Abstract
This study questioned whether the mechanisms of resistance to antiestrogens differ when acquired under premenopausal (Pre-M) vs. postmenopausal (PM) conditions and whether structurally diverse antiestrogens induce adaptation of differing signaling pathways. To address this issue, we conducted systematic studies under Pre-M vs. PM culture conditions with long-term exposure to different antiestrogens and examined the resultant “specific biologic signatures” of the various resistant cells. Estradiol stimulated growth and inhibited apoptosis of “pre-menopausal” antiestrogen-resistant cells but exerted opposite effects on their “post-menopausal” counterparts. Under Pre-M conditions, tamoxifen (TAM)-resistant cells exhibited a marked translocation of estrogen receptor α from the nucleus into the cytoplasm, whereas this occurred to a lesser extent under PM conditions. MCF-7 cells exposed to PM but not Pre-M conditions exhibited up-regulation of basal epidermal growth factor (EGF) receptor (EGFR) levels, an effect exaggerated in cells exposed to 4-hydroxytamoxifen. Differing effects occurred in response to structurally divergent antiestrogens. Long-term treatment with both 4-hydroxytamoxifen and ICI182,780 increased EGFR levels, but this was not seen in response to TAM. Surprisingly, EGF administration slightly increased cell number in TAM-resistant cells, whereas only increasing cell weight and decreasing cell number in EGFR overexpressing-resistant cells. To assess potential differences among various parental cell lines, we induced resistance in cell lines obtained from other laboratories and confirmed the results from our own parental cells with minor differences. Together, these data demonstrate that culture of breast cancer cells under Pre-M and PM conditions and structurally diverse antiestrogens results in adaptive responses with differing biological signatures.
Phenotypes of acquired resistance to anti-estrogen therapy in hormone-dependent breast cancer cells are determined by levels of estrogens in culture media.
Women with hormone-dependent breast cancer initially respond to antiestrogens but later relapse on continuing treatment, a major therapeutic problem. Human breast cancer cells have a remarkable ability to adapt to the environment in which they are grown and to up-regulate pathways to allow circumvention of any form of imposed growth inhibition. Any changes in adaptive pressure may cause different pathways to be modulated to facilitate continued growth. In an attempt to determine the mechanistic causes of acquired resistance, numerous in vitro models using long-term exposure to antiestrogens have been established (1,2,3,4). The parental cells and culture methods used to develop resistant cell lines have varied substantially among laboratories as have the results reported (5,6,7,8,9). During careful examination of these data, we noted that these in vitro studies had not questioned whether the mechanisms of resistance differ under premenopausal (Pre-M) vs. postmenopausal (PM) conditions, thus providing a partial explanation for the differences observed. Our previous studies had provided preliminary data suggesting that exposure to a low estrogen environment dramatically alters estrogen receptor (ER) levels and functionality (10,11,12). We postulate that the estrogen milieu in which cells develop resistance might be critically important because the mechanisms causing relapse during tamoxifen (TAM) therapy in women might differ substantially between Pre-M and PM women.
In Pre-M women treated with TAM, total plasma estrogen levels are stimulated through the negative feedback effects of this agent on the hypothalamic/pituitary/ovarian axis, and estradiol (E2) reaches levels as high as 1000–2000 pmol/liter (300–600 pg/ml) (13). In PM women, negative feedback from ovarian steroidogenesis is not operative, and estrogen levels remain at levels of 2–5 pg/ml when measured by ultrasensitive and highly specific assays (14,15,16). We postulated that these major differences in circulating hormone levels could result in substantial alterations in the specific adaptive mechanisms leading to antiestrogen resistance. To examine this possibility, it was necessary to use in vitro culture conditions that simulated the Pre-M and PM hormonal status. We reasoned that media containing charcoal-stripped serum would mimic PM conditions and nonstripped serum, Pre-M. A recent study demonstrated that media containing 5% fetal bovine serum (FBS) resulted in E2 concentrations of 0.2 nmol/liter (i.e. 60 pg/ml) (17). This resulted from the fact that conjugated estrogens in nonstripped serum were enzymatically converted to free estrogen and produced higher than the levels expected from 5% serum (18). In addition, phenol red can act as an estrogen in tissue culture media and exerts effects similar to approximately 0.3 nmol/liter (i.e. 90 pg/ml) E2 (19,20). Accordingly, culture medium containing 5% nonstripped serum and phenol red contains estrogenic activity equivalent to 150 pg/ml E2. The average E2 in Pre-M women sampled daily for 1 month is 130 pg/ml, a similar value (21).
To determine whether the development of antiestrogen resistance differs under Pre-M vs. PM conditions, we conducted systematic studies using several antiestrogens and examined a wide range of cellular properties. The cDNA array analyses had previously indicated major differences among the various antiestrogens, including TAM, 4-hydroxytamoxifen (4-OH TAM), and fulvestrant [ICI182,780 (ICI)], suggesting that mechanistic effects of various antiestrogens differ substantially (22,23,24). A major mediator of TAM action is 4-OH TAM, which has had a similar affinity for the ERα as E2 (25). However, relative binding assays have shown that the affinity of antiestrogens for ERα is not always representative of their inhibitory activity (26). The ability of estrogen and antiestrogens to manifest divergent biological activities in different cells is determined in part by differential ERα conformational changes (26,27,28). Finally, ICI clearly has a different mechanism of action from other antiestrogens because it acts both by binding to ERα but also by enhancing its rate of degradation (29).
Prior studies of antiestrogen-resistant cells reported divergent results with respect to growth factor and receptor expression. Our previous findings suggested that an increased association between ERα and epidermal growth factor (EGF) receptor (EGFR) represented an important mechanism underlying TAM resistance (1). Other data also suggested that bidirectional cross talk between ERα and EGFR signaling pathways regulated growth of TAM-resistant (TAM-R) cells (30,31). For this reason, our studies focused primarily on EGFR-mediated pathways but also examined more global biological signatures.
Based upon the considerations discussed previously, we sought to determine whether various antiestrogens resulted in differential patterns of resistance when examined under Pre-M vs. PM conditions. Accordingly, we have performed a comprehensive analysis of the mechanisms of resistance occurring during long-term TAM, 4-OH TAM, and ICI exposure. The results of these studies clearly demonstrated that the adaptive changes induced by various antiestrogens differ when comparing cells grown under Pre-M and PM conditions. Key factors in this process are the altered functionalities of the E2/ER and EGF/EGFR pathways. Together, these data suggest that systematic studies now need to be performed in women to determine whether the mechanisms of antiestrogen resistance similarly differ in Pre-M and PM women.
Materials and Methods
Materials
TAM and 4-OH TAM were purchased from Sigma-Aldrich Corp. (St. Louis, MO), E2 from Steraloids, Inc. (Whilton, NH), EGF from Collaborative Biomedical Products (Bedford, MA), and ICI 182,780 from Tocris (Park Ellisville, MO). Sources of antibodies for Western analysis are as follows: ERα antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); and EGFR sheep polyclonal antibody (06-129) was from Upstate Biotechnology Inc. (Lake Placid, NY). Total MAPK antibody was purchased from Zymed Laboratories, Inc. (South San Francisco, CA). The phosphorylated MAPK antibody was from Cell Signaling Technologies (Beverly, MA). Secondary antibodies conjugated with horseradish peroxidase were purchased from GE Healthcare Bio-Sciences Corp. (Piscataway, NJ). Alexa Fluor 568 phalloidin and Alexa Fluor 488 goat antirabbit IgG were purchased from Molecular Probes, Inc. (Eugene, OR). All chemicals were obtained from Sigma-Aldrich. Wild-type MCF-7 cells were kindly provided by Dr. R. Bruggemeier (Ohio State University, Columbus, OH) and routinely grown in phenol red supplemented, improved MEM (IMEM) containing 5% FBS.
Cell culture conditions
Rationale
To mimic Pre-M and PM conditions, we cultured cells in “high” and “low” estrogen environments. Pre-M plasma levels of total E2 range from 40–600 pg/ml (early follicular to midcycle peak), and over 1 month of daily measurements average approximately 130 pg/ml (21). PM plasma, in contrast, contains 50- to 75-fold lower levels of total E2 (i.e. 2–5 pg/ml) when measured by ultrasensitive bioassays or gas chromatography/tandem mass spectrometry assays (GC/MS/MSs) (14,15,16). Recent studies of culture media used to grow MCF-7 cells reported the levels of E2 to be 60 pg/ml when medium contained 5% FBS. As shown by our previous data, this resulted from the conversion of estrogen sulfates to free estrogen by sulfatase and conversion of estrone to E2 by 17β-hydroxysteroid dehydrogenase in MCF-7 cells (18).
Pre-M culture conditions
Based on the data reviewed previously, medium containing 5% FBS and phenol red provides the biological equivalent of E2 activity of 150 pg/ml, which mimics the plasma estrogen levels in Pre-M women. This calculation considers the activities of sulfatase and 17β-hydroxysteroid dehydrogenase, but not aromatase, whose levels are very low in MCF-7 cells (32).
PM culture conditions
Media containing charcoal-stripped serum and lacking phenol red contain at least 50- to 75-fold lower levels of E2. Precise quantification of the exact levels is difficult because of limitation of assay sensitivity. Based on these considerations, we considered charcoal-stripped media lacking phenol red to mimic PM conditions.
Exposure to antiestrogens
Cells were exposed to various antiestrogens for 3–24 months under Pre-M or PM culture conditions. The concentrations of antiestrogens used were based on those reported by other investigators (1,2,3,4,5,6,7,8,9). Using Pre-M or PM culture conditions, our MCF-7 cells were continuously exposed to TAM (10−7 mol/liter), 4-OH TAM (10−7 mol/liter), ICI (10−8 mol/liter), or 0.1% ethanol as a vehicle control, respectively, until they were resistant to each antiestrogen. We also studied MCF-7 TAM-R cells kindly provided by Dr. Rachel Schiff (Baylor College of Medicine, Houston, TX). This cell line was originally generated by Dr. Planas-Silva (Penn-State College of Medicine, Hershey, PA). Another wild-type MCF-7 cell line was from Dr. Nicholson’s laboratory (Tenovus Center for Cancer Research, UK). A uniform nomenclature to describe these cells, their culture conditions, and properties is in Table 1.
Table 1.
Information on cell lines used in this paper
| Sources | Cell name | Culture conditions | Properties |
|---|---|---|---|
| Santen laboratory | TAM-R Pre-M | Pre-M | TAM resistant |
| TAM-R PM | PM | TAM resistant | |
| 4-OHT-R Pre-M | Pre-M | 4-OH TAM resistant | |
| 4-OHT-R PM | PM | 4-OH TAM resistant | |
| ICI-R Pre-M | Pre-M | ICI resistant | |
| ICI-R PMa | PM | ICI resistant | |
| Nicholson laboratory | 4-OHT-Rn Pre-Mb | Pre-M | 4-OH TAM resistant |
| 4-OHT-Rn PM | PM | 4-OH TAM resistant | |
| Planas-Silva laboratory | TAM-Rp PM | PM | TAM resistant |
ICI-R PM cells are in progress and will not be fully described in this paper.
4-OHT-Rn Pre-M resistant cells are established in the Santen laboratory.
General properties of cell lines
Doubling times of TAM and 4-OH TAM-resistant (4-OHT-R) cells were slightly longer than that of control cells, whereas doubling times of ICI-resistant (ICI-R) cells were much longer than control cells, especially in the early stage. The duration of time necessary for the development of resistance differed for each antiestrogen treatment. TAM, 4-OH TAM, and ICI resistance occurred after 1 yr, 6 month, and 3 month continuous treatment, respectively.
Growth assays
Cells were plated in six-well plates at a density of 60,000 cells per well. Two days later, the cells were treated as described in the figure legends. At the end of treatment, cells were rinsed twice with saline. Nuclei were prepared and counted using a Coulter counter (Beckman Coulter, Inc., Fullerton, CA) as described in Ref. 1.
Cell weight assays
Cells were plated in two six-well plates and treated as for cell counting. At the end of treatment, one plate was used for cell counting as described previously. Cells in the other plate were lysed in 0.1 n sodium hydroxide solution, and protein concentrations of the lysates were determined using the Lowry method. Assuming that cell weight is proportional to protein concentration, cell weight was expressed as microgram protein per 100,000 cells (μg/100,000 cells).
Immunoblotting
The detailed method was described in Ref. 1.
Immunofluorescent microscopy
Cells grown on coverslips in six-well plates were fixed in 4% paraformaldehyde at room temperature for 20 min. Cells were permeabilized in cold (−20 C) acetone for 2–4 min. After background blocking with 5% normal goat serum, the cells were incubated with the anti-ERα antibody (H-184) overnight at 4 C, followed by 1 h incubation with Alexa Fluor 488-labeled secondary antibody at room temperature and counterstaining of F actin with Alexa Fluor 568-labeled phalloidin. The cells incubated with secondary antibody only served as the control for antibody specificity. Immunofluorescent images were captured using a Zeiss immunofluorescent microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
Apoptosis ELISA assay
Apoptosis was measured using the Cell Death Detection ELISA kit (Roche Diagnostics Corp., Indianapolis, IN) according to our previously published and extensively characterized method (11).
Subcellular protein fractionation
Plasma membrane protein was extracted using Mem-PER Eukaryotic Membrane Protein Extraction Reagent kit, and nuclear and cytoplasmic proteins were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents kit following the manufacturer’s instruction. The kits were purchased from Pierce (Rockford, IL).
Statistical analysis
All reported values are the means ± se. Statistical comparisons were determined with two-tailed Student’s t tests. Results were considered statistically significant if the P value was less than 0.05.
Results
Specific biological signatures of MCF-7 cells cultured under Pre-M and PM conditions
We initially questioned whether MCF-7 cells alter their responses to E2 under Pre-M vs. PM culture conditions and systematically examined a series of characteristics measuring the various properties outlined in supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. For clarity of presentation, only key differences are described in Results.
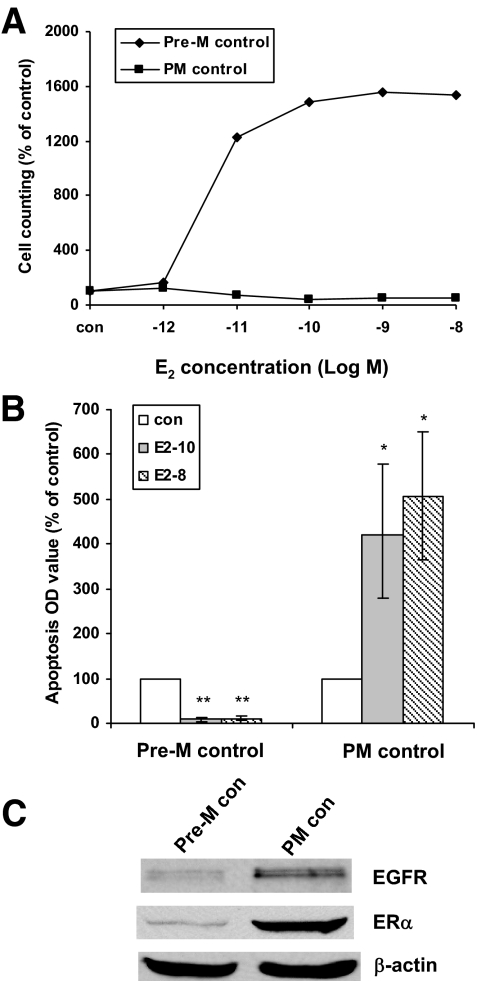
Under Pre-M conditions, E2 stimulated cell growth and blocked apoptosis. However, in marked contrast, E2 inhibited growth and enhanced apoptosis under PM conditions (Fig. 1, A and B). In addition, ERα levels increased markedly, and EGFR expression level slightly increased under PM compared with Pre-M conditions (Fig. 1C). All other parameters did not differ between Pre-M and PM cells (supplemental Fig. S1). Together, these data demonstrated that some of the biological signatures of MCF-7 cells differ when they were grown under Pre-M vs. PM conditions.
Figure 1.
Baseline differences in Pre-M and PM control (con) cells. Parental MCF-7 cells treated with vehicle ethanol in Pre-M and PM conditions. A, Pre-M control and PM control cells were treated with different concentrations of E2 in 5% dextran-coated charcoal-stripped serum (DCC) IMEM phenol red-free medium for 5 d. B, Apoptosis was detected by ELISA kit. Pre-M control and PM control cells were plated in a 12-well plate, respectively, 2 d later the cells were treated with different concentrations of E2 in 5% dextran-coated charcoal-stripped serum (DCC) IMEM phenol red-free medium for 72 h. *, P < 0.05 vs. PM control cells; **, P < 0.001 vs. Pre-M control cells. C, Cell lysates were prepared from Pre-M and PM control cells. EGFR and ERα were examined by immunoblotting with primary antibodies against them, respectively. Immunoblotting for β-actin was detected for loading control.
Specific biological signatures induced by three antiestrogens under Pre-M and PM conditions
Several investigative groups have developed antiestrogen-resistant MCF-7 cell lines as a method to identify specific mechanistic pathways involved, but functional characteristics of these cell lines differ in several respects (1,2,3,4,5,6,7,8,9). We postulated that exposure of these cells to Pre-M vs. PM conditions might be the predominant reason for the divergent results and that different antiestrogens might also produce varying effects. To address this issue, we compared the mechanisms of acquired antiestrogen resistance in cells grown long-term under Pre-M and PM conditions. We focused on the altered functionalities of the E2/ERα and EGF/EGFR pathways.
E2 effects on proliferation and apoptosis
Pre-M conditions
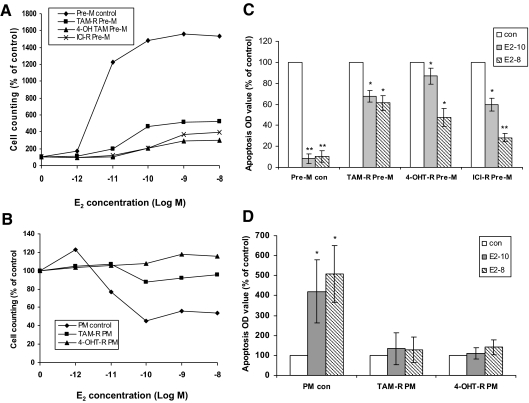
We initially evaluated the effects of three antiestrogens (TAM, 4-OH TAM, and ICI) on the specific biological signatures induced under Pre-M conditions. As shown in Fig. 2A, E2 continued to stimulate cell growth after long-term exposure to each antiestrogen, but the percent increase compared with control cells was diminished by each agent. The antiestrogens caused a shift of E2 dose response curves to the right, indicating reduced sensitivity to E2 (supplemental Fig. S2). This effect was not due to the persistence of antiestrogens in the media or nuclei because similar responses were seen when the experiment was repeated 2 wk after the antiestrogens were extensively washed out (data not shown). Cell number represents an integrated effect of both proliferation and apoptosis. To elucidate whether the different growth responses to E2 of resistant cells cultured in different culture conditions were partly due to differential effects of E2 on apoptosis, we examined this parameter. The apoptosis level was suppressed by E2 treatment under Pre-M conditions (Fig. 2C).
Figure 2.
Differences in specific biological signatures in response to long-term antiestrogen administration. A, Pre-M resistant cells’ response to E2. B, PM resistant cells’ response to E2. Various resistant cells and control (con) cells cultured under Pre-M and PM conditions were treated with different concentrations of E2 in 5% dextran-coated charcoal-stripped serum (DCC) IMEM phenol red-free medium for 5 d, respectively. C, E2 effects on apoptosis in Pre-M resistant cells. Apoptosis was detected by ELISA kit. D, E2 effects on apoptosis in PM resistant cells. Various resistant cells and control cells cultured under PM conditions were plated in a 12-well plate, respectively. 2 d later the cells were treated with different concentrations of E2 in 5% DCC IMEM phenol red-free medium for 72 h. *, P < 0.05 vs. control cells; **, P < 0.01 vs. control cells.
PM conditions
In contrast to cells grown under Pre-M conditions, E2 did not stimulate growth of TAM-R PM and 4-OH TAM-R (4-OHT-R) PM cells. The number of the control cells was reduced by E2 (Fig. 2B). High-dose E2 treatment no longer protected against apoptosis, and showed a trend toward enhancing apoptosis in TAM-R PM and 4-OHT-R PM but without statistical significance (Fig. 2D). ICI-R PM cells also lost the response to E2 (10−9 m) (data not shown).
Basal apoptosis
Although apoptosis induced by estrogen differed in Pre-M and PM-resistant cells, the basal apoptotic levels of all resistant cells were higher than that of corresponding control cells under either Pre-M or PM conditions due to the apoptotic effects of these antiestrogens (supplemental Fig. S3).
ER localization
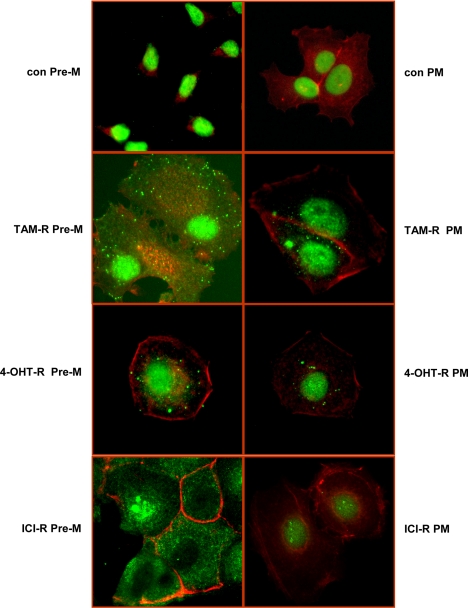
Our previous findings indicated that long-term TAM treatment could cause redistribution of ERα from the nucleus to extranuclear areas, which facilitated its association with EGFR and increased nongenomic function of ERα in TAM-R cells (1). This study was prompted by the need to extend this observation by comparing effects of different antiestrogens on ERα localization.
Pre-M conditions
ERα translocated to the cytoplasm to the greatest extent in TAM-R cells and to a lesser degree in 4-OHT-R cells. ICI could also induce translocation of ERα from the nucleus to extranuclear areas. However, the translocation pattern differed from that induced by TAM and 4-OH TAM, in that ICI resulted in a complete absence of nuclear ERα, which presumably resulted from nuclear receptor degradation (29) (Fig. 3).
Figure 3.
The TAM, 4-OH TAM, and ICI influent on ERα localization in various resistant cells. The various resistant and control (con) cells cultured on coverslips for 2 d were fixed and incubated with anti-ERα antibody overnight at 4 C. After a thorough wash with PBS, the cells were incubated with Alexa Fluor 488-labeled secondary antibody (green) for 1 h. Actin was stained with Alexa Fluor 546-labeled phalloidin (red). Immunofluorescence images were captured under a Zeiss immunofluorescent microscope (×40 objective). Control experiments omitting anti-ERα antibody revealed no background staining.
PM conditions
TAM-R cells still exhibited a redistribution of ERα to the cytoplasm but to a much lower extent than that under Pre-M conditions. 4-OH TAM-treated cells also exhibited relocalization of ERα to the cytoplasm, and this also occurred to a much lesser extent than under Pre-M conditions. There was almost no ERα redistribution in ICI-treated cells under PM conditions. However, nuclear ERα staining was clearly reduced when compared with control cells (Fig. 3).
ER and EGFR expression
Pre-M conditions
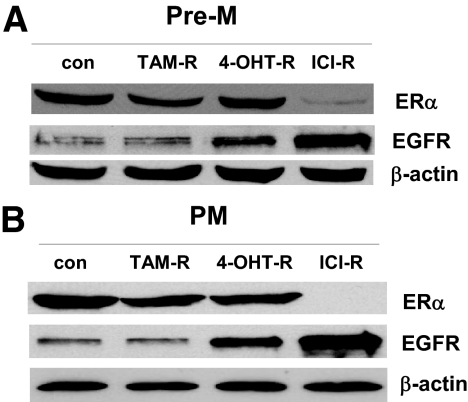
There were no substantial changes in ERα levels in TAM-R and 4-OHT-R cells, but as expected, ERα levels were markedly reduced in ICI-R cells (Fig. 4A). Dramatic differences were observed in EGFR concentrations. EGFR levels were increased in 4-OHT-R and ICI-R cells but were unchanged in TAM-R cells. The ICI-R cells had the highest EGFR level among these three Pre-M-resistant cell lines (Fig. 4A).
Figure 4.
EGFR and ERα expression in various resistant cells. A, Cell lysates were prepared from TAM, 4-OH TAM, and ICI Pre-M resistant cells and control (con) cells. ERα and EGFR were examined by immunoblotting with primary antibodies against them. Immunoblotting for β-actin was detected for loading control. B, Cell lysates were prepared from TAM, 4-OH TAM, and ICI PM resistant cells and matched control cells. ERα and EGFR were examined by immunoblotting with primary antibodies against them. All data were representative of three repeatable experiments.
PM conditions
The ERα levels were down-regulated in the three PM-resistant cells. However, ICI-R had the lowest ERα level. EGFR levels were unchanged in the TAM-R cells but markedly up-regulated in the 4-OHT-R and ICI-R cells (Fig. 4B).
Changes of EGFR functionality
Pre-M conditions
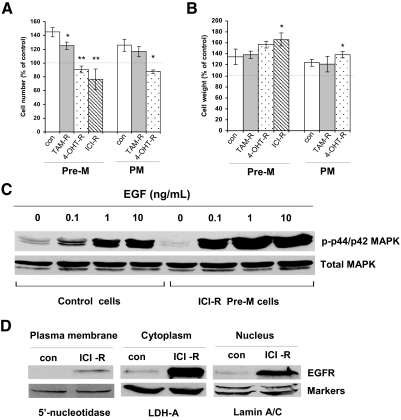
Because different antiestrogen-resistant cells expressed divergent levels of EGFR, we considered it essential to assess the EGF responsiveness of these resistant cells. EGF (100 ng/ml) treatment increased the cell number of TAM-R cells by 20%, an increase lower than that of control cells (40% increase) (Fig. 5A). Although both 4-OHT-R and ICI-R cells overexpressed EGFR, treatment with EGF decreased the cell number by 20 and 50%, respectively. However, EGF did not induce apoptosis in these two cell lines (data not shown). Because the cells on EGF were much lager in size, we measured the protein content as an indicator of cell weight and found that EGF stimulated cell growth by increasing cell weight rather than cell number in 4-OHT-R and ICI-R cells (Fig. 5B). One dramatic difference between the control and ICI-R cells was that ICI-R cells displayed enhanced sensitivity to EGF stimulation of MAPK phosphorylation (Fig. 5C). The EGFR inhibitor AG1478 significantly inhibited cell number in all three resistant cell lines (supplemental Fig. S4). The mechanisms as to why EGF increased cell weight but not cell number in these EGFR overexpression-resistant cells were unclear. Emerging evidence suggests the existence of a new mode of the EGFR signaling pathway in which activated EGFR undergoes nuclear translocalization. It was interesting to find that overexpressed EGFR translocated from cell membrane to cytosol and nuclear areas, which may change the function of traditional EGFR (Fig. 5D).
Figure 5.
Various resistant cells and their response to EGF. A, The cell number changes after EGF treatment. Different resistant and control (con) cells cultured under Pre-M and PM conditions were treated with EGF (100 ng/ml) in 5% Dextran-coated charcoal-stripped serum (DCC) IMEM phenol red-free medium for 5 d, respectively. Cell number was assayed. *, P < 0.05 vs. control cells; **, P < 0.01 vs. control cells. B, The cell weight changes after EGF treatment. Different resistant and control cells cultured under Pre-M and PM conditions were treated with EGF (100 ng/ml) in 5% DCC IMEM phenol red-free medium for 5 d, respectively. Cell weight was assayed. *, P < 0.05 vs. control cells; **, P < 0.01 vs. control cells. C, EGF increased phosphor-MAPK level in EGFR overexpressed-resistant cells. The ICI-R Pre-M and control cells were treated with a different concentration of EGF for 10 min in 5% DCC IMEM phenol red-free medium. Cell lysates were harvested. The total and phosphor-MAPKs were examined by immunoblotting with primary antibodies against them, respectively. D, EGFR was translocated to the cytoplasm and nucleus in EGFR overexpressing-resistant cells. Cell fractionations were isolated from ICI-R Pre-M and control cells, respectively. EGFR was examined by immunoblotting with a primary antibody against it. Immunoblotting for different markers were detected for loading control.
PM conditions
EGF also increased cell number in the TAM-R cells but inhibited this effect in 4-OHT-R cells and at the same time increased cell weight (Fig. 5, A and B). With respect to EGFR functionality, AG1478 caused a substantially greater reduction in cell number in TAM-R and 4-OHT-R cells (supplemental Fig. S4).
Specific signatures in different parental cell lines
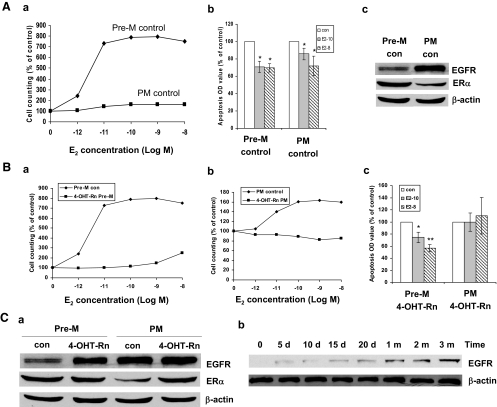
We considered the possibility that differences in parental cell lines might provide an additional explanation for the alterations in specific signatures observed. Accordingly, we subjected a set of parental cells obtained from Dr. Nicholson (MCF-7n) to the same conditions as used with our parental MCF-7 cells, and examined the effect of Pre-M and PM conditions as well as that of different antiestrogens. These cells also changed their biological signature when switched to PM conditions. Similar to our MCF-7 cells, growth responses of MCF-7n PM to E2 were markedly decreased (Fig. 6, Aa and Ab). The major differences in biological signatures between these two parental lines were up-regulation of EGFR and down-regulation of ERα in MCF-7n but not in our MCF-7 cells under PM conditions (Fig. 6Ac).
Figure 6.
Differences in parental cell lines. A, Parental MCF-7n cells treated with vehicle ethanol under Pre-M and PM conditions. a, Pre-M control (con) and PM control cells were treated with different concentrations of E2 in 5% Dextran-coated charcoal-stripped serum (DCC) IMEM phenol red-free medium for 5 d. b, Apoptosis was detected by ELISA kit. Pre-M control and PM control cells were treated with different concentrations of E2 using the same method as described previously. *, P < 0.05 vs. respective control. c, Cell lysates were prepared from Pre-M control and PM control cells. EGFR and ERα were examined by immunoblotting with primary antibodies against them, respectively. Immunoblotting for β-actin was detected for loading control. B, Nicholson’s 4-OHT-Rn cells cultured under Pre-M and PM condition. a, The 4-OHT-Rn Pre-M and control cells were treated with different concentrations of E2 in 5% DCC IMEM phenol red-free medium for 5 d, respectively. b, The 4-OHT-Rn PM and control cells were treated with different concentrations of E2 in 5% DCC IMEM phenol red-free medium for 5 d, respectively. c, Apoptosis was detected by ELISA kit. 4-OHT-Rn Pre-M and 4-OHT-Rn PM cells were treated with different concentrations of E2 in 5% DCC IMEM phenol red-free medium for 72 h. *, P < 0.05 vs. respective control; **, P < 0.01 vs. respective control. C, EGFR and ERα expression in Nicholson’s 4-OH TAM-resistant cells cultured under Pre-M and PM conditions a, Cell lysates were prepared from 4-OHT-Rn Pre-M, 4-OHT-Rn PM, and matched control cells. EGFR and ERα were examined by immunoblotting with primary antibodies against them. b, Cell lysates were prepared from MCF-7n cells treated with 4-OH TAM (10−7mol/liter) for different times under Pre-M conditions. EGFR was examined by immunoblotting with a primary antibody against it.
Exposure to 4-OH TAM also altered the biological signatures of MCF-7n cells by reducing proliferative responses to E2 and abrogating the pro-apoptosis suppressing effect of estrogen, effects similar to those observed in our MCF-7 cells (Fig. 6, Ba–c. There was no change in ERα levels under Pre-M conditions, but ERα levels increased under PM conditions after 4-OH TAM treatment (Fig. 6Ca). The EGFR level was enhanced clearly after 5 d treatment with 4-OH TAM (Fig. 6Cb). EGF effects on proliferation and on cell weight also occurred in a similar fashion as our 4-OHT-R cells (supplemental Fig. S5). These data confirmed the conclusion that different parental cells adapt under PM conditions with some differences, but the antiestrogen-resistant process was similar between different parental cells.
We also compared TAM-R PM with TAM-Rp PM cells. In both cell lines, E2 caused no increase in cell number but induced apoptosis instead. There were no changes in EGFR expression in TAM-Rp PM cells, which was similar to findings in TAM-R PM cells. These additional studies strengthen our conclusion that most, but not all of the adaptive changes, are similar in different parental cell lines (supplemental Fig. S6).
Because we considered our original observation of ERα translocation surprising (1), we wished to confirm this in cells obtained from other laboratories as well. Accordingly, we examined this parameter under the conditions shown in Fig. 3. Only in the Nicholson’s MCF-7 cells treated with 4-OH TAM under PM conditions did the ERα remain in the nucleus (supplemental Fig. S7). However, if these parental cells were long-term treated with TAM, ERα was also translocated out from nucleus to extranuclear areas.
Discussion
Secondary resistance to antiestrogens develops in both Pre-M and PM women treated for advanced hormone-dependent breast cancer. We questioned whether the mechanisms of resistance might involve up-regulation of different pathways under these two conditions because the estrogen milieu differs so substantially. In addition, we queried whether various antiestrogens might cause different adaptive mechanisms leading to resistance. To address these issues, we conducted systematic studies of three different antiestrogens under Pre-M and PM conditions, and used multiple endpoints to define a “specific biologic signature” for each. Substantial adaptive differences were observed when comparing cells grown under Pre-M and PM conditions without addition of antiestrogens. These variances were accentuated by long-term exposure to each antiestrogen. Key differences involved: the up-regulation of EGFR levels, the effects of estrogen on cell proliferation and apoptosis, and EGF on cell weight. In contrast, a nearly uniform hallmark of antiestrogen resistance was the translocation of ERα from the nucleus to the cytoplasm and cell membrane. To assess possible confounding effects of differing parental cells, we reexamined “specific biologic signatures” induced in parental cells obtained from other laboratories. Although minor differences were observed, the major adaptive events occurred because of the differences in estrogen milieu and antiestrogens used. These results provide preclinical support for the hypothesis that the development of resistance to antiestrogens probably differs substantially in Pre-M vs. PM women.
The growth of cells under Pre-M vs. PM conditions without administration of antiestrogens exerted profound effects on adaptive signatures, but additional effects occurred as a result of using different antiestrogens. A major difference reflecting the type of antiestrogen was the pattern of translocation of ERα observed in “pre-menopausal cells” treated with ICI. Here, there was a near absence of ERα in the nucleus but a dramatic enhancement of cytoplasmic and plasma membrane translocation. The reason for the predominant ERα localization in the cytoplasm of ICI-resistant cells is not clear. Several possibilities exist, including resistance to down-regulation in the cytoplasm, disruption of ER nucleo-cytoplasmic shuttling, impairment of receptor dimerization, and increased receptor degradation in the nucleus (29,33,34).
It has been appreciated for many years that MCF-7 cell lines differ as a result of an adaptive “drift” since their initial generation in 1972 (35,36). For this reason we assessed the differences in biological signatures in parental cells obtained from other laboratories (2,3). Although the majority of the biological signature patterns were similar to those of our MCF-7 cells, the Nicholson’s MCF-7n cells grown under PM conditions with 4-OH TAM did not exhibit ER translocation to the cytoplasm. This is the only condition in which ER translocation has not been observed, and we do not currently have a mechanistic explanation for this finding. However, ERα translocation out of the nucleus occurred in the same parental line after long-term treatment with TAM. It implied that TAM and 4-OH TAM had different effects on ER translocation.
In the various published studies examining antiestrogen resistance, the findings regarding expression and function of growth factor receptors were inconsistent. Although up-regulation of growth factor receptor signaling is a common feature of several studies, not all laboratories have found this to be the case. For example, EGFR was reported to be 10-fold higher in one antiestrogen-resistant model (4) but to have no significant change in another (1,37). Our data suggest that this difference may relate to the striking differential effects of TAM compared with 4-OH TAM. The 4-OH TAM-R and ICI-R cells overexpressed EGFR, but this was not seen in TAM-R cells. This was not a dose effect because we got the same result when using a 10-fold higher concentration of TAM, which was supposedly in similar potency as 4-OH TAM (data not shown). To provide further evidence for the hypothesis that overexpression of EGFR resulted from 4-OH TAM but not TAM, we used another parental cell line, Nicholson’s MCF-7n cells, and examined the effects of long-term treatment with TAM or 4-OH TAM under Pre-M or PM conditions, respectively. 4-OH TAM treatment in both Pre-M and PM conditions up-regulated EGFR compared with matched control cells, but the TAM treatment did not (Fig. 6C and supplemental Fig. S8). Notably, enhancement of EGFR could be observed after 5 d treatment with 4-OH TAM when cells were still sensitive to this antiestrogen. This finding suggested that overexpression of EGFR resulted from 4-OH TAM treatment but not TAM treatment. We could further conclude that overexpression of EGFR alone did not result in resistance because this finding temporally occurred before resistance was observed (37). Other results also indicated that overexpression of HER2/neu mRNA alone might not be sufficient to explain TAM-R breast cancer (38). Nonetheless, overexpression of EGFR could promote the more rapid development of resistance and provide an explanation as to why 4-OH TAM and ICI resistance occurred earlier than that due to TAM (Materials and Methods).
To explore further the effects of EGFR, we investigated the functional changes of overexpressed EGFR. Surprisingly, EGF treatment increased cell number in TAM-R cells that had a normal complement of EGFR but decreased cell number in EGFR overexpressing ICI-R cells or 4-OHT-R cells. The decrease in cell number did not result from apoptosis or inhibition of cell growth by EGF, whereas stimulation of MAPK phosphorylation did occur. Notably, EGF increased cell size and caused a statistically significant increase in cell weight. The reasons why EGF increased cell weight but not cell number in these EGFR overexpression-resistant cells were unclear but potentially could reflect mammalian target of rapamycin (mTOR) activation. It was interesting to find that overexpressed EGFR translocated from cell membrane to cytosol and nuclear areas, which may change the function of traditional EGFR (39,40).
A potential problem in our experimental design was that our PM culture media may have contained lower levels of E2 than found in PM women. Charcoal stripping has been widely used to remove steroids from serum for use in culture media, but E2 assays are not sufficiently sensitive to quantify precisely the residual E2 present. We attempted to do this by using an ultrasensitive and specific GC/MS/MS methodology as previously published (14,15,16). With this assay, levels of E2 (<0.625 pg/ml) and E1S (<3.13 pg/ml) were undetectable in charcoal-stripped serum, suggesting that culture media levels were below PM levels. However, measurements in PM women represent total E2, and non-SHBG bound E2 concentrations are even lower. Nonetheless, our results probably reflect the effects of quite low E2 concentrations.
Another problem is that charcoal stripping can lower the levels of growth factors present in sera. This can confound interpretation by suggesting that any resultant effects come about through reduction of E2, whereas growth factor reduction could be responsible. This problem applies to nearly all previous studies using charcoal-stripped serum, a generally standard approach. We have conducted preliminary studies indicating that growth factor deprivation is unlikely to have affected our findings (supplemental Fig. S9).
A more difficult issue is whether breast cancer tissue in PM women contains lower levels of estrogen than in Pre-M women, as would be expected if tumor levels reflected primarily uptake from plasma rather than local synthesis in the tumor. Miller and colleagues (41) provided quantitative data on uptake vs. synthesis using isotopic techniques in breast tumor tissue from PM women. Uptake was the exclusive source of tissue E2 in one third of patients with local synthesis in another third and combined uptake and synthesis in the remainder. Uptake from plasma into the tumor was observed in all subjects with tissue plasma gradients of 2- to 4-fold (41). We had previously conducted a study in nude mice that allowed a quantitative distinction between uptake and local synthesis (42,43). At PM levels of E2, uptake was equal to synthesis as a determinant of tissue E2 level. Increasing concentrations of E2 led to increasing tissue concentrations. Accordingly, with infusion of E2 to achieve Pre-M levels of E2, the E2 levels would be much higher in the tumors than with infusion of PM levels (42,43). Based on this physiology, we would expect breast tumors in Pre-M women to be much higher than in PM women. However, this issue is controversial because several studies demonstrated equivalent levels of E2 in tumors of Pre-M and PM women (44). The absolute levels of E2 differed substantially among studies, and highly specific GC/MS/MSs were not used. Our interpretation of the data is that uptake plays a considerable role in at least a substantial fraction of patients, and that tumor estrogen exposure is likely to differ between Pre-M and PM breast tumors, the thesis underlying this study.
Although these issues represent potential problems in interpretation, they do not invalidate our conclusion that mechanisms of resistance differ when developing in high vs. low estrogen conditions. This previously unrecognized phenomenon highlights the critical importance of considering this factor when studying the development of resistance and the need to consider resistance to antiestrogens to be potentially different in Pre-M vs. PM women, a postulate that now requires intensive testing.
Supplementary Material
Footnotes
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 29, 2009
Abbreviations: E2, Estradiol; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ER, estrogen receptor; FBS, fetal bovine serum; GC/MS/MS, gas chromatography/tandem mass spectrometry assay; ICI, ICI182,780; ICI-R, ICI-resistant; IMEM, improved MEM; 4-OH TAM, 4-hydroxytamoxifen; 4-OHT-R, 4-OH TAM-resistant; PM, postmenopausal; Pre-M, premenopausal; TAM, tamoxifen; TAM-R, TAM-resistant.
References
- Fan P, Wang J, Santen RJ, Yue W 2007 Long-term treatment with tamoxifen facilitates translocation of estrogen receptor α out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res 67:1352–1360 [DOI] [PubMed] [Google Scholar]
- Kilker RL, Hartl MW, Rutherford TM, Planas-Silva MD 2004 Cyclin D1 expression is dependent on estrogen receptor function in tamoxifen-resistant breast cancer cells. J Steroid Biochem Mol Biol 92:63–71 [DOI] [PubMed] [Google Scholar]
- Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI 2003 Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 144:1032–1044 [DOI] [PubMed] [Google Scholar]
- McClelland RA, Barrow D, Madden TA, Dutkowski CM, Pamment J, Knowlden JM, Gee JM, Nicholson RI 2001 Enhanced epidermal growth factor receptor signaling in MCF7 breast cancer cells after long-term culture in the presence of the pure antiestrogen ICI 182,780 (Faslodex). Endocrinology 142: 2776–2788 [DOI] [PubMed] [Google Scholar]
- Pink JJ, Jordan VC 1996 Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res 56:2321–2330 [PubMed] [Google Scholar]
- Katzenellenbogen BS 1991 Antiestrogen resistance: mechanisms by which breast cancer cells undermine the effectiveness of endocrine therapy. J Natl Cancer Inst 83:1434–1435 [DOI] [PubMed] [Google Scholar]
- Brünner N, Boysen B, Jirus S, Skaar TC, Holst-Hansen C, Lippman J, Frandsen T, Spang-Thomsen M, Fuqua SA, Clarke R 1997 MCF7/LCC9: an antiestrogen-resistant MCF-7 variant in which acquired resistance to the steroidal antiestrogen ICI 182,780 confers an early cross-resistance to the nonsteroidal antiestrogen tamoxifen. Cancer Res 57:3486–3493 [PubMed] [Google Scholar]
- Lykkesfeldt AE, Madsen MW, Briand P 1994 Altered expression of estrogen-regulated genes in a tamoxifen-resistant and ICI 164,384 and ICI 182,780 sensitive human breast cancer cell line, MCF-7/TAMR-1. Cancer Res 54:1587–1595 [PubMed] [Google Scholar]
- Osborne CK, Coronado E, Allred DC, Wiebe V, DeGregorio M 1991 Acquired tamoxifen resistance: correlation with reduced breast tumor levels of tamoxifen and isomerization of trans-4-hydroxytamoxifen. J Natl Cancer Inst 83:1477–1482 [DOI] [PubMed] [Google Scholar]
- Jeng MH, Shupnik MA, Bender TP, Westin EH, Bandyopadhyay D, Kumar R, Masamura S, Santen RJ 1998 Estrogen receptor expression and function in long-term estrogen-deprived human breast cancer cells. Endocrinology 139:4164–4174 [DOI] [PubMed] [Google Scholar]
- Song RX, Mor G, Naftolin F, McPherson RA, Song J, Zhang Z, Yue W, Wang J, Santen RJ 2001 Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17β-estradiol. J Natl Cancer Inst 93:1714–1723 [DOI] [PubMed] [Google Scholar]
- Yue W, Wang JP, Conaway M, Masamura S, Li Y, Santen RJ 2002 Activation of the MAPK pathway enhances sensitivity of MCF-7 breast cancer cells to the mitogenic effect of estradiol. Endocrinology 143:3221–3229 [DOI] [PubMed] [Google Scholar]
- Sawka CA, Pritchard KI, Paterson AH, Sutherland DJ, Thomson DB, Shelley WE, Myers RE, Mobbs BG, Malkin A, Meakin JW 1986 Role and mechanism of action of tamoxifen in premenopausal women with metastatic breast carcinoma. Cancer Res 46:3152–3156 [PubMed] [Google Scholar]
- Lee JS, Ettinger B, Stanczyk FZ, Vittinghoff E, Hanes V, Cauley JA, Chandler W, Settlage J, Beattie MS, Folkerd E, Dowsett M, Grady D, Cunnings SR 2006 Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab 91:3791–3797 [DOI] [PubMed] [Google Scholar]
- Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S 2007 Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids 72:666–671 [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, Lee JS, Santen RJ 2007 Standardization of steroid hormone assays: why, how and when? Cancer Epidemiol Biomarkers Prev 16:1713–1719 [DOI] [PubMed] [Google Scholar]
- Spink DC, Katz BH, Hussain MM, Pentecost BT, Cao Z, Spink BC 2003 Estrogen regulates Ah responsiveness in MCF-7 breast cancer cells. Carcinogenesis 24:1941–1950 [DOI] [PubMed] [Google Scholar]
- Santner SJ, Ohlsson-Wilhelm B, Santen RJ 1993 Estrone sulfate promotes human breast cancer cell replication and nuclear uptake of estradiol in MCF-7 cell cultures. Int J Cancer 54:119–124 [DOI] [PubMed] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS 1986 Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA 83:2496–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Wolf MF, Murphy CS, Jordan VC 1988 Estrogenic activity of phenol red. Mol Cell Endocrinol 57:169–178 [DOI] [PubMed] [Google Scholar]
- Wu CH, Motohashi T, Abdel-Rahman HA, Flickinger GL, Mikhail G 1976 Free and protein-bound plasma estradiol-17β during the menstrual cycle. J Clin Endocrinol Metab 43:436–445 [DOI] [PubMed] [Google Scholar]
- Fan M, Yan PS, Hartman-Frey C, Chen L, Paik H, Oyer SL, Salisbury JD, Cheng AS, Li L, Abbosh PH, Huang TH, Nephew KP 2006 Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res 66:11954–11966 [DOI] [PubMed] [Google Scholar]
- Frasor J, Chang EC, Komm B, Lin CY, Vega VB, Liu ET, Miller LD, Smeds J, Bergh J, Katzenellenbogen BS 2006 Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res 66:7334–7340 [DOI] [PubMed] [Google Scholar]
- Meijer D, van Agthoven T, Bosma PT, Nooter K, Dorssers LC 2006 Functional screen for genes responsible for tamoxifen resistance in human breast cancer cells. Mol Cancer Res 4:379–386 [DOI] [PubMed] [Google Scholar]
- Borgna JL, Rochefort H 1980 High-affinity binding to the estrogen receptor of [3H]4-hydroxytamoxifen, an active antiestrogen metabolite. Mol Cell Endocrinol 20:71–85 [DOI] [PubMed] [Google Scholar]
- Wijayaratne AL, Nagel SC, Paige LA, Christensen DJ, Norris JD, Fowlkes DM, McDonnell DP 1999 Comparative analyses of mechanistic differences among antiestrogens. Endocrinology 140:5828–5840 [DOI] [PubMed] [Google Scholar]
- Beekman JM, Allan GF, Tsai SY, Tsai MJ, O'Malley BW 1993 Transcriptional activation by the estrogen receptor requires a conformational change in the ligand binding domain. Mol Endocrinol 7:1266–1274 [DOI] [PubMed] [Google Scholar]
- Michalides R, Griekspoor A, Balkenende A, Verwoerd D, Janssen L, Jalink K, Floore A, Veld A, van't Veer L, Neefjes J 2004 Tamoxifen resistance by a conformational arrest of the estrogen receptor α after PKA activation in breast cancer. Cancer Cell 5:597–605 [DOI] [PubMed] [Google Scholar]
- Dauvois S, Danielian PS, White R, Parker MG 1992 Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci USA 89:4037–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton DJ, Hutcheson IR, Knowlden JM, Barrow D, Giles M, McClelland RA, Gee JM, Nicholson RI 2006 Bidirectional cross talk between ERα and EGFR signalling pathways regulates tamoxifen-resistant growth. Breast Cancer Res Treat 96:131–146 [DOI] [PubMed] [Google Scholar]
- Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R 2004 Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96:926–935 [DOI] [PubMed] [Google Scholar]
- Yue W, Berstein LM, Wang JP, Clark GM, Hamilton CJ, Demers LM, Santen RJ 2001 The potential role of estrogen in aromatase regulation in breast. J Steroid Biochem Mol Biol 79:157–164 [DOI] [PubMed] [Google Scholar]
- Fawell SE, White R, Hoare S, Sydenham M, Page M, Parker MG 1990 Inhibition of estrogen receptor-DNA binding by the “pure” antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci USA 87:6883–6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvois S, White R, Parker MG 1993 The antiestrogen ICI 182,780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci 106:1377–1388 [DOI] [PubMed] [Google Scholar]
- Brooks SC, Locke ER, Soule HD 1973 Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem 248:6251–6253 [PubMed] [Google Scholar]
- Soule HD, Vazguez J, Long A, Albert S, Brennan M 1973 A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst 51:1409–1416 [DOI] [PubMed] [Google Scholar]
- Larsen SS, Egeblad M, Jäättelä M, Lykkesfeldt AE 1999 Acquired antiestrogen resistance in MCF-7 human breast cancer sublines is not accomplished by altered expression of receptors in the ErbB-family. Breast Cancer Res Treat 58:41–56 [DOI] [PubMed] [Google Scholar]
- Osipo C, Gajdos C, Cheng D, Jordan VC 2005 Reversal of tamoxifen resistant breast cancer by low dose estrogen therapy. J Steroid Biochem Mol Biol 93:249–256 [DOI] [PubMed] [Google Scholar]
- Hsu SC, Hung MC 2007 Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J Biol Chem 282:10432–10440 [DOI] [PubMed] [Google Scholar]
- Lo HW, Ali-Seyed M, Wu Y, Bartholomeusz G, Hsu SC, Hung MC 2006 Nuclear-cytoplasmic transport of EGFR involves receptor endocytosis, importin β1 and CRM1. J Cell Biochem 98:1570–1583 [DOI] [PubMed] [Google Scholar]
- Larionov AA, Berstein LM, Miller WR 2002 Local uptake and synthesis of oestrone in normal and malignant postmenopausal breast tissues. J Steroid Biochem Mol Biol 81:57–64 [DOI] [PubMed] [Google Scholar]
- Yue W, Wang JP, Hamilton CJ, Demers LM, Santen RJ 1998 In situ aromatization enhances breast tumor estradiol levels and cellular proliferation. Cancer Res 58:927–932 [PubMed] [Google Scholar]
- Santner SJ, Pauley RJ, Tait L, Kaseta J, Santen RJ 1997 Aromatase activity and expression in breast cancer and benign breast tissue stromal cells. J Clin Endocrinol Metab 82:200–208 [DOI] [PubMed] [Google Scholar]
- van Landeghem AA, Poortman, J, Nabuurs M, Thijssen JH 1985 Endogenous concentration and subcellular distribution of estrogens in normal and malignant human breast tissue. Cancer Res 45:2900–2906 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.