The synthesis and secretion of thyrotropin-releasing hormone (TRH) is absolutely required for intact thyroid function in humans and rodents. Furthermore, feedback regulation of TRH production by thyroid hormone allows for tight maintenance of circulating thyroid hormone levels and thus establishes the hypothalamic-pituitary-thyroid (H-P-T) axis. Although the TRH gene is expressed in many regions of the brain, its regulation by thyroid hormone is restricted to neurons in the paraventricular nucleus of the hypothalamus (PVH) (1). This discrete set of neurons in the PVH, termed hypophysiotropic, project to the median eminence where mature TRH peptide is released into the portal capillary system bound for TRH-receptors present in the anterior pituitary (Fig. 1). Whereas it is clear that thyroid hormone regulates TRH production in the PVH both at the level of gene expression and posttranslational processing, in this issue of Endocrinology, Sanchez et al. (2) provide evidence that thyroid hormone also controls TRH peptide degradation by regulating the enzyme pyroglutamyl peptidase II (PPII). This enzyme is expressed in tanycytes; glial cells that line the third ventricle in the hypothalamus whose cytoplasmic processes extend into the median eminence. Thus, PPII appears to regulate TRH peptide bioavailability upstream of the pituitary.
Figure 1.
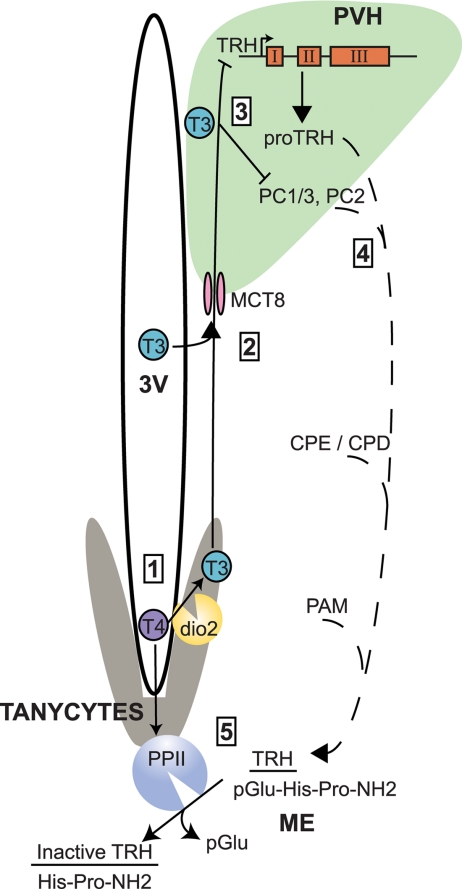
Thyroid hormone regulates TRH transcription, posttranslational modification, and degradation through the assistance of tanycytes. 1, Tanycytes take up T4, which is converted to T3 by dio2. 2, Hypophysiotropic TRH neurons in the PVH receive T3 from tanycytes or from circulating thyroid hormone through the MCT8. 3, TRH, PC1/3, and PC2 are inversely regulated by T3. 4, Posttranslational modifications to proTRH by PC1/3 and PC2, carboxypeptidases E and D (CPE and CPD), and peptidyl α-amidating monooxygenase (PAM) occur as it travels down the axon (dashed line). 5, TRH is released in the median eminence (ME) where it can be degraded by tanycyte-bound PPII, which is positively regulated by thyroid hormone.
The importance of this discovery is best highlighted by an understanding of the unique anatomic and biologic mechanisms by which TRH is regulated. As outlined, TRH is widely expressed in the hypothalamus but its production is only regulated by thyroid hormone in hypophysiotropic neurons present in the PVH. This observation suggests that these neurons possess either a singular molecular regulatory mechanism or, more likely, are uniquely positioned to sense thyroid hormone levels. Indeed, recent evidence suggests that local hypothalamic T3 can be produced from circulating T4 by the type 2 deiodinase (dio2) that is also expressed in tanycytes (3,4). Locally produced T3 is then available for uptake by hypophysiotropic neurons to regulate TRH both transcriptionally and posttranslationally via specific transporters such as the monocarboxylate transporter 8 (MCT8). The physiological importance of MCT8 has been confirmed by mutations found in humans and in mouse knockout studies in which the regulation of TRH mRNA expression by thyroid hormone is impaired in the absence of MCT8 (5,6,7).
In addition to regulating TRH mRNA expression, thyroid hormone also regulates the production of the mature TRH tripeptide that is delivered to the pituitary. The TRH gene in humans and rodents encodes for multiple copies of TRH (pGlu-His-Pro-NH2). After its transcription and translation, proTRH, a 26-kDa protein, is sequentially modified by prohormone convertases 1/3 (PC1/3) and 2 (PC2) to a variety of cleavage products including TRH precursors (8,9,10). Importantly, these two prohormone convertases are negatively regulated by thyroid hormone such that their levels are high when thyroid hormone levels are low, which increases TRH production (11). After the actions of PC1/3 and PC2, TRH precursors are further modified by carboxypeptidases-E and -D (12). Finally, the immediate precursor to TRH undergoes cyclization at its N terminus and is amidated at its carboxyl terminus (13,14,15). Although all of these steps ensure the appropriate production of TRH, Sanchez et al. (2) demonstrate another mechanism by which thyroid hormone regulates TRH, through degradation by PPII.
PPII, a membrane-bound metallopepitdase with an extracellular active site, is widely distributed in the brain and in some peripheral tissues including the pituitary (16,17,18,19,20,21). PPII has high specificity for TRH (unlike the related PPI) hydrolyzing its pyroglutamyl-histidyl peptide bond. The findings of Sanchez et al. (2) update previous findings on the localization of PPII expression. Previously, PPII inactivation of TRH was thought to occur in the anterior pituitary; however, expression of PPII is limited to lactotrophs (22,23). Although prior work suggested that PPII was expressed in neurons (21,22,24), elegant use of in situ hybridization by Sanchez et al. (2) reveals a PPII mRNA expression pattern indicative of tanycyte localization. Earlier studies have shown that thyroid hormone could regulate PPII expression in the pituitary and frontal cortex (25,26,27). Now, Sanchez et al. (2) demonstrate that both PPII expression and enzymatic activity are strongly up-regulated in tanycytes in the presence of increased T4 levels. Furthermore, confocal microscopy shows that axon terminals containing TRH in the median eminence are closely positioned to tanycytes, allowing for a model in which thyroid hormone levels dictate the amount of TRH peptide that reaches the portal circulation through the PPII-mediated degradation of TRH. The importance of these findings is accentuated by the fact that tanycytes also express dio2, suggesting that these cells function as key sensors of circulating thyroid hormone levels and are able to influence TRH production, either in the PVH by regulating T3 levels via dio2 or by degradation in the median eminence by the actions of PPII.
Collectively, Sanchez et al. (2) demonstrate a novel and potentially critical mechanism by which thyroid hormone feeds back to regulate TRH production. This exciting observation now raises a number of questions including one posed by the authors; is the regulation of PPII expression and activity the first step in decreasing TRH action on the H-P-T axis after an increase in thyroid hormone? In addition, as TRH mRNA expression and peptide production play a key role in the response of the H-P-T axis to critical illness and nutritional deprivation, is PPII expression regulated in these states? Indeed, dio2 is strongly up-regulated by bacterial lipopolysaccharide in tanycytes, and a recent study demonstrated that hypothalamic dio2 responds to leptin in food-restricted rats (28,29). Thus, PPII may be poised to respond to these pathways and allow for rapid down regulation of thyroid hormone levels in these states. Lastly, do PPII mRNA expression and activity decrease in response to low thyroid hormone levels, and what is the molecular mechanism by which tanycytes sense thyroid hormone levels? The answers to these questions will allow for a better understanding of the complete role that PPII expression plays in regulating TRH action and will give new insight into the role of tanycytes in the regulation of the H-P-T axis.
Footnotes
This work was supported by National Institutes of Health Grants DK056123 and DK078090 (to A.N.H.) and T32 DK07516 (to K.R.V.).
Disclosure Summary: The authors have nothing to disclose.
For article see page 2283
Abbreviations: dio2, Type 2 deiodinase; H-P-T, hypothalamic-pituitary-thyroid; MCT8, monocarboxylate transporter 8; PC, prohormone convertase; PPII, pyroglutamyl peptidase II; PVH, paraventricular nucleus of the hypothalamus; TRH, thyrotropin releasing hormone.
References
- Segerson TP, Kauer J, Wolfe HC, Mobtaker H, Wu P, Jackson IM, Lechan RM 1987 Thyroid hormone regulates TRH biosynthesis in the paraventricular nucleus of the rat hypothalamus. Science 238:78–80 [DOI] [PubMed] [Google Scholar]
- Sánchez E, Vargas MA, Singru PS, Pascual I, Romero F, Fekete C, Charli J-L, Lechan RM 2009 Tanycyte pyroglutamyl peptidase II contributes to regulation of the hypothalamic-pituitary-thyroid axis through glial-axonal associations in the median eminence. Endocrinology 150:2283–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu HM, Kim SW, Salvatore D, Bartha T, Legradi G, Larsen PR, Lechan RM 1997 Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology 138:3359–3368 [DOI] [PubMed] [Google Scholar]
- Guadaño-Ferraz A, Escámez MJ, Rausell E, Bernal J 1999 Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory systems. J Neurosci 19:3430–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S 2004 A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer H, Maier MK, Iden S, Mittag J, Friesema EC, Visser TJ, Bauer K 2005 The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology 146:1701–1706 [DOI] [PubMed] [Google Scholar]
- Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H 2007 Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillni EA, Friedman TC, Todd RB, Birch NP, Loh YP, Jackson IM 1995 Pro-thyrotropin-releasing hormone processing by recombinant PC1. J Neurochem 65:2462–2472 [DOI] [PubMed] [Google Scholar]
- Nillni EA, Luo LG, Jackson IM, McMillan P 1996 Identification of the thyrotropin-releasing hormone precursor, its processing products, and its coexpression with convertase 1 in primary cultures of hypothalamic neurons: anatomic distribution of PC1 and PC2. Endocrinology 137:5651–5661 [DOI] [PubMed] [Google Scholar]
- Schaner P, Todd RB, Seidah NG, Nillni EA 1997 Processing of prothyrotropin-releasing hormone by the family of prohormone convertases. J Biol Chem 272:19958–19968 [DOI] [PubMed] [Google Scholar]
- Perello M, Friedman T, Paez-Espinosa V, Shen X, Stuart RC, Nillni EA 2006 Thyroid hormones selectively regulate the posttranslational processing of prothyrotropin-releasing hormone in the paraventricular nucleus of the hypothalamus. Endocrinology 147:2705–2716 [DOI] [PubMed] [Google Scholar]
- Nillni EA, Xie W, Mulcahy L, Sanchez VC, Wetsel WC 2002 Deficiencies in pro-thyrotropin-releasing hormone processing and abnormalities in thermoregulation in Cpefat/fat mice. J Biol Chem 277:48587–48595 [DOI] [PubMed] [Google Scholar]
- Busby Jr WH, Quackenbush GE, Humm J, Youngblood WW, Kizer JS 1987 An enzyme(s) that converts glutaminyl-peptides into pyroglutamyl-peptides. Presence in pituitary, brain, adrenal medulla, and lymphocytes. J Biol Chem 262:8532–8536 [PubMed] [Google Scholar]
- Eipper BA, Stoffers DA, Mains RE 1992 The biosynthesis of neuropeptides: peptide α-amidation. Annu Rev Neurosci 15:57–85 [DOI] [PubMed] [Google Scholar]
- Perello M, Nillni EA 2007 The biosynthesis and processing of neuropeptides: lessons from prothyrotropin releasing hormone (proTRH). Front Biosci 12:3554–3565 [DOI] [PubMed] [Google Scholar]
- O'Connor B, O'Cuinn G 1984 Localization of a narrow-specificity thyroliberin hydrolyzing pyroglutamate aminopeptidase in synaptosomal membranes of guinea-pig brain. Eur J Biochem 144:271–278 [DOI] [PubMed] [Google Scholar]
- Garat B, Miranda J, Charli JL, Joseph-Bravo P 1985 Presence of a membrane bound pyroglutamyl amino peptidase degrading thyrotropin releasing hormone in rat brain. Neuropeptides 6:27–40 [DOI] [PubMed] [Google Scholar]
- Charli JL, Cruz C, Vargas M, Joseph-Bravo P 1988 The narrow specificity pyroglutamate animopeptidase degrading TRH in brain is an ectoenzyme. Neurochem Int 13:237–242 [DOI] [PubMed] [Google Scholar]
- Vargas M, Mendez M, Cisneros M, Joseph-Bravo P, Charli JL 1987 Regional distribution of the membrane-bound pyroglutamate amino peptidase-degrading thyrotropin-releasing hormone in rat brain. Neurosci Lett 79:311–314 [DOI] [PubMed] [Google Scholar]
- Vargas MA, Herrera J, Uribe RM, Charli JL, Joseph-Bravo P 1992 Ontogenesis of pyroglutamyl peptidase II activity in rat brain, adenohypophysis and pancreas. Brain Res Dev Brain Res 66:251–256 [DOI] [PubMed] [Google Scholar]
- Heuer H, Ehrchen J, Bauer K, Schäfer MK 1998 Region-specific expression of thyrotrophin-releasing hormone-degrading ectoenzyme in the rat central nervous system and pituitary gland. Eur J Neurosci 10:1465–1478 [DOI] [PubMed] [Google Scholar]
- Bauer K, Carmeliet P, Schulz M, Baes M, Denef C 1990 Regulation and cellular localization of the membrane-bound thyrotropin-releasing hormone-degrading enzyme in primary cultures of neuronal, glial and adenohypophyseal cells. Endocrinology 127:1224–1233 [DOI] [PubMed] [Google Scholar]
- Cruz R, Vargas MA, Uribe RM, Pascual I, Lazcano I, Yiotakis A, Matziari M, Joseph-Bravo P, Charli JL 2008 Anterior pituitary pyroglutamyl peptidase II activity controls TRH-induced prolactin release. Peptides 29:1953–1964 [DOI] [PubMed] [Google Scholar]
- Cruz C, Charli JL, Vargas MA, Joseph-Bravo P 1991 Neuronal localization of pyroglutamate aminopeptidase II in primary cultures of fetal mouse brain. J Neurochem 56:1594–1601 [DOI] [PubMed] [Google Scholar]
- Schomburg L, Bauer K 1995 Thyroid hormones rapidly and stringently regulate the messenger RNA levels of the thyrotropin-releasing hormone (TRH) receptor and the TRH-degrading ectoenzyme. Endocrinology 136:3480–3485 [DOI] [PubMed] [Google Scholar]
- Ponce G, Charli JL, Pasten JA, Aceves C, Joseph-Bravo P 1988 Tissue-specific regulation of pyroglutamate aminopeptidase II activity by thyroid hormones. Neuroendocrinology 48:211–213 [DOI] [PubMed] [Google Scholar]
- Lin J, Wilk S 1998 Quantitation and regulation of pyroglutamyl peptidase II messenger RNA levels in rat tissues and GH3 cells. Neuroendocrinology 67:197–208 [DOI] [PubMed] [Google Scholar]
- Sánchez E, Singru PS, Fekete C, Lechan RM 2008 Induction of type 2 iodothyronine deiodinase in the mediobasal hypothalamus by bacterial lipopolysaccharide: role of corticosterone. Endocrinology 149:2484–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo RL, Andrade BM, da Silva ML, Ferreira AC, Carvalho DP 10 February 2009 Tissue-specific deiodinase regulation during food restriction and low replacement dose of leptin in rats. Am J Physiol Endocrinol Metab 10.1152/ajpendo.90869.2008 [DOI] [PubMed] [Google Scholar]