Abstract
The fuel-sensing enzyme AMP-activated protein kinase (AMPK) has been implicated in central nervous system control of energy balance. Hypothalamic AMPK activity is increased by food deprivation, and this elevation is inhibited by refeeding or by leptin treatment. The contribution of extrahypothalamic AMPK activity in energy balance control has not been addressed. Here, we investigate the effects of physiological state on the AMPK activity in hindbrain nucleus tractus solitarius (NTS) neurons because treatments that reduce energy availability in these neurons trigger behavioral, endocrine, and autonomic responses to restore energy balance. Food-deprived rats showed significantly increased AMPK activity in both NTS- and hypothalamus-enriched lysates compared with those that were ad libitum fed. Pharmacological inhibition of AMPK activity in medial NTS neurons, by intraparenchymal injection of compound C, suppressed food intake and body weight gain compared with vehicle. Fourth ventricle (4th icv) compound C delivery increased heart rate and spontaneous activity in free-moving rats. Suppression of AMPK activity has been implicated in leptin’s anorectic action in the hypothalamus. Given the role of leptin signaling in food intake inhibition within the medial NTS, we also examined whether stimulation of hindbrain AMPK by 4th icv administration of 5-aminoimidazole-4-carboxamide-riboside (AICAR), an AMP-mimicking promoter of AMPK activity, could attenuate the inhibition of food intake by 4th icv leptin. The intake-suppressive effects of leptin (at 2 and 4 h) were completely reversed by AICAR. We conclude that 1) hindbrain AMPK activity contributes to energy balance control through regulation of food intake and energy expenditure, 2) leptin’s intake-reducing effects in the NTS are meditated by AMPK, and 3) central nervous system AMPK controls whole-body homeostasis at anatomically distributed sites across the neuraxis.
Hindbrain AMP-activated protein kinase (AMPK) activity contributes to energy balance control through regulation of energy intake and expenditure, with AMPK activity in the nucleus tractus solitarius mediating leptin’s intake-reducing effects.
The fuel-sensing enzyme AMP-activated protein kinase (AMPK) is an important component of central nervous system (CNS) control of energy balance (1,2). To date, studies have focused on the functional effects of AMPK activity in neurons of the arcuate and paraventricular nuclei (PVN) of the hypothalamus. Hypothalamic AMPK activity is increased by food deprivation (1,2,3) and by the energy-reducing effects of 2-deoxy-d-glucose or insulin treatment (4,5,6). The elevation in AMPK activity induced by food deprivation in the arcuate nucleus and PVN is inhibited by treatment with leptin or refeeding (1,2). Furthermore, inhibition of hypothalamic AMPK activity by leptin is necessary for leptin’s intake- and body weight-suppressive effects, because elevation in constitutive AMPK activity blocks the anorectic effects of leptin treatment (1). AMPK is expressed in brain regions other than the hypothalamus including those also implicated in the neural control of energy balance (7). The contribution of extrahypothalamic CNS AMPK activity to energy balance control has yet to be addressed.
Nucleus tractus solitarius (NTS) neurons are found within the dorsal hindbrain; they receive and integrate gastrointestinal (GI) (vagus nerve transmission) and blood-borne (e.g. leptin) signals and issue output commands that are essential to energy balance control (8,9,10,11,12,13,14). Medial NTS (mNTS) neurons at the area postrema (AP) level express the long form of the leptin receptor, and activation of these leptin-responsive (pSTAT3) neurons reduces food intake (8,10). The role of NTS AMPK signaling in mediating this effect is untested.
AMPK is a heterotrimer that consists of a catalytic α-subunit and regulatory β- and γ-subunit (1,15). AMPK has been termed the intracellular fuel gauge, because its activity is regulated by the cellular AMP/ATP ratio and by upstream kinases (15,16). AMPK activation represses ATP-consuming anabolic pathways and induces ATP-producing pathways through regulation of gene expression. Responses to alterations in CNS AMPK signaling may be mediated through transcriptional effects (15), actions on ion channels (17,18), or changes in cytosolic Ca2+ (3).
To address whether NTS AMPK contributes to energy balance control, experiments investigated whether energy status (food deprived vs. ad libitum-fed or refeeding after deprivation) alters AMPK activity in NTS-enriched lysates. Hindbrain [fourth intracerebroventricular (4th icv)] delivery of compound C, a selective pharmacological inhibitor of AMPK, was used to investigate the role of hindbrain AMPK activity in vivo in the control of energy intake, as well as energy expenditure in freely moving rats. To determine whether the effects of AMPK activity on food intake control would be neuron and/or site specific, inhibition of AMPK activity was targeted to the leptin receptor-expressing mNTS neurons at the level of the AP and to other, more rostral mNTS neurons that lack leptin signaling (10). To explore whether reduced food intake after hindbrain leptin delivery is mediated by a reduction in hindbrain AMPK activity, leptin was delivered to the fourth ventricle with and without pretreatment with 5-aminoimidazole-4-carboxamide-riboside (AICAR), an AMP-mimicking promoter of cellular AMPK activity. Collectively, results from these experiments provide support for the hypothesis that dorsal hindbrain AMPK activity contributes to energy balance control. When combined with findings from hypothalamic-targeted experiments (1,19,20), these data are consistent with the perspective that CNS AMPK activity is critical to the control of energy balance and establish that the contribution of CNS AMPK activity to energy balance control is anatomically distributed, rather than localized in the hypothalamus.
Materials and Methods
Subjects and materials
Adult male Sprague Dawley rats (275–300 g; Charles River, Wilmington, MA) were housed in individual cages in a room maintained at 23 C with a 12-h light, 12-h dark cycle. All rats had ad libitum access to rodent chow (Purina Rodent Chow 5001) and water during experimental testing except as noted.
The selective AMPK antagonist, compound C (Fisher Scientific, Pittsburgh, PA), and AMP-mimicking promoter of AMPK activity, AICAR (Acros Organics, Geel, Belgium), were chosen to examine hindbrain AMPK-mediated effects on energy balance regulation because responses generated by both of these pharmacological tools are reversible and the functional effects of AMPK inhibition/activation are induced rapidly (thereby enabling within-subject comparisons) (21). Recombinant mouse leptin was purchased from Dr. E. Parlow (National Institute of Diabetes and Digestive and Kidney Diseases, Torrance, CA) and was dissolved in 0.01 m sodium bicarbonate.
NTS and 4th icv cannulation and telemetric transponder surgery
Chronic indwelling guide cannulas (Plastics One, Roanoke, VA; 26-gauge) were implanted under ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) anesthesia and analgesia (Metacam 2 mg/kg) at the following coordinates: 4th icv, 2.0 mm above the fourth cerebral ventricle, 2.5 mm anterior to occipital suture, 4.5 mm ventral to dura, and on the midline; bilateral rostral mNTS, 2.0 mm above the mNTS, 0.0 mm from occipital crest, ±0.8 mm lateral to midline, and 5.9 mm ventral from skull surface (22,23);and bilateral caudal mNTS, 2.0 mm above the mNTS, −1.0 mm from occipital crest, ±0.7 mm lateral to midline, and 5.9 mm ventral from skull surface (23). Cannulas were cemented to four jeweler’s screws attached to the skull and closed with an obturator. The intended anatomical position of the 4th icv and mNTS injection sites were evaluated 1 wk after surgery by measurement of the sympathoadrenal-mediated glycemic effect of cytoglucopenia induced by icv/parenchymal injection of 210 μg/24 μg 5-thio-d-glucose in 2 μl/100 nl artificial cerebrospinal fluid (aCSF; Harvard Apparatus, Holliston, MA) (24). A hyperglycemic response (≥100% of basal glycemia) verified cannula placement and served as an inclusion criterion for the experiments. Parenchymal injection sites were confirmed via postmortem histological verification of the position of pontamine sky blue injections. In rats designated for recording core temperature, heart rate (HR), and spontaneous activity, a small midline abdominal incision was made and a telemetric transponder (HRC 4000 Mini-Mitter; VitalView, Bend, OR) was inserted into the abdominal cavity. Leads were then tunneled under the skin and secured to the chest muscles with metal sutures.
Dorsal hindbrain AMPK activity
Tissue collection
Rats fed ad libitum, food deprived for 24 or 48 h, or refed for 2 h after a 48-h fast (n = 4–6 per energy state for immunoblot and n = 6–7 per energy state for activity assay) were killed by decapitation 1 h into the light cycle (lights on at 0800 h). Brains were rapidly removed; tissue lysates were collected from NTS-enriched tissue of dorsal vagus complex (DVC), whole hypothalamus, and liver. Samples were then flash frozen in isopentane and stored at −80 C until processing. Briefly, to collect NTS-enriched tissue, brains were positioned with the dorsal surface up, and the cerebellum was carefully separated from the medulla. The calamus scriptorius (most caudal portion of the fourth ventricle) provides a landmark for isolating NTS-enriched dorsomedial medullary tissue. Coronal cuts were made 2 mm anterior and posterior to the calamus scriptorius. Bilateral sagittal cuts were made about 2 mm lateral to the midline. A horizontal cut was then made 0.5 mm ventral to the central canal. These methods are a modification of our mouse work (25).
Biochemistry
Tissue lysates were prepared as previously described (1). Briefly, lysates were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes for immunoblot analysis. Phospho- (p)AMPKα-Thr 172 rabbit monoclonal antibody (Cell Signaling Technology, Beverly, MA; catalog no. 2535S) was used to evaluate AMPK activity normalized to immunoblot analysis of total AMPKα (reactive against both the α1 and α2 subunits; Cell Signaling). Blots were quantified using NIH Image software.
AMPK activity assays were performed in parallel as described (1). Tissue was homogenized in 1.0 mm dithiothreitol and 0.2 mm AMP buffer (50 mm Tris-HCl, 150 mm NaCl, 50 mm NaF, 2 mm EDTA). AMPKα was immunoprecipitated from 300 μg lysates using AMPK antibody (reactive against both the α1 and α2 subunits; Cell Signaling) prebound to protein-G-Sepharose. AMPK activity in the immune complex was determined by phosphorylation of SAMS.a Immunoprecipitate was divided into three aliquots: two assayed for AMPK activity with reaction solution containing 0.1 μCi [γ-32P]ATP, 0.1 μl 100 mm cold ATP, 0.25 μl 1 m MgCl2, 10 μl 1 mm AMP, and 10 μl 0.1 mm SAMS peptide. The third aliquot served as a control for background. Samples were pipetted onto Whatman paper squares, and 32P incorporation was measured via a scintillation counter. AMPK activity was calculated from the difference of counts between SAMS-containing and SAMS-negative samples and expressed as nanomoles of ATP incorporated per minute per milligram sample peptide.
Hindbrain AMPK activity and food intake and energetic responses
Rats (n = 10) implanted with a 4th icv cannula received injections of the AMPK inhibitor, compound C (2.5 and 5.0 μg in 1 μl), or vehicle (dimethylsulfoxide) in a counterbalanced design before dark onset (lights off at 1000 h; dose selection from Ref. 21). Ten minutes after drug administration, rats were presented with preweighed maintenance chow. Intakes were recorded to the nearest 0.1 g at 1, 3, and 24 h after food presentation.
A separate group of ad libitum-fed rats (n = 12) had their food withdrawn at light onset (0800 h), and their plastic home cages were placed on transponder receivers on experimental testing days of energetic response recording. A minimum of 2 d separated each experimental day. At 0930 h on testing days, rats received a 4th icv injection of either compound C (5.0 μg in 1 μl; dose selection based on food intake results) or vehicle in a counterbalanced design. HR, core temperature, and spontaneous activity were recorded for a 4-h period after injections. Recording began 1 h before injections. HR was recorded every 30 sec, and core temperature and activity was recorded every 5 min. Spontaneous activity was recorded as cumulative activity counts on the x-y axis every 5 min (change in x-y position is equal to one count). Food was returned at the end of the recording period.
NTS AMPK activity and food intake
In a counterbalanced fashion, two groups of rats received unilateral intraparenchymal mNTS injections of compound C (1.0 μg in 100 nl) or vehicle aimed at either the caudal mNTS (n = 11; at the level of the AP) or more rostral mNTS (n = 7; at the level of the fourth ventricle) before dark onset (lights off at 1000 h). This dose of compound C was previously determined to be subthreshold for a feeding effect when delivered to the fourth ventricle. Food was presented 10 min after injections, and intakes were recorded to the nearest 0.1 g at 1, 3, 6, and 24 h after food presentation.
Hindbrain AMPK and the intake-reducing effects of hindbrain leptin
A separate group of ad libitum-fed rats (n = 5) received 4th icv injection of the AMP-mimicking promoter of AMPK activity, AICAR [300 μg in 3 μl; dose selection from Ref. 20) or aCSF vehicle followed 45min later by 4th icv injection of leptin (5.0 μg in 2 μl; dose selection based on intake suppression from Ref. 8) or PBS vehicle given immediately before dark onset (lights off at 1000 h). Food was presented, and intakes were recorded at 2 and 4 h after food presentation.
To directly assess the effects of hindbrain-directed leptin, AICAR and their combination on AMPK activity in the DVC and the hypothalamus, a separate group of ad libitum-fed rats (n = 12) received 4th icv injection of AICAR (300 μg in 3 μl) or aCSF vehicle followed 45 min later by 4th icv injection of leptin (5.0 μg in 2 μl) or PBS vehicle given immediately before dark onset (lights off at 1000 h) in a 2 × 2 design. Animals were killed 2 h after the second injection by decapitation. Brains were rapidly removed, DVC and whole hypothalamus were dissected, and tissue lysates were assessed for pAMPKα2 and total AMPKα levels as described above.
Data and statistical analyses
Data for each respective study were analyzed separately. Results are expressed as mean ± sem. For all experiments, comparisons between treatment means were analyzed by one- or two-way ANOVA and, if appropriate, post hoc, pair-wise comparisons were made using Tukey’s honestly significant difference test with P < 0.05 considered statistically significant. Analyses were made using PC-SAS (version 8.02; SAS Institute, Cary, NC) mixed procedure.
Results
Energy state alters hindbrain AMPK activity
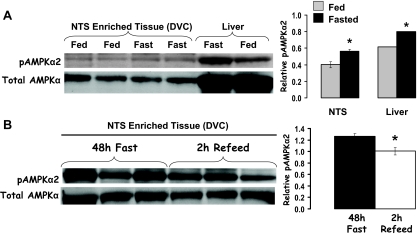
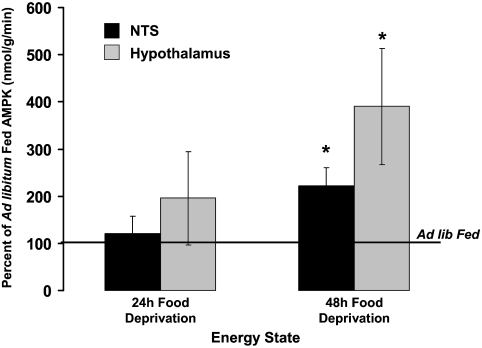
Food deprivation significantly increased pAMPKα levels in both NTS-enriched (DVC) and liver tissue (Fig. 1A). A 2-h refeed (food access after 48-h food deprivation) reduced DVC pAMPKα levels compared with 48-h food-deprived rats (Fig. 1B). Total AMPKα levels were equivalent. AMPKα activity assay results confirm that food deprivation significantly increased AMPKα activity in hypothalamic and DVC tissues similarly, with 48-h food deprivation significantly increasing activity in both tissues compared with AMPKα activity levels in ad libitum-fed rats (Fig. 2).
Figure 1.
A, Compared with rats fed ad libitum, 24 h food deprivation increased pAMPKα levels in NTS-enriched tissue (DVC). These data indicate that pAMPKα levels in DVC and liver tissues are similarly responsive to energy status, with food deprivation increasing pAMPKα in both the liver (control) and NTS by approximately 25%. B, Elevated pAMPKα levels in NTS-enriched tissue of 48-h-food-deprived rats is reduced after a 2-h reefed. Representative immunoblots for total AMPK and pAMPKα are shown. *, P < 0.05.
Figure 2.
Food deprivation for 48 h significantly increased AMPKα activity in NTS- and hypothalamus-enriched lysates compared with ad libitum-fed rats. *, P < 0.05 from respective tissue for ad libitum AMPKα activity.
Inhibition of hindbrain AMPK activity affects food intake and body weight gain
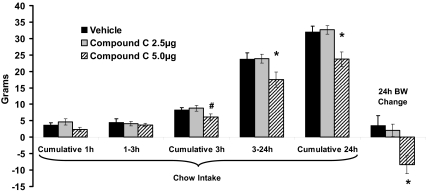
Inhibition of hindbrain AMPK activity by 4th icv administration of compound C produced a significant dose-dependent suppression of food intake compared with vehicle administration (Fig. 3). The 5.0-μg dose of compound C significantly suppressed food intake between 3 and 24 h and suppressed cumulative 24-h intake compared with vehicle injection, whereas the 2.5-μg dose had no effect. Similarly, only treatment with 5.0 μg compound C significantly suppressed 24-h body weight gain compared with vehicle injection, whereas the 2.5-μg dose had no significant effect.
Figure 3.
Inhibition of hindbrain AMPK activity (4th icv compound C, 5 μg) significantly suppressed cumulative food intake between 3 and 24 h as well as cumulative 24-h intake compared with intakes after vehicle injection. In addition, 4th icv compound C at 5 μg significantly suppressed 24-h body weight gain compared with vehicle injection. Chow intake and body weight (BW) were unaffected by the lower compound C dose (2.5 μg) for all time points. *, P < 0.05 from vehicle; #, P = 0.058.
Inhibition of hindbrain AMPK activity affects energetic responses
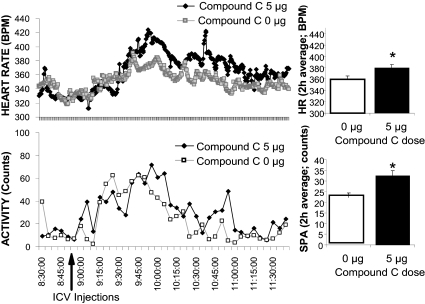
Inhibition of hindbrain AMPK activity (compound C, 5.0 μg) significantly increased 2-h HR average by about 20 beats/min compared with vehicle for 2 h after injection (Fig. 4A). This treatment also increased spontaneous activity by about 46% compared with vehicle for 2 h after injection (Fig. 4B). There was no significant effect on core temperature during the same time period (data not shown).
Figure 4.
[HR; Heart Rate beats per minute (BPM)] and spontaneous activity (counts) before and after 4th icv injection of compound C (5 μg) or vehicle in freely moving awake rats. The histograms represent 2-h averages of HR and activity and shows elevated HR and spontaneous activity in response to 4th icv compound C compared with vehicle injections. *, P < 0.05 from respective vehicle HR. SPA, Spontaneous activity.
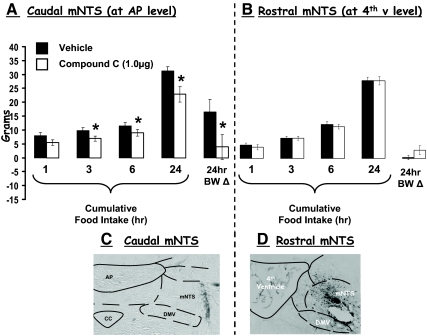
Inhibition of caudal and more rostral mNTS AMPK activity differentially affects food intake and body weight gain
Compound C delivered to the caudal mNTS at the AP level (histologically verified), at a dose (1 μg) that was without effect when delivered ventricularly, significantly suppressed food intake at 3, 6, and 24 h compared with within-subject intakes after vehicle injections (Fig. 5A). In addition, 24-h body weight gain was significantly reduced by this treatment compared with vehicle (Fig. 5A). By contrast, when the same treatment was delivered to a more rostral level of the mNTS (at the fourth ventricle level, histologically verified), neither food intake nor 24-h body weight gain was affected (Fig. 5B).
Figure 5.
A, Compound C (1.0 μg/100 nl) delivered to the caudal mNTS, at the AP level, significantly suppressed food intake at 3, 6, and 24 h as well as 24-h body weight (BW) gain compared with intakes and body weight after vehicle injections. B, By contrast, when compound C was delivered to the rostral mNTS [at the 4th ventricle (v) level], neither food intake nor 24-h body weight gain was affected. *, P < 0.05 from respective vehicle intakes and body weights. Photographs of representative histological sections are provided to show intraparenchymal injections of pontamine sky blue in the caudal mNTS (at the level of the AP) (C) and rostral mNTS (at the level of the fourth ventricle) (D). CC, Central canal; DMV, dorsal motor nucleus of the vagus.
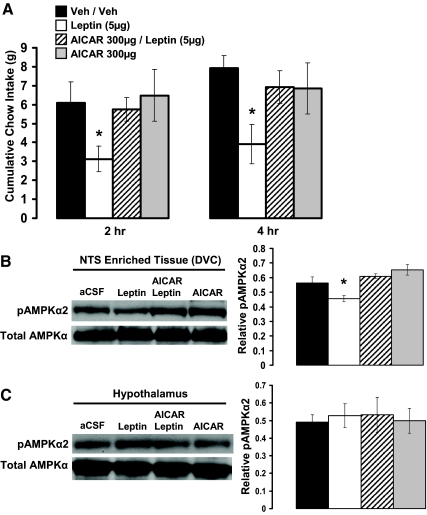
Hindbrain AMPK activity mediates the intake-reducing effects of hindbrain leptin
Hindbrain ventricular delivery of leptin (5 μg) significantly reduced food intake at 2 and 4 h compared with vehicle injection (Fig. 6A). Hindbrain delivery of the AMPK promoter AICAR (300 μg) alone had no effect on food intake at either 2 or 4 h compared with vehicle, whereas pretreatment with AICAR reversed the suppression of food intake by hindbrain leptin at 2 and 4 h.
Figure 6.
A, Increasing hindbrain AMPK activity (4th icv AICAR, 300 μg) reversed the suppression of cumulative food intake at 2 and 4 h after 4th icv leptin (5 μg) administration. B, In ad libitum-fed rats, 4th icv administration of leptin (5 μg) suppressed pAMPKα levels in NTS-enriched tissue (DVC) 2 h after 4th icv administration compared with control injections. This suppression in pAMPKα levels in the DVC was reversed by 4th icv administration of AICAR (300 μg) at a dose that was without effect on its own. C, Conversely, no alterations in pAMPKα levels were observed in hypothalamic lysates by 4th icv administration of leptin, AICAR, or their combination, thus indicating a hindbrain site of action for the 4th icv-administered compounds. Representative immunoblots for total AMPK and pAMPKα are shown. *, P < 0.05 from vehicle intakes and respective vehicle tissue.
Hindbrain delivery (4th icv) of leptin (5 μg) significantly decreased pAMPKα levels in the NTS-enriched DVC tissue but had no effect on pAMPKα levels in the hypothalamus compared with vehicle injection (Fig. 6, B and C). The 4th icv injection of AICAR (300 μg) alone had no effect on pAMPKα levels in either the DVC or hypothalamic tissues. Pretreatment with AICAR, however, reversed the suppression of pAMPKα levels in the DVC by hindbrain leptin and did not alter the pAMPKα levels in the hypothalamus.
Discussion
AMPK activity in the hypothalamus has been implicated in control of energy balance and in the mediation of the anorectic effects of leptin. The contribution of extrahypothalamic AMPK activity in energy balance control has not been addressed. Here, we investigate AMPK activity in hindbrain NTS neurons because 1) treatments that reduce energy availability in these neurons trigger behavioral, endocrine, and autonomic responses to restore energy balance and 2) mNTS neurons at the AP level express the long form of the leptin receptor and contribute to the control food intake (8,10). A set of experiments was performed to address the hypothesis that dorsal hindbrain AMPK activity contributes to the control of energy balance through regulation of food intake and energy expenditure. Results indicate that hindbrain AMPK activity is responsive to energy status, that pharmacological inhibition of AMPK in this brain region affects food intake and energy expenditure, and that the intake inhibition resulting from hindbrain leptin treatment is mediated by AMPK activity in the hindbrain. DVC AMPK activity, assessed by immunoblotting and activity assay, was increased by energy deprivation. Pharmacological inhibition of hindbrain AMPK activity by 4th icv compound C suppressed food intake and body weight gain. In addition, inhibition of hindbrain AMPK activity increased HR and spontaneous activity. Direct mNTS parenchymal injection of compound C confirmed the intake-suppressive effects of hindbrain ventricular injection and was mNTS site specific; inhibition of AMPK activity in mNTS neurons at the AP level resulted in intake suppression, but no effect resulted from targeting neurons at a more rostral level of the mNTS. The intake-reducing effects of hindbrain leptin delivery was mediated by AMPK signaling, because increased hindbrain AMPK activity by 4th icv AICAR administration reversed the suppression of food intake by hindbrain leptin delivery. Together with results from hypothalamic-targeted experiments (1,19,20), the current findings support the perspective that 1) CNS AMPK activity contributes to energy balance control, 2) the CNS AMPK activity of relevance to energy balance is anatomically distributed, with contributions arising from hindbrain and hypothalamic nuclei, and 3) the intake-suppressive effects of hindbrain leptin delivery are mediated at least in part by AMPK activity.
The regional NTS differences in the effects of AMPK activity on food intake highlight the need for further discussion of the role of NTS neurons in energy balance control. Based on the similar profile of responses, reduced body weight and food intake, to compound C delivered via 4th icv and intraparenchymal caudal mNTS injections, we conclude that the mNTS neurons at the AP level are likely responsible for the observed hindbrain icv compound C-driven responses. Explanations for these regional differences will require characterizations of the neurochemical phenotype of AMPK-expressing caudal mNTS neurons and/or from AMPK-mediated control over the integration and processing of ascending and descending projections involved in energy balance control that are received by this subset of mNTS neurons. Neurons of the mNTS at the AP level receive afferent vagal input from within-meal satiation signals such as gastric distension, cholecystokinin, and intra-intestinal nutrients (9,11,26,27,28). Previously, we showed that mNTS neurons at the AP level express the long form of the leptin receptor and that 40% of these leptin-responsive neurons are also activated by within-meal, intake-suppressive signals, e.g. gastric distension (10). Given the current finding showing that elevation in hindbrain AMPK activity attenuates the suppression of food intake by hindbrain leptin delivery, it is reasonable to assume that alterations in AMPK activity by leptin may enhance the responsivity of the intracellular signaling cascades triggered by other satiating stimuli (e.g. GI satiation signals) acting on the same neurons. This notion is supported by previous reports showing that AMPKα2 activity in the hypothalamus is necessary to mediate leptin’s energy balance effects (1,19,20) and by current findings showing that 2 h refeeding (a multimodal stimulus that includes GI loading and vagal afferent activity) after 48 h food deprivation reduces pAMPKα levels in the NTS. Thus, future investigations examining whether AMPK acts as a common intracellular signal in caudal brainstem and hypothalamic nuclei mediating the potentiation of the intake inhibition of GI signals by leptin is certainly warranted.
Although not directly tested, we did not observe any malaise-like responses (aversive oral motor behavior during chow feeding and the stretching posture associated with treatments that serve as unconditioned stimuli in taste aversion experiments) after compound C administration in any of our experiments. In addition, despite the suppression of intake by compound C, rats continued to feed. The collective findings from Kim et al. (21), showing that C75 (fatty acid synthase inhibitor) reduces food intake by inhibiting AMPK activity and a previous report showing that icv C75 administration reduces food intake without producing a conditioned taste aversion (29), go some way to reduce concern that the intake-inhibitory effect by inhibition of AMPK activity by compound C does not occur through an aversive response.
Results from various studies implicate a link between increased hypothalamic AMPK activity and activation of counterregulatory responses (4,6). These results suggest that alterations in central AMPK activity may also drive adjustments in energy expenditure mediated by central sympathetic nervous system outflows. However, no experiments have directly examined this relationship. Prominent among the responses driven by the central integration of energy deficit signals in response to food deprivation is the reduction in energy expenditure responses that include reduced oxygen consumption, HR, and physical activity as well as uncoupling protein-1 (UCP-1) activity and norepinephrine turnover in effector tissues. Conversely, the positive energy balance that results from diet-induced obesity (30,31) is associated with increases in these same variables. The case for AMPK exerting a central role in control of energy balance has focused exclusively on hypothalamic tissues (1,2). It is well known that treatments that reduce energy availability in NTS neurons trigger behavioral as well as endocrine and autonomic responses (24,32,33,34,35,36,37,38). Two recent studies showed that compensatory adjustments in energy expenditure responses, driven by food deprivation (39) or by cold exposure (40), are observed in chronically maintained decerebrate rats lacking neural connections between hypothalamus and caudal brainstem. This indicates that energy status signals are detected and integrated within the caudal brainstem and that efferent commands to sympathetic outflows can arise locally and do not depend on connections with the forebrain. The NTS is prominent among sites of caudal brainstem integration and efferent control (14). Complementing the suppression in food intake, pharmacological inhibition of hindbrain AMPK activity increased HR and physical activity, implicating a dual role of hindbrain AMPK to energy balance control, with hindbrain AMPK mediating energy intake as well as HR and activity expenditure responses. This dual role of hindbrain AMPK likely accounted for a coordinated reduction in body weight gain. Collectively, current data indicate that mNTS neurons receive, integrate, and issue behavioral, sympathetic, and parasympathetic commands controlling for energy balance through AMPK signaling.
A parallel set of AMPK-mediated energy balance responses are reported for the PVN and arcuate nuclei of the hypothalamus in mice (1) to the AMPK-mediated effects observed here in the caudal brainstem for rats. Suppression of either NTS or hypothalamic AMPK activity is sufficient to inhibit food intake and reduce body weight, emphasizing the physiological relevance of AMPK (1). Energy balance responses driven by alterations in central AMPK signaling are likely mediated through transcriptional effects (15), actions on ion channels (17,18), or changes in cytosolic Ca2+ (3). It is likely that AMPK in either of these nuclei is responsive to various anorexigenic inputs, including leptin. Using hypothalamic injections of a constitutively active AMPK adenovirus, Minokoshi et al. (1) demonstrated that the suppression of food intake by leptin is reduced when hypothalamic AMPK activity is in a constantly elevated state. Other investigators examining the role of AMPK in energy balance control have used pharmacological methods (e.g. icv AICAR or compound C delivery) to elevate or suppress CNS (or specifically hypothalamic) (20) AMPK activity, because responses generated by these compounds are reversible and the functional effects of AMPK activation are induced rapidly (thereby enabling within-subject comparisons) (20,21,41). We employed the pharmacological strategy to elevate hindbrain AMPK activity and found that AICAR pretreatment reversed the suppression of intake by hindbrain leptin. It is interesting to note that the conclusion that the anorectic effects of leptin signaling in the CNS depend upon local AMPK activity has been demonstrated for both the hypothalamus (1) and dorsal hindbrain using two different approaches (genetic and pharmacological).
Limitations exist for both genetic (e.g. adenovirus or chronic knockout) and pharmacological approaches to assessing the physiological role of CNS AMPK in control of energy balance. For genetic approaches, the chronic activation or suppression of AMPK activity may result in a compromised state of the neuron and/or whole animal, producing nonphysiological alterations in downstream signaling cascades that may also drive functional changes in CREB-mediated protein synthesis (42). The pharmacological approach may also be limited by alterations in downstream signaling pathways. Current feeding effects and previous findings from Kim et al. (21), after pharmacological inhibition of AMPK by compound C, likely involve alterations in gene transcription as intake suppression began approximately 3 h after injection and persisted for 24 h. However, unlike genetic approaches, these alterations in protein synthesis by pharmacological agents are acute and reversible. In addition, it has been suggested that pharmacological AMPK agents can alter other non-AMPK-derived intracellular signaling pathways (for review see Refs. 43 and 44), and therefore, the potential for non-AMPK pathways contributing to the effects observed cannot be overlooked. It is worth noting, however, that direction-appropriate changes in AMPK activity (i.e. increase or decrease in activity) have been reported for both AICAR and compound C, that alterations in AMPK activity by one drug are antagonized by the other, and that each pharmacological agent is widely used to examine AMPK-mediated effects both in vivo and in vitro (20,21,41,43,45,46,47,48).
In conclusion, the overall pattern of data indicates that hindbrain AMPK activity contributes to energy balance control through regulation of food intake and energy expenditure. The current data together with previous reports (1,2,19,20,21) demonstrate that contributions of CNS AMPK activity to energy balance control is distributed across the neuraxis (for review see Refs. 49,50,51) rather than localized to a specific brain region.
Acknowledgments
Thanks to Grace Lee, Lauren Bradley, Ashley Brandt, Derek Zimmer, Theresa Leichner, and Cait Kauffman for their technical assistance. Thanks to Dr. Ted Abel for use of his cryostat. Thanks to Dr. Bart De Jonghe for his editorial assistance.
Footnotes
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK21397 (H.J.G.), DK019525 (K.K.B.), and DK077484 (M.R.H.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 30, 2008
Synthetic peptide substrate with amino acid sequence HMRSAMSGLHLVKRR.
Abbreviations: aCSF, Artificial cerebrospinal fluid; AICAR, 5-aminoimidazole-4-carboxamide-riboside; AMPK, AMP-activated protein kinase; AP, area postrema; CNS, central nervous system; DVC, dorsal vagus complex; HR, heart rate; 4th icv, fourth intracerebroventricular; mNTS, medial nucleus tractus solitarius; p, phospho-; PVN, paraventricular nucleus.
References
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB 2004 AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574 [DOI] [PubMed] [Google Scholar]
- Xue B, Kahn BB 2006 AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol 574:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA 2003 Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J 371:761–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Lee KU 2005 Role of hypothalamic 5′-AMP-activated protein kinase in the regulation of food intake and energy homeostasis. J Mol Med 83:514–520 [DOI] [PubMed] [Google Scholar]
- Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU 2004 Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 10:727–733 [DOI] [PubMed] [Google Scholar]
- Han SM, Namkoong C, Jang PG, Park IS, Hong SW, Katakami H, Chun S, Kim SW, Park JY, Lee KU, Kim MS 2005 Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia 48:2170–2178 [DOI] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp BE, Mattson MP 2001 AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci 17:45–58 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG 2002 Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143:239–246 [DOI] [PubMed] [Google Scholar]
- Hayes MR, Covasa M 2006 Gastric distension enhances CCK-induced Fos-like immunoreactivity in the dorsal hindbrain by activating 5-HT3 receptors. Brain Res 1088:120–130 [DOI] [PubMed] [Google Scholar]
- Huo L, Maeng L, Bjorbaek C, Grill HJ 2007 Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology 148:2189–2197 [DOI] [PubMed] [Google Scholar]
- Ritter RC 2004 Gastrointestinal mechanisms of satiation for food. Physiol Behav 81:249–273 [DOI] [PubMed] [Google Scholar]
- Smith GP 1996 The direct and indirect controls of meal size. Neurosci Biobehav Rev 20:41–46 [DOI] [PubMed] [Google Scholar]
- Rinaman L, Card JP, Schwaber JS, Miselis RR 1989 Ultrastructural demonstration of a gastric monosynaptic vagal circuit in the nucleus of the solitary tract in rat. J Neurosci 9:1985–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Hayes MR, The NTS: a portal for visceral afferent signal processing, energy status assessment, and integrator of their combined effects on food intake. Int J Obes 33:S11–S15 [DOI] [PubMed] [Google Scholar]
- Hardie DG, Scott JW, Pan DA, Hudson ER 2003 Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546:113–120 [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG 2005 AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25 [DOI] [PubMed] [Google Scholar]
- Light PE, Wallace CH, Dyck JR 2003 Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation 107:1962–1965 [DOI] [PubMed] [Google Scholar]
- Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK 2000 Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 105:1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB 2006 Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem 281:18933–18941 [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ 2004 AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279:12005–12008 [DOI] [PubMed] [Google Scholar]
- Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV 2004 C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem 279:19970–19976 [DOI] [PubMed] [Google Scholar]
- Hayes MR, Covasa M 2006 Dorsal hindbrain 5-HT3 receptors participate in control of meal size and mediate CCK-induced satiation. Brain Res 1103:99–107 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1998 The rat brain in stereotaxic coordinates. San Diego: Academic Press [Google Scholar]
- Ritter RC, Slusser PG, Stone S 1981 Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213:451–452 [DOI] [PubMed] [Google Scholar]
- Huo L, Grill HJ, Bjorbaek C 2006 Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes 55:567–573 [DOI] [PubMed] [Google Scholar]
- Powley TL 2000 Vagal circuitry mediating cephalic-phase responses to food. Appetite 34:184–188 [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ 2004 Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol Behav 82:69–74 [DOI] [PubMed] [Google Scholar]
- Vrang N, Phifer CB, Corkern MM, Berthoud HR 2003 Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 285:R470–R478 [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Wortman MD, Benoit SC, McOsker CC, Seeley RJ 2002 Comparison of central and peripheral administration of C75 on food intake, body weight, and conditioned taste aversion. Diabetes 51:3196–3201 [DOI] [PubMed] [Google Scholar]
- Stock MJ 1989 Effects of low (LCD) and very low (VLCD) energy diets on metabolic rate and body composition in obese (fa/fa) Zucker rats. Int J Obes 13(Suppl 2):61–65 [PubMed] [Google Scholar]
- Stock MJ, Rothwell NJ 1986 The role of brown fat in diet-induced thermogenesis. Int J Vitam Nutr Res 56:205–210 [PubMed] [Google Scholar]
- Ritter S, Dinh TT 1994 2-Mercaptoacetate and 2-deoxy-d-glucose induce Fos-like immunoreactivity in rat brain. Brain Res 641:111–120 [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Ritter S 1992 Hypothalamic paraventricular nucleus lesions do not abolish glucoprivic or lipoprivic feeding. Brain Res 595:25–31 [DOI] [PubMed] [Google Scholar]
- Ritter S, Taylor JS 1990 Vagal sensory neurons are required for lipoprivic but not glucoprivic feeding in rats. Am J Physiol 258:R1395–R1401 [DOI] [PubMed] [Google Scholar]
- Jean A 1991 The nucleus tractus solitarius: neuroanatomic, neurochemical and functional aspects. Arch Int Physiol Biochim Biophys 99:A3–A52 [DOI] [PubMed] [Google Scholar]
- Dallaporta M, Himmi T, Perrin J, Orsini JC 1999 Solitary tract nucleus sensitivity to moderate changes in glucose level. Neuroreport 10:2657–2660 [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, Li AJ 2006 Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav 89:490–500 [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, Zhang Y 2000 Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res 856:37–47 [DOI] [PubMed] [Google Scholar]
- Harris RB, Kelso EW, Flatt WP, Bartness TJ, Grill HJ 2006 Energy expenditure and body composition of chronically maintained decerebrate rats in the fed and fasted condition. Endocrinology 147:1365–1376 [DOI] [PubMed] [Google Scholar]
- Nautiyal KM, Dailey M, Brito MN, DAB, Harris RB, Bartness TJ, Grill HJ 2008 Energetic responses to cold temperatures in rats lacking forebrain-caudal brainstem connections. Am J Physiol Regul Integr Comp Physiol 295:R789–R798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S 2008 UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454:846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Nian C, Widenmaier S, McIntosh CH 2008 Glucose-dependent insulinotropic polypeptide-mediated up-regulation of β-cell antiapoptotic Bcl-2 gene expression is coordinated by cyclic AMP (cAMP) response element binding protein (CREB) and cAMP-responsive CREB coactivator 2. Mol Cell Biol 28:1644–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P 2007 The selectivity of protein kinase inhibitors: a further update. Biochem J 408:297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Mollapour E, Choi JH, Shears SB 2008 Cellular energetic status supervises the synthesis of bis-diphosphoinositol tetrakisphosphate independently of AMP-activated protein kinase. Mol Pharmacol 74:527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyral-Castel S, Tosca L, Ferreira G, Jeanpierre E, Rame C, Lomet D, Caraty A, Monget P, Chabrolle C, Dupont J 2008 The effect of AMP-activated kinase activation on gonadotrophin-releasing hormone secretion in GT1-7 cells and its potential role in hypothalamic regulation of the oestrous cyclicity in rats. J Neuroendocrinol 20:335–346 [DOI] [PubMed] [Google Scholar]
- Kohno D, Sone H, Minokoshi Y, Yada T 2008 Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem Biophys Res Commun 366:388–392 [DOI] [PubMed] [Google Scholar]
- Thomson DM, Herway ST, Fillmore N, Kim H, Brown JD, Barrow JR, Winder WW 2008 AMP-activated protein kinase phosphorylates transcription factors of the CREB family. J Appl Physiol 104:429–438 [DOI] [PubMed] [Google Scholar]
- Fukuda T, Ishii K, Nanmoku T, Isobe K, Kawakami Y, Takekoshi K 2007 5-Aminoimidazole-4-carboxamide-1-β-4-ribofuranoside stimulates tyrosine hydroxylase activity and catecholamine secretion by activation of AMP-activated protein kinase in PC12 cells. J Neuroendocrinol 19:621–631 [DOI] [PubMed] [Google Scholar]
- Berthoud HR 2002 Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev 26:393–428 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM 2002 The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 23:2–40 [DOI] [PubMed] [Google Scholar]
- Watts AG 2000 Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Horm Behav 37:261–283 [DOI] [PubMed] [Google Scholar]