Abstract
Mutations of the gene expressing plasma membrane transporter for thyroid hormones MCT8 (SLC16A2) in humans lead to altered thyroid hormone levels and a severe neurodevelopmental disorder. Genetically engineered defect of the Mct8 gene in mice leads to similar thyroid hormone abnormalities but no obvious impairment of brain development or function. In this work we studied the relative role of the blood-brain barrier and the neuronal plasma cell membrane in the restricted access of T3 to the target neurons. To this end we compared the effects of low doses of T4 and T3 on cerebellar structure and gene expression in wild-type (Wt) and Mct8 null male mice [Mct8-/y, knockout (KO)] made hypothyroid during the neonatal period. We found that compared with Wt animals, T4 was considerably more potent than T3 in the Mct8KO mice, indicating a restricted access of T3, but not T4, to neurons after systemic administration in vivo. In contrast, T3 action in cultured cerebellar neurons was similar in Wt cells as in Mct8KO cells. The results suggest that the main restriction for T3 entry into the neural target cells of the mouse deficient in Mct8 is at the blood-brain barrier.
The main restriction for T3 entry into the neural target cells of the mouse deficient in the thyroid hormone transporter Mct8 is at the blood brain barrier.
The importance of plasma membrane transporters for the transfer of thyroid hormones from the extracellular milieu to the interior of the cell is now widely recognized. For many years it was thought that thyroid hormones enter the target cells by passive or facilitated diffusion. However mutations in a specific T4 and T3 transporter, the monocarboxylate transporter 8 (MCT8, SlC16A2), were found in patients with a severe neurodevelopmental defect and abnormal levels of iodothyronines in blood, consisting of decreased T4 and rT3 and increased T3 (1,2). These and subsequent findings revealed the physiological role of transporters in thyroid hormone action and their relevance to the brain (3,4,5).
The generation of Mct8 knockout (KO) mice demonstrated that absence of Mct8 impairs brain thyroid hormone uptake and metabolism, possibly due to a primary decreased uptake and degradation of T3 in target neurons (6,7). As a consequence, T3 concentrations increase in serum, with stimulation of Dio1 expression in liver and other tissues. It is postulated that the increased Dio1 activity increases conversion of T4 to T3, thereby decreasing T4 and further increasing T3 in serum. On the other hand, circulating rT3 is also decreased, which might be due to increased degradation by Dio1 and/or decreased formation from T4 by inner ring deiodination.
However, whereas the absence of Mct8 in mice reproduces the endocrine changes characteristic for humans with MCT8 gene mutations, the mutant mice do not show signs of neurological impairment, which contrasts with the observations in humans. It is logical to think that the neurological syndrome is due to impaired T3 action in neurons as a consequence of restricted uptake. However, no histological changes suggestive of cerebral hypothyroidism in the mutant mice have been found, and only a moderately decreased expression of thyroid hormone regulated genes such as neurogranin (Nrgn, also known as RC3) could be related to the decreased T3 uptake (6,7). At least in part, this could be interpreted as if the mice brains were in a state of locally compensated hypothyroidism because Dio2 activity is increased in the brain due to the decreased concentration of circulating T4 (6,7).
Early studies on Mct8 gene expression in rodents indicated that the gene is expressed predominantly in the choroid plexuses and in neurons (8). Recent studies have shown that Mct8 is also expressed in the blood-brain barrier (9). Other thyroid hormone transporters are expressed in the blood-brain barrier, such as organic anion transporters and L-type amino acid transporters (10,11). In the absence of Mct8, the restriction to T3 transport through the blood-brain barrier or through the neuronal plasma membrane would depend on the presence of alternative transporters.
In this work, we studied the relevance of Mct8 gene expression in neurons for T3 action. We have analyzed the relative effects of low doses of T4 and T3 on two T3 target genes, expressed in the striatum (Nrgn) and cerebellum [Hairless (Hr)]. We found that in male Mct8KO mice, when compared with wild-type (Wt) mice, these genes are less responsive to T3 than T4, indicating a restricted entry of plasma T3 but not T3 derived from T4. On the other hand, the action of T3 in primary cultures of cerebellar granular cells was little affected in the absence of Mct8. The data suggest that the critical restriction to T3 transport in the absence of Mct8 is located at the blood-brain barrier rather than at the plasma membrane of individual neurons.
Materials and Methods
Animals
Protocols for animal handling were approved by the local institutional Animal Care Committee, and followed the rules of the European Union. Animals were housed in temperature- (22 ± 2 C) and light (12 h light, 12 h dark cycle; lights on at 0700 h)-controlled conditions and had free access to food and water. Mct8KO mice were originally produced by Dumitrescu et al. (6) by homologous recombination. Experiments were carried out on Wt (Mct8+/y) and KO (Mct8-/y) male litter mates derived from the third and fourth back crossing of heterozygous females (Mct8−/+) with Wt (Mct8+/y) males of the C57BL/6J strain. The genotype was confirmed by PCR of tail DNA (38 cycles at 55 C annealing temperature) using the following primers: forward common, 5′-ACAACAAAA AGCCAAGCATT-3′; reverse Wt specific, 3′-GAGAGCAGCGTAAGGACAAA-5′; reverse knockout specific, 3′-CTCCCA AGCCTGATTTCTAT-5′. Using this procedure the Wt allele generated a 476-bp products and the null allele a 239-bp PCR product.
Induction of hypothyroidism and thyroid hormone treatments
After crossing with Wt male mice, Mct8+/− pregnant dams were given either drinking water or a solution containing 0.02% 1-methyl-2-mercapto-imidazol (Sigma Chemical Co., St. Louis, MO) plus 1% KClO4 ad libitum. These antithyroid drugs were given from gestational d 17, and throughout the lactating period, until the end of the experiment on postnatal day (P) 21. The pups were genotyped on P11 to select for Mct8+/y and Mct8−/y mice from the same litters. For simplicity, these animals will be referred to as Wt and KO mice, respectively, throughout this paper. The hypothyroid pups were then divided into three groups receiving no hormonal treatment, 20 ng T4 per gram body weight, or 3 ng T3 per gram body weight respectively. The hormones were administered in PBS containing 0.1% BSA, as daily single ip injections from P16 to P20. The following groups were thus prepared: euthyroid (n = 7) and hypothyroid (n = 6) Wt mice; euthyroid (n = 8) and hypothyroid (n = 6) KO mice; hypothyroid Wt mice treated with either T4 (n = 5) or T3 (n = 6), and hypothyroid KO mice treated with either T4 (n = 6) or T3 (n = 6). The pups were killed by decapitation 24 h after the last T4 or T3 injection, on P21. The striatum and cerebellum were rapidly dissected out, frozen on dry ice, and kept at −80 C until RNA isolation.
Histological methods
Examination of stained sections of the cerebellum and in situ mRNA hybridization analysis were performed on pups perfused with paraformaldehyde under anesthesia. Methods for perfusion, sectioning, staining, and in situ hybridization have been previously described in detail (12,13).
PCR
Total RNA was extracted using the TRIZOL reagent (Invitrogen, Carlsbad, CA). cDNA was prepared from 250 ng RNA using the high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). For quantitative PCR, a cDNA aliquot corresponding to 5 ng of the starting RNA was used, with Taqman Assay-on-Demand primers and the Taqman universal PCR master mix, No Amp Erase UNG (Applied Biosystems), on a 7900HT fast real-time PCR system (Applied Biosystems). The PCR program consisted in a hot start of 95 C for 10 min, followed by 40 cycles of 15 sec at 95 C and 1 min at 60 C. PCRs were performed in triplicates, using the 18S gene as internal standard and the 2-cycle threshold method for analysis. For quantitative assays, a standard curve was generated after amplification of known amounts of specific templates for each gene including 18S to calculate the number of mRNA copies in each sample.
Primary granular cell cultures
All media were purchased from Invitrogen. The cerebella were dissected from P6-P7 Wt and Mct8KO mice in Hanks’ balanced sodium salt solution, without Ca2+ and Mg2+, supplemented with 1 mm Na pyruvate and 10 mm HEPES (pH 7.4). The tissue was disaggregated by passing through a 0.9-mm syringe, rinsed in Hanks’ balanced sodium salt solution/pyruvate/HEPES and resuspended in serum-free culture medium (neurobasal medium supplemented with 2% B27, 0.5 mm glutamine, 10 U/ml penicillin, and 10 U/ml streptomycin) before seeding on poly-l-ornithine (Sigma)-coated 12-well multiwells (Sigma; 2.5 × 105 cells/well). After 4 d, the granular cells were incubated for 24 h in the absence or presence or T3 (Sigma) (from 0.2 to 5 nm) in the same medium containing 0.1% newborn calf serum deprived of thyroid hormones. Astrocyte contamination of the cultures was 3% as determined by immunofluorescence. Cells plated on glass coverslips were fixed with 4% paraformaldehyde for 5 min and permeabilized with 0.2% Triton X-100 in PBS for 5 min and then with methanol at −20 C for 2 min. After blocking with 5% nonimmune serum (Vector Laboratories, Burlingame, CA), the cells were doubly stained by overnight incubation at 4 C with the following combination of primary antibodies diluted 1:2000: rabbit polyclonal antiglial fibrillary acidic protein (Dako, Glostrup, Denmark) for astrocytes and mouse monoclonal anti-NeuN (Chemicon, Temecula, CA) for neurons. Nuclei were labeled with the nuclear stain 4′, 6-diamidino-2-phenylindole dihydrochloride.
Statistical calculations
Differences between means were obtained by two-way ANOVA, with the two factors being genotype and thyroidal state. As post hoc test, we used the Bonferroni tests using the Graph-Pad Prism software (http://www.graphpad.com/prism/).
Results
In this study we examined the relative effects of low doses of T4, and T3, administered to hypothyroid Wt and Mct8-deficient mice. The goal of this study was to evaluate the relative role of Mct8 in the transport of T4 and T3 in the brain in vivo by determining the effects of the hormones on cerebellar structure and the expression of thyroid hormone-regulated genes. Preliminary studies using morphological techniques failed to reveal consistent differences between age-matched, Wt, and KO mice during development that could be related to deficient thyroid hormone transport into the brain. Although not shown in this paper, we examined the laminar structure of the cerebral cortex, myelin protein expression, maturation of glial cells and different classes of interneurons and Purkinje cells, and number of interneuron precursors in the cerebellum and found no consistent deficits in the KO mice. These observations agree with previous studies reporting no obvious phenotype of cerebral hypothyroidism in these mice (6,7).
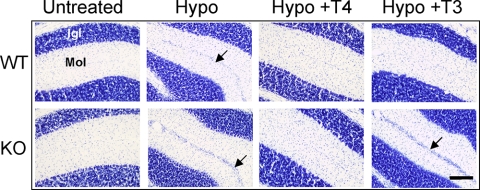
The next question we addressed was whether the absence of the Mct8 transporter impaired the biological activities of exogenous T4 and T3 selectively. Wt and KO mice were made hypothyroid by administration of antithyroid drugs and received 20 ng/g T4 or 3 ng/g T3 daily for 5 d before the animals were killed on P21. This dosage schedule was sufficient to completely correct the delayed migration of granular cells in the cerebellum of hypothyroid mice, as shown in Fig. 1: Cerebellar sections from each group of mice were stained and examined by optical microscopy. Migration of granular cells was already completed in the euthyroid Wt animals by P21 so that the external germinal layer (EGL) was absent. As expected, the EGL was still present in the hypothyroid Wt mice at this age. Both T4 and T3 treatments were equally able to prevent the effects of hypothyroidism in the Wt mice.
Figure 1.
Effects of hypothyroidism (Hypo) and thyroid hormone treatment on the structure of the cerebellar cortex of Wt and KO mice. The figure shows photomicrographs of lobule 7 from toloudine-stained sagittal slices of P21 mice. Igl, Internal granular layer; Mol, molecular layer. The arrows show the external germinal layer. Scale bar, 100 μm.
In the absence of Mct8, the structure of the cerebellar cortex in the untreated KO mice was identical with that of the Wt mice, with no EGL remaining, illustrating the lack of morphological developmental abnormalities. As in the hypothyroid Wt mice, the EGL was still present in the hypothyroid KO P21 mice. T4 treatment prevented the effects of hypothyroidism. However, in contrast to the effect on Wt mice, T3 treatment did not correct the migration abnormality.
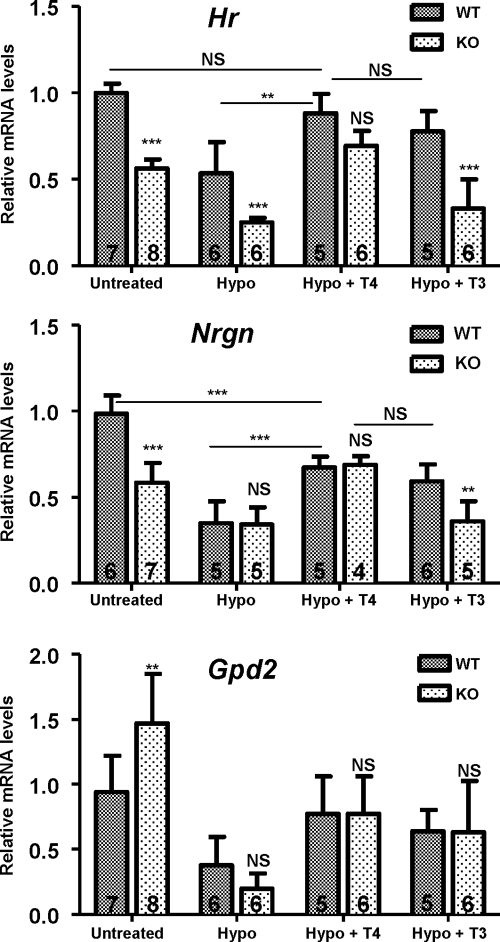
To examine the effects of T4, and T3 on gene expression, two well-known thyroid hormone target genes, Nrgn in the striatum, and Hr in the cerebellum, were examined by real-time PCR. The results are shown in Fig. 2. Interestingly and despite the lack of morphological impairment, Hr expression was decreased in the cerebellum of untreated KO mice with respect to the Wt mice, with levels similar to those present in WT hypothyroid mice. Induction of hypothyroidism decreased Hr expression further in the KO mice. T4 treatment significantly increased Hr expression in the hypothyroid Wt mice to levels that were similar to the untreated Wt mice. The effect of T4 treatment in the hypothyroid KO mice was not different from the hypothyroid Wt mice. The response to T3 was similar to that of T4 in the Wt mice but was significantly different when the hypothyroid Wt and KO mice were compared. T3 was without effect in the hypothyroid KO mice.
Figure 2.
Influence of Mct8 deficit on the response to T4 and T3. Wt and KO mice were made hypothyroid (Hypo) from the late fetal period up to P21, and treated with either vehicle or T4 or T3 for 5 d before the animals were killed. Data are means ± sd. Hr and RC3/Nrgn expression was analyzed by real-time PCR in the cerebellum and striatum, respectively. The data were analyzed by two-way ANOVA and the Bonferroni posttest to compare the data from each KO condition with the corresponding Wt. **, P < 0.01; ***, P < 0.001. For Hr expression, there was a significant effect of genotype (F1,41 = 116.01, P < 0.0001) and thyroidal status (F3,41 = 37.84, P < 0.0001), with a significant interaction (F3,41 = 3.88, P = 0.0156). Also for Nrgn, there was a significant effect of genotype (F1,35 = 24.68, P < 0.0001) and thyroidal status (F3,35 = 41.16, P < 0.0001), with a significant interaction (F3,35 = 10.30, P < 0.0001). The lower panel shows Gpd2 expression in the liver. There was a significant effect of thyroidal status (F3,41 = 23.67, P < 0.0001) but not genotype (F3,41 = 1.065, P = 0.308), with a significant interaction (F3,41 = 3.936, P < 0.0148). NS, Not significant.
Nrgn expression was also lower in the untreated KO than the Wt mice and decreased further with hypothyroidism. In contrast to Hr, the hypothyroid Wt and KO mice had similar Nrgn mRNA levels. Although neither T4 nor T3 treatment were able to fully normalize Nrgn expression, again there was a significant difference between the responses to T3 between the Wt and the KO mice but no difference in the responses to T4.
The liver mRNA Gpd2 (encoding mitochondrial α-glycerol phosphate dehydrogenase) was increased in the untreated KO mice relative to the untreated Wt mice. Hypothyroidism decreased Gpd2 expression in both genotypes (P < 0.001). T4 and T3 significantly increased the Gpd2 mRNA level compared with hypothyroid mice (P < 0.05). This increase was of similar magnitude in both genotypes and with both hormones.
To correlate the effects of thyroid hormones on Hr and Nrgn gene expression with Mct8 gene expression, we performed in situ mRNA hybridization (Fig. 3). The Mct8 gene was heavily expressed in the choroid plexus (Fig. 3, A–C) and the ependymal lining of the third ventricle (Fig. 3B). Other sites of expression were the upper layers of the cerebral cortex, especially the cingulate, visceral, and piriform cortices; the pyramidal and granular layers of the hippocampus; and the amygdala (Fig. 3B). In the cerebellum, besides expression in the choroid plexus, Mct8 mRNA had low but detectable abundance in the cerebellar cortex. In the striatum Mct8 was poorly expressed (Fig. 3A). Figure 3D shows Nrgn mRNA, which is abundantly present after a lateral-medial gradient, contrasting with the poor expression of Mct8. The effect of Mct8 gene deletion (Fig. 3E) did not affect the Nrgn mRNA signal gradient, in contrast to the effect of hypothyroidism (not shown, but see Ref. 14), which results in a total suppression of the gradient. Interestingly, Dio2 mRNA distribution in the striatum (Fig. 3F) also followed a similar gradient, with no changes in the pattern of distribution in the Mct8 KO mice (not shown). The lack of correlation between the sites of expression of Mct8 with that of the T3-target genes, Nrgn and Hr, indicate that Mct8 might be playing a minor role in thyroid hormone transport through the plasma membrane of cerebellar granular cells and striatal neurons in vivo.
Figure 3.
35S in situ hybridization for Mct8 (A–C), Nrgn (D and E) and Dio2 (F) mRNAs. The slices are from coronal sections at the level of the caudate (A, B, and D–F) and sagittal section of the cerebellum (C). All slices are from P21 Wt mice except for E, which shows the typical Nrgn expression in a P21 KO mouse. The arrows in A–C show heavy Mct8 expression in the choroid plexus. The asterisks show the caudate nucleus in A, with low hybridization signal, and the faint but detectable hybridization in the cerebellar cortex in C. 3V, Third ventricle.
To address this question more directly, we studied transporter expression and Hr induction by T3 in primary cultures of neurons. Granular cells from newborn mice cerebella were cultured. To analyze the effect of Mct8 deficit on the effect of T3 on Hr gene induction, T3 was added to granular cells from Wt and KO mice, and Hr mRNA was measured by quantitative PCR. One representative experiment using different concentrations of T3 is shown in Fig. 4. Starting at the lowest concentration used, 0.2 nm, all T3 concentrations gave a significant stimulation of Hr expression (P < 0.001) in both the Wt and KO cells. There were no significant differences in the effect of T3 in the KO mice as compared with the Wt except for the 1.25 nm T3 concentration in this particular experiment.
Figure 4.
Hr expression in primary cultures of granular cells from Wt or Mct8-deficient mice, as a function of T3 added to the cultures. Differences in Hr expression between the cells without T3 added and the 0.2 nm T3 concentration were P < 0.001 (a). Differences between the Wt and KO cells at each T3 concentration, by two-way ANOVA were not significant, except for the 0.25 nm T3, with P < 0.05 (b).
We also examined the profile of transporter expression in the same cultures used to analyze the effect of T3 on Hr. We measured the amounts of mRNA of organic anion-transporting polypeptide (Oatp)-2 (Slco1a4), Oatp14 (Slc1c1), and Mct8 (Table 1). Granular cells from Wt cells expressed predominantly Mct8 (591 ± 130 mRNA copies, relative to 18S RNA), which was undetectable in the KO cells. Oatp2 and Oatp14 were expressed at much lower levels (27.0 ± 9.1 and 7.4 ± 3.4, respectively). There were no changes in the KO compared with the Wt, and T3 treatment had no effects on transporter expression, except for the higher dose, which decreased Oatp14 mRNA in the KO mice.
Table 1.
Effect of T3 treatment on transporter expression in cultured granular cells
| Transporter mRNA | No T3
|
2.5 nm T3
|
5.0 nm T3
|
|||
|---|---|---|---|---|---|---|
| Wt | KO | Wt | KO | Wt | KO | |
| Mct8 | 591 ± 130 | 596 ± 48 | 621 ± 109 | |||
| Oatp2 | 27.0 ± 9.1 | 28.0 ± 8.5 | 29.3 ± 5.4 | 23.9 ± 6.1 | 28.3 ± 1.6 | 19.5 ± 4.7 |
| Oatp14 | 7.4 ± 3.4 | 6.8 ± 2.4 | 4.9 ± 0.9 | 2.8 ± 0.4 | 5.9 ± 1.7 | 2.5 ± 0.3a |
Primary cultures of granular cells from the cerebella of Wt and Mct8 KO mice were incubated in the presence of 0, 0.2, 0.5, 1.25, 2.5, and 5.0 nm T3 for 24 h. Expression of Mct8 (Slc16a2), Oatp2 (Slco1a4), and Oatp14 (Slc1c1) was quantified by real-time PCR using TaqMan probes. Shown are the data (mean number of RNA copies relative to 18S RNA ± sd) from cells incubated without added T3 or in the presence of 2.5 and 5.0 nm only. Mct8 mRNA was not detected in the KO cells. The cells used in this experiment are the same as for Hr mRNA quantification shown in Fig. 4. Two-way ANOVA using the data from all T3 concentrations revealed that there was no difference of genotype or treatment, except for the highest T3 dose that decreased Oatp14 mRNA in the KO cells.
P < 0.05.
Discussion
The main finding of the present work is that the brain of animals lacking the thyroid hormone transporter Mct8 do not readily respond to a low dose of T3, whereas the sensitivity to an equally low dose of T4 is similar to that of Wt animals. Five nanograms per gram T3 were previously shown to normalize circulating TSH and hypothalamic TRH transcripts in hypothyroid Wt but not hypothyroid Mct8KO mice (7). We used low doses of T4 and T3 to avoid inhibition of Dio2 by T4 and the use of low-affinity transporters by T3. Although, based on Gpd2 gene expression, the doses used did not fully restore euthyroidism in the Wt mice liver, they were sufficient to normalize cerebellar development, EGL migration, and Hr expression in the Wt hypothyroid mice. In contrast, they were insufficient for Nrgn mRNA normalization.
In normal animals, T3 reaches the extracellular fluid of the brain parenchyma from the circulation through the blood-brain barrier and acts directly on the neurons. T4 may exert some extranuclear actions, but the bulk of genomic responses are mediated by its conversion to T3 by Dio2. In the brain, this reaction takes place predominantly in glial cells, namely astrocytes, and IV ventricle tanycytes, although Dio2 expression has also been observed in some cerebral cortex interneurons as a response to hypothyroidism (13,15).
In the developing cerebellum, T4 to T3 conversion takes place in the protoplasmic astrocytes located within the granular layer in close association to the granular cells. Because there was no difference in the effects of T4 in KO vs. Wt mice, the results suggest that, in the doses used, T4 could reach the cerebellar astrocytes of Mct8-deficient mice in sufficient amount to produce the effects observed in Wt mice. This event is further facilitated by the great increase of Dio2 activity in the brain of Mct8 KO mice (6,7). In addition, the results also suggest that the T3 produced in astrocytes can access the granular cell nuclear receptors with little restriction at the neuronal cell membrane.
The effects of T3 on granular cells in culture agree with the above conclusion. Trajkovic et al. (7) also showed that T3 was effective in inducing Purkinje cell differentiation in vitro in the presence or absence of Mct8. In the context of these findings, it was surprising that in isolated granular cells the Mct8 gene was by far the more abundantly expressed transporter. However, its absence in Mct8−/y cells caused only a minimal impairment of T3 action at the nuclear level, as evidenced by Hr gene expression, with a trend toward a lower effect at intermediate doses in the KO cells. Although expression of other transporters was much lower, it was enough to elicit almost identical responses to T3 in the absence as in the presence of Mct8. The presence of other transporters is also likely the cause for the similar effect of T4 in vivo in Wt and KO mice. The relative effects of T4 and T3 on the expression of the Nrgn gene in the striatum suggests a similar conclusion.
These results agree with the preferential accumulation of administered T4, relative to the restricted accumulation of administered T3 in the brain of Mct8 KO mice (6,7). Brain T3 concentrations in the KO mice were about two thirds of normal. Given the restriction to T3 entry, most T3 in the brain of these animals must be derived from T4.
The main site of Mct8 expression is the choroid plexus. The consequences of the absence of Mct8 in the choroid plexus are not known. Intrathecally administered T4 and T3 can access brain structures (16). However, most studies on the routes of thyroid hormone entry to the brain agree that the cerebrospinal fluid allows only limited access of thyroid hormone to the brain parenchyma, preferentially reaching cells located near the surface of the ventricles (17,18). Therefore, the main access of thyroid hormone to the brain parenchyma is through the blood-brain barrier. In keeping with this concept, our data suggest that the restriction of T3 entry in Mct8-deficient mice is at the blood-brain barrier. Indeed Mct8 has been recently demonstrated in the membrane of the brain parenchyma capillaries (9). The presence of other transporters such as Oatp14 and Oatp2, with more affinity for T4 than T3 may explain the different sensitivities to T4 and T3. Whereas normal T4 uptake may preserve the compensated phenotype in mice, the lack of alternative transporters in the human blood-brain barrier would be the reason for the neurological impairment (9).
In conclusion, the data show that the main restriction to T3 action in the absence of Mct8 is at the level of the blood-brain barrier. The thyroid hormone transport role of Mct8 in the plasma membrane of neurons, at least in the striatum and the cerebellum, seems to be minimal.
Acknowledgments
We thank Eulalia Moreno and Ana Torrecilla for the technical help.
Footnotes
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 15, 2009
Abbreviations: EGL, External germinal layer; Gpd2, α-glycerol phosphate dehydrogenase; Hr, hairless; KO, knockout; MCT, monocarboxylate transporter; Nrgn, neurogranin; Oatp, organic anion-transporting polypeptide; P, postnatal day; Wt, wild type.
References
- Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S 2004 A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ 2004 Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364:1435–1437 [DOI] [PubMed] [Google Scholar]
- Refetoff S, Dumitrescu AM 2007 Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab 21:277–305 [DOI] [PubMed] [Google Scholar]
- Grüters A 2007 Thyroid hormone transporter defects. Endocr Dev 10:118–126 [DOI] [PubMed] [Google Scholar]
- Visser WE, Friesema EC, Jansen J, Visser TJ 2008 Thyroid hormone transport in and out of cells. Trends Endocrinol Metab 19:50–56 [DOI] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S 2006 Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology 147:4036–4043 [DOI] [PubMed] [Google Scholar]
- Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H 2007 Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer H, Maier MK, Iden S, Mittag J, Friesema EC, Visser TJ, Bauer K 2005 The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology 146:1701–1706 [DOI] [PubMed] [Google Scholar]
- Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, Grindstaff KK, Mengesha W, Raman C, Zerangue N 2008 Expression of the thyroid hormone transporters MCT8 (SLC16A2) and OATP14 (SLCO1C1) at the blood-brain barrier. Endocrinology 149:6251–6261 [DOI] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y 2003 Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem 278:43489–43495 [DOI] [PubMed] [Google Scholar]
- Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM 1999 Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci USA 96:12079–12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J, Guadaño-Ferraz A 2002 Analysis of thyroid hormone-dependent genes in the brain by in situ hybridization. Methods Mol Biol 202:71–90 [DOI] [PubMed] [Google Scholar]
- Guadaño-Ferraz A, Escámez MJ, Rausell E, Bernal J 1999 Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory systems. J Neurosci 19:3430–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano J, Morte B, Scanlan TS, Bernal J 2003 Differential effects of triiodothyronine and the thyroid hormone receptor beta-specific agonist GC-1 on thyroid hormone target genes in the brain. Endocrinology 144:5480–5487 [DOI] [PubMed] [Google Scholar]
- Guadaño-Ferraz A, Obregón MJ, St. Germain DL, Bernal J 1997 The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc Natl Acad Sci USA 94:10391–10396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palha JA, Nissanov J, Fernandes R, Sousa JC, Bertrand L, Dratman MB, Morreale de Escobar G, Gottesman M, Saraiva MJ 2002 Thyroid hormone distribution in the mouse brain: the role of transthyretin. Neuroscience 113:837–847 [DOI] [PubMed] [Google Scholar]
- Blay P, Nilsson C, Owman C, Aldred A, Schreiber G 1993 Transthyretin expression in the rat brain: effect of thyroid functional state and role in thyroxine transport. Brain Res 632:114–120 [DOI] [PubMed] [Google Scholar]
- Dratman MB, Crutchfield FL, Schoenhoff MB 1991 Transport of iodothyronines from bloodstream to brain: contributions by blood:brain and choroid plexus:cerebrospinal fluid barriers. Brain Res 554:229–236 [DOI] [PubMed] [Google Scholar]