Abstract
γ-Aminobutyric acid (GABA) is one of the most important neurotransmitters that regulate the excitability of GnRH neurons. Numerous studies have shown that GABA activates Cl− currents in GnRH neurons, and these effects are antagonized by GABAA receptor antagonists. The GABAB receptor is a heterodimer composed of GABAB R1 and R2, and although both subunits have been localized in GnRH neurons, nothing is known about the cellular signaling of this Gαi,o-coupled receptor in GnRH neurons. Using whole-cell recordings from mouse enhanced green fluorescent protein-GnRH neurons, we found that the GABAB receptor agonist baclofen hyperpolarized GnRH neurons through activation of an inwardly rectifying K+ current in a concentration-dependent manner. The effects of baclofen were antagonized by the selective GABAB receptor antagonist CGP 52432 with a Ki (inhibitory constant) of 85 nm. Furthermore, in the presence of the GABAA receptor antagonist picrotoxin, GABA hyperpolarized GnRH neurons in a similar manner. Treatment with 17β-estradiol as compared with oil vehicle did not significantly alter either the EC50 for the baclofen-induced response (0.8 ± 0.1 vs. 1.0 ± 0.1 μm, respectively) or the maximal outward current (10.8 ± 1.7 pA vs. 11.4 ± 0.6 pA, respectively) in GnRH neurons. However, the outward current (and membrane hyperpolarization) was abrogated by submaximal concentrations of the G protein-coupled receptor 54 (GPR54) agonist kisspeptin-10 in both groups, indicating that Gαq-coupled (GPR54) can desensitize the GABAB receptor-mediated response. Therefore, the activation of GABAB receptors in GnRH neurons may provide increased inhibitory tone during estrogen-negative feedback states that is attenuated by kisspeptin during positive feedback.
The activation of GABAB receptors in GnRH neurons may provide increased inhibitory tone during estrogen negative feedback states that are attenuated by kisspeptin during positive feedback.
GABA (γ-aminobutyric acid) is the predominant inhibitory neurotransmitter in the mammalian central nervous system, and its actions are mediated by two classes of GABA receptors, the GABAA and the GABAB receptors. The GABAA receptor, which was the first identified class, is a ligand-gated chloride channel that is expressed in virtually every central nervous system neuron (1,2). On the other hand, the GABAB receptor is a metabotropic receptor that is Gαi,o-coupled to postsynaptic activation of K+ channels (3) or presynaptic inhibition of Ca2+ channel activity (4). Despite the early recognition (1981) of the GABAB receptor and the identification of β-p-chlorophenyl GABA (baclofen) as a selective ligand, it was not until 1997 that the first GABAB receptor (GABAB R1) was cloned by Kaupmann and colleagues (5). Although GABA binds to GABAB R1, fully functional activity was not realized until GABAB R2 was cloned and coexpressed as a heterodimer with the GABAB R1 subunit (6,7,8). Both GABAB R1 and R2 subunits are expressed throughout the mammalian brain (2), and functional GABAB receptors have been demonstrated in numerous hypothalamic neurons (9,10,11,12,13,14).
Recent studies have shown that GABA activates Cl− currents in GnRH neurons, and these effects are blocked by the GABAA receptor antagonist bicuculline (15,16,17,18,19,20). However, activation of GABAA receptors has been reported to depolarize and excite GnRH neurons in some studies (17,20) but hyperpolarize and inhibit GnRH neurons in other studies (18,19). Interestingly, although GABAB R1 and R2 subunits have been localized in GnRH neurons (21), a definite role for GABAB receptor-mediated signaling in GnRH neurons has not been established.
Kisspeptin is a recently discovered neurotransmitter/neuromodulator that plays a critical role in the regulation of GnRH and LH secretion (22,23,24,25). Recently there have been numerous publications about the vital role of kisspeptins in controlling pubertal development (26,27,28,29). Centrally administered kisspeptin stimulates GnRH and gonadotropin secretion in prepubertal and adult animals, presumably by an action in GnRH neurons (30,31,32). Kisspeptin, when perfused onto GnRH neurons in vitro, potently activates these neurons and causes increased neuronal firing (29,33,34,35). Kisspeptin neurons are located in the arcuate nucleus and anteroventral periventricular nucleus of the preoptic area (POA) and are differentially regulated by estrogen (36). Kisspeptins (e.g. kisspeptin-54, -14, -13, and -10) have been identified as endogenous ligands for the orphan G protein-coupled receptor (GPR)-54 (37,38). Upon binding to kisspeptins, GPR54 activates Gαq/11, which causes an increase in phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis, calcium mobilization, and MAPK phosphorylation (37). Our recent study showed that kisspeptin depolarizes GnRH neurons through Gαq/11-phospholipase C signaling mediated inhibition of an inwardly rectifying potassium (Kir) channel as well as activation of a Canonical Transient Receptor Potential (TRPC)-like cationic channel (35).
Importantly, Gαq/11-coupled receptors have been shown to modulate Gαi/o-coupled receptor activation of G protein-coupled inwardly rectifying potassium (GIRK) channels through PIP2 hydrolysis (i.e. heterologous desensitization), attenuating the Gαi/o--coupled receptor-mediated hyperpolarization (39,40,41). Therefore, in the present study, we designed experiments to characterize the kisspeptin modulation of the baclofen (GABAB)-mediated activation of GIRK channels in GnRH neurons.
Materials and Methods
Animals and treatments
All animal treatments described in this study are in accordance with institutional guidelines based on National Institutes of Health standards and were performed with institutional Animal Care and Use Committee approval at the Oregon Health and Science University. Transgenic female mice expressing enhanced green fluorescent protein (EGFP) under the control of the GnRH promoter (EGFP-GnRH) were used in these studies (42). Animals were group housed until surgery after which time they were housed individually. All animals were maintained under controlled temperature and photoperiod (lights on at 0600 h and off at 1800 h) and given free access to food and water.
Adult females were ovariectomized under a ketamine/xylazine (10 and 2 mg, respectively) anesthesia, implanted with an oil capsule or 17β-estradiol (E2) capsule for 4–7 d and killed at 1000–1100 h at which time the uterus was removed and weighed. This oil and E2 treatment yielded plasma E2 levels of 5.4 ± 1.7 and 25.4 ± 4.5 pg/ml, respectively, and uterine weights of 18.3 ± 1.4 and 120.3 ± 7.1 mg, respectively, in oil- and E2-treated animals.
Preparation of POA-GnRH slices
Mice were killed by decapitation. The brain was rapidly removed from the skull and a block containing the diagonal band-POA (DB-POA) was immediately dissected. The DB-POA block was submerged in cold (4 C) oxygenated (95% O2-5% CO2) high sucrose cerebrospinal fluid (CSF; in millimoles): 208 sucrose, 2 KCl, 26 NaHCO3, 10 glucose, 1.25 NaH2PO4, 2 MgSO4, 1 MgCl2, 10 HEPES (pH 7.4). Coronal slices (200 μm) from the DB-POA were cut on a vibratome during which time (10 min) the slices were bathed in high sucrose CSF at 4 C. The slices were then transferred to an auxiliary chamber in which they were kept at room temperature (25 C) in artificial CSF (aCSF) consisting of (in millimoles): 124 NaCl, 5 KCl, 2.6 NaH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, 10 HEPES, 10 glucose (pH 7.4), until recording (recovery for 2 h). A single slice was transferred to the recording chamber at a time and was kept viable by continually perfusing with warm (35 C) oxygenated aCSF at 1.5 ml/min.
Visualized whole-cell patch recording using epifluorescence and infrared differential interference contrast videomicroscopy
Whole-cell patch recordings were made under a Axioskop FS (Zeiss, Jena, Germany) outfitted with epifluorescence (fluorescein isothiocyanate filter set) and IR-DIC video microscopy. The EGFP-tagged GnRH neurons in a slice were visualized through a ×40 water immersion objective (Achroplan; Zeiss). Patch pipettes (A-M Systems, Seattle, WA; 1.5 mm outer diameter borosilicate glass) were pulled on a Brown/Flaming puller (Sutter Instrument Co., Navato, CA; model P-97). Pipette resistances were 4–6 mΩ when filled with pipette solutions. In whole-cell configuration, access resistance was 15–30 mΩ. In experiments to measure the current-voltage (I-V) relationship and reversal potential of baclofen- or kisspeptin-activated currents, the access resistance was kept less than 20 mΩ and was 80% compensated. Current-clamp and voltage-clamp experiments were performed with an Axopatch 1D amplifier (Axon Instruments, Foster City, CA). Electrophysiological signals were digitized with Digidata 1322A (Axon Instruments). Steady-state I-V plots were generally constructed with step command potentials from −50 to −120 mV with steps of 5 mV (holding potential was −60 mV) and durations of 0.5–1 sec. Time-dependent, baclofen-induced currents were measured at a holding potential of −60 mV.
Electrophysiological solutions/drugs
Modified aCSF (in millimoles: 124 NaCl, 5 KCl, 2.6 NaH2PO4, 2 MgCl2, 2 CaCl2, 26 NaHCO3, 10 HEPES, 10 glucose) was used in most cases for electrophysiological recording. The pipette solution contained (in mm): 125 potassium gluconate, 10 NaCl, 1 MgCl2, 11 EGTA, 10 HEPES, 2 MgATP, 2 K2ATP, 0.25 GTP; adjusted to pH 7.3 with KOH; 295 mOsm. In whole-cell current-clamp and voltage-clamp recordings, 0.5–1 μm tetrodotoxin (TTX) was used to eliminate the effect of presynaptic input.
The different drug stock solutions were diluted 1000-fold in aCSF to their final concentrations in 20 ml syringes and were delivered by a Mini-Plus pump (Gilson, Middleton, WI) with a perfusion rate of 1.5 ml/min. The ion channel blockers/activators that were used were the following [Sigma (St. Louis, MO) unless otherwise noted]: kisspeptin-10 (mouse KiSS-1 ((110–119)-NH2); Phoenix Pharmaceuticals, Inc., Burlingame, CA); baclofen (0–100 μm), CGP52432 (5–10 μm); BaCl2 (300 μm); and TTX (0.5–1 μm; Alomone Laboratories, Jerusalem, Israel).
Electrophysiology data analysis
Data were analyzed using p-Clamp software (version 9.2; Axon Instruments). Comparisons between different treatments were performed using an unpaired Student’s t test or a one-way ANOVA. Differences were considered significant if the probability of error was less than 5%. The Ki (inhibitory constant) of CGP52432 was calculated with the method reported by Barlow (43).
Cell harvesting of dispersed GnRH neurons and single-cell RT-PCR
Two to three 300-μm DB-POA slices from intact EGFP-GnRH female mice (n = 5) were cut on a vibratome and placed in an auxiliary chamber containing oxygenated aCSF. The slices were allowed to recover for 1–2 h in the chamber before dispersion. A discrete region of the diagonal band-rostral POA was microdissected and incubated in 5–10 ml of aCSF containing 1 mg/ml protease for about 17 min at 37 C. The tissue was then washed four times in low calcium CSF (1.0 mm CaCl2) and two times in aCSF. The cells were isolated by trituration with flame-polished Pasteur pipettes. The cells were dispersed onto a 60-mm glass-bottom petri dish and were visualized under an inverted microscope equipped with fluorescence (Nikon, Tokyo, Japan). Fluorescent cells or adjacent nonfluorescent cells were patched and then harvested into the patch pipette by applying negative pressure. The contents of the pipette were expelled into a siliconized microcentrifuge tube containing 1 μl 5× Colorless GoTaq Flexi buffer (Promega Corp., Madison, WI), 15 U RNasin, 0.5 μl 100 mm dithiothreitol, and diethylpyrocarbonate-treated water in a total volume of 5 μl. Each harvested cell was reverse transcribed as described previously (35,44). Briefly, the harvested cell solution was denatured for 5 min at 65 C and then cooled on ice for 5 min. Also, 25 ng of hypothalamic total RNA in 5 μl was similarly treated and used as positive control. Single-stranded cDNA was synthesized from cellular RNA by adding 50 U murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA), 3 μl 5× Colorless GoTaq Flexi buffer, 5 mm MgCl2, 0.625 mm deoxynucleotide triphosphates, 15 U RNasin, 10 mm dithiothreitol, and 100 ng random hexamers in a total of 15 μl diethylpyrocarbonate-treated water for a final volume of 20 μl. Cells and tissue RNA used as negative controls were processed as described above but without murine leukemia virus reverse transcriptase.
The reaction mixtures were incubated at 42 C for 60 min, denatured at 95 C for 5 min, and cooled on ice for 5 min. PCR was performed using 2.75 μl cDNA template from each reverse transcription reaction in a 30-μl PCR mix containing the following: 6 μl 5× buffer (Promega), 2–3 mm MgCl2, 0.33 mm deoxynucleotide triphosphate, 0.33 μm forward and reverse primers, 2 U TaqDNA polymerase, and 0.22 μg TaqStart antibody (CLONTECH, Palo Alto, CA). TaqDNA polymerase and TaqStart antibody were combined and incubated at room temperature for 5 min, and the remainder of the reaction content was added to the tube. Primers for GnRH and GABAB R1 and R2 were designed using known mouse sequences as described below and were synthesized by Invitrogen (Carlsbad, CA). Each reaction was amplified for 50 cycles using a PTC-100 thermocycler (MJ Research, Waltham, MA) in 0.5 ml thin-walled PCR tubes according to protocols optimized for each primer pair. Ten microliters of PCR product were visualized with ethidium bromide on a 2% agarose gel.
Design of primers
Primers were designed using the Clone Manager software (Sci Ed Software, Cary, NC). Both GnRH and GABAB R1 and R2 primer pairs were designed to cross intron-exon boundaries to distinguish between genomic DNA and RNA products. In addition, primers were tested for sensitivity through serial dilutions (1:10 to 1:5000) and for efficiency using a SYBR Green real-time PCR assay as described previously (44). Primers were also subjected to magnesium and temperature gradient analysis to further determine the optimal conditions for amplification. The primers were as follows: mouse GnRH (239-bp product) accession no. NM_008145, forward primer 21–40 bp, reverse primer 240–259 bp (regression slope −3.28; efficiency 100%); mouse GABAB R1 (243-bp product) accession no. NM_019439, forward primer 2545–2562 bp, reverse primer 2770–2787 bp (regression slope −3.29; efficiency 100%); mouse GABAB R2 (230-bp product) accession number NM_001081141, forward primer 2473–2494 bp, reverse primer 2681–2702 bp (regression slope −3.45; efficiency 96%). Single cell PCR products from two GnRH-positive cells that also expressed GABAB R1 and R2 were confirmed by sequencing. Moreover, real-time PCR analysis of four individual GnRH neurons, with melting curve conformation of real products, revealed that GABAB R1 could be detected at 34 cycles and GABAB R2 at 37 cycles of amplification.
Data analysis
Forty-three individual GnRH neurons were harvested from five intact females. Four to 12 cells were harvested from each animal, and the mean number of cells expressing GABAB R1, GABAB R2, or both transcripts was determined for each animal and used for additional analysis of mean, sem, and percentage expression.
Results
Baclofen hyperpolarizes GnRH neurons through activation of GIRK channels
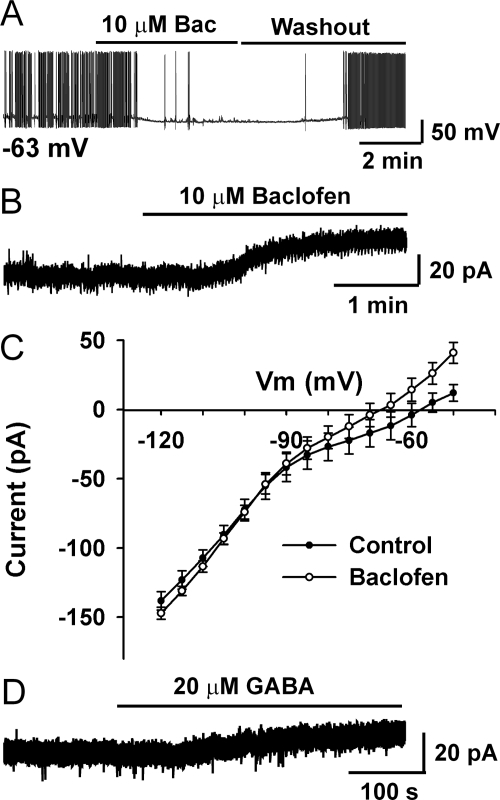
Previous studies have shown that GABAA receptors are expressed in GnRH neurons (45) and GABA or muscimol excite/inhibit GnRH neurons (17,18). However, GABAB receptor function has not been explored in GnRH neurons. As shown in Fig. 1A, we found that baclofen, a GABAB receptor agonist, hyperpolarized GnRH neurons and inhibited cell firing. To exclude potential presynaptic effects, TTX (1 μm) was preperfused into the bath, and the effects of baclofen were studied under voltage clamp conditions. As illustrated in Fig. 1B, baclofen induced a robust outward current at a holding potential of −60 mV (essentially the resting membrane potential of GnRH neurons) (35,44). The I-V relationship illustrates that baclofen induced an outward current (mean 10.0 ± 0.6 pA, n = 11) that reversed at −95 mV (n = 4), which is close to EK+ (−90 mV) (Fig. 1C). This indicates that baclofen hyperpolarizes GnRH neurons through activating GIRK channels. To test whether a physiological concentration of GABA has similar effects, GABA was applied to GnRH neurons in the presence of GABAA receptor antagonist picrotoxin (100 μm). As shown in Fig. 1D, GABA (20 μm) induced an outward current of 7.5 pA. The mean amplitude of GABA-induced outward current was less (4.2 ± 1.7 pA, n = 6) than for the full agonist baclofen but probably reflects rapid uptake of GABA by glial cells (46). Therefore, it appears that GABA has direct inhibitory effects on GnRH neurons through activation of GIRK channels, a response found in more than 90% of GnRH neurons (n = 94).
Figure 1.
Baclofen hyperpolarizes GnRH neurons and inhibits firing through activating a GIRK channel. A, A representative current-clamp recording shows that baclofen (Bac; 10 μm) hyperpolarizes a GnRH neuron by 10 mV and inhibits firing. The resting membrane potential (Vm) was −63 mV. B, A representative voltage-clamp recording shows that baclofen induced an outward current in a GnRH neuron in the presence of TTX. The holding potential was −60 mV. C, The mean I-V plot shows that baclofen-induced currents reversed at −95 mV, which is close to EK+ (−90 mV), indicating the activation of a GIRK channel. Cell number n = 4. D, In the presence of GABA-A receptor antagonist picrotoxin (100 μm), GABA (20 μm) also induced outward currents in GnRH neurons under voltage clamp conditions. The holding potential was −60 mV.
Baclofen hyperpolarizes GnRH neurons through activation of GABAB receptors
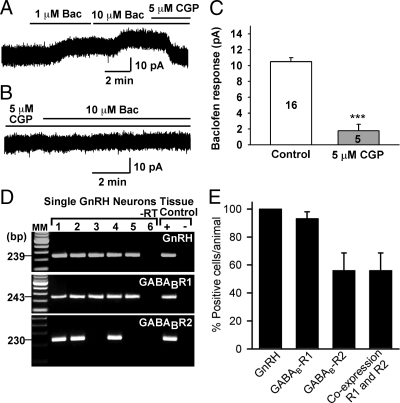
To confirm that the hyperpolarizing effects of baclofen were mediated by the activation of GABAB receptors, the actions of baclofen were examined in the presence of CGP52432, a selective antagonist of GABAB receptors (47). As shown in Fig. 2, CGP52432 (5 μm) antagonized the effects of baclofen. In the presence of CGP52432, the baclofen-induced outward currents were reduced by 84% (10.7 pA in control vs. 1.8 pA in the presence of CGP52432, n = 5). The calculated Ki of CGP52432 antagonism of the response was 85 nm, which agrees with previous published values (11,47).
Figure 2.
Baclofen (Bac) inhibits GnRH neurons through activation of GABAB receptors. A and B, The baclofen-induced outward current in GnRH neurons was antagonized by the selective GABAB receptor antagonist CGP52432 (CGP; Ki = 85 nm). C, Summary of the blockade effect of CGP52432 on baclofen-induced outward current. ***, P < 0.001 (t test), baclofen response in CGP52432-treated compared with untreated (control) neurons. Cell numbers are indicated. D, A representative gel illustrating the mRNA expression of GABAB receptor R1 and R2 subtypes in GnRH neurons (also confirmed by sequencing). As a negative control, a cell reacted without reverse transcriptase (−RT) did not express any of the transcripts. POA-positive (+, with RT) and-negative (− without RT) tissue controls were also included. MM, Molecular markers. E, Quantitative analysis of the expression of GABAB R1 and R2 as well as coexpression of these transcripts in GnRH neurons. Forty-three GnRH neurons from five individual females were analyzed. Bars and vertical lines indicate the mean ± sem of the percent GnRH neurons expressing each of the transcripts.
GABAB receptor subunits mRNA are expressed in GnRH neurons
To further document the involvement of GABAB receptors in baclofen-mediated effects, mRNA transcripts of GABAB receptor subunits were measured by single-cell RT-PCR in 43 GnRH neurons obtained from five females. Indeed, GABAB R1 mRNA was detected in 93 ± 5% of the cells and GABAB R2 mRNA in 56 ± 12% of the cells (Fig. 2, D and E). Most importantly, all of the GnRH neurons that expressed GABAB R2 also expressed GABAB R1 (Fig. 2, D and E).
The baclofen response is not modulated by in vivo E2 treatment
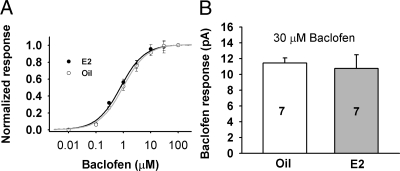
Because it is known that estrogen feedback may influence GABA input to GnRH neurons (48,49,50), we investigated the potential postsynaptic changes in the GABAB response. To examine the effects of estrogen feedback on the GABAB receptor coupling, baclofen-induced outward currents were measured from ovariectomized, oil- and E2-treated females. Figure 3A illustrates that baclofen dose-dependently induced an outward current in both oil- and E2-treated females. However, the EC50 for the baclofen-induced response was not significantly different between oil- and E2-treated animals (1.0 ± 0.1 vs. 0.8 ± 0.1 μm, respectively, n = 5–11), which indicates that the potency of baclofen is not altered by in vivo E2 treatment. To examine the effect of E2 treatment on the efficacy of the baclofen-induced response, the baclofen-induced maximal responses at 30 μm were compared. As shown in Fig. 3B, the baclofen-induced maximal outward currents in oil- and E2-treated animals were not significantly different (11.4 ± 0.6 pA, n = 7 for oil vs. 10.8 ± 1.7 pA, n = 7 for E2 treated). This indicates that the postsynaptic GABAB response was not modulated by in vivo E2 treatment.
Figure 3.
GABAB receptor-mediated (baclofen) response is not affected by E2 treatment. A, Normalized dose-response curve of the baclofen-induced outward currents in GnRH neurons (n = 5–11 cells per concentration). Curves were fitted with a logistic equation. EC50: 1.0 ± 0.1 μm for oil-treated and 0.8 ± 0.1 for E2-treated. B, The maximal outward current induced by baclofen (30 μm) in GnRH neurons from oil- and E2-treated females were not significantly different (t test).
Baclofen response is reversed by kisspeptin
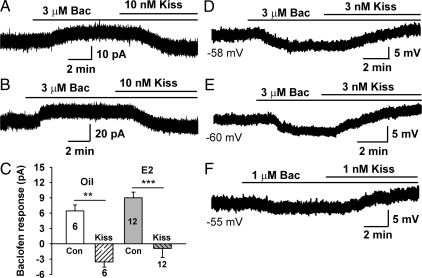
During the positive feedback of estrogen, the GABAergic synaptic input to GnRH neurons is increased (48), which may lead to the activation of GABAB receptor-coupled GIRK channels and thus dampen the excitability of GnRH neurons. However, during positive feedback the inhibitory GABAergic tone must be attenuated by excitatory drive to increase neuronal excitability and hence the firing rate of GnRH neurons. Therefore, we hypothesized that kisspeptin would provide this excitatory input, in part, through attenuating the inhibitory effects of GABA at the GABAB receptor and/or other Gi/o-coupled neurotransmitter receptors. Thus, submaximal concentrations of kisspeptin-10 (10 nm) and baclofen (3 μm) were coperfused, and both membrane current (voltage clamp) and membrane polarization (current clamp) were measured. As we hypothesized, the baclofen-induced outward currents in both oil- and E2-treated GnRH neurons were completely abolished by kisspeptin-10 (Fig. 4, A–C). In addition, even EC50 concentrations of kisspeptin-10 (3 nm) reversed the hyperpolarizing effects of submaximal concentrations of baclofen (Figs. 4, D–F, and 5D).
Figure 4.
Kisspeptin (Kiss) inhibits the baclofen (Bac)-induced outward current and reverses the hyperpolarization of GnRH neurons from both oil- and E2-treated animals. A and B, Representative recordings show that kisspeptin-10 inhibited the baclofen-induced outward current in oil- (A) and E2 (B)-treated animals. C, Summary of the effects of kisspeptin-10 (10 nm) on baclofen (3 μm)-induced outward current as shown in A and B. **, P < 0.01 and ***, P < 0.001 (t test), baclofen responses with coperfusion of kisspeptin-10 compared with baclofen only (control). Cell numbers are indicated. Con, Control. D–F, Representative recordings showing that kisspeptin-10 reversed the baclofen-induced hyperpolarization at even lower concentrations in both E2- (D) and oil- (E and F)-treated animals. The initial resting membrane potentials are indicated.
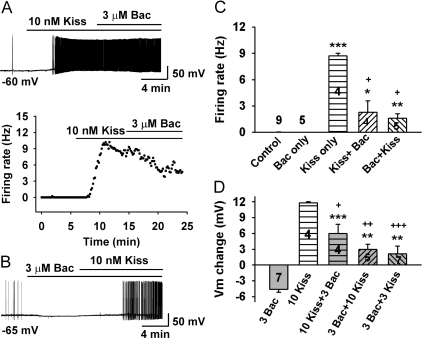
Figure 5.
Baclofen attenuates kisspeptin-induced depolarization and firing of GnRH neurons. A, A representative recording shows that baclofen (Bac) attenuated kisspeptin-induced depolarization and firing. Kisspeptin-10 (10 nm) depolarized a GnRH neuron by about 12 mV and increased the firing to 9 Hz, which was then partially reversed by coapplication of baclofen (3 μm). The original recording is shown on the top panel and the firing rate analysis in the bottom panel showing the time course of the firing rate change. The initial membrane potential (Vm) was −60 mV. B, A representative recording shows that kisspeptin-10 (10 nm) reversed the baclofen (3 μm)-induced hyperpolarization and increased the firing rate to 3 Hz. The initial Vm was −65 mV. C, Summary of the firing rate of GnRH neurons under control, baclofen (Bac, 3 μm) only, kisspeptin-10 (Kiss, 10 nm) only, and kisspeptin-10 followed by baclofen (Kiss + Bac) or baclofen followed by kisspeptin-10 (Bac + Kiss). *, P < 0.05, **, P < 0.01, and ***, P < 0.001 (t test), compared with resting condition (no drug) control group. +, P < 0.001 (t test), compared with Kiss-only group. Cell numbers are indicated. D, Summary of the membrane potential change with baclofen (Bac, 3 μm) only, kisspeptin-10 (Kiss, 10 nm) only, and kisspeptin-10 followed by baclofen (10 Kiss + 3 Bac) or baclofen followed by kisspeptin-10 (3 Bac + 10 Kiss and 3 Bac + 3 Kiss). *, P < 0.05, **, P < 0.01, and ***, P < 0.001 (t test), compared with baclofen (3 Bac) group; +, P < 0.05, ++, P < 0.01, and +++, P < 0.001 (t test), compared with kisspeptin-10 (10 Kiss) group. Cell numbers are indicated.
To further examine the interaction of kisspeptin and baclofen, we reversed the order of baclofen and kisspeptin-10 application. As shown in Fig. 5, A, C, and D, coapplication of baclofen after kisspeptin-10 attenuated but did not completely reverse the kisspeptin-induced firing and depolarization.
Discussion
In the present study, we have shown that GABAB R1 and R2 receptor transcripts are expressed in GnRH neurons, and activation of GABAB receptors by the selective agonist baclofen hyperpolarized GnRH neurons through opening of GIRK channels in a concentration-dependent manner. Moreover, the selective GABAB receptor antagonist CGP52432 blocked the actions of baclofen. Most importantly, the GABAB-mediated hyperpolarization was abrogated by kisspeptin activation of the Gαq-coupled GPR54 receptor, which is congruent with previous findings showing that kisspeptin inhibits K+ channel activity and activates TRPC-like cationic channels in GnRH neurons (34,35,51).
GABA is one of the most important neurotransmitters that regulate the excitability of GnRH neurons. Multiple studies have shown that GABA activates Cl− currents in GnRH neurons, and these effects are reversed by GABAA receptor antagonists (15,16,17,18,19,20). Although GABAB R1 and GABAB R2 subunits have been localized in GnRH neurons (21), this is the first study to show that GABA activates GABAB receptors in GnRH neurons. Moreover, as has been demonstrated in numerous other hypothalamic neurons (9,10,11,12,13,14), GABAB receptors are coupled (Gαi.o) to activation of GIRK channels, resulting in a robust hyperpolarization of GnRH neurons.
The lack of E2 effect on GABAB receptor-GIRK channel coupling in the present study confirms our previous findings in guinea pig GnRH neurons (52). Female GnRH neurons sit at a relatively negative resting membrane potential (−63 mV) that is due, in part, to the activity of another class of Kir channels, ATP-sensitive potassium (Kir 6.2) channels (44). Interestingly, ATP-sensitive potassium channel activity in mouse GnRH neurons is highly regulated by a similar E2 treatment to that used in the present study and that did not have an effect on the coupling of the GABAB receptors to GIRK channels. In contrast, in arcuate proopiomelanocortin neurons in both mouse and guinea pig the GABAB receptor coupling to GIRK channels is highly sensitive to E2 (10,14,53). The mechanism behind this cell-specific difference is currently unknown but will be investigated in future experiments. However, we do know that GABA release is regulated by E2 through presynaptic mechanisms (9,11,49,50) that affects GnRH neuronal activity (48,52).
The significance of the GABAB regulation is highlighted by the fact that GnRH neurons also express a time-dependent, hyperpolarization-activated, cation (h) current (pacemaker current) that contributes to rhythmic firing (44,52,54,55). In addition, T-type calcium channels are expressed in GnRH neurons (56) (our unpublished findings) and the T-type calcium current contributes to burst firing (55,57). However, the activation of these conductances is dependent on the membrane (hyper) polarization (55,57), which is critical for removing the inactivation of T-type calcium channels and activating the h-current. Both GABA (via GABAB) and opioids (via μ-opioid receptors) provide this input to GnRH neurons (52 and present findings). The question then becomes what provides the added excitatory drive during the preovulatory phase of GnRH neurons? One possibility is that kisspeptin via its cognate receptor (GPR54) provides the strong excitatory drive during E2-positive feedback (34,36). Indeed, the present and other findings demonstrate that kisspeptin counters the hyperpolarizing effects of activation of GABAB receptors (baclofen), μ-opioid receptors (our unpublished findings), and other Ba2+-sensitive Kir channels in general (34,35,51). Moreover, there is precedence in the literature for Gαq/11-coupled receptors desensitizing (i.e. heterologous desensitization) Gαi/o-coupled receptors through PIP2 hydrolysis and attenuating the GIRK-mediated hyperpolarization (39,40,41). This is, in part, how kisspeptin is acting in GnRH neurons to attenuate the GABAB-Gαi/o-mediated hyperpolarization through activation of a Gαq/11-phospholipase C signaling pathway (29,34,35,51). In addition, kisspeptin activates nonselective cation (TRPC-like) channels to cause further depolarization and the pronounced excitatory effects on GnRH neurons (35,51).
In summary, we have identified a robust inhibitory effect of GABA on GnRH neurons via activation of GABAB receptors. These receptors are Gαi,o coupled to activation of GIRK channels, which hyperpolarize the cell membrane beyond the relatively negative resting membrane potential. However, this hyperpolarization would facilitate the recruitment of channels (e.g. T-type calcium channels) that are necessary for phasic bursting activity. Most importantly, kisspeptin is able to inhibit Kir channel activity and activate TRPC channels and thus to robustly depolarize GnRH neurons to rapidly fire. Future experiments will elucidate the signaling molecules that modulate these vital channels involved in GnRH neuronal excitability.
Acknowledgments
We thank Dr. Suzanne Moenter (University of Virginia, Charlottesville, VA) for providing the transgenic EGFP-GnRH mice and Elizabeth Rick for her excellent technical assistance.
Footnotes
This work was supported by Public Health Service Grants NS43330, NS38809, and DK68098.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 22, 2009
Abbreviations: aCSF, Artificial CSF; CSF, cerebrospinal fluid; DB-POA, diagonal band-POA; E2, 17β-estradiol; EGFP, enhanced green fluorescent protein; GABA, γ-aminobutyric acid; GABAB R, GABAB receptor; GIRK, G protein-coupled inwardly rectifying potassium; GPR, G protein-coupled receptor; I-V, current-voltage; Kir, inwardly rectifying potassium; PIP2, phosphatidylinositol 4,5-bisphosphate; POA, preoptic area; TTX, tetrodotoxin.
References
- Krnjevic K 1974 Chemical nature of synaptic transmission in vertebrates. Physiol Rev 54:419–518 [Google Scholar]
- Emson PC 2007 GABA(B) receptors: structure and function. In: Tepper JM, Abercrombie ED, Bolam JP, eds. GABA and the basal ganglia. 160th ed. Amsterdam: Elsevier; 43–57 [Google Scholar]
- Dutar P, Nicoll RA 1988 A physiological role for GABAB receptors in the central nervous system. Nature 332:156–158 [DOI] [PubMed] [Google Scholar]
- Bowery NG 1993 GABAB receptor pharmacology. Annu Rev Pharm Toxicol 33:109–147 [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Held J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Froestl W, Bettler B 1997 Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature 386:239–246 [DOI] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao W-J, Johnson M, Gunwaldsen C, Huang L-Y, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C 1998 GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature 396:474–479 [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B 1998 GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature 396:683–687 [DOI] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH 1998 Heterodimerization is required for the formation of a functional GABAB receptor. Nature 396:679–682 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Rønnekleiv OK 1992 Estrogen suppresses μ-opioid and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci 12:2745–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Wagner EJ, Rønnekleiv OK, Kelly MJ 1996 Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinology 64:114–123 [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Bosch MA, Kelly MJ 2001 Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J Neurosci 21:2085–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Li Y, Richards DS 2002 Postsynaptic GABA(B) receptors in supraoptic oxytocin and vasopressin neurons. Prog Brain Res 139:121–125 [DOI] [PubMed] [Google Scholar]
- Slugg RM, Zheng SX, Fang Y, Kelly MJ, Rønnekleiv OK 2003 Baclofen inhibits guinea pig magnocellular neurones via activation of an inwardly rectifying K+ conductance. J Physiol (Lond) 551:295–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ 2003 Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 23:9529–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH 1999 GABA-and glutamate activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Pape JR, Herbison AE 2000 Late postnatal reorganization of GABAA receptor signalling in native GnRH neurons. Eur J Neurosci 12:3497–3504 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM 2002 Activation of A-type γ-aminobutyric receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2872–2891 [DOI] [PubMed] [Google Scholar]
- Han SK, Abraham IM, Herbison AE 2002 Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 143:1459–1466 [DOI] [PubMed] [Google Scholar]
- Han SK, Todman MG, Herbison AE 2004 Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology 145:495–499 [DOI] [PubMed] [Google Scholar]
- Yin C, Ishii H, Tanaka N, Sakuma Y, Kato M 2008 Activation of A-type γ-amino butyric acid receptors excites gonadotrophin-releasing hormone neurones isolated from adult rats. J Neuroendocrinol 20:566–575 [DOI] [PubMed] [Google Scholar]
- Sliwowska JH, Billings HJ, Goodman RL, Lehman MN 2006 Immunocytochemical colocalization of GABA-B receptor subunits in gonadotropin-releasing hormone neurons of the sheep. Neuroscience 141:311–319 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Clifton DK, Steiner RA 2006 Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol 254–255:91–96 [DOI] [PubMed] [Google Scholar]
- Plant TM 2006 The role of KiSS-1 in the regulation of puberty in higher primates. Eur J Endocrinol 155:S11–S16 [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M 2006 Kiss-1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology 83:275–281 [DOI] [PubMed] [Google Scholar]
- Kuohung W, Kaiser UB 2006 GPR54 and KiSS-1: role in the regulation of puberty and reproduction. Rev Endocr Metab Disord 7:257–263 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MBL, Crowley WF, Aparicio SAJR, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- De Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS 1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL 2003 The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 312:1357–1363 [DOI] [PubMed] [Google Scholar]
- Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE 2005 Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA 2004 A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA 2004 Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuronendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio SAJR 2005 Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaynor S, Hu L, Leung PK, Feng H, Mores N, Krsmanovic LZ, Catt KJ 2007 Expression of a functional GPR54-kisspeptin autoregulatory system in hypothalamic GnRH neurons. Mol Endocrinol 21:3062–3070 [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM 2008 Kisspeptin acts directly and indirectly to increase GnRH neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK 2008 Kisspeptin depolarizes GnRH neurons through activation of TRPC-like cationic channels. J Neurosci 28:4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden J-M, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M 2001 The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276:34631–34636 [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M 2001 Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411:613–617 [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Zhang H 1999 Gating of G protein-sensitive inwardly rectifying K+ channels through phosphatidylinositol 4,5-bisphosphate. J Physiol (Lond) 520:630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrinsky E, Mirshahi T, Zhang H, Jin T, Logothetis DE 2000 Receptor-mediated hydrolysis of plasma membrane messenger PIP2 leads to K+-current desensitization. Nat Cell Biol 2:507–514 [DOI] [PubMed] [Google Scholar]
- Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ 2007 Serotonin 5HT2c receptor signaling in hypothalamic POMC neurons: role in energy homeostasis in females. Mol Pharm 72:885–896 [DOI] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin J-P, Saunders JT, Dudek FE, Moenter SM 2000 Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- Barlow RB 1995 Use of an antagonist for estimating the degree of agonist stimulation during physiological release. Trends Pharmacol Sci 16:262–264 [DOI] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ 2007 Gonadotropin-releasing hormone neurons express KATP channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27:10153–10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape JP, Skynner MJ, Sim JA, Herbison AE 2001 Profiling gamma-aminobutyric acid (GABAA) receptor subunit mRNA expression in postnatal gonadotropin-releasing hormone (GnRH) neurons of the male mouse with single cell RT-PCR. Neuroendocrinology 74:300–308 [DOI] [PubMed] [Google Scholar]
- Shen K-Z, Johnson SW 2001 Potentiation of GABAA receptor agonists by GABA uptake inhibitors in the rat ventral midbrain. Eur J Pharmacol 428:1–7 [DOI] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ 2002 International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev 54:247–264 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM 2007 Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 27:1913–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE 1997 Estrogen regulation of GABA transmission in rat preoptic area. Brain Res Bull 44:321–326 [DOI] [PubMed] [Google Scholar]
- Jackson GL, Kuehl D 2002 γ-Aminobutyric acid (GABA) regulation of GnRH secretion in sheep. Reproduction 59:15–24 [PubMed] [Google Scholar]
- Liu X, Lee K, Herbison AE 2008 Kisspeptin excites gonadotropin-releasing hormone (GnRH) neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149:4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ 1995 Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology 136:2341–2344 [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ 2006 A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci 26:5649–5655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM 2002 Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci 22:2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA 1992 Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol 68:1373–1383 [DOI] [PubMed] [Google Scholar]
- Kato M, Ui-Tei K, Watanabe M, Sakuma Y 2003 Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology 144:5118–5125 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ 2002 GnRH neurons and episodic bursting activity. Trends Endocrinol Metab 13:409–410 [DOI] [PubMed] [Google Scholar]