Abstract
In female rats, estradiol (E2) and suckling induce prolactin (PRL) secretion. This involves inhibition of hypothalamic dopaminergic tone and stimulation by a PRL-releasing hormone, possibly oxytocin (OT). Infusing an OT antagonist (OTA) iv, we evaluated the role of OT on suckling- and E2-induced PRL secretion. Three days after parturition at 0900 h, lactating dams were fitted with 24-h osmotic minipumps filled with saline or OTA. On d 5 of lactation, pups were separated from their dams for 6 h. Immediately or 20 min after the resumption of suckling, dam trunk blood was collected. Also, ovariectomized (OVX) rats were treated with E2 (OVE) and OTA at 1000 h on d 1. Blood samples were obtained from 1300 to 2100 h on d 2 for PRL measurements. Additionally, OVX rats were evaluated on d 2 after receiving progesterone (P4). OTA blocked suckling and E2-induced release of PRL but not that induced by E2+P4. Pups from treated dams failed to gain weight when allowed to nurse for 20 min on d 5 but gained more than 7 g when nursed on d 7 of lactation, indicating that the OTA was active 48 h later. Western blot analysis showed that E2 treatment increased OT receptors in the anterior pituitary when compared with OVX animals. No further increase was observed in response to the P4, suggesting that the enhancing effect of P4 on E2-induced PRL release may act through mechanisms independent of OT. These data demonstrate the role of OT in the control of suckling and steroid-induced PRL secretion.
Oxytocin controls the steroid-induced and suckling-induced release of prolactin.
In the female rat, elevated prolactin (PRL) secretion occurs in response to three physiological stimuli: estradiol (E2), suckling, and mating (1). In response to rising blood levels of E2 throughout the 4-d estrous cycle, cycling rats display a surge of PRL on the afternoon of proestrus (2). In ovariectomized (OVX) animals, treatment with E2 (OVE) induces a surge of PRL, which is similar to that secreted on proestrus (3). The administration of E2 + progesterone (P4) (OVEP) amplifies and prolongs this surge (4). The suckling of rat pups initiates immediate release of PRL in the lactating rat, which persists as long as the suckling stimulus is maintained (5). In response to the mating stimulus, the female rat secretes a nocturnal and a diurnal surge of PRL, which recurs for 10 d if the mating was fertile (6,7). Twice-daily surges can also be artificially induced in OVX cervically stimulated (CS) female rats, and these surges recur for 10–12 d (7,8). Although the secretory patterns of PRL are different in each of these paradigms, they are all modulated by hypothalamic inputs.
Dopamine released into hypophyseal portal blood from tuberoinfundibular, periventricular hypophyseal and tuberohypophyseal neurons of the hypothalamus tonically inhibits the secretion of PRL from lactotrophs in the anterior pituitary (for review see Refs. 1, 9). Physiological or exteroceptive stimuli cause a diminution of dopamine release (10,11,12) into the portal vasculature, but pharmacological reduction of dopaminergic tone is not enough to account for a full surge of PRL (13). Therefore, the physiological and exteroceptive stimuli may also allow a PRL-releasing factor to exert its effect facilitating the secretion of PRL.
Several neuropeptides have been identified as possible physiological PRL-releasing factors (for review see Ref. 1). Among these, oxytocin (OT) has proven to be the strongest candidate. OT, a nonapeptide classically known for its role in milk ejection and parturition, is produced in neurons of the paraventricular and supraoptic nuclei of the hypothalamus and is released from the posterior pituitary into the peripheral circulation to stimulate contractions of the uterine myometrium or myoepithelium of the mammary glands (for review see Ref. 14). OT is also transported to the anterior pituitary via the portal vasculature (15), in which the cell membrane of lactotrophs bear OT receptors (16).
A rise of OT in the peripheral plasma precedes the increase in PRL observed after suckling (17,18). Likewise, an increase in OT in the portal blood precedes the increase of PRL observed on the afternoon of proestrus (15). Evidence suggests that OT is involved in the facilitation of PRL release both in vivo and in vitro (19,20,21,22,23). Immunoneutralization of endogenous OT attenuates the proestrous, suckling, and E2-induced surges of PRL (24,25), whereas pharmacological blockade of OT receptors prevents PRL release (21,26,27,28,29). However, this is not without controversy because immunoneutralization has been shown to attenuate the suckling-induced PRL surge (24), whereas other laboratories in which an OT antagonist (OTA) has been used have not observed this attenuation (28).
Our laboratory has previously described a mathematical model for the mechanism controlling CS-induced PRL surges (30). According to the model, CS induces a surge of OT, which inhibits dopaminergic tone. This reduction in dopaminergic tone coupled with stimulation of lactotrophs by OT induces twice-daily surges of PRL, which recur for several days. To test this, our laboratory has recently shown that 24 h after the infusion of the selective OTA, desGly-NH2-d(CH2)5[d-Tyr2,Thr4]OVT, the CS-induced nocturnal and diurnal surges of PRL are abolished (29). The present study was designed to determine whether the same mechanism would apply for the suckling- and ovarian steroid-induced PRL surges.
Materials and Methods
Animals
Adult female Sprague Dawley rats (>60 d of age) weighing 250–300 g (Charles River Laboratories, Raleigh, NC) were kept in standard rat cages under a 12-h light, 12-h dark cycle (lights on at 0600 h) with water and rat chow available ad libitum. Animals receiving hormone replacement were OVX bilaterally under isoflurane anesthesia (Butler Animal Health Supply, Dublin, OH) and then allowed to recover for 10 d. All animal procedures were approved by the Florida State University Animal Care and Use Committee.
Jugular vein catheter implantation and OTA infusion
Animals were anesthetized with isoflurane (Butler) and micro-Renathane tubing (outer diameter 0.040, inner diameter 0.025, MRE-025; Braintree Scientific, Braintree, MA) was inserted into both jugular veins. The tubing, filled with heparinized saline (30 U/ml), was fitted sc and exteriorized at the back of the animal’s neck. A daily flush with heparinized saline kept the right line patent until the start of blood collection.
The selective OTA desGly-NH2-d(CH2)5[d-Tyr2,Thr4]OVT (GenScript Corp., Scotch Plains, NJ), which does not cross the blood-brain barrier, was dissolved in sterile saline and infused via osmotic minipumps (Alzet osmotic pumps, model AP-2001D, rate of 8 μl/h, duration 24 h; Braintree Scientific). The osmotic pumps were filled with the OTA or sterile saline and connected to the left jugular vein catheter.
RIA
The concentration of rat PRL in serum was determined in duplicate by RIA using National Institute of Diabetes and Digestive and Kidney Diseases materials (supplied by Dr. A. F. Parlow, National Hormone and Pituitary Program, Torrance, CA). Serum concentrations of PRL are expressed as nanograms per milliliter in terms of the rat PRL RP-3 standard. Assay sensitivity was 1 ng/ml and the intraassay coefficient of variation was 5%. To prevent interassay variation, all samples were analyzed in a single RIA.
Western blot analysis and immunodetection of OT receptors
Animals maintained under a standard 12-h light, 12-h dark cycle were injected with E2 or corn oil on d 1 and then with P4 or corn oil on d 2. Animals were then decapitated at 1700 h on d 2 (time of E2 induced PRL surge) under hypercapnic conditions. The anterior pituitary was removed and stored at −80 C until protein extraction. Tissues were thawed on ice in chilled homogenization buffer containing (in millimoles) 320 sucrose, 10 Tris base, 50 KCl, and 1 EDTA. A protease inhibitor cocktail (P8340; Sigma-Aldrich, St. Louis, MO) and pepstatin A (2 μg/ml) were added to the homogenizing buffer before use. Tissue samples were sonicated on ice with a minihomogenizer and disposable pestle (Kimble-Kontes, Vineland, NJ) using 10–15 strokes (3–4 sec/stroke). After centrifugation at 25,000 × g for 10 min, supernatant was transferred to a fresh tube and recentrifuged at 100,000 × g for 1.5 h. Supernatant was decanted and the pellet was resuspended in homogenizing buffer. The amounts of protein in samples were measured using a micromodified form of the DC protein assay kit (Bio-Rad, Hercules, CA). Samples were mixed with equal volume of 2× sample buffer containing: 100 mm Tris-HCl (pH 6.8), 4% sodium dodecyl sulfate (SDS), 20% glycerol, 5% β-mercaptoethanol, 2 mm EDTA, and 0.1 mg/ml bromophenol blue and warmed at 95 C for 5 min. Samples were cooled on ice for 5 min before being briefly centrifuged. Samples and DualVue molecular weight marker (GE Healthcare Life Sciences, Piscataway, NJ) were loaded into a 10-well 8% polyacrylamide gel using the Miniprotean II system (Bio-Rad) and electrophoresis was carried out using constant 150 V for 1 h in Tris-glycine-SDS buffer (8 mm Tris-base, 40 mm glycine, 0.1% SDS).
Electroblotting was carried out in transfer buffer (Tris-glycine-SDS-methanol, 8 mm Tris-base, 40 mm glycine, 0.1% SDS, and 20% methanol) using 100 V for 90 min. Transfer to nitrocellulose blots was verified by visual inspection after brief staining with Ponceau S protein stain. Blots were then placed in 4% nonfat milk for 30 min at room temperature, followed by primary antibody (goat anti-OTR, sc-8102; Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1:500) incubation for 24 h at 4 C. Blots were rinsed three times for 10 min with Tris-buffered saline containing 0.1% Tween 20 and placed in secondary antibody (bovine antigoat IgG conjugated to horseradish peroxidase; Jackson ImmunoResearch, West Grove, PA; dilution 1:3000) for 90 min at room temperature. After two 10-min washes in Tris-buffered saline containing 0.1% Tween 20 and two 10-min washes in Tris-buffered saline, OT receptor protein levels were visualized using enhanced chemiluminescence (GE Healthcare Life Sciences) exposure on Amersham Hyperfilm ECL (GE Healthcare Life Sciences). The molecular size of the OT receptor in the anterior pituitary was approximately 66 kDa, in agreement with the size estimate in rat cervix (31) and mammary gland (32). Blots were stripped of primary/secondary antibody complex according to the protocol provided by Pierce (Pierce, Rockford, IL) using their stripping and reprobing buffer (Pierce). Blots were then reprobed with antiactin mouse monoclonal primary antibody (Abcam, Cambridge, MA; dilution: 1:1000) and placed in secondary antibody (goat antimouse IgG conjugated to horseradish peroxidase; Jackson ImmunoResearch; dilution 1:3000) for 90 min at room temperature. All film was analyzed by quantitative densitometry using the Gel Logic 100 system (Kodak, Rochester, NY).
Statistical analysis
Data are presented as the mean ± sem. Data analysis was conducted using a two-way ANOVA for the comparison of differences between treatment groups, followed by Bonferroni comparison. A one-way ANOVA was used for comparison of time differences within treatment groups, followed by Bonferroni comparison. A one-tailed paired Student t test was used to determine significant pup weight gain. Graph Pad Prism 4.0 (GraphPad Software, Inc., San Diego, CA) was used for statistical analyses and graph preparation. Differences were considered significant when P < 0.05.
Experiments
Effects of OTA on suckling-induced PRL secretion
Lactating mothers, each with ten 3-d-old pups, were fitted with 24-h osmotic minipumps for iv infusion of saline or OTA (1.25 or 12.5 μg/μl). Forty-eight hours later, at 0900 h on d 5 of lactation, pups were separated for 6 h before suckling. Immediately (0 min) or 20 min after the resumption of suckling, lactating rats were killed and trunk blood was collected. At the time of resumption of suckling, the delivery of saline or OTA from the minipumps had been exhausted. PRL levels were measured by RIA.
In an additional experiment, the weight of suckling pups was determined to evaluate the presence of milk and thus the degree of blockade of OT receptors by OTA. Pup weight was determined immediately before and after resumption of suckling on d 5 and 7 of lactation.
Effects of OTA on E2-induced PRL secretion
Ten days after ovariectomy, animals were injected sc with 20 μg of E2-17β (Sigma, St. Louis, MO) in corn oil at 1000 h (d 1). This paradigm results in PRL levels similar to those found on the afternoon of proestrus (3). Immediately after the E2-17β administration, animals were fitted with 24-h osmotic minipumps for iv infusion of saline or OTA (1.25, 3.75, or 12.5 μg/μl). On the following day (d 2), 400 μl of blood were collected in heparinized syringes at 2-h intervals beginning at 1300 h and ending at 2100 h. Blood lost during sampling was replaced with sterile saline. Samples were stored at 4 C until centrifuged (4000 × g). Serum was collected and stored at −20 C until analysis for PRL concentration by RIA.
Effects of OTA on OVEP-induced PRL secretion
Ten days after ovariectomy, animals were injected sc with 20 μg of E2-17β (Sigma) in corn oil at 1000 h (d 1). Immediately after the E2-17β administration, animals were fitted with 24-h osmotic minipumps for iv infusion of saline or OTA (1.25, 12.5, or 62.5 μg/μl). On d 2, all animals were injected sc with 1.25 mg of P4 (Sigma) in corn oil at 1100 h. As described in the previous experiment, sampling began at 1300 h on d 2 and continued every 2 h until 2100 h.
In a second experiment, the schedule of treatment was changed to allow exposure to E2 before the administration of OTA and P4. Ten days after ovariectomy, animals were injected sc with 20 μg of E2-17β (Sigma) in corn oil at 1200 h (d 1). The following day (d 2), all animals were fitted with 24-h osmotic minipumps for iv infusion of saline or OTA (12.5 μg/μl) at 0800 h. All animals were injected sc with 1.25 mg of P4 (Sigma) in corn oil at 1100 h on d 2. Blood sampling began at 1300 h and continued every 2 h until 2100 h.
Effects of steroid treatment on oxytocin receptor density in the anterior pituitary
Ten days after ovariectomy, animals were divided into two groups. Group 1 received a sc injection of corn oil and group 2 received a sc injection of 20 μg of E2-17β (Sigma) in corn oil at 1000 h. At 1000 h on the following day (d 2), half of group 2 was injected sc with 1.25 mg of P4 (Sigma) in corn oil. At 1700 h (time of peak E2 induced secretion of PRL) animals were decapitated under hypercapnic conditions. The anterior pituitary was removed and stored at −80 C until protein extraction for Western blot analysis.
Results
Suckling-induced PRL secretion is dependent on OT
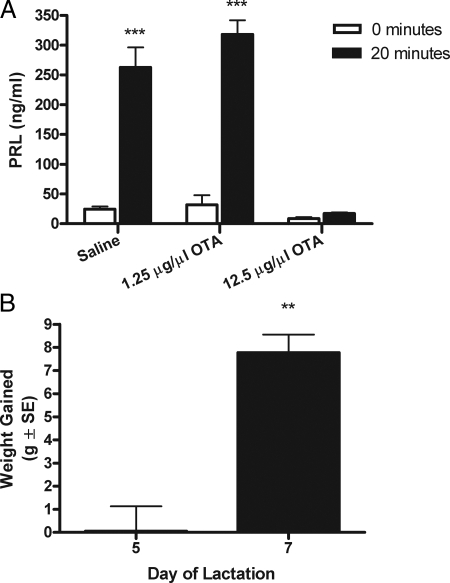
The effect of OTA on the suckling-induced secretion of PRL was evaluated 5 d after parturition after the infusion of OTA or saline on d 3 (Fig. 1A). Replacement of suckling pups induced a significant rise in the secretion of PRL at 20 min from dams infused with saline. Dams treated with 1.25 μg/μl OTA presented a similar increase in PRL secretion by 20 min of suckling on d 5. This dose is capable of blocking the surges of PRL observed in CS-OVX rats (29). Increasing the dose of OTA to 12.5 μg/μl resulted in prevention of the suckling-induced secretion of PRL on d 5 (***, P < 0.001, Fig. 1A).
Figure 1.
A, Mean concentration (nanograms per milliliter ± sem) of serum PRL in lactating animals 5 d after parturition (n = 6) after the infusion of saline or OTA (1.25 or 12.5 μg/μl). Upon pup replacement, 12.5 μg/μl of OTA prevented the suckling-induced rise in PRL secretion by 20 min (two-way ANOVA, followed by Bonferroni’s test; ***, P < 0.001). B, Mean (grams ± sem) pup weight gain on d 5 and 7 of lactation after infusion of OTA on d 3. When OTA was infused on d 3 of lactation, OT receptors were still affected on d 5 as determined by a lack of gain in pup weight. By d 7, pup weight gain was significantly greater, suggesting clearance of OTA and subsequent release of milk (one-tailed paired student t test; **, P < 0.01).
By d 5 of lactation (2 d after treatment with OTA), dams treated with 12.5 μg/μl OTA failed to eject enough milk to increase their pup’s weight (Fig. 1B). However, by d 7 of lactation, milk ejection resulted in a significant increase in the weight of pups (**, P < 0.01, Fig. 1B).
E2-induced PRL secretion is dependent on OT
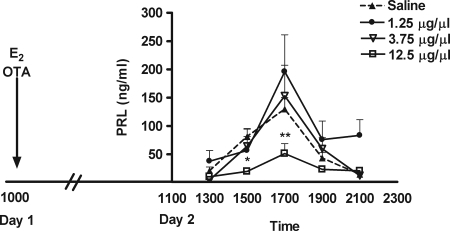
The effect of OTA on the E2-induced secretion of PRL was evaluated in OVX rats treated with E2 on d 1 (Fig. 2). The infusion of OTA or saline began on d 1 and blood sampling occurred on d 2. Administration of E2 followed by infusion of saline resulted in a significant rise in the secretion of PRL on d 2. Interestingly, the dose of OTA capable of blocking the surges of PRL observed in CS-OVX rats (1.25 μg/μl) (29) was insufficient to block the secretion of PRL in the E2-treated rat. Similarly, treatment with an intermediate dose of OTA (3.75 μg/μl) also was ineffective. To successfully suppress the E2-induced surge of PRL, the OTA had to be given at a dose of 12.5 μg/μl. The concentration of PRL at 1500 h (*, P < 0.05) and 1700 h (**, P < 0.01) was significantly lower than saline controls.
Figure 2.
Mean concentration (nanograms per milliliter ± sem) of serum PRL in OVE animals (n = 6) after the infusion of OTA (solid line) or saline (dotted line). A 24-h infusion of 12.5 μg/μl of OTA attenuated the OVE-induced PRL surge at 1700 h, whereas 1.25 and 3.75 μg/μl did not affect the PRL surge (two-way ANOVA, followed by Bonferroni’s test; *, P < 0.05; **, P < 0.01).
OVEP-induced PRL secretion is not dependent on OT
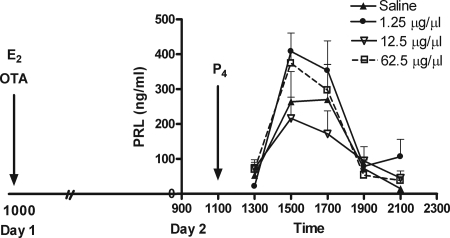
In contrast to that observed in CS-OVX, suckling- and E2-induced PRL secretion, OVEP-induced PRL secretion was not affected by OTA (Figs. 3 and 4). In the first experiment, E2 was administered on d 1 and the infusion of saline or OTA began immediately thereafter. Administration of P4 and blood sampling occurred on d 2. The dose of OTA capable of blocking the suckling- and E2-induced PRL surge, 12.5 μg/μl was not capable of blocking the OVEP-induced PRL surge (Fig. 3). Increasing the dose of OTA to 62.5 μg/μl was still ineffective at blocking the surge (Fig. 3).
Figure 3.
Mean concentration (nanograms per milliliter ± sem) of serum PRL in OVEP animals (n = 6) after the infusion of OTA (solid line) or saline (dotted line). A 24-h infusion of OTA (1.25, 12.5, or 62.5 μg/μl) failed to block the OVEP-induced PRL surge.
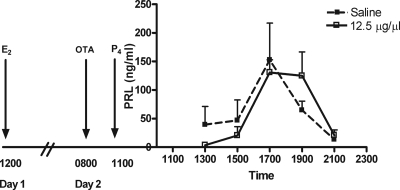
Figure 4.
Mean concentration (nanograms per milliliter ± sem) of serum PRL obtained from OVEP animals (n = 6) after an infusion of OTA (dotted line) or saline (solid line). An infusion of OTA (12.5 μg/μl) beginning on d 2 (before P4 treatment) did not block the OVEP-induced PRL surge.
In a second experiment, E2 was administered on d 1 and the infusion of saline or OTA began at 0800 h on d 2 before administration of P4 (allowing longer exposure to E2 before the OTA and P4 treatments). This too was ineffective in preventing the OVEP-induced surge of PRL (Fig. 4). Likewise, infusion of OTA begun on d 2 in the E2-only-treated group was similarly ineffective (data not shown).
P4 does not further increase E2-induced up-regulation of OT receptor density in the anterior pituitary
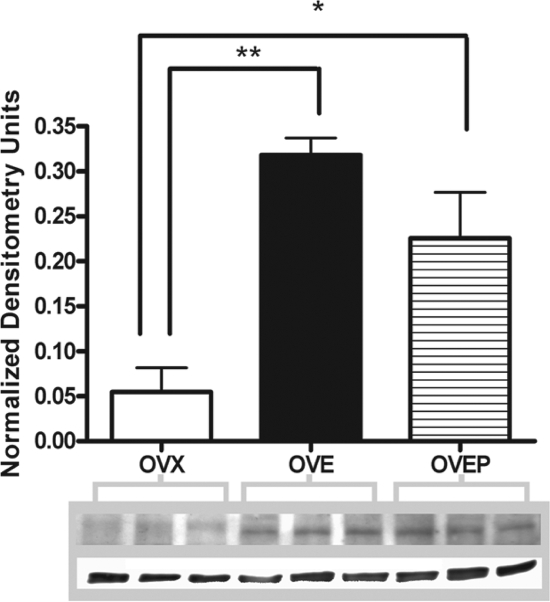
To determine why P4 prevented the decrease in the release of PRL after OTA, OT receptor density was determined in OVX, OVE, and OVEP. OVX animals treated with corn oil exhibited OT receptor density just above the level of detection. Both E2- (**, P < 0.01) and E2+ P4 treatment (*, P < 0.05) administered to OVX rats significantly increased OT receptor density when compared with untreated OVX rats (Fig. 5). However, no difference was observed between E2- and E2+progesterone-treated animals.
Figure 5.
Ovarian steroid treatment increases OT receptor density in the anterior pituitary. Administration of 20 μg of 17-β E2 significantly (**, P < 0.01) increased the amount of OT receptor protein in the anterior pituitary compared with untreated OVX rats. Likewise, the administration of E2 + P4 significantly (*, P < 0.05) increased the amount of OT receptor protein in the anterior pituitary compared with OVX rats. No significant difference was observed between OVE- and OVEP-treated rats.
Discussion
The data presented further support the hypothesis that OT stimulates PRL release from lactotrophs of the anterior pituitary. Previous work has shown that OTA is capable of blocking the nocturnal and diurnal surge of PRL observed in CS-OVX rats (29). However, this dose of OTA capable of blocking the release of PRL in CS-OVX rats (1.25 μg/μl) was incapable of blocking the suckling-, E2-, and OVEP-induced surges of PRL in the present study. Increasing the dose of OTA to 12.5 μg/μl blocked the suckling- and E2-induced surges but not the OVEP-induced surge of PRL. This effect of P4 is not likely due to an increase in OT receptor density. E2 treatment in OVX rats increases OT receptor density when compared with untreated OVX animals. However, in response to E2+ P4 treatment, there was no further increase compared with the E2-treated group, suggesting that P4 is acting through another mechanism to stimulate the secretion of PRL.
Immunoneutralization of OT attenuates the suckling- induced rise in PRL secretion (24). However, a second study in which OTA was used does not support these findings (28). In agreement with Samson et al. (24), the present study found that raising the dose of OTA effectively blocked the suckling-induced rise in PRL secretion. In the current study, OTA was infused for 24 h, whereas in the previous study, a single bolus injection (25 or 45 μg/kg) was administered. Whereas our high dose was 12.5 μg/μl (infusion rate of 8 μl/h), the animals were presumably exposed to a greater amount of OTA for a prolonged period. Additionally, because the OTA was infused and not injected, the possibility of a missed critical time window is not a factor in the current study. Furthermore, the current study began OTA infusions on d 3 of lactation, whereas in the previous study (28), OTA was administered on d 12 of lactation. Plasma P4 is low postpartum and begins to increase by d 4 of lactation (33), remains high until d 14 of lactation, and declines thereafter (34). Therefore, if P4 is acting through another mechanism to stimulate the secretion of PRL, the high levels of P4 on d 12 may offer an explanation as to why the suckling-induced surge was not blocked in the previous study.
It is well established that OT is influenced by E2; plasma OT concentrations increase in response to E2 administration (35) and plasma OT levels are highest in hypophyseal portal blood on proestrus (15) when E2 levels are highest (36). Likewise, in the supraoptic nucleus, OT gene expression increases throughout the estrous cycle before peaking on estrus (37). In OVX rats, OT immunoreactivity and Fos-related antigen expression is E2 dependent in the periventricular nucleus but not the paraventricular nucleus (38,39). Indeed, the majority of neurophysin-positive neurons in the paraventricular nucleus (which concentrate E2) are found in the posterior paraventricular nucleus (40). These magnocellular neurons may not be involved in the secretion of PRL as they project to the brainstem and spinal cord but not the pituitary (41,42). However, a high level of E2 binding is found along the third ventricle (43) in which the periventricular nucleus’ OT neuronal population is found.
In the rat (44) and mouse (45), OT receptors are influenced by E2, and their promoters contain an estrogen response element. In the periphery, uterine OT receptor mRNA increases in response to E2 treatment (46,47,48). Additionally, uterine OT receptor mRNA also increases throughout gestation and during the estrous cycle, peaking on proestrus before returning to baseline values on estrus (46,49). However, OT receptor mRNA is not influenced by E2 in the mammary gland (47).
Centrally, OT receptors are found throughout the brain, many of which are regulated by E2 (50,51). One of the well-established areas of steroid-sensitive OT receptors is the ventromedial nucleus of the hypothalamus (VMN). Here OT receptors are induced by E2 (52,53), with the highest levels of induction in the caudal ventrolateral VMN (54,55). These OT receptors are then transported laterally to an area of increased E2-induced OT immunoreactivity outside the VMN (56,57,58,59). E2 acts at the level of gene expression as OT receptor mRNA increases in the VMN during the estrous cycle (60) and in OVX rats treated with E2 (61).
In the anterior pituitary, OT receptor mRNA has been localized to lactotrophs, and E2 has been shown to increase this population of OT receptor mRNA 6-fold in OVX rats (16,62). Because OT receptors are robustly up-regulated in response to E2, it is not surprising that a lower dose of OTA is necessary to block the surges of PRL in CS-OVX rats (29).
P4 affects OT receptors in a complex tissue-specific manner. In the cervix, E2-induced up-regulation of OT receptor mRNA is antagonized by P4 (31). In contrast, P4 administration has no effect on the E2-induced rise in OT receptor mRNA in the uterus, but it does decrease binding affinity, suggesting a nongenomic mechanism (46,48). In the ventrolateral region of the caudal VMN, P4 further increases the region of E2-induced OT receptors as well as OT receptor binding (56,58,63), and because of the time course, P4 is believed to act nongenomically (63). In the pituitary, OT receptor gene expression increases in response to E2, but no further increase is observed in response to P4 (64). In the anterior pituitary, in which OT receptors have been localized to lactotrophs (62), the current data show P4 had no effect on E2-induced up-regulation of OT receptor density. Similar studies on the effect of P4 in the anterior pituitary have not yet been presented, and because of the great divergence in other tissue, it is hard to hypothesize what effect P4 may have, if any, on OT receptors. The possibility exists that, in this region, P4 may increase OT receptor binding sites or affinity without affecting receptor density, suggesting a possible mechanism through which the high dose of OTA may have been ineffective in the OVEP group.
Throughout gestation, P4 is necessary to maintain uterine quiescence, and the mechanism through which it does so may be nongenomic. Grazzini et al. (65) have shown that P4 binds directly to OT receptors, preventing OT from binding and inhibiting inositol phosphate production and calcium mobilization. Because pituitary and uterine OT receptors are suggested to be structurally similar (16), we were surprised that the 12.5 μg/μl dose of OTA was incapable of blocking the E2+ P4-induced increase in PRL release. If P4 is sterically hindering OT binding to its ligand binding domain, a smaller dose should have been sufficient to prevent the PRL surge because P4 should have acted as an antagonist of OT binding. The possibility does exist that the mechanism is not the same in the lactotroph and that, instead, P4 binding to OT receptors exerts agonist effects in the anterior pituitary. Future work will address this possibility.
Ovarian steroids are known modulators of hypothalamic dopamine. In tuberoinfundibular dopaminergic neurons, E2 inhibits dopamine synthesis and tyrosine hydroxylase activity (the rate limiting enzyme in catecholamine synthesis) (66,67). Furthermore, E2 also decreases the release of dopamine into the portal vasculature (68). Likewise, short-term P4 treatment decreases dopamine synthesis and release as well as tyrosine hydroxylase activity and mRNA (69,70,71,72,73,74,75,76,77). This results in the prolongation and amplification of the PRL surge (69,72,73). Whereas P4 receptors are not localized to lactotrophs in the rat (78), they have been localized to tyrosine hydroxylase-positive neurons, which are found in the periventricular and arcuate nucleus of the hypothalamus (78,79,80). Tuberoinfundibular, periventricular hypophyseal, and tuberohypophyseal neurons (81) arise from these regions and secrete dopamine into hypophyseal blood, which binds to D2 receptors found on lactotrophs and inhibits the secretion of PRL (for review see Ref. 9).
Arbogast and Ben-Jonathan (69) showed that P4 maintains the plateau phase of the secretion of PRL by reducing the activity of hypothalamic dopaminergic neurons. They further hypothesized that the early peak phase of the proestrous surge of PRL depends on an interaction between vasoactive intestinal peptide and a PRL-releasing factor from the posterior pituitary, which we are suggesting is OT. Therefore, the possibility exists that P4 is not affecting OT receptors. Instead, it is possible that it is acting through the main point of PRL regulation, hypothalamic dopamine.
In female rats, P4 has been shown to phase advance the decrease in median eminence 3, 4-dihydroxyphenylacetic acid (a reliable index of dopamine activity), thereby advancing the onset of the E2-induced PRL surge (73). These effects appear to be mediated through dephosphorylation of tyrosine hydroxylase in the median eminence (82). In fetal hypothalamic cells, P4 decreases tyrosine hydroxylase activity within 1 h, and this decreased activity is sustained for at least 6 h (71). Additionally, this effect is dependent on pretreatment with E2. In the present study, P4 was administered at 1100 h. Presumably, the decrease in tyrosine hydroxylase activity was achieved by 1200 h and remained low until at least 1700 h, possibly removing any source of tonic inhibition on the lactotroph increasing the secretion of PRL.
In summary, this study demonstrates that OT antagonism at the lactotroph influences PRL release. However, the effective amount of OTA is dependent on the hormonal milieu. In the current study, both lactating and E2-treated females required higher doses of OTA than CS-OVX females have in previous work from our laboratory (29). In the presence of P4, OTA is not capable of inhibiting the E2-induced PRL surge. This effect is most likely due to inhibition of dopaminergic tone because progesterone does not change the receptor density in the anterior pituitary. A great amount is left to be explored in terms of the effect of ovarian steroids on OT receptor expression at the lactotroph; the temporal course required for sensitizing OT receptors, the effect of P4 on OT binding affinity, or possibly ligand-independent receptor activation. Further elucidation of this mechanism would provide clarity into the control of the secretion of PRL by OT at the lactotroph.
Note Added in Proof
The original description of the synthesis and activity of the OT antagonist may be found in Manning et al. (83).
Acknowledgments
We thank Albert Parlow, Ph.D., and The National Hormone and Pituitary Program for supplying the rat PRL RIA reagents. We also thank Ruth Cristancho-Gordo, Bonnie Dodge, and Cindy-Sue Turco for their excellent technical assistance.
Footnotes
This work was supported by National Institutes of Health Grants DA-19356 and DK-43200.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 23, 2008
Abbreviations: CS, Cervically stimulated; E2, estradiol; OT, oxytocin; OTA, OT antagonist; OVE, treatment with OVX and E2; OVEP, treatment with E2 + P4; OVX, ovariectomized; P4, progesterone; PRL, prolactin; SDS, sodium dodecyl sulfate; VMN, ventromedial nucleus of the hypothalamus.
References
- Freeman ME, Kanyicska B, Lerant A, Nagy G 2000 Prolactin: structure, function, and regulation of secretion. Physiol Rev 80:1523–1631 [DOI] [PubMed] [Google Scholar]
- Neill JD, Freeman ME, Tillson SA 1971 Control of the proestrus surge of prolactin and luteinizing hormone secretion by estrogens in the rat. Endocrinology 89:1448–1453 [DOI] [PubMed] [Google Scholar]
- Neill JD 1972 Sexual differences in the hypothalamic regulation of prolactin secretion. Endocrinology 90:1154–1159 [DOI] [PubMed] [Google Scholar]
- Caligaris L, Astrada JJ, Taleisnik S 1974 Oestrogen and progesterone influence on the release of prolactin in ovariectomized rats. J Endocrinol 60:205–215 [DOI] [PubMed] [Google Scholar]
- Grosvenor CE, Mena F, Whitworth NS 1979 The secretion rate of prolactin in the rat during suckling and its metabolic clearance rate after increasing intervals of nonsuckling. Endocrinology 104:372–376 [DOI] [PubMed] [Google Scholar]
- Butcher RL, Fugo NW, Collins WE 1972 Semicircadian rhythm in plasma levels of prolactin during early gestation in the rat. Endocrinology 90:1125–1127 [DOI] [PubMed] [Google Scholar]
- Smith MS, Neill JD 1976 Termination at midpregnancy of the two daily surges of plasma prolactin initiated by mating in the rat. Endocrinology 98:696–701 [DOI] [PubMed] [Google Scholar]
- Freeman ME, Smith MS, Nazian SJ, Neill JD 1974 Ovarian and hypothalamic control of the daily surges of prolactin secretion during pseudopregnancy in the rat. Endocrinology 94:875–882 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hnasko R 2001 Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 22:724–763 [DOI] [PubMed] [Google Scholar]
- de Greef WJ, Klootwijk W, Karels B, Visser TJ 1985 Levels of dopamine and thyrotrophin-releasing hormone in hypophysial stalk blood during an oestrogen-stimulated surge of prolactin in the ovariectomized rat. J Endocrinol 105:107–112 [DOI] [PubMed] [Google Scholar]
- de Greef WJ, Neill JD 1979 Dopamine levels in hypophysial stalk plasma of the rat during surges of prolactin secretion induced by cervical stimulation. Endocrinology 105:1093–1099 [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Neill JD 1982 The decrease in hypothalamic dopamine secretion induced by suckling: comparison of voltammetric and radioisotopic methods of measurement. Endocrinology 110:691–696 [DOI] [PubMed] [Google Scholar]
- Arey BJ, Averill RL, Freeman ME 1989 A sex-specific endogenous stimulatory rhythm regulating prolactin secretion. Endocrinology 124:119–123 [DOI] [PubMed] [Google Scholar]
- Kiss A, Mikkelsen JD 2005 Oxytocin—anatomy and functional assignments: a minireview. Endocr Regul 39:97–105 [PubMed] [Google Scholar]
- Sarkar DK, Gibbs DM 1984 Cyclic variation of oxytocin in the blood of pituitary portal vessels of rats. Neuroendocrinology 39:481–483 [DOI] [PubMed] [Google Scholar]
- Chadio SE, Antoni FA 1989 Characterization of oxytocin receptors in rat adenohypophysis using a radioiodinated receptor antagonist peptide. J Endocrinol 122:465–470 [DOI] [PubMed] [Google Scholar]
- Grosvenor CE, Shyr SW, Goodman GT, Mena F 1986 Comparison of plasma profiles of oxytocin and prolactin following suckling in the rat. Neuroendocrinology 43:679–685 [DOI] [PubMed] [Google Scholar]
- Wakerley JB, O'Neill DS, ter Haar MB 1978 Relationship between the suckling-induced release of oxytocin and prolactin in the urethane-anaesthetized lactating rat. J Endocrinol 76:493–500 [DOI] [PubMed] [Google Scholar]
- Chadio SE, Antoni FA 1993 Specific oxytocin agonist stimulates prolactin release but has no effect on inositol phosphate accumulation in isolated rat anterior pituitary cells. J Mol Endocrinol 10:107–114 [DOI] [PubMed] [Google Scholar]
- Egli M, Bertram R, Toporikova N, Sellix MT, Blanco W, Freeman ME 2006 Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. Am J Physiol Endocrinol Metab 290:E566–E572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson WK, Bianchi R, Mogg RJ, Rivier J, Vale W, Melin P 1989 Oxytocin mediates the hypothalamic action of vasoactive intestinal peptide to stimulate prolactin secretion. Endocrinology 124:812–819 [DOI] [PubMed] [Google Scholar]
- Salisbury RL, Krieg Jr RJ, Seibel HR 1980 Effects of arginine vasotocin, oxytocin, and arginine vasopressin on steroid-induced surges of luteinizing hormone and prolactin in ovariectomized rats. Acta Endocrinol (Copenh) 94:166–173 [DOI] [PubMed] [Google Scholar]
- Lumpkin MD, Samson WK, McCann SM 1983 Hypothalamic and pituitary sites of action of oxytocin to alter prolactin secretion in the rat. Endocrinology 112:1711–1717 [DOI] [PubMed] [Google Scholar]
- Samson WK, Lumpkin MD, McCann SM 1986 Evidence for a physiological role for oxytocin in the control of prolactin secretion. Endocrinology 119:554–560 [DOI] [PubMed] [Google Scholar]
- Sarkar DK 1988 Immunoneutralization of oxytocin attenuates preovulatory prolactin secretion during proestrus in the rat. Neuroendocrinology 48:214–216 [DOI] [PubMed] [Google Scholar]
- Arey BJ, Freeman ME 1989 Hypothalamic factors involved in the endogenous stimulatory rhythm regulating prolactin secretion. Endocrinology 124:878–883 [DOI] [PubMed] [Google Scholar]
- Arey BJ, Freeman ME 1990 Oxytocin, vasoactive-intestinal peptide, and serotonin regulate the mating-induced surges of prolactin secretion in the rat. Endocrinology 126:279–284 [DOI] [PubMed] [Google Scholar]
- Johnston CA, Negro-Vilar A 1988 Role of oxytocin on prolactin secretion during proestrus and in different physiological or pharmacological paradigms. Endocrinology 122:341–350 [DOI] [PubMed] [Google Scholar]
- McKee DT, Poletini MO, Bertram R, Freeman ME 2007 Oxytocin action at the lactotroph is required for prolactin surges in cervically stimulated ovariectomized rats. Endocrinology 148:4649–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram R, Egli M, Toporikova N, Freeman ME 2006 A mathematical model for the mating-induced prolactin rhythm of female rats. Am J Physiol Endocrinol Metab 290:E573–E582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umscheid CA, Wu WX, Gordan P, Nathanielsz PW 1998 Up-regulation of oxytocin receptor messenger ribonucleic acid and protein by E2 in the cervix of ovariectomized rat. Biol Reprod 59:1131–1138 [DOI] [PubMed] [Google Scholar]
- Muller M, Soloff MS, Fahrenholz F 1989 Photoaffinity labelling of the oxytocin receptor in plasma membranes from rat mammary gland. FEBS Lett 242:333–336 [DOI] [PubMed] [Google Scholar]
- Thomas A, Crowley RS, Amico JA 1995 Effect of progesterone on hypothalamic oxytocin messenger ribonucleic acid levels in the lactating rat. Endocrinology 136:4188–4194 [DOI] [PubMed] [Google Scholar]
- Valdez SR, Penissi AB, Deis RP, Jahn GA 2007 Hormonal profile and reproductive performance in lactation deficient (OFA hr/hr) and normal (Sprague-Dawley) female rats. Reproduction 133:827–840 [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Akaishi T, Negoro H 1979 Effect of estrogen treatment on plasma oxytocin and vasopressin in ovariectomized rats. Endocrinol Jpn 26:197–205 [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD 1975 The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96:219–226 [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Bolwerk EL, Liu B, Burbach JP 1988 Oxytocin and vasopressin gene expression in the hypothalamo-neurohypophyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology 122:945–951 [DOI] [PubMed] [Google Scholar]
- Kennett JE, Poletini MO, Freeman ME 2008 Vasoactive intestinal polypeptide modulates the E2-induced prolactin surge by entraining oxytocin neuronal activity. Brain Res 1196:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirikowski GF, Caldwell JD, Pedersen CA, Stumpf WE 1988 E2 influences oxytocin-immunoreactive brain systems. Neuroscience 25:237–248 [DOI] [PubMed] [Google Scholar]
- Rhodes CH, Morrell JI, Pfaff DW 1982 Estrogen-concentrating neurophysin-containing hypothalamic magnocellular neurons in the vasopressin-deficient (Brattleboro) rat: a study combining steroid autoradiography and immunocytochemistry. J Neurosci 2:1718–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong WE, Warach S, Hatton GI, McNeill TH 1980 Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience 5:1931–1958 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG 1980 The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194:555–570 [DOI] [PubMed] [Google Scholar]
- Pfaff D, Keiner M 1973 Atlas of E2-concentrating cells in the central nervous system of the female rat. J Comp Neurol 151:121–158 [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM 1997 Cloning, novel promoter sequence, and estrogen regulation of a rat oxytocin receptor gene. Endocrinology 138:1151–1158 [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kimura T, Hashimoto K, Tokugawa Y, Nobunaga K, Azuma C, Saji F, Murata Y 1996 Structure and expression of the mouse oxytocin receptor gene. Mol Cell Endocrinol 124:25–32 [DOI] [PubMed] [Google Scholar]
- Larcher A, Neculcea J, Breton C, Arslan A, Rozen F, Russo C, Zingg HH 1995 Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology 136:5350–5356 [DOI] [PubMed] [Google Scholar]
- Breton C, Di Scala-Guenot D, Zingg HH 2001 Oxytocin receptor gene expression in rat mammary gland: structural characterization and regulation. J Mol Endocrinol 27:175–189 [DOI] [PubMed] [Google Scholar]
- Zingg HH, Rozen F, Breton C, Larcher A, Neculcea J, Chu K, Russo C, Arslan A 1995 Gonadal steroid regulation of oxytocin and oxytocin receptor gene expression. Adv Exp Med Biol 395:395–404 [PubMed] [Google Scholar]
- Murata T, Narita K, Honda K, Higuchi T 2003 Changes of receptor mRNAs for oxytocin and estrogen during the estrous cycle in rat uterus. J Vet Med Sci 65:707–712 [DOI] [PubMed] [Google Scholar]
- Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Barberis C, Jard S 1992 Oxytocin receptors in the central nervous system. Distribution, development, and species differences. Ann NY Acad Sci 652:29–38 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Voorhuis DA, Boschma Y, Elands J 1986 E2 modulates density of putative ‘oxytocin receptors’ in discrete rat brain regions. Neuroendocrinology 44:415–421 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Voorhuis TAM, Elands J 1985 E2 induces oxytocin binding sites in rat hypothalamic ventromedial nucleus. Eur J Pharmacol 118:185–186 [DOI] [PubMed] [Google Scholar]
- Johnson AE, Ball GF, Coirini H, Harbaugh CR, McEwen BS, Insel TR 1989 Time course of the E2-dependent induction of oxytocin receptor binding in the ventromedial hypothalamic nucleus of the rat. Endocrinology 125:1414–1419 [DOI] [PubMed] [Google Scholar]
- Johnson AE, Coirini H, Ball GF, McEwen BS 1989 Anatomical localization of the effects of 17β-E2 on oxytocin receptor binding in the ventromedial hypothalamic nucleus. Endocrinology 124:207–211 [DOI] [PubMed] [Google Scholar]
- Coirini H, Johnson AE, McEwen BS 1989 E2 modulation of oxytocin binding in the ventromedial hypothalamic nucleus of male and female rats. Neuroendocrinology 50:193–198 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Frankfurt M, McEwen BS 1989 Localized actions of progesterone in hypothalamus involve oxytocin. Proc Natl Acad Sci USA 86:6798–6801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AE, Harbaugh CR, Gelhard RE 1991 Projections from the ventromedial nucleus of the hypothalamus contain oxytocin binding sites. Brain Res 567:332–336 [DOI] [PubMed] [Google Scholar]
- Coirini H, Schumacher M, Flanagan LM, McEwen BS 1991 Transport of estrogen-induced oxytocin receptors in the ventromedial hypothalamus. J Neurosci 11:3317–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Johnson AE, Flanagan LM, Frankfurt M, Pfaff DW, McEwen BS 1993 The oxytocin receptor: a target for steroid hormones. Regul Pept 45:115–119 [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM, Johnston CA 1995 Oxytocin receptor mRNA expression in the ventromedial hypothalamus during the estrous cycle. J Neurosci 15:5058–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM 1995 Sex differences in and effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the ventromedial hypothalamus. Endocrinology 136:27–32 [DOI] [PubMed] [Google Scholar]
- Breton C, Pechoux C, Morel G, Zingg H 1995 Oxytocin receptor messenger ribonucleic acid: characterization, regulation, and cellular localization in the rat pituitary gland. Endocrinology 136:2928–2936 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Pfaff DW, McEwen BS 1990 Behavioral effects of progesterone associated with rapid modulation of oxytocin receptors. Science 250:691–694 [DOI] [PubMed] [Google Scholar]
- Breton C, Zingg HH 1997 Expression and region-specific regulation of the oxytocin receptor gene in rat brain. Endocrinology 138:1857–1862 [DOI] [PubMed] [Google Scholar]
- Grazzini E, Guillon G, Mouillac B, Zingg HH 1998 Inhibition of oxytocin receptor function by direct binding of progesterone. Nature 392:509–512 [DOI] [PubMed] [Google Scholar]
- Jones EE, Naftolin F 1990 Estrogen effects on the tuberoinfundibular dopaminergic system in the female rat brain. Brain Res 510:84–91 [DOI] [PubMed] [Google Scholar]
- Arita J, Kimura F 1987 Direct inhibitory effect of long term E2 treatment on dopamine synthesis in tuberoinfundibular dopaminergic neurons: in vitro studies using hypothalamic slices. Endocrinology 121:692–698 [DOI] [PubMed] [Google Scholar]
- Cramer OM, Parker Jr CR, Porter JC 1979 Estrogen inhibition of dopamine release into hypophysial portal blood. Endocrinology 104:419–422 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Ben-Jonathan N 1990 The preovulatory prolactin surge is prolonged by a progesterone-dependent dopaminergic mechanism. Endocrinology 126:246–252 [DOI] [PubMed] [Google Scholar]
- Morrell JI, Rosenthal MF, McCabe JT, Harrington CA, Chikaraishi DM, Pfaff DW 1989 Tyrosine hydroxylase mRNA in the neurons of the tuberoinfundibular region and zona incerta examined after gonadal steroid hormone treatment. Mol Endocrinol 3:1426–1433 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Voogt JL 2002 Progesterone induces dephosphorylation and inactivation of tyrosine hydroxylase in rat hypothalamic dopaminergic neurons. Neuroendocrinology 75:273–281 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Voogt JL 1994 Progesterone suppresses tyrosine hydroxylase messenger ribonucleic acid levels in the arcuate nucleus on proestrus. Endocrinology 135:343–350 [DOI] [PubMed] [Google Scholar]
- Yen SH, Pan JT 1998 Progesterone advances the diurnal rhythm of tuberoinfundibular dopaminergic neuronal activity and the prolactin surge in ovariectomized, estrogen-primed rats and in intact proestrous rats. Endocrinology 139:1602–1609 [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Livingstone JD, Freeman ME 2000 Ovarian steroids influence the activity of neuroendocrine dopaminergic neurons. Brain Res 879:139–147 [DOI] [PubMed] [Google Scholar]
- Babu GN, Vijayan E 1984 Hypothalamic tyrosine hydroxylase activity and plasma gonadotropin and prolactin levels in ovariectomized-steroid treated rats. Brain Res Bull 12:555–558 [DOI] [PubMed] [Google Scholar]
- Beattie CW, Rodgers CH, Soyka LF 1972 Influence of ovariectomy and ovarian steroids on hypothalamic tyrosine hydroxylase activity in the rat. Endocrinology 91:276–279 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Murai I, Ben-Jonathan N 1989 Differential alterations in dopamine turnover rates in the stalk-median eminence and posterior pituitary during the preovulatory prolactin surge. Neuroendocrinology 49:525–530 [DOI] [PubMed] [Google Scholar]
- Fox SR, Harlan RE, Shivers BD, Pfaff DW 1990 Chemical characterization of neuroendocrine targets for progesterone in the female rat brain and pituitary. Neuroendocrinology 51:276–283 [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Blaustein JD 2004 Immunocytochemical investigation of nuclear progestin receptor expression within dopaminergic neurones of the female rat brain. J Neuroendocrinol 16:534–543 [DOI] [PubMed] [Google Scholar]
- Sar M 1988 Distribution of progestin-concentrating cells in rat brain: colocalization of [3H]ORG. 2058, a synthetic progestin, and antibodies to tyrosine hydroxylase in hypothalamus by combined autoradiography and immunocytochemistry. Endocrinology 123:1110–1118 [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Zelena D, Vecsernyes M, Nagy GM, Freeman ME 1998 The effect of neurointermediate lobe denervation on hypothalamic neuroendocrine dopaminergic neurons. Brain Res 806:89–94 [DOI] [PubMed] [Google Scholar]
- Liu B, Arbogast LA 2008 Phosphorylation state of tyrosine hydroxylase in the stalk-median eminence is decreased by progesterone in cycling female rats. Endocrinology 149:1462–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Miteva K, Pancheva S, Stoev S, Wo NC, Chan WY 1995 Design and synthesis of highly selective in vitro and in vivo uterine receptor antagonists of oxytocin: comparisons with Atosiban. Int J Pept Protein Res 46:244–252 [DOI] [PubMed] [Google Scholar]