Abstract
The primary induced isoflavones in soybean, the glyceollins, have been shown to be potent estrogen antagonists in vitro and in vivo. The discovery of the glyceollins’ ability to inhibit cancer cell proliferation has led to the analysis of estrogenic activities of other induced isoflavones. In this study, we investigated a novel isoflavone, glycinol, a precursor to glyceollin that is produced in elicited soy. Sensitive and specific in vitro bioassays were used to determine that glycinol exhibits potent estrogenic activity. Estrogen-based reporter assays were performed, and glycinol displayed a marked estrogenic effect on estrogen receptor (ER) signaling between 1 and 10 μm, which correlated with comparable colony formation of MCF-7 cells at 10 μm. Glycinol also induced the expression of estrogen-responsive genes (progesterone receptor and stromal-cell-derived factor-1). Competitive binding assays revealed a high affinity of glycinol for both ERα (ΙC50 = 13.8 nm) and ERβ (ΙC50 = 9.1 nm). In addition, ligand receptor modeling (docking) studies were performed and glycinol was shown to bind similarly to both ERα and ERβ. Taken together, these results suggest for the first time that glycinol is estrogenic and may represent an important component of the health effects of soy-based foods.
Glycinol, a pterocarpan precursor to glyceollin that is produced in elicited soy, demonstrates potent estrogenic activity.
Epidemiologic studies support the view that consumption of soy prevents certain hormonally induced cancers and other diseases associated with estrogen deficiency (1,2,3,4,5,6). Asian women who consume a traditional low-fat soy diet have a 4- to 6-fold lower risk of developing breast cancer when compared with women living in the industrialized Western world (1,2,3,4,5,6). Many of the health benefits of soybean have been attributed, in part, to the presence of isoflavones (3,6,7,8,9,10). The primary isoflavones in soybean are genistein, daidzein, glycitein, and their respective β-glucosides. In most soy foods, isoflavones are present primarily as β-glucosides, esterified with malonic or acetic acid (11). However, in fermented soy foods, higher levels of the aglycone form of isoflavones are formed from microorganism-induced fermentation and hydrolysis.
Isoflavones are considered phytoalexins, low-molecular-weight antimicrobial compounds and are synthesized de novo, accumulating in different plant tissues in response to stress, physical stimuli, or infectious agents (12,13,14,15,16,17). In soybean, several significant changes in isoflavone concentration occur in response to stress or elicitor treatment. The predominant phytoalexins produced in soybean are glyceollins I, II, and III, and these compounds demonstrate antifungal activity against several plant pathogens (13,14,15,16,17,18). Recent research in our laboratory focused on the antiestrogenic activity of the glyceollins in vitro (19) and in vivo (20,21). Our results have led to the examination of other soybean phytoalexins for estrogenic and antiestrogenic activities.
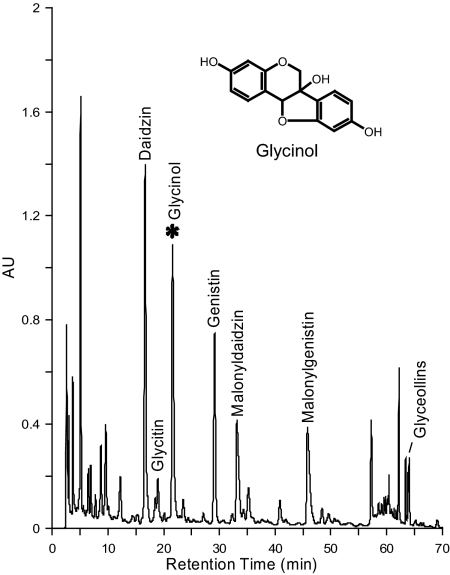
Several other phytoalexins that are structurally similar to the glyceollins, occur at low concentrations including glyceollidin (22,23), glyceocarpin (22,23,24), and glycinol (22,23,24,25,26,27) shown in Fig. 1. Glycinol, a precursor in the biosynthetic pathway of the glyceollins, was first isolated by Lyne and Mulheirn (25). In a study by Weinstein et al. (24), glycinol inhibited the growth of all six bacteria examined and the three fungi Phytophthora megasperma f. sp glycinea (race 1), Saccharomyces cerevisae, and Cladosporium cucumerinium. These results suggested that glycinol possessed antimicrobial and antifungal activity; however, no other biological activity was examined. Because of the structural similarity to daidzein and coumestrol in addition to various other environmental estrogens studied (28, 29), it was hypothesized that glycinol would have estrogenic activity.
Figure 1.
HPLC chromatogram of soybean seeds after treatment with silver nitrate. Predominant constitutive isoflavones are daidzin, genistin, malonyldaidzin, and malonylgenistin. Induced isoflavone phytoalexins are glycinol and glyceollins (I, II, and III). AU, Absorbance units.
In this study, we examined the estrogenic activity of the induced soybean isoflavone glycinol using several in vitro assays. Glycinol’s effect on estrogen receptor (ER) activity was analyzed using an ER-positive MCF-7 human breast carcinoma cell line transfected with an estrogen response element (ERE)-luciferase reporter. We also examined glycinol’s effect on expression levels of two estrogen-responsive genes by semiquantitative real time RT-PCR: stromal-cell-derived factor (SDF)-1 and progesterone receptor (PgR). Furthermore, viability and proliferative properties exerted by MCF-7 cells was explored using a colony formation assay. ERα and ERβ binding assays were conducted to determine the binding affinities of glycinol to both ER subtypes. In addition, ligand-receptor docking studies using computer modeling were performed to analyze the interaction of glycinol with the ERα and ERβ ligand binding domains. These in vitro studies support our hypothesis and demonstrate for the first time that glycinol has potent estrogenic activity and may prove beneficial for the use of postmenopausal women requiring hormone replacement therapy.
Materials and Methods
Chemicals and plasmids
ICI 182,780 (Fulvestrant) was purchased from Tocris Bioscience (Ellisville, MO). 17β-Estradiol was purchased from Sigma (St. Louis, MO). The solvents acetonitrile (HPLC grade), methanol, and ethanol were purchased from Aldrich Chemical Co. (St. Louis, MO). H2O treated with a Millipore system (Bedford, MA) was used during sample preparation procedures and HPLC analyses.
ERβ cDNA was generously provided by Jan-Åke Gustafsson (Karolinska Institute, Stockholm, Sweden) in pBluescript. ERα and ERβ expression vectors were constructed by inserting the ERα and ERβ cDNA respectively into pcDNA 3.1 vector (Invitrogen, Carlsbad, CA). ERα cDNA (2090 bp) was cleaved from plasmid (pBluescript) with BamHI/EcoRI and then ligated into the pcDNA3.1. ERβ cDNA (1460 bp) was cleaved from Plasmid (pBluescript) with HindIII/BamHI and then ligated into the pcDNA3.1. Each construct was verified by detailed restriction mapping.
HEK 293 cells were cultured in 5% charcoal-stripped (CS) DMEM and seeded into a 24-well plate at a density of 50,000 cells/well and allowed to attach overnight. Cells were transfected with 0.2 μg ER(2)-luc plasmid (Panomics, Fremont, CA), 0.1 μg pcDNA, 0.1 μg pcDNA3.1B-ERβ, or 0.1 μg pcDNA3.1B-ERα plasmids the next day using Effectene transfection reagent (QIAGEN, Valencia, CA) according to the manufacturer’s protocol. After a 6-h transfection, cells were treated with compounds [dimethylsulfoxide (DMSO), estradiol (E2), glycinol, or fulvestrant] overnight. On the following day, the cells were lysed with 150 μl of the M-Per mammalian extraction reagent (Pierce, Rockford, IL). After 18 h cell lysates were measured for luciferase activity. One hundred microliters of the cell extract were assayed using the Bright-glo luciferase assay substrate (Promega, Madison, WI) and determined in a AutoLumat Plus lunimometer (Berthold, Bad Wildbad, Germany).
Isolation of glycinol
Glycinol was isolated using a procedure developed by Qi et al. (27). Seeds from the soybean [Glycine max (L.)] cultivar Asgrow 5902 were obtained from Helena Chemical Co. (Thibodaux, LA). Seeds were surface sterilized for 3 min in 70% EtOH followed by a quick deionized H2O rinse and two 2-min rinses in deionized H2O. Seeds were presoaked in sterile deionized H2O for 4–5 h before placement into treatment chambers (10 g/chamber). Each chamber consisted of a petri dish (100 × 15 mm, four compartments), each compartment lined with two autoclaved filter papers (Whatman, Middlesex, UK) moistened with 0.5 ml distilled H2O. Seeds were cut and treated with a solution of 0.01 mol/liter silver nitrate. All chambers were stored at 25 C in the dark for 3 d and then transferred to −70 C. Soy extracts were extracted from 10 g finely ground seeds in 20 ml methanol.
HPLC analyses were performed on a Waters 2695 combined with a Waters UV-visible 2996 photodiode array detector (Waters Associated, Milford, MA). Isoflavones were separated using a Luna II C18 reverse-phase column (4.6 × 250 mm; 5 μm; Phenomenex, Torrance, CA). A guard column containing the same packing was used to protect the analytical column. The injection volume of sample was 20 μl with a flow rate of 1.0 ml/min with the following solvent system: A = 0.1% acetic acid/water, B = acetonitrile; 15% B for 8 min, then to 58% B in 50 min, then to 90% B in 10 min followed by holding at 90% B for 10 min. The spectra were collected between 220 and 400 nm by photodiode array detector.
Glycinol was isolated using semipreparative HPLC using a Whatman ODS-2 10 mm × 500 mm column at a flow rate of 3.0 ml/min with the following solvent system: A = acetonitrile, B = water; 5% A for 15 min, then 5% A to 90% A in 40 min followed by holding at 90% A for 20 min. Glycinol was confirmed by UV-visible spectrophotometry, mass spectrometry, and nuclear magnetic resonance analyses. The solvents acetonitrile (HPLC grade) and methanol were purchased from Aldrich. Water was obtained using a Millipore system and used during sample preparation procedures and HPLC analyses.
Cell culture
MCF-7 breast cancer cells and human embryonic kidney, HEK 293, cells were cultured in 150-cm2 culture flasks in DMEM supplemented with 10% fetal bovine serum (FBS) (Life Technologies, Inc.-BRL, Gaithersburg, MD), basal medium Eagle (BME) and MEM amino acids, l-glutamine, sodium pyruvate and penicillin-streptomycin, and porcine insulin (10−8 m) (Sigma). The culture flasks were maintained in 5% CO2 at 37 C.
Luciferase assays
MCF-7 breast cancer cells were cultured in 5% charcoal-stripped media overnight. Cells were plated in 24-well plates in 5% charcoal-stripped phenol red-free media overnight. Cells were transfected with 0.3 μg of ER(2)-luc plasmid (Panomics) for 6 h according to the manufacturer’s protocol using Effectene (QIAGEN) and treated with vehicle (DMSO) or glycinol (0.01–10 μm) overnight. Media were removed and cells were lysed with reporter lysis buffer. Relative light units were measured in an Opticomp II luminometer (MGM Laboratories, Hamden, CT) using luciferase reagent (Promega).
HEK 293 cells were cultured in 5% FBS-DMEM and seeded into a 24-well plate at a density of 50,000 cells/well and allowed to attach overnight in 5% CS-FBS. Cells were transfected with 0.2 μg ER(2)-luc plasmid (Panomics), 0.1 μg pcDNA, 0.1 μg pcDNA3.1B-ERβ, or 0.1 μg pcDNA3.1B-ERα plasmids the next day using Effectene transfection reagent (QIAGEN) according to the manufacturer’s protocol. After a 6-h transfection, cells were treated with compounds (DMSO, E2, glycinol, or fulvestrant) overnight. On the following day, the cells were lysed with 150 μl of the M-Per mammalian extraction reagent (Pierce). After 18 h cell lysates were measured for luciferase activity. One hundred microliters of the cell extract were assayed using the Bright-glo luciferase assay substrate (Promega) and determined in a Berthold AutoLumat Plus lunimometer.
RNA extraction and semiquantitative real-time RT-PCR
MCF-7 cells were seeded at a density of 2 × 106 cells per 25 cm2 culture flask in phenol red containing 5% FBS-DMEM. On the following day, cells were washed in PBS and media were changed to phenol red-free media supplemented with 5% CS-DMEM and grown to 50–80% confluency for 48 h before treatment with DMSO vehicle, E2, glycinol, or fulvestrant. RNA was extracted using QiaShredders (QIAGEN) and purified on RNeasy columns (QIAGEN) according to the manufacturer’s protocol. RNA quality and concentration were determined by absorbance at 260 and 280 nm. Total RNA was reverse transcribed using the iScript kit (Bio-Rad Laboratories, Hercules, CA). The levels of ERα, SDF-1, and PgR transcripts were determined using real-time semiquantitative PCR. The primer sequences for PgR, SDF-1 and ERα are (sense and antisense, respectively): PgR, 5′-TACCCGCCCTATCTCAACTACC-3′, 5′-TGCTTCATCCCCACAG-ATTAAACA-3′; SDF-1, 5′-AGTCAGGTGGTGGCTTAACAG-3′, 5′-AGAGGAGGTGAAGGCAGTGG-3′; and ERα, 5′-GGCATGGTGGAGATCTTCGA-3′, 5′-CCTCTCCCTGCAGATTCATCA-3′. PCR mix contained optimal concentrations of primers, cDNA, and SYBR Green PCR master mix (Bio-Rad). β-Actin, PgR, SDF-1, and ERα genes were amplified in triplicate. Quantification and relative gene expression was calculated by comparing PgR, SDF-1, and ERα relative gene expression to internal β-actin control. The ratio between these values obtained provided the relative gene expression levels.
ERα and ERβ binding assays
Receptor binding determinations of glycinol were achieved using the method of Bolger (30). In this method, recombinant ER is in equilibrium with a fluorescent estrogen ligand (ES2; Panvera, Madison, WI) and a concentration of the competitor (glycinol). The relative displacement of the ES2 is measured as a change in polarization anisotropy. Serial dilutions of competitors (glycinol and estradiol) were prepared from DMSO stock solutions in screening buffer at the desired concentrations. The ER and ES2 were combined with each competitor aliquot to a final concentration of 2 nm ER and 3 nm ES2, respectively. In addition, both a no-binding control (ER + ES2 only, equivalent to 0% competitor inhibition) and a 100% binding control (only free ES2, no ER, equivalent to 100% competitor inhibition) were prepared. All competitor and controls were prepared in duplicate within a binding experiment. After a 2-h incubation at room temperature, the anisotropy value for each sample and control were measured using the Beacon 2000 (Invitrogen, Carlsbad, CA). Anisotropy values were converted to percent inhibition using the following formula: I% = (A0 − A)/(A0 − A100) × 100, where I% is the percent inhibition, A0 is 0% inhibition, A100 is 100% inhibition, and A represents the observed value. This conversion to percent inhibition makes the data more intuitive and normalizes the day-to-day differences of multiple experiments. The percent inhibition vs. competitor concentration curves was analyzed by nonlinear least-squares curve fitting (Prism 5.0a; GraphPad Software, San Diego CA, www.graphpad. com) to yield IC50 values (the concentration of competitor needed to displace half of the bound ligand). To compare binding affinities of the test compounds to those reported in the literature, IC50 values were converted to relative binding affinities (RBAs) using E2 as a standard. The E2 RBA was set equal to 100 RBA = (IC50/IC50 of E2) × 100.
Colony formation assay
Cells were cultured in 5% FBS-DMEM and media were changed to phenol red-free 5% CS-DMEM 2 d before assay. Cells were seeded at a density of 3000 cells/well in a six-well plate. The cells were allowed to attach overnight and treated on the following day with DMSO vehicle or 1 nm E2, glycinol, or glycinol + fulvestrant. Media were replaced every 7 d and treated with appropriate drug for 3 wk. After 3 wk the media were removed and the cells were fixed with formaldehyde and dried overnight. The cells were then washed and stained with crystal violet and dried. The colonies were counted.
Docking models of glycinol to ERα and ERβ
The glycinol used in this study possessed two chiral centers within its structure, resulting in four possible enantiomers of the compound. Of these, configurations found in nature were selected for ligand-receptor modeling (docking) studies. The SS configuration or (−)-glycinol structure was converted to a unique SMILE string with ChemDraw (CambridgeSoft, cambridgesoft.com) and then converted to a three-dimensional structure using CONCORD (R. S. Pearlman, distributed by Tripos International, St. Louis, MO). The initial three-dimensional model was then optimized in Sybyl 8.0 (Tripos International) using the MMFF94 force field and the conjugated gradient method with a termination of 0.005 kcal/mol. After optimization, the glycinol structure was assigned AM1 charges using MOPAC 6.0 (Colorado Springs, CO) distributed with Sybyl (Tripos International, St. Louis, MO).
Docking and scoring of glycinol was performed using Surflex-Dock in Sybyl 8.0. Crystal structure from the Protein Data Bank (pdb) of the human ERα ligand-binding domain in complex with E2 (pdb:1ERE) and human ERβ ligand-binding domain in complex with E2 (pdb: 2×7R) were used for the docking studies. For this study, only the A chain of each crystal structure was retained for docking. Docking preparation of the receptor included extraction of E2 from each of the crystal structures, addition of hydrogens to the protein, and the generation of a protomol surface of the binding cavity. The glycinol model was then docked into the crystal structure protein model with Surflex-Dock (default settings with reign flexibility sampled) and the resulting 10 poses best were sorted by the Surflex-Dock scoring function in −log10 [Kd (affinity constant)] units to simulate binding affinities. The best scoring pose of glycinol docked with the α- and β-forms of the ER were retained for this study.
Results
Isolation of glycinol
Several constitutive isoflavones are responsible for the estrogenic activity observed in soy, including daidzein, genistein, and glycitein. These isoflavones, as well as coumestrol and other flavonoids, predominantly act as estrogenic chemicals but may also exhibit antiestrogenic activity in a dose-dependent manner (17,18,31). We have shown that under stress conditions or elicitor treatment, the concentrations of the constitutive isoflavones increase and several isoflavone phytoalexins are produced. Our previous results showed that several soy isoflavone phytoalexins are induced with elicitor treatments (19,32). Coumestrol, the glyceollins, and several other phytoalexins are induced to higher concentrations as a result of secondary metabolism within the plant tissue. These studies resulted in the identification of the induced pterocarpan glycinol shown in Fig. 1. The isolated compound was identified as glycinol by comparison with its spectroscopic data [1H and 13C nuclear magnetic resonance; atmospheric pressure chemical ionization (APCI) and electron ionization mass spectrometry (EI MS)] with those in the literature (24,25,27).
Effect of glycinol on ER transcriptional activation
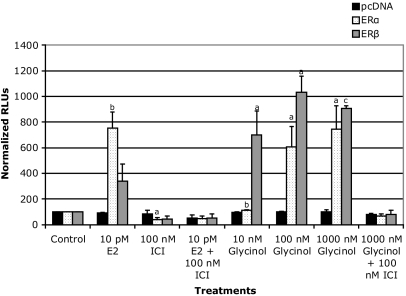
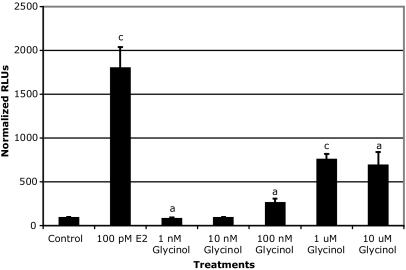
Based on the similarity of the chemical structure of glycinol to other isoflavones, ERE-based reporter assays were performed to examine the potential estrogenicity of glycinol. Initially, we chose to use HEK 293 cells lacking endogenous ERα and ERβ for this purpose. Estrogen-responsive reporter gene assays were performed by transiently transfecting the cells with the ERE reporter construct in addition to ERα or ERβ. Glycinol exhibited potent estrogenic activity (Fig. 2). Treatment with glycinol at 100 nm displayed moderate activity equivalent to 35% of E2 (1 nm) (Fig. 2). However, at 1000 nm, estrogenic activity increased to 90% of E2 (1 nm), which was blocked by the ER down-regulator, fulvestrant, suggesting an ER-dependent mechanism. When compared with glycinol-treated HEK 293 cells transfected with ERα, ERβ-transfected cells exhibited a higher response. We then used the MCF-7 breast cancer cells to further examine the estrogenicity of glycinol in a system endogenously expressing both ERs. ER-positive MCF-7 cells were also used to examine the potential of glycinol to induce the ER-mediated transcriptional activity. Glycinol dose-response studies were performed, and doses ranging from 100 nm to 10 μm enhanced the ER-mediated transcriptional activity to levels that were 250–750% above the control, whereas lower doses did not have any effect on activity (Fig. 3).
Figure 2.
HEK 293 cells ERE-luciferase reporter assay with glycinol treatments. Cells were cultured in 5% CS-DMEM and seeded into a 24-well plate at a density of 50,000 cells/well and allowed to attach overnight. Cells were transfected with 0.2 μg ERE-luciferase plasmid, and 0.1 μg pcDNA, 0.1 μg ERβ or 0.1 μg ERα the next day using Effectene (QIAGEN) according to the manufacturer’s protocol. After a 6-h transfection, cells were treated with compounds (DMSO, E2, glycinol or ICI) and incubated overnight. On the following day, the cells were lysed with 150 μl of the M-Per mammalian extraction reagent (Pierce). Luciferase activity for 100 μl of the cell extract was assayed using the Bright-glo luciferase assay substrate (Promega) and determined in a Berthold AutoLumat Plus lunimometer. Data are represented as relative light units (RLUs) normalized to untreated vector control (100 ± sem), and the values are the means and the ses of triplicates from a single experiment and representative for at least two independent experiments. a, Significant difference from vector control, P < 0.05; b, significant difference from vector control, P < 0.01; c, significant difference from vector control, P < 0.005, Tukey test.
Figure 3.
MCF-7 ERE-luciferase reporter assay. Cells were cultured in 5% CS-DMEM and seeded into a 24-well plate and allowed to attach overnight. Cells were transfected with ERE-luciferase plasmid the next day using Effectene (QIAGEN) according to the manufacturer’s protocol. After a 6-h transfection, cells were treated with compounds (DMSO or glycinol) and incubated overnight. On the following day, the cells were lysed with 150 μl of the M-Per mammalian extraction reagent (Pierce). Luciferase activity for 100 μl of the cell extract was assayed using the Bright-glo luciferase assay substrate (Promega) and determined in a Berthold AutoLumat Plus lunimometer. Data are represented as relative light units (RLUs) normalized to untreated vector control (100 ± sem), and the values are the means and the ses of triplicates from a single experiment and representative for at least two independent experiments. a, Significant difference from vector control, P < 0.05; b, significant difference from vector control, P < 0.01; c, significant difference from vector control, P < 0.005, Tukey test.
Regulation of ERα, SDF-1, and PgR mRNA levels after glycinol treatment
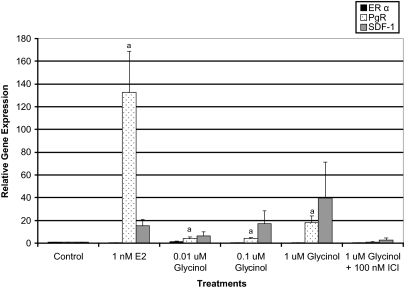
Three differentially expressed genes were selected for analysis of expression levels by semiquantitative real-time RT-PCR: ERα, SDF-1, and PgR. We chose to examine ERα mRNA levels as a control. SDF-1 and PgR, both estrogen-responsive genes, which have been shown to have functions that are associated with cell proliferation and/or tumorigenesis. SDF-1 has been identified as a novel ER-regulated gene involved in metastasis and a mediator of the mitogenic effects of estrogen in ovarian and breast cancer cells (33). In the present study, we examined the effect of glycinol treatment on the mRNA expression of SDF-1, ERα, and PgR in MCF-7 cells.
To determine the effect of glycinol on expression of PgR, SDF-1, and ERα genes, MCF-7 cells were treated with glycinol at varying concentrations (0.01–1 μm) for 4 h (Fig. 4). ERα mRNA expression remained relatively unchanged with all treatments, suggesting that the effects observed by glycinol are not due to changes in ERα mRNA expression. However, estrogen induced PgR and SDF-1 gene expression to levels greater than 100- and 15-fold (respectively) above the control. Glycinol treatments (0.01–1 μm) elicited a dose-dependent increase in SDF-1 expression, which ranged from 6- to 39-fold above the control, whereas PgR gene expression increased from 4- to 18-fold above the control. As expected, fulvestrant at 100 nm effectively inhibited the up-regulation of PgR and SDF-1 expression by glycinol indicating a direct ER effect.
Figure 4.
ERα, PgR, and SDF-1 gene expression in MCF-7 breast cancer cells. Total RΝA was isolated from MCF-7 cells, reverse transcribed into cDNA, and subjected to real-time PCR analysis for quantification. Treatment was as follows: DMSO vehicle control, 1 nm E2, glycinol ± fulvestrant. The values are the means and the ses of triplicates from a single experiment and representative for at least two independent experiments. a, Significant difference from vector control, P < 0.05; b, significant difference from vector control, P < 0.01; c, significant difference from vector control, P < 0.005, Tukey test.
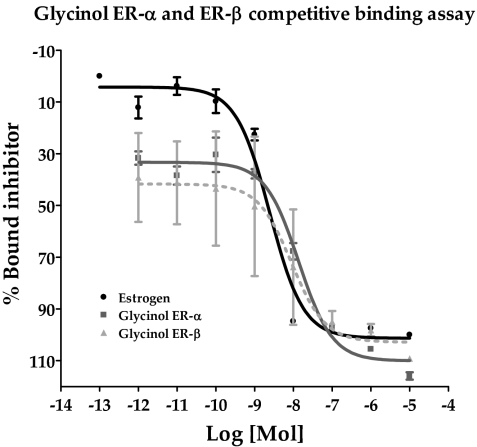
Competitive binding of glycinol to ERα and ERβ
Several studies have demonstrated that phytoestrogens exert their stimulatory effect on the ER by binding to the same site as E2 with some differences in ligand binding specificity and transactivation between ERα and ERβ (34,35,36,37). Of particular interest was the observation that certain isoflavones may bind with higher affinity and possess higher agonistic activity toward ERβ (35,36,37). Additionally we found that glycinol induces a greater ERβ transcriptional response compared with ERα in ERE transcriptional reporter assays (Fig. 2). Therefore, we chose to assess the ability of glycinol to bind ERα and ERβ using a competitive binding assay with fluorescent detection. Figure 5 details the results for the competitive binding assay. Glycinol produced a displacement of E2 bound ERα (50%), which occurred at a concentration of 2.46 nm. The IC50 of glycinol for ERα was 13.75 nm. However, the IC50 of glycinol for ERβ was 9.05 nm. This indicated that the ability of glycinol to act as an ER agonist occurred through receptor binding, and only slightly greater affinity for ERβ vs. ERα was observed, which may account in part for the greater glycinol-induced ERβ transcriptional response in HEK 293 cells.
Figure 5.
Competitive binding curves of glycinol and ERα/ERβ. Increasing concentrations of glycinol were added to ERα or ERβ complex and compared with E2. Data points and error bars represent the mean ± sem of three independent experiments for glycinol and E2 treatment for each concentration tested.
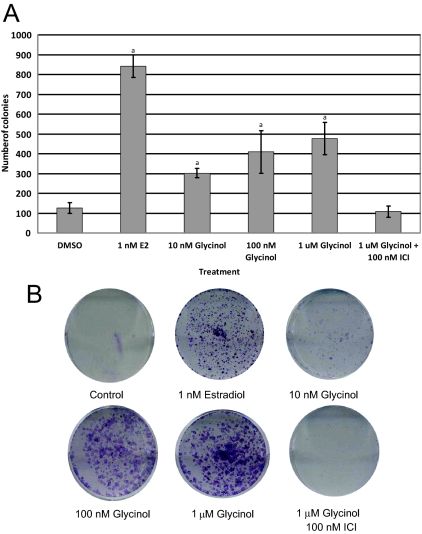
Glycinol induces colony formation in MCF-7 cells
Upon establishing the ability of glycinol to bind both ERα and ERβ with high affinity, we then examined the biology of glycinol on cell viability and proliferation using the colony formation assay (Fig. 6). MCF-7 cells were treated with 1 nm E2, glycinol (0.01–1 μm), and 1 μm glycinol + 100 nm fulvestrant, a classical ER down-regulator. Cells were treated once a week and colonies formed after 5 wk of treatment. Upon formation of colonies, cells were fixed, stained, and counted. MCF-7 cells treated with vehicle exhibited few colonies; however, treatment with 1 nm E2 treatment enhanced the number of colonies greater than 600% above the control. Cells treated with glycinol exhibited a dose-dependent increase in colony formation reaching a maximum of approximately 300% above control levels. In addition to an increase in colony formation, there was a difference in the morphology, size, and staining intensity of the glycinol-treated colonies compared with the estrogen-treated colonies. Glycinol-treated cells stained more intensely and exhibited an increase in colony size compared with estrogen. Furthermore, the combination of glycinol plus fulvestrant led to a marked decrease in colony formation compared with glycinol alone.
Figure 6.
A, Effects of glycinol on colony formation on MCF-7 cells placed in phenol red-free DMEM supplemented with 5% dextran-coated charcoal-treated FBS for 48 h before plating. Then 3000 cells/well were plated in six-well plates. Forty-eight hours later, cells were treated with glycinol or vehicle. B, The picture is a representative of the best of three experiments. The values are the means and the ses of triplicates from a single experiment and representative for at least two independent experiments. a, Significant difference from vector control, P < 0.05; Tukey test.
Glycinol docks to ERα and ERβ similarly
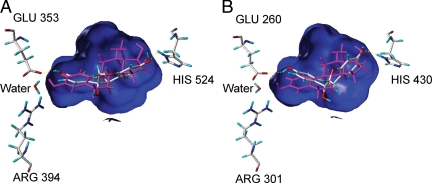
Evaluation of the ligand-receptor docking and scoring of glycinol to both the ERα and ERβ ligand binding domains illustrates that glycinol can bind similarly to each of these receptor subtypes in a binding interaction that might involve fewer hydrogen bond interactions than E2 (Fig. 7). A Connolly channel, colored blue, which represents the surface of the binding cavity of the ER was constructed, and only the three essential amino acids within the binding cavity are presented. In Fig. 7A, these are arginine 394 and glutamate 353 (left panel), with histidine 524 [right panel, from pdb:1ERE (ERα)]. At the same time [Fig. 7B, pdb:2×7R (ERβ)], the same conserved amino acids are arginine 301 and glutamate 260 (Fig. 7, left panel), with histidine 430 (Fig. 7, right panel). Within Fig. 7A, it is possible to see glycinol makes the same interactions with glutamate 353 and histidine 524 of ERα as does E2. However, this simulation suggests that glycinol may not interact with arginine 394 or the water molecule within the binding cavity. Figure 7B represents how glycinol can make the same interactions with glutamate 260 and histidine 430 of ERβ as does E2, whereas interactions with arginine 301 and the water molecule are excluded as observed in the ERα docking model. The docking simulations suggest that glycinol may bind the ERα and ERβ with different ligand conformations. The ERα docking pose maintains the more flat glycinol conformation, whereas the best ERβ docking pose takes on the bent conformation. Thus, glycinol docking simulations result in a slightly higher interaction score for ERα binding (5.29) than for ERβ binding (4.63). If realistic, these receptor subtype-specific binding modes may contribute to the subtype-specific activity observed in cells.
Figure 7.
Glycinol docked to ERα and ERβ. A, Glycinol bound to ERα with endogenous estrogen, E2. Conserved amino acids arginine (ARG) 394 and glutamate (GLU) 353 (left) with a water molecule hydrogen binds to glycinol, whereas histidine (HIS) 524 (right) hydrogen bonds to a hydroxyl of glycinol. E2 is pictured in magenta as found within the crystal structure of ERα, and glycinol is atom colored. B, Glycinol bound to ERβ with endogenous estrogen E2. Amino acids arginine 301 and glutamate 260 (left) with a water molecule hydrogen binds to glycinol, whereas histidine 430 (right) hydrogen bonds to a hydroxyl of glycinol. E2 is pictured in magenta as found within the crystal structure of ERβ, and glycinol is atom colored.
Discussion
Soy is an excellent source of the constitutive isoflavones daidzein, genistein, and glycitein. Numerous studies have been conducted detailing the estrogenic and antiestrogenic activities of isoflavones and their many health-promoting properties (1,2,3,4,5,6,7,8,9,10). Isoflavones mimic the shape and polarity of the steroid hormone estradiol, and their structural similarity leads to the competitive binding of isoflavones with estradiol for the ERs. Recently after concerns were raised regarding the risks of hormone therapy, there has been increased interest in identifying new natural plant-derived agents to treat menopausal symptoms. This research has led to the discovery of many new estrogenic and antiestrogenic natural compounds derived from soy and other plants. The induced soy glyceollins displayed antiestrogenic activity in both in vitro and in vivo experiments using both breast and ovarian cancer cells (19,20,21). The discovery of an inducible isoflavone with selective ER modulator activity has led to the identification of a new soy phytoalexin, glycinol with estrogenic activity. Glycinol accumulates in high concentrations in soybean under conditions of stress or elicitor treatment. However, little is known about the hormonal effects of glycinol in mammalian systems. Therefore, glycinol was examined in a variety of hormone-responsive systems and, in contrast to the glyceollins, demonstrated potent estrogenic effects in each system.
Studies with MCF-7 cells showed glycinol-induced gene transactivation and proliferation when administered in a dose-dependent manner. Glycinol induced a high level of ER transactivation between 100 nm and 10 μm, and significant MCF-7 cell proliferation was observed at 1 μm. Compared with earlier results, this observed estrogenic activity is greater than that observed with daidzein and genistein and slightly lower than that observed for coumestrol (19).
Consistent with previous results, genistein, coumestrol, and daidzein demonstrated a dose-dependent activation of estrogen response in MCF-7 cells (19), with coumestrol showing the greatest activity (90% at 100 nm) followed by genistein (110% at 1 μm) and daidzein (150% at 10 μm).
Genistein binds both ERα and ERβ; however, it preferentially binds ERβ. Its binding affinity to ERα is only 4% compared with E2, whereas the affinity to ERβ is 87% (38). The relative affinities of glycinol for ERα and ERβ were calculated by dividing the IC50 of unlabeled E2 by the IC50 of glycinol and then multiplying by 100 (E2 arbitrarily set to 100%). The relative binding affinity of glycinol calculated was 27.2% for ERβ and 17.9% for ERα. This relative binding affinity is comparable with the binding affinity of coumestrol. In earlier work by Nikov et al. (39), the relative binding affinity of coumestrol calculated was 34% for ERβ and 12% for ERα. The binding affinity of another isoflavone, genistein, has even greater affinity for ERβ.
Several phytoalexins containing the pterocarpan structure are synthesized by soybean tissues when treated with elicitors or during stress and insect damage. Glyceollins I, II, and III in addition to smaller amounts of glyceollin IV, glyceollidin I, and glyceollidin II (glyceocarpin) are used for plant defense. We have shown that glyceollins I, II, and III all bind to the ER and display antagonist activity using both in vitro and in vivo assays in the presence of E2. Further research in our laboratory has determined that glyceollin I is the most active antagonist among the three glyceollin isomers tested. Glycinol also accumulates in elicitor-treated soybean and is a precursor in the biosynthetic pathway of all the glyceollins. Glycinol is derived from daidzein via a pterocarpan by cyclization and 6α-hydroxylation with retention of configuration (18,40). For the synthesis of the glyceollins, glycinol is prenylated to produce glyceollidin I and II, followed by cyclization to the corresponding glyceollin (41). The prenylation and cyclization steps are key in converting the estrogenic pterocarpan glycinol into the antiestrogenic pterocarpans glyceollins. These structural changes affect the binding within the ER pocket, and our modeling data present ligand receptor models to describe this difference in activity.
Other isoflavonoids are inducibly formed and synthesized in greater amounts by stressed soy, including daidzein, isoformononetin, glyceofuran, and coumestrol. Coumestrol is a coumestan-derived phytoalexin present in several legumes, certain foods, and forages (31,42,43,44,45). Coumestrol has been well characterized for its estrogenic activity (46,47,48,49) because it interacts with ERs in vertebrates and acts as an ER agonist (49,50). The IC50 value of coumestrol is 109 nm for ERα and 35 nm for ERβ (39). These IC50 values are higher than those of glycinol: 13.75 nm for ERα and 9.05 nm for ERβ, suggesting glycinol binds ERα and ERβ with higher affinity compared with coumestrol.
The ability of glycinol to increase the expression of an ERE-LUC reporter gene in MCF-7 cells suggests that it may exert its estrogenic activity, at least in part, via an ERE-dependent mechanism. In this context, we confirmed that glycinol up-regulates endogenous E2-responsive gene transcription, i.e. SDF-1 and PgR in a dose-dependent manner. Glycinol’s ability to up-regulate gene transcription was inhibited by the pure ER antagonist fulvestrant and colony assays demonstrated that fulvestrant inhibited the estrogenic effects of glycinol. It was therefore concluded that the agonist effects of glycinol were mediated primarily through ER.
When glycinol is docked to ERα or ERβ and compared with E2, it does fit with the binding cavity and can interact with key residues. However, these models suggest that glycinol does not share the same hydrogen bonding network as E2. The docking simulations also suggest that glycinol may bind ERα and ERβ with different ligand ring conformations that this differential binding mode may be the basis for ER subtype-specific effects observed in cells. Taken together these results demonstrate for the first time the isolation of a novel phytoalexin. We demonstrate glycinol to be estrogenic in breast cancer cells and may prove beneficial for patients who may require hormone replacement therapy.
Footnotes
This work was supported by the National Institutes of Health (NIH) Digestive Kidney Disease Research Grant DK 059389 (to M.E.B), Susan G. Komen Breast Cancer Foundation (BCTR0601198) to M.E.B., NIH Research Supplements to Promote Diversity in Health-Related Research (to M.E.B. and S.L.T.), NIH National Research Service Award Training Grant T32-HL-07973-05 (to S.L.T), Office of Naval Research N00014-06-1-1136 (to J.A.M. and M.E.B.), and United States Department of Agriculture 59-6435-7-188 (to J.A.M. and M.E.B.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 30, 2008
Abbreviations: CS, Charcoal stripped; DMSO, dimethylsulfoxide; E2, estradiol; ER, estrogen receptor; ERE, estrogen response element; FBS, fetal bovine serum; PgR, progesterone receptor; RBA, relative binding affinity; SDF, stromal-cell-derived factor.
References
- Adlercreutz H 1990 Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest 50:3–23 [PubMed] [Google Scholar]
- Wu AH, Ziegler RG, Horn-Ross PL, Nomura AM, West DW, Kolonel LN, Rosenthal JF, Hoover RN, Pike MC 1996 Tofu and risk of breast cancer in Asian-Americans. Cancer Epidemiol Biomarkers Prev 5:901–906 [PubMed] [Google Scholar]
- Fournier DB, Erdman Jr JW, Gordon GB 1998 Soy, its components, and cancer prevention: a review of the in vitro, animal and human data. Cancer Epidemiol Biomarkers Prev 7:1055–1065 [PubMed] [Google Scholar]
- Baird DD, Umbach DM, Lansdell L, Hughes CL, Setchell KD, Weinberg CR, Haney AF, Wilcox AJ, McLachlan JA 1995 Dietary intervention study to assess estrogenicity of dietary soy among postmenopausal women. J Clin Endocrinol Metab 80:1685–1690 [DOI] [PubMed] [Google Scholar]
- Adlercreutz H 1995 Phytoestrogens: epidemiology and a possible role in cancer protection. In: McLachlan JA, Korach KS, eds. Estrogens in the environment, III: global health implications. Environ Health Perspect 103(Suppl 7):103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham DM, Gardner CD, Haskell WL 1998 Clinical review 97: potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab 83:2223–2235 [DOI] [PubMed] [Google Scholar]
- Murkies AL, Wilcox G, Davis SR 1998 Clinical review 92: phytoestrogens. J Clin Endocrinol Metab 83:297–303 [DOI] [PubMed] [Google Scholar]
- Cline JM, Hughes CL 1998 Phytochemicals for the prevention of breast and endometrial cancer. In: Foon KA, Muss HB, eds. Biological and hormonal therapies of cancer. Boston: Klewer Academic Publishers; 107–134 [DOI] [PubMed] [Google Scholar]
- Humfrey CDN 1998 Phytoestrogens and human health effects: weighing up the current evidence. Nat Toxins 6:51–59 [DOI] [PubMed] [Google Scholar]
- Bingham SA, Atkinson C, Liggins J, Bluck L, Coward A 1998 Phyto-estrogens: where are we now? Br J Nutr 79:393–406 [DOI] [PubMed] [Google Scholar]
- Coward L, Smith M, Kirk M, Barnes S 1998 Chemical modification of isoflavones in soyfoods during cooking and processing. Am J Clin Nutr 68:1486S–1491S [DOI] [PubMed] [Google Scholar]
- Dakora FD, Phillips DA 1996 Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol Mol Plant Physiol 49:1–20 [Google Scholar]
- Darvill AG, Albersheim P 1984 Phytoalexins and their elcitors—a defense against microbial infection in plants. Ann Rev Plant Physiol 35:243–275 [Google Scholar]
- Graham TL, Kim JE, Graham MY 1990 Role of constitutive isoflavone conjugates in the accumulation of glyceollin in soybean infected with Phytophthora megasperma. Mol Plant Microbe Interact 3:157–166 [Google Scholar]
- Paxton JD 1991 Biosynthesis and accumulation of legume phytoalexins in Mycotoxins and Phytoalexins. In: Sharma RP, Salunkhe DK, eds. Boca Raton, FL: CRC Press; 485–499 [Google Scholar]
- Rivera-Vargas LI, Schmitthenner AF, Graham TL 1993 Soybean flavonoid effects on and metabolism by Phytophthora sojae. Phytochem 32:851–857 [Google Scholar]
- Graham TL, Graham MY 1991 Glyceollin elicitors induce major but distinctly different shifts in isoflavonoid metabolism in proximal and distal soybean cell populations. Mol Plant Microbe Interact 4:60–68 [Google Scholar]
- Banks SW, Dewick PM 1983 Biosynthesis of glyceollins I, II, and III in soybean. Phytochemistry 22):2729–2733 [Google Scholar]
- Burow ME, Boué SM, Collins-Burow BM, Melnik LI, Duong BN, Carter-Wientjes CH, Li S, Wiese TE, Cleveland TE, McLachlan JA 2001 Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor α and β. J Endocrinol 86:1750–1758 [DOI] [PubMed] [Google Scholar]
- Salvo VA, Boué SM, Fonseca JP, Elliott S, Corbitt C, Collins-Burow BM, Curiel TJ, Srivastav SK, Shih BY, Carter-Wientjes C, Wood CE, Erhardt PW, Beckman BS, McLachlan JA, Cleveland TE, Burow ME 2006 Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res 12:7159–7164 [DOI] [PubMed] [Google Scholar]
- Wood CE, Clarkson TB, Appt SE, Franke AA, Boué SM, Burow ME, McCoy T, Cline JM 2006 Effects of soybean glyceollins and estradiol on postmenopausal female monkeys. Nutr Cancer 56:74–81 [DOI] [PubMed] [Google Scholar]
- Moesta P, Hahn MG, Grisebach H 1983 Development of a radioimmunoassay for the soybean phytoalexin glyceollin I. Plant Physiol 73:233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez WS, Martins D, Garcez FR, Marques MR, Pereira AA, Oliveira LA, Rondon JN, Peruca AD 2000 Effect of spores of saprophytic fungi on phytoalexin accumulation in seeds of frog-eye leaf spot and stem canker-resistant and -susceptible soybean (Glycine max L.) cultivars. J Agric Food Chem 48:3662–3665 [DOI] [PubMed] [Google Scholar]
- Weinstein LI, Hahn MG, Albersheim P 1981 Host-Pathogen Interactions XVIII. Isolation and biological activity of glycinol, a pterocarpan phytoalexin synthesized by soybeans. Plant Physiol 69:358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyne RL, Mulheirn LH 1978 Minor pterocarpinoids of soybean. Tetrahedron Lett 34:3127–3128 [Google Scholar]
- Weinstein LI, Albersheim P 1983 Host-Pathogen Interactions XXIII. The mechanism of the antibacterial action of glycinol, a pterocarpan phytoalexin synthesized by soybeans. Plant Physiol 72:557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Moco S, Boeren S, de Vos C, Bovy A 2005 Isolation and identification of glycinol form glycine max [L.] Merri. Chin J Chromatogr 23:353–357 [PubMed] [Google Scholar]
- Burow ME, Tang Y, Collins-Burow BM, Krajewski S, Reed JC, McLachlan JA, Beckman BS 1999 Effects of environmental estrogens on tumor necrosis factor α-mediated apoptosis in MCF-7 cells. Carcinogenesis 20:2057–2061 [DOI] [PubMed] [Google Scholar]
- Klotz DM, Beckman BS, Hill SM, McLachlan JA, Walters MR, Arnold SF 1996 Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect 104:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger R, Wiese TE, Ervin K, Nestich S, Checovich W 1998 Rapid screening of environmental chemicals for estrogen receptor binding capacity. Environ Health Perspect 106:551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewick PM, Barz W, Griesbach H 1970 Biosynthesis of coumestrol in Phaseolus aureus. Phytochemistry 9:775–783 [Google Scholar]
- Boué SM, Carter CH, Ehrlich KC, Cleveland TE 2000 Induction of the soybean phytoalexins coumestrol and glyceollin by Aspergillus. J Agric Food Chem 48:2167–2172 [DOI] [PubMed] [Google Scholar]
- Hall JM, Korach KS 2003 Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol 17:792–803 [DOI] [PubMed] [Google Scholar]
- Collins-Burow BM, Burow ME, Duong BN, McLachlan JA 2000 The estrogenic and antiestrogenic activities of flavonoid phytochemicals through estrogen receptor binding dependent and independent mechanisms. Nutr Cancer 38:229–244 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA 1997 Comparison of the ligand binding specificity and transcript distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S 1998 Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists. Mol Pharmacol 54:105–112 [DOI] [PubMed] [Google Scholar]
- McInerney EM, Weis KE, Sun J, Mosselmam S, Katzenellenbogen BS 1998 Transcription activation by the human estrogen receptor subtype β (ERβ) studied with ERβ and ERα receptor chimeras. Endocrinology 139:4513–4522 [DOI] [PubMed] [Google Scholar]
- Wolters M, Hahn A 2004 Soy isoflavones—a therapy for menopausal symptoms? Wien Med Wochenschr 154:334–341 [DOI] [PubMed] [Google Scholar]
- Nikov G, Hopkins NE, Boue S, Alworth W 2000 Interactions of dietary estrogens with human estrogen receptors and the effect of estrogen receptor-estrogen response element complex formation. Environ Health Perspect 108:867–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann ML, Heller W, Grisebach H 1984 Induction of phytoalexin synthesis in soybean. Stereospecific 3,9-dihydropterocarpan 6a-hydrolase from elicitor-induced soybean cell cultures. Eur J Biochem 142:127–131 [DOI] [PubMed] [Google Scholar]
- Zähringer U, Schaller E, Grisebach H 1981 Induction of phytoalexin synthesis in soybean. Structure and reactions of naturally occurring and enzymatically prepared prenylated pterocarpans from elicitor-treated cotyledons and cell cultures of soybean. Z. Naturforsch 36:234–241 [Google Scholar]
- Oldfield JE, Fox CW, Bahn AV, Bickoff EM, Kohler GO 1966 Coumestrol in alfalfa as a factor in growth and carcass quality in lambs. J Anim Sci 25:167–174 [DOI] [PubMed] [Google Scholar]
- Wong E, Latch GCM 1971 Coumestans in diseased white clover. Phytochemistry 10:466–468 [Google Scholar]
- O'Neil M, Adesnaya SA, Roberts MF, Pantry I 1986 Inducible isoflavonoids from the lima bean, Phaseolus lunatus. Phytochemistry 25:1315–1332 [Google Scholar]
- Gutendorf B, Westendorf J 2001 Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology 166:79–89 [DOI] [PubMed] [Google Scholar]
- Bickoff EM, Livingston AL, Hendrickson AP, Booth AN 1962 Relative potencies of several estrogen-like compounds found in forages. J Agric Food Chem 10:410–412 [Google Scholar]
- Dodge JA, Glaesbbrook AL, Magee DE, Philips DL, Sato M, Short L, Bryant HU 1996 Environmental estrogens: effect of cholesterol lowering and bone in the ovariectomized rat. J Steroid Biochem Mol Biol 59:155–161 [DOI] [PubMed] [Google Scholar]
- Martin PM, Horwitz KB, Ryan DS, McGuire WL 1978 Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology 103:1860–1867 [DOI] [PubMed] [Google Scholar]
- Whitten PL, Patisaul HB, Young LJ 2002 Neurobehavioral actions of coumestrol and related flavonoids in rodents. Neurotoxicol Teratol 24:47–54 [DOI] [PubMed] [Google Scholar]
- Markaverich BM, Webb B, Densmore CL, Gregory RR 1995 Effects of coumestrol on estrogen receptor function and uterine growth in ovariectomized rats. Environ Health Perspect 103:574–581 [DOI] [PMC free article] [PubMed] [Google Scholar]