Abstract
Animals shipped from commercial suppliers to laboratories are exposed to a wide variety of stressors. Female C57Bl6/J mice shipped during the peripubertal/adolescent period (6 wk old) display lower levels of female sexual behavior in response to estradiol and progesterone injections after ovariectomy when tested in adulthood than female mice shipped in adulthood (12 wk old). These shipping-induced reductions in female sexual behavior appear to be limited to a vulnerable period around the time of puberty. Likewise, male mice shipped at 6 wk of age express lower levels of masculine sexual behavior in response to testosterone treatment as adults than do mice shipped when 12 wk old. RIA of corticosterone levels in response to behavior testing revealed that, upon first exposure to testing, mice shipped at 6 wk of age have reduced corticosterone levels. These results suggest that during the peripubertal/adolescent period, mice of both sexes are susceptible to the effects of stressors associated with shipping. Furthermore, they suggest that stress during this period has enduring, negative influences on behavioral responses to estradiol and progesterone in females and to testosterone in males, and it induces changes in response of the hypothalamic-pituitary-adrenal axis. These results suggest that age at shipping is a critical variable that may influence many endocrinological studies, and they suggest that the peripubertal/adolescent period is a period of vulnerability to some stressors.
Mice shipped during the peripubertal/adolescent period are less responsive to the behavioral effects of gonadal hormones when tested in adulthood.
Laboratory animals, as well as animals used for agricultural purposes, are often shipped to other locations at various stages of development. During shipping, animals are likely to be exposed to noise, vibrations, temperature and humidity changes, predator odors and other noxious smells, light-dark changes, food and water deprivation, as well as many pathogens (1). It can be inferred that shipping is a stressful experience because many of these factors are actually used as independent variables in stress research. Thus, shipping animals can be considered a multidimensional stressor.
In rodents, shipping increases several markers of the hypothalamo-pituitary-adrenal (HPA) axis. Shipping mice via a commercial shipper increases corticosterone levels and decreases immune function (2). Likewise, shipping mice from a breeder causes significant weight loss and dehydration, changes that are evident even if food and moisture are available during transit (3). Even short-term transportation of mice within a research facility increases serum corticosterone for up to 24 h (4), and it causes behavioral changes that can last for a few days after the termination of exposure. Some of these behavioral changes include increases in feeding behavior, exploratory behaviors, hiding, and aggressive sexual/dominance behaviors (4). It is likely, then, that mice shipped from commercial breeders to laboratories experience transient changes in HPA axis activity as well as changes in immune function.
Most studies that have assessed the physiological influences of shipping have focused on the effects of shipping domestic animals for breeding or agricultural purposes (5,6,7). In addition to changes in HPA axis function, metabolic changes related to the transportation of other species have also been reported (6,7). In goats, commercial shipping increases levels of glucose and blood urea nitrogen. In addition, increases in the neutrophil to leukocyte ratio are positively correlated with duration of the exposure to shipping, suggesting that the immune system is activated during shipping (6). Long-distance transport of horses temporarily causes dehydration and increases electrolyte osmolality (7). In pigs, stress of shipping interacts with social status to affect natural killer cell activity, making socially dominant pigs less vulnerable to viral infections after shipping. Furthermore, shipping-induced weight loss is negatively correlated with cortisol levels (5). Rabbits, shipped either in air-conditioned trucks or by air, become anorectic, develop high blood glucose levels, decreased leukocyte counts, and increased neutrophil counts, as well as increased cortisol concentrations that persist for at least 2 d after the end of the shipping (8).
Because experimental animals are shipped in filtered cages, it is likely that they are exposed to fewer pathogens than animals shipped for agricultural or commercial purposes, and thus, less likely to develop shipping-induced pathogenic infection. The factors associated with shipping that are responsible for inducing specific changes are for the most part unknown. However, exposure to vibration and noise related to shipping increases respiration rate in pregnant female rabbits (9) and increases ACTH levels in female guinea pigs (10). Nevertheless, although the likelihood of experimental animals being exposed to pathogens during shipping might be minimal, stress-induced activation of the immune system is likely to occur as a result of shipping.
Exposure to experimental stressors during particular developmental periods has long-term effects on the brain, behavior, and physiology. During the perinatal period, stress exposure has long-term consequences for behavior and reproductive capacity (11,12,13), and on the sexual differentiation of adult behaviors (14,15,16,17). Shipping of rats during pregnancy may induce long-lasting changes in the offspring, such as elimination of the typical asymmetry in the thickness of the cerebral cortex in male rats (18) as well as decreases in surgery related mortality of rat pups (19). Exposure to experimental stressors in the prepubertal period is associated with a reduction in reproductive capacity (20) as well as delay in puberty onset in female mice (21). Little is known about the long-term effects of stress exposure during the peripubertal/adolescent period on physiology and behavior.
Although the perinatal period has long been recognized as a critical period in sexual differentiation, recently, the peripubertal/adolescent period has also been recognized as critical in the sexual differentiation of the hypothalamo-pituitary-gonadal axis and reproductive behaviors (22,23,24). This period also appears to be important in the maturation of the HPA axis because stress exposure during that time leads to changes in HPA axis response not observed in adult animals (25). Prepubertal female rats have a prolonged stress-induced corticosterone release response relative to adults (26). Likewise, prepubertal male rats have a prolonged stress-induced adrenal progesterone response relative to adult males (27). Prepubertal animals also display greater stress-induced neuronal activation in CRH-containing neurons (28). In BALB/c mice the maximal corticosterone response to an immunogenic stressor occurs during the peripubertal/adolescent period in females (29).
We became interested in the long-term effects of exposing female mice to shipping stress when we observed that female mice shipped from suppliers at 6 wk of age expressed reduced levels of female sexual behavior after ovariectomy and treatment with exogenous ovarian steroid hormones. As a result, we tested the hypothesis that shipping mice during the peripubertal/adolescent period reduces the expression of female sexual behavior in response to estradiol and progesterone in adulthood and on masculine sexual behavior in male mice.
Materials and Methods
Animals (experiments 1–4)
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Amherst. Female C57Bl/6 mice were used. With the exception of one group of mice that was bred in the laboratory from mice obtained from Taconic Farms (Hudson, NY) or Charles River Laboratories (Kingston, NY) (experiment 2), all animals were obtained from Charles River Laboratories (experiments 1 and 3) or Taconic Farms (experiment 2). Shipped mice were transported in filter-top crates (∼20 mice per crate), and arrived at our animal facility between 24 and 48 h after they were shipped from the supplier. During shipment, all mice had access to food pellets and gel moisture packs.
Upon arrival in the laboratory, mice were placed in a cage (30.5 × 19.5 × 12.0 cm polycarbonate with wire lid), and they were left undisturbed in the colony room for 7–10 d with free access to food (Harlan Teklad 2014, low phytoestrogen rodent chow; Harlan Teklad, Madison, WI) and water. Animals were housed three to five per cage in a colony room under a controlled environment, with a consistent temperature (∼24 ± 2 C) and reversed 14-h light, 10-h dark cycle (lights off at 1000 h). When handling occurred during the dark phase of the cycle, dim red illumination was used. A combination of wood shavings and CareFRESH (International Absorbents, Inc., North Vancouver, British Columbia, Canada) bedding was used, and a fresh Nestlet (Ancare Corp., Bellmore, NY) was placed in the cage when bedding was changed.
Animals (experiment 5)
A total of 36 male mice was shipped either at 6 (n = 24) or 12 wk of age (n = 12) from a supplier (Charles River Laboratories). Half of the animals shipped at 6 wk of age were randomly assigned to be orchiectomized 1 wk after arrival in the laboratory, and the others were left undisturbed until surgery at 13 wk old.
Ovariectomy
Female mice were anesthetized by ip injection of Avertin (3,3,3-tribromoethanol, 32 mg/g body weight; Winthrop Laboratories, Winthrop, MA) and ear tagged before the bilateral removal of the ovaries. Incisions to the skin and the muscle layer were made. After locating the uterine horns and ovaries, each uterine horn was tied with absorbable suture just below the ovary, and each ovary was then removed. The muscle layer was sutured, and the skin layer was fastened with wound clips. After the procedure the mice were placed on a heating pad until fully alert. Mice were returned to their home cage, and allowed access to an additional water bottle containing a 3% Children’s Tylenol solution (32 mg acetaminophen/ml; McNEIL-PPC, Inc., Parsippany, NJ) for 48 h.
Orchiectomy
Male mice were anesthetized as described previously. An incision was made in the scrotum, followed by an incision in the tunica of the first testis. The testis, vas deferens, and attached testicular fat pad were pulled out of the incision, the blood vessels sutured, and the testis, vas deferens, and fatty tissue severed immediately below the site of the suture. The same procedure was then followed for the contralateral testis. The scrotal incision was closed using wound clips.
Female sexual behavior testing
Seven to 10 d after ovariectomy, mice were tested for the expression of sexual receptivity in response to a sc injection of 2 μg estradiol benzoate (EB) (dissolved in 0.1 ml sesame oil), followed 44 h later by 100 μg progesterone (dissolved in 0.2 ml sesame oil containing 5% benzyl benzoate and 15% benzyl alcohol), as previously published (30,31). These are the minimal doses that reliably induce feminine sexual behavior in our laboratory. Approximately 6 h after progesterone injection, mice were tested for female sexual behavior in response to a sexually vigorous male mouse. A sexually experienced stimulus male was placed in a Plexiglas arena (18 × 38 cm) (32) on a mirror stand, which allowed a ventral view of the behavioral interactions with one female until 20 mounts had occurred. All testing was done under dim red illumination. Mounts, defined as both of the male’s forepaws on the female’s hind region, as well as intromissions, were scored. Scoring a lordosis response required that the female hold a rigid posture with slight arching of the back, and elevation of the hindquarters to facilitate mount and intromission by the male (33). The lordosis quotient (LQ) was calculated as the number of lordosis responses/number of mounts × 100 as an index of sexual receptivity. Because female mice often become nonreceptive after receiving an ejaculation from a male, testing was terminated if the female received an ejaculation. Testing was performed with scorers blind to the treatment that each animal received. Weekly tests continued for 5 wk.
Corticosterone RIA
Mice were euthanized with a lethal dose of chloropent, and quickly decapitated in a separate room from that containing the experimental animals. Trunk blood was collected immediately after decapitation into EDTA-coated blood collection tubes (Microvette CB 300; Sarstedt, Newton, NC), and placed on ice until sample collection was complete. Blood was centrifuged for 15 min at 3000 rpm at 4 C, serum was collected, and stored at −20 C, until corticosterone levels were assessed by RIA. Serum corticosterone levels were determined using a corticosterone RIA (MP Biomedicals, Irvine, CA) with an intraassay coefficient of variability of 7.1%, and an interassay coefficient of variability of 6.5%.
Statistical analysis
Two-way, repeated measures ANOVAs were performed to assess the effects of treatments on sexual receptivity in the females over the five test sessions. When appropriate, post hoc tests (Bonferroni comparisons) were performed to obtain pair-wise comparisons. Student’s t tests were used to assess group differences in the males.
Experiment 1: effect of peripubertal shipping on female sexual behavior
Female mice were either shipped from a supplier (Charles River Laboratories) or were bred in our laboratory (n = 5). Mice were shipped either at 6 wk of age (n = 11) or at 12 wk of age (n = 10). The mice were ovariectomized either at 7 or 13 wk (i.e. 1 wk after arrival to our facility). Mice bred in the laboratory were ovariectomized at 7 wk of age. Beginning 1 wk after ovariectomy, animals were injected weekly with EB and progesterone, and tested for sexual receptivity for 5 consecutive weeks.
Experiment 2: effect of peripubertal shipping on adult female sexual behavior in adulthood
To determine whether shipping mice during the peripubertal/adolescent period had an enduring effect on sexual receptivity in adulthood, C57Bl/6 female mice (Taconic Farms) were shipped at two different ages: 6 wk (during the peripubertal/adolescent period; n = 20); or 12 wk (in adulthood; n = 10). Upon arrival in our laboratory, the mice were housed in groups of five per cage and were kept under the same conditions described previously for the mice bred in our laboratory. The mice shipped at 6 wk of age were randomly assigned to one of two groups: shipped at 6 wk and ovariectomized 1 wk later at 7 wk old (n = 10); or ovariectomized at 13 wk of age (n = 10), the age at which mice shipped at 12 wk underwent the same treatment. One week after ovariectomy, the mice were injected with EB and progesterone, and tested for female sexual receptivity in five weekly sessions.
Experiment 3: effect of age at shipping on adult female sexual behavior
A total of 115 female mice was shipped from a commercial supplier (Charles River Laboratories) at: 3 wk (weaning; n = 12); 4 wk (n = 13); 5 wk (n = 13); 6 wk (n = 13); 7 wk (n = 13); 8 wk (n = 13); 9 wk (n = 13); 10 wk (n = 13); or 12 wk (n = 12) of age. All of the animals were group housed, three to four per cage, from their arrival in our laboratory until the end of the experiment. At 13 wk of age, all animals were ovariectomized and tested for female sexual behavior in five weekly tests as described previously, beginning at 14 wk of age.
Experiment 4: effect of peripubertal shipping on serum corticosterone levels during female sexual behavior testing
It is not clear why ovariectomized mice require multiple episodes of hormone priming followed by behavior testing before they show high levels of female sexual behavior. However, because suppression of synthesis of corticosterone (34) increases the expression of female sexual behavior in hormone-primed, ovariectomized, mice, it is possible that corticosterone release at the time of testing might inhibit female sexual behavior. In this experiment we determined whether female mice shipped at 6 wk of age have elevated levels of corticosterone in response to testing relative to mice shipped in adulthood.
A total of 90 female mice (45, 6-wk-old mice, and 45, 12-wk-old mice) was shipped from a supplier (Charles River Laboratories). One week later, all 90 animals were ovariectomized and prepared to be tested for female sexual receptivity. On each testing week, nine animals were randomly selected from each group, and trunk blood from these animals was collected immediately after behavior testing or after the animal had been in the testing arena for 20 min, whichever came first. Mice were decapitated at 2–3 min after a lethal injection of chloropent. The samples were collected at 1500–1600 h (lights off at 1000 h). All samples were taken between 15 and 20 min, and the timing did not differ between the groups. Serum corticosterone levels were determined by RIA.
Experiment 5: effect of peripubertal shipping on masculine sexual behavior
Male mice were shipped from a supplier (Charles River Laboratories) when either 6 or 12 wk old. At 13 wk of age, all mice were orchiectomized, and SILASTIC brand capsules (Dow Corning, Midland, MI; outer diameter, 2.16 mm; inner diameter, 1.02 mm; and inner length, 5 mm) filled with 60% testosterone (Sigma Chemical Co., St. Louis, MO) diluted with cholesterol (Steraloids, Newport, RI) were implanted sc between the shoulder blades as previously described (35). Five days after surgery, the animals were singly housed. Approximately 2 wk after surgery (15 wk old), the animals were tested for masculine sexual behavior in response to an estradiol- and progesterone-treated, sexually receptive female mouse placed in the experimental animal’s home cage (36). The tests lasted until the animals ejaculated or up to 60 min if no sexual behavior was observed. The mice were tested once a week for 3 consecutive weeks. Mount latency, number of mounts, and intromission/pelvic thrust latency were calculated. Ejaculation latency and frequency were calculated by adding the data for up to three test sessions.
Results
Experiment 1: effect of peripubertal shipping on female sexual behavior
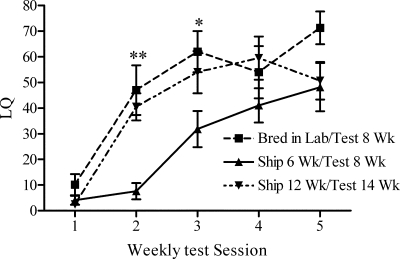
Mice shipped at 6 wk expressed lower levels of sexual receptivity than those bred in our laboratory or shipped in adulthood. Statistical analyses revealed a significant main effect of test session (F4,92 = 31.48; P < 0.0001) and treatment (F2,92 = 6.487; P < 0.01), and an interaction between test session and treatment (F8,92 = 2.192; P ≤ 0.05). All groups showed increasing levels of hormone-dependent sexual receptivity over the five consecutive test sessions (Fig. 1). Bonferroni posttests revealed that female mice shipped at 6 wk displayed lower levels of female sexual behavior on tests 2 and 3 than female mice that were bred in our laboratory or shipped in adulthood. There were no significant differences between mice bred in our laboratory and mice shipped from the supplier at 12 wk.
Figure 1.
LQs (mean ± sem) of female mice bred in this laboratory or shipped at two different ages during development. *, P < 0.05; **, P < 0.01.
Experiment 2: effect of peripubertal shipping on female sexual behavior in adulthood
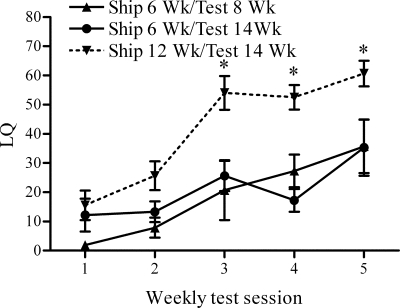
The results confirmed that females shipped from a supplier when 6 wk old expressed significantly lower levels of sexual receptivity than mice shipped during adulthood (Fig. 2), even when the mice shipped at 6 wk old were tested as adults. They confirm that the effects of exposing female mice to the stress of shipping during the peripubertal/adolescent period causes enduring behavioral changes in the response of ovariectomized female mice to estradiol and progesterone treatment.
Figure 2.
LQs (mean ± sem) of female mice shipped during the peripubertal period. Statistically significant differences in receptivity were observed between mice shipped at 6 wk and tested at 14 wk, and those shipped at 12 wk and tested at 14 wk during test sessions 3–5. *, P < 0.05.
A two-way, repeated measures ANOVA revealed significant main effects for test session (F4,88 = 16.55; P < 0.0001) and treatment group (F2,88 = 19.35; P < 0.0001), confirming that mean LQ increased over consecutive test sessions, and that the mice shipped at 6 wk were less receptive than those shipped at 12 wk. Post hoc tests indicated that mice shipped at 6 wk and tested in adulthood displayed lower levels of sexual receptivity than those shipped at 12 wk during sessions 3 (t13 = 4.23; P < 0.001), 4 (t13 = 3.38; P < 0.01), and 5 (t13 = 3.47; P < 0.01). In contrast, there were no statistically significant differences in sexual receptivity between the two groups of mice shipped at 6 wk of age during any of the test sessions.
Experiment 3: effect of age at shipping on adult female sexual behavior
The defeminizing effects of shipping are primarily restricted to a vulnerable period at 5–6 wk of age (Fig. 3). Female mice are more sensitive to the defeminizing effects of shipping around the age of 5–6 wk, and mice shipped after this age are relatively insensitive to the defeminizing effects of shipping. Mice shipped earlier (3–4 wk) were not as affected by the defeminizing effects of shipping in that, by the fifth test, they displayed levels of sexual receptivity comparable to those of female shipped after 6 wk of age.
Figure 3.
LQs (mean ± sem) over five consecutive weekly test sessions in adulthood of female mice shipped at different ages. Significant differences between animals shipped at 6 wk and other age groups are noted for each test session. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Statistical analysis confirmed a main effect of test session (F4,424 = 30.20; P < 0.0001), a main effect of age at shipping (F8,424 = 10.93; P < 0.0001), and an interaction between the two main factors of age and shipping (F32,424 = 4.89; P < 0.005). Bonferroni post hoc analyses were used to contrast LQ at each test session, with female mice shipped at 6 wk used as the control group. When contrasted with mice shipped when 6 wk old, mice shipped at 7 wk or older were more receptive (P < 0.05) at nearly every test session with the exception of test 1.
Animals shipped at either 3 or 4 wk were significantly more receptive than animals shipped at 6 wk on the final test (3 vs. 6 wk: t23 = 3.31, P < 0.01; 4 vs. 6 wk: t24 = 2.90, P < 0.05), suggesting that, unlike animals shipped at 5 or 6 wk, female mice shipped in the prepubertal period are less impeded in their response to estradiol and progesterone.
Experiment 4: effect of peripubertal shipping on serum corticosterone levels during female sexual behavior testing
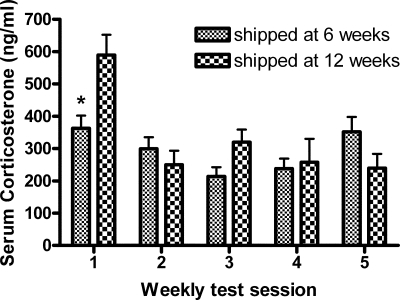
The results support the hypothesis that corticosterone levels during testing decrease after repeated exposure to the testing conditions. However, our results do not support the hypothesis that peripubertally shipped female mice have higher corticosterone levels than the control mice during testing (Fig. 4). Corticosterone levels were decreased after repeated exposures to behavior testing, and corticosterone levels during behavior testing were lower in peripubertally shipped female mice on test session 1 only.
Figure 4.
Serum corticosterone levels (mean ± sem) in serum taken 15–20 min after the start of female sexual behavior testing in female mice shipped at 6 or 12 wk. *, P < 0.01.
Statistical analysis revealed that the corticosterone secretion response during female sexual behavior testing decreased over the five test sessions (F4,79 = 8.06; P < 0.0001) and that, whereas there was no main effect of age at shipping (F1,79 = 1.66; P > 0.05), there was a significant interaction effect between the two main factors (F4,79 = 4.12; P < 0.005). Bonferroni tests revealed that shipping female mice at 6 wk decreased the level of corticosterone during sexual behavior testing contrasted with mice shipped during adulthood (t16 = 3.45; P < 0.01) (Fig. 4).
Experiment 5: effect of peripubertal shipping on masculine sexual behavior
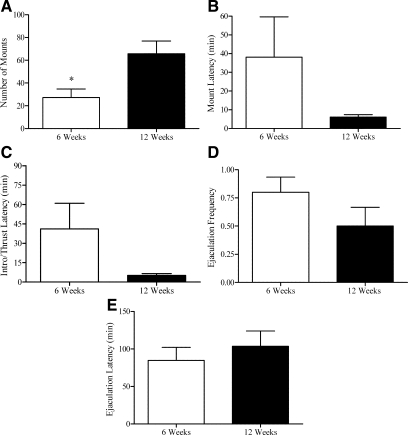
Males shipped at 6 wk old displayed fewer mounts (t16 = 2.73; P < 0.02) than mice shipped in adulthood (Fig. 5). Although mount latency was not significantly longer, there was a strong trend toward longer intromission latencies in mice shipped at 6 wk than in those shipped at 12 wk (t16 = 2.04; P < 0.05, one-tailed test). There was no statistically significant difference in latency to ejaculation, or ejaculation frequency, between males shipped during the peripubertal/adolescent period and the control males shipped at 12 wk, but ejaculations were infrequent (less than one).
Figure 5.
Number of mounts (mean ± sem) (A), mount latency (mean ± sem) (B), intromission (Intro)/thrust latency (mean ± sem) (C), ejaculation frequency (mean ± sem) (D), and latency to ejaculation (mean ± sem) (E) in male mice shipped during the peripubertal period. *, P < 0.02.
Discussion
Shipping during the peripubertal/adolescent period causes a long-term suppression of ovarian steroid-induced female sexual behavior in female mice. In addition, because the effects of shipping on female sexual behavior are limited to the peripubertal/adolescent period, the results suggest that the peripubertal period is a critical period of stress vulnerability. Although stress exposure during critical periods of development can have long-term effects on rodent sexual behavior and sexual differentiation, most previous experiments have examined the effects of stress exposure during the neonatal period (17).
The defeminizing effects of peripubertal shipping on female sexual behaviors were more pronounced in experiment 2 than in experiment 1. Due to animal availability issues, the mice in the two experiments were shipped from different suppliers. Mice of the same strain, but from different shippers, are genetically dissimilar; some of these differences could account for the slight discrepancy. However, this difference demonstrates the robustness of the effects of shipping at 6 wk old because it was observed with mice shipped from different suppliers. It also should be noted that shipping is a multifactor, and potentially variable, stressor.
In experiment 3, we observed a period of vulnerability to shipping in inducing the decreased response to estradiol and progesterone. It must be noted that to keep the age at testing constant, the shipping to ovariectomy/testing latency differed among the groups shipped at different ages. However, experiment 2 suggested that this was not a relevant variable.
We also found that exposure to peripubertal/adolescent shipping decreased response of some aspects of masculine sexual behavior in adult male mice. Although the demasculinizing effects of stress exposure during development are well established (37,38,39,40), previous experiments examined the effects of stress exposure during the prenatal, not the peripubertal, period.
It is unclear if the effects of peripubertal/adolescent exposure to shipping occur in other species or strains of mice with all types of shipping. The fact that these deleterious effects of shipping have been duplicated in mice shipped from different suppliers in multiple shipments during different seasons indicates that the effects are robust. However, the absence of a precise understanding of the specific stressors to which mice are exposed to during shipping, and the potential lack of reproducibility among shipments makes it difficult to use shipping as an independent variable in experiments examining the effects of peripubertal stressor exposure on neuroendocrine function. Specific conditions within the trucks used for each shipment are not available (e.g. temperature, other species in the truck, amount of vibration, etc.). Nevertheless, these results suggest that, at least in C57Bl/6 mice, exposure to commercial shipping during the peripubertal period induces a decrease in female sexual behavior in females and in masculine sexual behavior in males.
Female mice require multiple weekly exposures to both exogenous hormones and testing conditions to display high levels of female sexual receptivity under specific testing conditions (41). Corticosterone synthesis and release in response to the sexual behavior testing situation have been implicated in the initial lack of response to ovarian steroid-induced female sexual behavior in ovariectomized mice because suppressing corticosterone synthesis potentiates the behavioral response (34). Experiments examining the importance of habituation to the testing environment have also revealed that male mice are more likely to display copulatory behaviors, regardless of androgen status, if they are repeatedly tested in the same environment (42).
We hypothesized that the present results could be due to long-term changes in HPA axis regulation in mice shipped during the peripubertal/adolescent period, which might in turn have negative effects on expression of sexual behavior. However, although we expected that mice shipped at 6 wk might have a greater corticosterone response to testing, mice shipped at 6 wk of age had a decreased corticosterone response at 15–20 min after the start of the test. Considering the stimulatory actions of estrogens on the HPA axis in females, it is plausible that decreased corticosterone levels are due to reductions in ovarian hormones associated with shipping stress (43). The lower corticosterone response of female mice shipped during the peripubertal period suggests that exposure to a peripubertal stressor has long-term effects on an animal’s response to stressors.
Reduced testing-related corticosterone release is consistent with differential responsiveness to shipping stress in the group shipped at 6 wk. In rats, reduced post-stress ACTH and corticosterone release and enhanced glucocorticoid feedback sensitivity are observed at long time intervals after exposure to repeated or intense stressors (44,45). Enhanced glucocorticoid feedback is also associated with traumatic stress exposure (posttraumatic stress disorder) in humans (46). The data suggest that female mice may be particularly susceptible to stressful stimuli during this developmental period, resulting in long-term changes in stress response profile as well as behavior.
For logistical reasons, we did not examine the acute effects of shipping exposure on the HPA axis of female mice during shipping or immediately after arrival at the animal facility. However, it is a near certainty that shipping mice causes acute activation of the HPA axis. Exposure to other stressors has been shown to induce maximal HPA axis activation in 45-d-old female mice (29). Thus, we cannot exclude the possibility that the reason that the greatest effect of shipping occurred at 6 wk of age is because this is the age at which shipping induces the largest activation of the HPA axis.
Based on behavioral phenotype, we have referred to the decrease in expression of female sexual behavior as defeminization. However, it is not clear whether the effects of peripubertal shipping are primarily on behavioral response to sex steroid hormones, perturbations in the HPA regulation, or an interaction between the two. For example, exposure to stressors during the peripubertal period has been associated with changes in HPA axis function during adulthood that include an enhancement of the stress response upon repeated exposures to a stressor during adulthood (47,48). Female mice appear to be particularly vulnerable to stress-induced disruption of the HPA axis around the time of puberty, and exposing prepubertal and peripubertal female mice to immobilization stress causes a persistent shift in the peak of the daily corticosterone secretion rhythm and even a loss of daily rhythmic variations in corticosterone secretion (49). It is possible that mice exposed to shipping stress during the peripubertal period display a modified stress response when exposed to the testing conditions.
Besides inducing the release of corticosteroids, stress-induced activation of the HPA axis is accompanied by the secretion of other steroids, such as androgens, estrogens (50), and progestins (51). It is also possible that the effects of shipping stress on adult behavioral response are mediated by sex hormone-induced changes in subsequent response to these hormones. Prenatal or postnatal exposure to estrogens and/or androgens can defeminize sexual behavior in female guinea pigs (52), mice (53), and rats (54,55,56). Like prepubertal exposure to testosterone in female rats (57), exposure to sex steroid hormones during the peripubertal/adolescent period influences neural circuits and sexual differentiation of adult behavior (58). Therefore, it is possible that the enduring influences of shipping on adult behavioral response to sex steroid hormones are mediated by stress-induced release of sex steroids during the peripubertal/adolescent period.
Stress exposure also causes the synthesis and release of cytokines (e.g. IL-6) in the brain and periphery (59). Cytokines have potent actions on the brain and behavior (60,61,62), and may affect the development of neurocircuitry responsible for control of sex behavior, even at levels that do not elicit frank illness or inflammation.
The results presented here suggest that peripubertal/adolescent shipping-induced reductions in female sexual behavior are long lasting. No attempt has been made yet to determine how long into adulthood this decrease in response to estradiol and progesterone persists, but the final test occurred at 18 wk of age.
These findings are also limited inasmuch as all of the mice were treated with the same hormone regimen; it is not known if different doses of hormones might have unmasked an effect in animals shipped at ages other than during the peripubertal/adolescent period or enhanced the differences among groups shipped at different ages.
In summary, shipping mice during adolescence causes enduring decreases in the response to gonadal hormones in adulthood and a decrease in the HPA response to testing. Besides the scientific questions of the mediating factors and of effects on other behaviors and physiological end-points, the results suggest that age at shipping is a critical factor that must be considered in all experiments. It adds to the already well-documented issues of prenatal stress in the offspring of dams shipped during pregnancy (19) and stress associated with nearby construction (63) as critical variables that must be considered in the design and interpretation of endocrinological research.
Acknowledgments
We thank Brooke Middlebrook and Matthew Riportella for their technical assistance, as well as Golien Safain, Kelly Goodwin, Cole Tucker, and Mihoko Yamamoto for their help with behavior testing. We also thank Kristin Olesen and Nafissa Ismail for critical comments on the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants NS 19327 and MH 67630, and a grant from the Isis Fund of the Society for Women’s Health Research.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 8, 2009
Abbreviations: EB, Estradiol benzoate; HPA, hypothalamo-pituitary-adrenal; LQ, lordosis quotient.
References
- Fisher AD, Stewart M, Duganzich DM, Tacon J, Matthews LR 2005 The effects of stationary periods and external temperature and humidity on thermal stress conditions within sheep transport vehicles. N Z Vet J 53:6–9 [DOI] [PubMed] [Google Scholar]
- Landi MS, Kreider JW, Lang CM, Bullock LP 1982 Effects of shipping on the immune function in mice. Am J Vet Res 43:1654–1657 [PubMed] [Google Scholar]
- Wallace ME 1976 Effects of stress due to deprivation and transport in different genotypes of house mouse. Lab Anim 10:335–347 [DOI] [PubMed] [Google Scholar]
- Tuli JS, Smith JA, Morton DB 1995 Stress measurements in mice after transportation. Lab Anim 29:132–138 [DOI] [PubMed] [Google Scholar]
- McGlone JJ, Salak JL, Lumpkin EA, Nicholson RI, Gibson M, Norman RL 1993 Shipping stress and social status effects on pig performance, plasma cortisol, natural killer cell activity, and leukocyte numbers. J Anim Sci 71:888–896 [DOI] [PubMed] [Google Scholar]
- Kannan G, Terrill TH, Kouakou B, Gazal OS, Gelaye S, Amoah EA, Samaké S 2000 Transportation of goats: effects on physiological stress responses and live weight loss. J Anim Sci 78:1450–1457 [DOI] [PubMed] [Google Scholar]
- Friend TH 2000 Dehydration, stress, and water consumption of horses during long-distance commercial transport. J Anim Sci 78:2568–2580 [DOI] [PubMed] [Google Scholar]
- Toth LA, January B 1990 Physiological stabilization of rabbits after shipping. Lab Anim Sci 40:384–387 [PubMed] [Google Scholar]
- Stephens DB, Adams CE 1982 Observations on the effects of vibration stress and sound on pregnancy, parturition and respiration in the rabbit. Lab Anim 16:341–347 [DOI] [PubMed] [Google Scholar]
- Bailey KJ, Stephens DB, Delaney CE 1986 Observations on the effects of vibration and noise on plasma ACTH and zinc levels, pregnancy and respiration rate in the guinea pig. Lab Anim 20:101–108 [DOI] [PubMed] [Google Scholar]
- Herrenkohl LR, Politch JA 1978 Effects of prenatal stress on the estrous cycle of female offspring as adults. Experientia 34:1240–1241 [DOI] [PubMed] [Google Scholar]
- Herrenkohl LR 1979 Prenatal stress reduces fertility and fecundity in female offspring. Science 206:1097–1099 [DOI] [PubMed] [Google Scholar]
- Herrenkohl LR 1983 Prenatal stress may alter sexual differentiation in male and female offspring. Monogr Neural Sci 9:176–183 [DOI] [PubMed] [Google Scholar]
- Zielinski WJ, Vandenbergh JG, Montano MM 1991 Effects of social stress and intrauterine position on sexual phenotype in wild-type house mice (Mus musculus). Physiol Behav 49:117–123 [DOI] [PubMed] [Google Scholar]
- Sachser N, Kaiser S 1996 Prenatal social stress masculinizes the females’ behaviour in guinea pigs. Physiol Behav 60:589–594 [DOI] [PubMed] [Google Scholar]
- Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN 2004 Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology 145:3778–3787 [DOI] [PubMed] [Google Scholar]
- Ward IL 1984 The prenatal stress syndrome: current status. Psychoneuroendocrinology 9:3–11 [DOI] [PubMed] [Google Scholar]
- Stewart J, Kolb B 1988 The effects of neonatal gonadectomy and prenatal stress on cortical thickness and asymmetry in rats. Behav Neural Biol 49:344–360 [DOI] [PubMed] [Google Scholar]
- Sachs BD, Lumia AR 1981 Is stress due to shipment of animals a confounding variable in developmental research? Dev Psychobiol 14:169–171 [DOI] [PubMed] [Google Scholar]
- Paris AL, Ramaley JA 1973 Effects of short-term stress upon fertility. I. Before puberty. Fertil Steril 24:540–545 [DOI] [PubMed] [Google Scholar]
- Ramaley JA 1976 The role of adrenal rhythmicity in puberty: effects of intermittent steroid replacement. Biol Reprod 15:396–401 [DOI] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Romeo RD, Morris JA, Lookingland KJ, Sisk CL 2003 Medial preoptic area dopaminergic responses to female pheromones develop during puberty in the male Syrian hamster. Brain Res 988:139–145 [DOI] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL 2004 Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav 45:242–249 [DOI] [PubMed] [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL 2006 Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Horm Behav 50:477–483 [DOI] [PubMed] [Google Scholar]
- Almeida SA, Anselmo-Franci JA, Rosa e Silva AA, Carvalho TL 1998 Chronic intermittent immobilization of male rats throughout sexual development: a stress protocol. Exp Physiol 83:701–704 [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS 2004 Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology 80:387–393 [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, McEwen BS 2005 Stress-induced progesterone secretion and progesterone receptor immunoreactivity in the paraventricular nucleus are modulated by pubertal development in male rats. Stress 8:265–271 [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS 2006 Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology 147:1664–1674 [DOI] [PubMed] [Google Scholar]
- Spinedi E, Chisari A, Pralong F, Gaillard RC 1997 Sexual dimorphism in the mouse hypothalamic-pituitary-adrenal axis function after endotoxin and insulin stresses during development. Neuroimmunomodulation 4:77–83 [DOI] [PubMed] [Google Scholar]
- Ring JR 1944 The estrogen-progesterone induction of sexual receptivity in the spayed female mouse. Endocrinology 34:269–275 [Google Scholar]
- Blaustein JD, Wade GN 1977 Concurrent inhibition of sexual behavior, but not brain [3H]estradiol uptake, by progesterone in female rats. J Comp Physiol Psychol 91:742–751 [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF 2003 Double oestrogen receptor α and β knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol 15:978–983 [DOI] [PubMed] [Google Scholar]
- Mani SK, Allen JM, Lydon JP, Mulac-Jericevic B, Blaustein JD, DeMayo FJ, Conneely O, O'Malley BW 1996 Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol 10:1728–1737 [DOI] [PubMed] [Google Scholar]
- de Catanzaro D, Lee PC, Kerr TH 1985 Facilitation of sexual receptivity in female mice through blockade of adrenal 11β-hydroxylase. Horm Behav 19:77–85 [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Rissman EF 2000 Dopamine activates masculine sexual behavior independent of the estrogen receptor α. J Neurosci 20:4248–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Scordalakes EM, Bodo C, Gustafsson JA, Rissman EF 2003 Lack of functional estrogen receptor β gene disrupts pubertal male sexual behavior. Horm Behav 44:427–434 [DOI] [PubMed] [Google Scholar]
- Ward IL 1972 Prenatal stress feminizes and demasculinizes the behavior of males. Science 175:82–84 [DOI] [PubMed] [Google Scholar]
- Rhees RW, Lephart ED, Eliason D 2001 Effects of maternal separation during early postnatal development on male sexual behavior and female reproductive function. Behav Brain Res 123:1–10 [DOI] [PubMed] [Google Scholar]
- Politch JA, Herrenkohl LR 1984 Effects of prenatal stress on reproduction in male and female mice. Physiol Behav 32:95–99 [DOI] [PubMed] [Google Scholar]
- Rhees RW, Fleming DE 1981 Effects of malnutrition, maternal stress, or ACTH injections during pregnancy on sexual behavior of male offspring. Physiol Behav 27:879–882 [DOI] [PubMed] [Google Scholar]
- Thompson ML, Edwards DA 1971 Experiential and strain determinants of the estrogen-progesterone induction of sexual receptivity in spayed female mice. Horm Behav 2:299–305 [Google Scholar]
- Wee BE, Clemens LG 1989 Environmental influences on masculine sexual behavior in mice. Physiol Behav 46:867–872 [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER 1995 The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol 144:311–321 [DOI] [PubMed] [Google Scholar]
- Liberzon I, Krstov M, Young EA 1997 Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology 22:443–453 [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP 2006 Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology 147:2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Giller EL, Southwick SM, Lowy MT, Mason JW 1991 Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biol Psychiatry 30:1031–1048 [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Gleason E, Kelsey JE 2004 Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Horm Behav 46:458–466 [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE 2005 Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav 48:64–74 [DOI] [PubMed] [Google Scholar]
- Paris AL, Ramaley JA 1974 Adrenal-gonadal relations and fertility: the effects of repeated stress upon the adrenal rhythm. Neuroendocrinology 15:126–136 [DOI] [PubMed] [Google Scholar]
- Edery M, Carreau S, Drosdowsky A 1980 In vitro pregnenolone metabolism by mouse adrenal gland: I-estrogen synthesis. Steroids 35:381–388 [DOI] [PubMed] [Google Scholar]
- Balfour WE, Comline RS, Short RV 1957 Secretion of progesterone by the adrenal gland. Nature 180:1480–1481 [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC 1959 Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382 [DOI] [PubMed] [Google Scholar]
- Edwards DA 1971 Neonatal administration of androstenedione, testosterone or testosterone propionate: effects on ovulation, sexual receptivity and aggressive behavior in female mice. Physiol Behav 6:223–228 [DOI] [PubMed] [Google Scholar]
- Thomas DA, Howard SB, Barfield RJ 1983 Influence of androgen on the development of sexual behavior in the rat. II. Time and dosage of androgen administration during the neonatal period and masculine and feminine copulatory behavior in females. Horm Behav 17:308–315 [DOI] [PubMed] [Google Scholar]
- Hoepfner BA, Ward IL 1988 Prenatal and neonatal androgen exposure interact to affect sexual differentiation in female rats. Behav Neurosci 102:61–65 [DOI] [PubMed] [Google Scholar]
- Barraclough CA, Gorski RA 1962 Studies on mating behaviour in the androgen-sterilized female rat in relation to the hypothalamic regulation of sexual behaviour. J Endocrinol 25:175–182 [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Mills R, Gale S 1995 Prepubertal testosterone treatment of female rats: defeminization of behavioral and endocrine function in adulthood. Neurosci Biobehav Rev 19:177–186 [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL 2005 Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 26:163–174 [DOI] [PubMed] [Google Scholar]
- Song DK, Suh HW, Huh SO, Jung JS, Ihn BM, Choi IG, Kim YH 1998 Central GABAA and GABAB receptor modulation of basal and stress-induced plasma interleukin-6 levels in mice. J Pharmacol Exp Ther 287:144–149 [PubMed] [Google Scholar]
- Masotto C, Caspani G, De Simoni MG, Mengozzi M, Scatturin M, Sironi M, Carenzi A, Ghezzi P 1992 Evidence for a different sensitivity to various central effects of interleukin-1β in mice. Brain Res Bull 28:161–165 [DOI] [PubMed] [Google Scholar]
- Craft TK, DeVries AC 2006 Role of IL-1 in poststroke depressive-like behavior in mice. Biol Psychiatry 60:812–818 [DOI] [PubMed] [Google Scholar]
- Armario A, Hernández J, Bluethmann H, Hidalgo J 1998 IL-6 deficiency leads to increased emotionality in mice: evidence in transgenic mice carrying a null mutation for IL-6. J Neuroimmunol 92:160–169 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bell ME, Bhatnagar S, Choi S, Chu A, Gomez F, Laugero K, Soriano L, Viau V 1999 Warning! Nearby construction can profoundly affect your experiments. Endocrine 11:111–113 [DOI] [PubMed] [Google Scholar]