoe T, Gery S, Yin D, O'Kelly J, Binderup L, Lemp N, Taguchi H, Koeffler HP 2005 CCAAT/enhancer-binding protein Δ: a molecular target of 1,25-dihydroxyvitamin D3 in androgen-responsive prostate cancer LNCaP cells. Cancer Res 65:4762–4768 [DOI] [PubMed] [Google Scholar]
- Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D 2005 Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res 65:7917–7925 [DOI] [PubMed] [Google Scholar]
- Stewart LV, Weigel NL 2005 Role of insulin-like growth factor binding proteins in 1α,25-dihydroxyvitamin D(3)-induced growth inhibition of human prostate cancer cells. Prostate 64:9–19 [DOI] [PubMed] [Google Scholar]
- Narayanan R, Edwards DP, Weigel NL 2005 Human progesterone receptor displays cell cycle-dependent changes in transcriptional activity. Mol Cell Biol 25:2885–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart LV, Lyles B, Lin MF, Weigel NL 2005 Vitamin D receptor agonists induce prostatic acid phosphatase to reduce cell growth and HER-2 signaling in LNCaP-derived human prostate cancer cells. J Steroid Biochem Mol Biol 97:37–46 [DOI] [PubMed] [Google Scholar]
- Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A 2001 Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J Cell Biol 154:369–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiariello M, Marinissen MJ, Gutkind JS 2001 Regulation of c-myc expression by PDGF through Rho GTPases. Nat Cell Biol 3:580–586 [DOI] [PubMed] [Google Scholar]
- Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, Gonzalez L, Porque PG, Leon J, Martin-Perez J 2004 Prolactin induces c-Myc expression and cell survival through activation of Src/Akt pathway in lymphoid cells. Oncogene 23:7378–7390 [DOI] [PubMed] [Google Scholar]
- Xie S, Lin H, Sun T, Arlinghaus RB 2002 Jak2 is involved in c-Myc induction by Bcr-Abl. Oncogene 21:7137–7146 [DOI] [PubMed] [Google Scholar]
- Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H, Ishida N, Okumura F, Nakayama K, Nakayama KI 2004 Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J 23:2116–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nupponen NN, Kakkola L, Koivisto P, Visakorpi T 1998 Genetic alterations in hormone-refractory recurrent prostate carcinomas. Am J Pathol 153:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J 1998 Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res 58:2720–2723 [PubMed] [Google Scholar]
- Wu Z, Gioeli D, Conaway M, Weber MJ, Theodorescu D 2008 Restoration of PTEN expression alters the sensitivity of prostate cancer cells to EGFR inhibitors. Prostate 68:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM 2005 Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310:644–648 [DOI] [PubMed] [Google Scholar]
- Sun C, Dobi A, Mohamed A, Li H, Thangapazham RL, Furusato B, Shaheduzzaman S, Tan SH, Vaidyanathan G, Whitman E, Hawksworth DJ, Chen Y, Nau M, Patel V, Vahey M, Gutkind JS, Sreenath T, Petrovics G, Sesterhenn IA, McLeod DG, Srivastava S 2008 TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene 27:5348–5353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SS, Madsen MW, Lukas J, Binderup L, Bartek J 2001 Inhibitory effects of 1αa,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol Endocrinol 15:1370–1380 [DOI] [PubMed] [Google Scholar]
- Feldman D, Zhao XY, Krishnan AV 2000 Vitamin D and prostate cancer. Endocrinology 141:5–9 [DOI] [PubMed] [Google Scholar]
- Peehl DM, Krishnan AV, Feldman D 2003 Pathways mediating the growth-inhibitory actions of vitamin D in prostate cancer. J Nutr 133:2461S–2469S [DOI] [PubMed] [Google Scholar]
- Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D 1994 Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res 54:805–810 [PubMed] [Google Scholar]
- Blutt SE, Weigel NL 1999 Vitamin D and prostate cancer. Proc Soc Exp Biol Med 221:89–98 [DOI] [PubMed] [Google Scholar]
- Peehl DM, Feldman D 2003 The role of vitamin D and retinoids in controlling prostate cancer progression. Endocr Relat Cancer 10:131–140 [DOI] [PubMed] [Google Scholar]
- Wiman KG 1993 The retinoblastoma gene: role in cell cycle control and cell differentiation. FASEB J 7:841–845 [DOI] [PubMed] [Google Scholar]
- Reed SI 1997 Control of the G1/S transition. Cancer Surv 29:7–23 [PubMed] [Google Scholar]
- Zetterberg A, Larsson O, Wiman KG 1995 What is the restriction point? Curr Opin Cell Biol 7:835–842 [DOI] [PubMed] [Google Scholar]
- Saunders DE, Christensen C, Wappler NL, Schultz JF, Lawrence WD, Malviya VK, Malone JM, Deppe G 1993 Inhibition of c-myc in breast and ovarian carcinoma cells by 1,25-dihydroxyvitamin D3, retinoic acid and dexamethasone. Anticancer Drugs 4:201–208 [DOI] [PubMed] [Google Scholar]
- Tong WM, Kallay E, Hofer H, Hulla W, Manhardt T, Peterlik M, Cross HS 1998 Growth regulation of human colon cancer cells by epidermal growth factor and 1,25-dihydroxyvitamin D3 is mediated by mutual modulation of receptor expression. Eur J Cancer 34:2119–2125 [DOI] [PubMed] [Google Scholar]
- Balaji KC, Koul H, Mitra S, Maramag C, Reddy P, Menon M, Malhotra RK, Laxmanan S 1997 Antiproliferative effects of c-myc antisense oligonucleotide in prostate cancer cells: a novel therapy in prostate cancer. Urology 50:1007–1015 [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J 2001 Pathways governing G1/S transition and their response to DNA damage. FEBS Lett 490:117–122 [DOI] [PubMed] [Google Scholar]
- Zhuang SH, Burnstein KL 1998 Antiproliferative effect of 1α,25-dihydroxyvitamin D3 in human prostate cancer cell line LNCaP involves reduction of cyclin-dependent kinase 2 activity and persistent G1 accumulation. Endocrinology 139:1197–1207 [DOI] [PubMed] [Google Scholar]
- Leung JY, Ehmann GL, Giangrande PH, Nevins JR 2008 A role for Myc in facilitating transcription activation by E2F1. Oncogene 27:4172–4179 [DOI] [PubMed] [Google Scholar]
- Sears R, Ohtani K, Nevins JR 1997 Identification of positively and negatively acting elements regulating expression of the E2F2 gene in response to cell growth signals. Mol Cell Biol 17:5227–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TR, Cole MD 1987 Rapid cytoplasmic turnover of c-myc mRNA: requirement of the 3′ untranslated sequences. Mol Cell Biol 7:4513–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts PH, Watson JV, Lamond A, Forster A, Stinson MA, Evan G, Fischer W, Atherton E, Sheppard R, Rabbitts TH 1985 Metabolism of c-myc gene products: c-myc mRNA and protein expression in the cell cycle. EMBO J 4:2009–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoorts J, Luscher-Firzlaff J, Luscher B 2006 The ins and outs of MYC regulation by posttranslational mechanisms. J Biol Chem 281:34725- 34729 [DOI] [PubMed] [Google Scholar]
- Shin S, Bosc DG, Ingle JN, Spelsberg TC, Janknecht R 2008 Rcl is a novel ETV1/ER81 target gene upregulated in breast tumors. J Cell Biochem 105:866–874 [DOI] [PubMed] [Google Scholar]
- Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR 2000 Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev 14:2501–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears RC 2004 The life cycle of C-myc: from synthesis to degradation. Cell Cycle 3:1133–1137 [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H 1997 Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784–1787 [DOI] [PubMed] [Google Scholar]
- Verras M, Sun Z 2006 Roles and regulation of Wnt signaling and β-catenin in prostate cancer. Cancer Lett 237:22–32 [DOI] [PubMed] [Google Scholar]
- Li X, Bijur GN, Jope RS 2002 Glycogen synthase kinase-3beta, mood stabilizers, and neuroprotection. Bipolar Disord 4:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia K, Huppi K, Spangler G, Siwarski D, Iyer R, Magrath I 1993 Point mutations in the c-Myc transactivation domain are common in Burkitt’s lymphoma and mouse plasmacytomas. Nat Genet 5:56–61 [DOI] [PubMed] [Google Scholar]
- Smith-Sorensen B, Hijmans EM, Beijersbergen RL, Bernards R 1996 Functional analysis of Burkitt’s lymphoma mutant c-Myc proteins. J Biol Chem 271:5513–5518 [DOI] [PubMed] [Google Scholar]