Abstract
Serine/arginine-rich (SR) proteins play essential roles in the constitutive and regulated splicing of precursor mRNAs. Phosphorylation of the arginine/serine dipeptide-rich (RS) domain by SR protein kinases such as Cdc2-like kinases (Clk/Sty) modulates their subcellular localization and activation. However, it remains unclear how these kinases and their target SR proteins are regulated by extracellular signals. Regulation of protein kinase C βII (PKCβII) pre-mRNA alternative splicing via exon inclusion by Akt2, a central kinase in insulin action, involves phosphorylation of SR proteins. Here we showed that Akt2, in response to insulin, resulted in phosphorylation of Clk/Sty, which then altered SR protein phosphorylation in concert with Akt2. Insulin-stimulated PKCβII pre-mRNA splicing was blocked by Clk/Sty and phosphatidylinositol-3-kinase inhibitors, and diabetic Akt2-null mouse tissues had impaired phospho-Clk/Sty, SR protein phosphorylation, and PKCβII expression. Furthermore, we observed that Akt2 phosphorylated several SR proteins distinct from Clk/Sty in response to insulin. Akt2-catalyzed phosphorylation of Clk/Sty and SR proteins revealed a role for both kinases in splicing regulation indicating dual functions for Akt2 in response to insulin in this pathway.
Insulin regulates Cdc2-like kinases (Clk/Sty) via phosphorylation by Akt2, thereby promoting the pivotal role of both kinases in insulin-mediated RNA alternative splicing and glucose uptake.
Alternative pre-mRNA splicing provides a versatile mechanism in gene expression and proteome diversity. It is also a means of diversifying signaling pathways when kinases, such as protein kinase C (PKC) β, are the target genes. Phosphorylated serine/arginine-rich (SR) proteins function as exon splicing enhancers and repressors within the spliceosomal complex. This family of proteins plays pivotal roles in constitutive and regulated splicing (1), because they are located at the center of the regulatory pathways that lead to the selection of alternative splice sites. The various kinases that regulate the phosphorylation, intranuclear distribution, and protein-protein interactions of SR proteins play key roles in selection of alternative splice sites (1,2,3). Recently, Akt2 or PKB, an oncogene originally isolated from retrovirus AKT8-induced mouse thymic lymphoma, was shown to regulate alternative splicing (4,5). Additionally, SR protein-specific kinases (SRPK), CDC2-like kinases (Clk), cAMP-dependent protein kinase (PKA), and PKC all phosphorylate splicing factors containing Arg/Ser repeat motifs and have the potential to regulate splicing (6,7,8). The Clk kinases participate in a network of regulatory mechanisms that enable SR proteins to control RNA splicing in response to phosphorylation (7,8,9,10).
Clk/Sty kinase controls splicing during cellular proliferation and differentiation (11,12,13). Clk/Sty (Clk1) and Clk2, Clk3, and Clk4 are phosphorylated on serine/threonine and tyrosine residues (7), and they contain Akt-substrate motifs, implying that Clk could be regulated by the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and that this alters their substrate specificity. However, no upstream kinase has been shown to regulate Clk/Sty function, thus limiting our understanding of its regulatory roles.
The unique mechanism that underlies alternative splicing of PKCβ pre-mRNA has been well documented in mammalian cells and animal models (14,15,16,17); these studies implicated Clk/Sty in splicing of PKCβII mRNA, a step necessary for insulin action (18,19,20). Insulin stimulates the inclusion of an exon encoding the C-terminal domain of PKCβII; PKCβI is the other splice variant. The pathway involves PI3K/Akt2-mediated phosphorylation of multiple SR proteins, including SRp40 (5). SR proteins, within their Arg/Ser domains, contain the highest number of Akt consensus motifs found in proteins (21). Regulation of alternative splicing and gene expression by PDK/Akt provides a unique modulation of growth factor action at the level of extracellular signaling (22,23,24). Because Clk/Sty contains two Akt consensus motifs, Arg-Xaa-Arg-Xaa-Xaa-(Ser/Thr), we hypothesized that its activity might be regulated by insulin and, furthermore, that Akt phosphorylation of Clk/Sty was necessary for the regulation of PKCβII exon inclusion.
Materials and Methods
Materials
Tissue culture medium was purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Norcross, GA). Porcine insulin was from Sigma Chemical Co. (St. Louis, MO). Reagents for polyacrylamide gel electrophoresis were from Bio-Rad (Hercules, CA). SRp75 polyclonal antibody was raised in rabbits to the synthetic peptide NH2-(GC)KDHAEDKLQNNDSAGKAK-COOH (residues 119–136), SRp30b/SC35 antibody to the synthetic peptide NH2-CRRVGDVYIPRDRYTKE-COOH (residues 38–53), and SRp55 antibody to the synthetic peptide NH2-CGERVIVEHARGPRRDRD-COOH (residues 61–78) by Bio-Synthesis, Inc. (Louisville, TX). Antibodies were characterized alongside unreactive preimmune antisera and were shown to recognize rat, mouse, and human cell line proteins. Antibody to a phospho-epitope in all SR proteins (mAb104) was obtained from hybridoma cells (CRL 2067; American Type Culture Collection, Manassas, VA). Anti-phospho-Akt (Ser473 and Akt antibody) and anti-Akt substrate (RXRXXS/T) antibody were from Cell Signaling (Beverly, MA). Anti-Clk/Sty antibody was kindly provided by Dr. James Manley (Columbia University, New York, NY). Enhanced chemiluminescence reagents were from Pierce (Rockford, IL). Taq Platinum polymerase was from PerkinElmer Life Sciences (Norwalk, CT). Lipofectin was from Invitrogen. PI3K inhibitor LY294002 and Src kinase inhibitor PP1 were purchased from Calbiochem (La Jolla, CA). All other chemicals and reagents were purchased from the usual vendors. PP1 is preincubated with cells for 30 min before addition of insulin. LY294002 and PP1 were preincubated with cells for 30 min before addition of insulin.
Cell lines and Akt2-null tissues
L6 rat skeletal myoblasts were obtained from Dr. Amira Klip (The Hospital for Sick Children, Toronto, Canada) and were grown in α-MEM containing 10% FBS at 37 C in a humidified 5% CO2, 95% air atmosphere in either six-well or 100-mm plates. Cells were grown to 80% confluency and induced to differentiate into multinucleated myotubes by reducing the FBS to 2% for 6 d before the experiment. Cells were then washed with PBS and incubated in serum-free α-MEM for 6 h before each experiment. Porcine insulin (Sigma) was used at a final concentration of 10 nm and, unless otherwise stated, was added to the cells 30 min before cell lysis. COS7 cells were grown in DMEM containing 10% FBS as stated above.
Akt2-null mouse tissues were provided by Dr. Morris J. Birnbaum (Howard Hughes Medical Institute, Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA). Akt2-null and wild-type tissues were obtained from fasted 24 week old male animals raised at the Chemerie Mouse Facility at University of Pennsylvania, an AALAC accredited facility. Akt2-null mice were impaired in the ability of insulin to lower blood glucose because of defects in the action of the hormone on liver and skeletal muscle (25).
Transient transfection of plasmid DNA or small interfering RNA (siRNA)
Hemagglutinin (HA)-Clk/Sty-expressing cDNA plasmid was obtained from Dr. James Manley (Department of Biology, Columbia University, New York, NY) (13). HA-Akt2 has been described previously (5,26). Plasmid DNA or siRNA (Ambion, Austin, TX; series 1: Clk1 ID#161218, Akt2 ID#200808; series 2: Alk1 ID#16704 and Akt2 ID#200807) was transfected into cells at 60–80% confluency using Lipofectin (Invitrogen) or siPORT Amine (Ambion) for 5 h as per manufacturer’s instructions. Cells were then maintained in α-MEM with 2% FBS for 30 h and serum starved 6 h before insulin addition. Expression of exogenous genes was demonstrated by Western blot analysis using the appropriate antibody. The transfection efficiency of L6 myotubes was routinely shown to be above 60% (19).
Immunoprecipitation
L6 myotubes, COS7 cell pellets, or mouse tissue samples were lysed in 20 vol 150 mm NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 50 mm Tris (pH 8.0) with the following protease inhibitors: benzamidine HCl, 16 μg/ml; aprotinin, 10 μg/ml; leupeptin; 10 μg/ml; and phenylmethylsulfonyl fluoride, 1 mm. The mixtures were placed on ice for 30 min, and insoluble material was pelleted at 12,000 × g for 10 min at 4 C. An aliquot (500 μg) of lysate was incubated at a final concentration of 1 μg/μl with anti-Clk/Sty polyclonal antibody followed by agitation at 4 C for 2 h. A 40-μl aliquot of protein A-Sepharose beads in a 1:1 suspension with the lysis buffer was added and incubated again for 1 h. After centrifugation at 10,000 × g, beads were washed four times with 1 ml lysis buffer containing 0.5 m NaCl, followed by a wash in lysis buffer with no NaCl. After adding 50 μl SDS-PAGE sample buffer, the precipitate was boiled for 5 min and centrifuged at 1000 rpm for 5 min before loading on the gel followed by Western blot analysis.
Western blot
Cell or tissue lysates (40 μg) or immunoprecipitates obtained from 500 μg lysates were separated on 10% SDS-PAGE. Proteins were electrophoretically transferred to nitrocellulose membranes, blocked with Tris-buffered saline-0.1% Tween 20 containing 5% nonfat dried milk, washed, and incubated with a polyclonal antibody against SRp75, SRp55, SRp30b, or mAb104, a monoclonal antibody against the phospho-epitope (RS domain) of SR proteins (27), Akt, anti-phospho-Akt, anti-phospho-ERK1/2 (Cell Signaling), anti-HA (Roche, Indianapolis, IN), or anti-Clk/Sty (Dr. James Manley, Columbia University, New York, NY). Anti-phosphotyrosine antibody 4G10 was purchased from Upstate (Lake Placid, NY). After incubation with secondary antirabbit or antimouse IgG-horseradish peroxidase, detection was performed using enhanced chemiluminescence (Pierce). In all cases, Western blot results were verified in two to four separate experiments.
RT-PCR analysis for PKCβII
Splice products were detected as reported earlier (5). Briefly, total RNA (1 μg) was used to synthesize first-strand cDNA using an oligo(dT) primer and Omniscript II reverse transcriptase (QIAGEN, Valencia, CA). Inclusion of the PKCβII-specific exon was detected using a sense primer corresponding to the V3 domain 5′-ATGAAACTGACCGATTTTAACTT-3′ and antisense primer (βII specific) for V5 domain (5′-CGGAGGTCTACAGATCTACTTAGCTCT-3′). After 35 cycles of the PCR amplification program (95 C for 30 sec, 54 C for 30 sec, and 72 C for 45 sec) using Taq Platinum DNA polymerase (PerkinElmer), 5% of the PCR was resolved by electrophoresis on 6% polyacrylamide gels, and fragments were detected by silver staining. The resulting product was 1044 bp. The reaction was optimized for linear range amplification. The Kodak (Rochester, NY) Digital Analysis System 120 was used to visualize PCR products. The results were verified in two separate experiments. PCR fragments were quantified using UnScan software (Silk Scientific, Orem, UT).
Measurement of insulin-stimulated glucose transport by 2-deoxyglucose uptake
L6 myoblasts were grown and differentiated as described above in 24-well plates. Cells were then washed with PBS and incubated in serum-free α-MEM for 4 h before each experiment. The inhibitor was added 2 h before each experiment. Cells were then washed and incubated in PBS with 1% BSA at 37 C with inhibitors and/or 50 nm insulin (Sigma) 30 min before the addition of 10 nmol [3H]2-deoxyglucose (50–150 μCi/μmol) and incubation for 6 min at 37 C. Cells were then washed three times with ice-cold PBS and lysed in 1% sodium dodecyl sulfate. Radioactivity was determined by liquid scintillation counting.
Results
A role for Clk/Sty in splicing of PKCβ mRNA
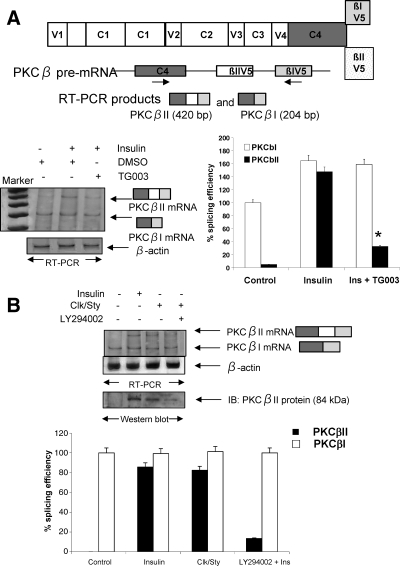
PKCβI and PKCβII are the products of alternative splicing of PKCβ pre-mRNA. The last common exon of the C4 domain (exon 16) splices to the βIV5 exon (exon 18) to produce PKCβI mRNA, which is constitutively expressed. Insulin regulates the inclusion of PKCβII exon (exon 17) between the C4 and βIV5 domains, thereby producing mature PKCβII mRNA. The βI exon then becomes 3′-untranslated region on PKCβII mRNA due to a stop codon within the βII exon. Insulin exerts some of its effects on alternative splicing via Akt2 phosphorylation of SRp40, a nuclear splicing factor (5). The notion that Clk/Sty might also be involved in the insulin response was suggested from the observation that the splicing of PKCβII pre-mRNA is accompanied by phosphorylation of multiple SR proteins (14), and Clk/Sty influences the activity of SR proteins (11,12,13). Our previous studies using a heterologous PKCβII splicing minigene demonstrated that βII exon inclusion is regulated by insulin and minigene exon inclusion is mimicked by Clk/Sty overexpression (14,15,16,17). Here, the selective Clk/Sty inhibitor TG003 was used to block its activity in L6 myotubes to demonstrate a role for the kinase in insulin action. TG003, a benzothiazole kinase inhibitor that targets Clk/Sty, is capable of suppressing dissociation of nuclear speckles, altering splicing patterns, and rescues embryonic defects induced by excessive Clk activity (28). Dimethylsulfoxide (DMSO), the solvent control, did not affect PKCβII splicing. TG009, the control compound (28), also did not affect splicing (data not shown). TG003 blocked insulin-stimulatory effects on βII exon inclusion (Fig. 1A). This suggested that Clk/Sty was involved in the splicing signal cascade.
Figure 1.
Role of Clk/Sty and Akt2 in PKCβII exon inclusion. A, Schematic shows PKCβ domains and C-terminal alternative exons. Arrow below exons indicates the primer positions on PKCβ pre-mRNA. The primer set allowed for simultaneous detection of endogenous PKCβI mRNA and βII mRNA (in which βII exon is included between C4 and βI exon). After serum starvation for 6 h, differentiated L6 myotubes were treated with TG003 (10 μm) for 60 min before insulin addition (10 nm, 30 min). DMSO served as the solvent control. Total RNA was extracted and 2 μg analyzed for PKCβI and PKCβII by RT-PCR using primers corresponding to the C4 and βI/V5 exon as shown on the diagram. β-Actin was amplified as an internal control. Clk/Sty inhibitor blocked insulin-stimulated PKCβII exon inclusion. The graph represents three separate experiments analyzed by RT-PCR. *, P < 0.05 by Student’s t test. B, Clk/Sty-induced PKCβII exon inclusion was blocked by the PI3K inhibitor LY294002. L6 myotubes were transfected with Clk/Sty cDNA-expressing construct for 36 h and treated with insulin (Ins; 10 nm, 30 min) in the presence or absence of LY294002 (10 μm, 30 min). Endogenous PKCβI and PKCβII mRNA levels were amplified by RT-PCR as described in A. Cell lysates from duplicate plates were analyzed for protein levels of PKCβII by Western blot analysis after SDS-PAGE. The graph is representative of three RT-PCR analyses. Percent splicing efficiency is calculated as a ratio of PKCβII/βI mRNA with control PKCβI mRNA assigned a value of 100%.
Cells overexpressing Clk/Sty increased exon 17 (βII exon) inclusion (Fig. 1B). PKCβII protein expression (Western blot in Fig. 1B) was also elevated in a manner similar to that of insulin. When cells overexpressing Clk/Sty were treated with LY294002, an inhibitor known to block Akt2 activation and Akt2-mediated exon inclusion (11), βII exon inclusion was blocked (Fig. 1B), suggesting that Akt2 was acting upstream of Clk/Sty. Densitometric scans of the PCR products indicated the block of exon inclusion by LY294002. PKCβI levels were not changed, as noted in earlier studies demonstrating the primary effect of insulin to be on exon inclusion rather than transcription (14).
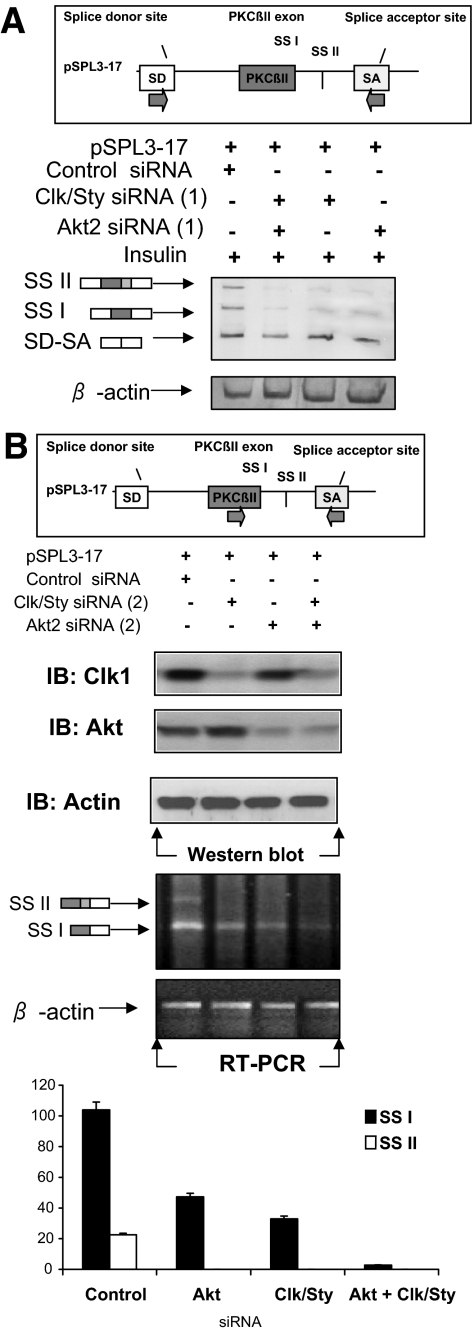
Splicing was also assessed with a heterologous minigene containing the PKCβII exon and its flanking intronic sequences. To provide evidence that the Clk/Sty inhibitor effects may be explained by its inhibitory actions on Clk/Sty, Clk/Sty and Akt2 siRNA were used to lower intracellular kinase levels in combination and alone in the presence of insulin. Insulin-induced inclusion of the PKCβII exon in the heterologous minigene was blocked more than 90% in the presence of both Clk/Sty and Akt2 siRNA and more than 75% in the presence of either kinase siRNA alone (Fig. 2A). Insulin also activates a second splice site, SSII, which is easier to detect with the minigene (17). The combined siRNA reduced levels of SSII product entirely. This suggested that there are differences in splice site regulation with regard to Akt2 and Clk/Sty. A second series of Clk/Sty and Akt2 siRNA confirmed the siRNA lowered the respective kinase levels, and the effects on splicing were not likely due to off-target knockdown by the complementary strand of DNA (Fig. 2B). The partial activation of an alternative 5′ splice site was still noted with Clk/Sty and Akt2 siRNA (series 2), suggesting roles for both kinases. Activation of both 5′ splice sites is also regulated by insulin (17), although only one protein is translated because there is a stop codon upstream of the first splice site. The longer mRNA is predicted to be more stable because it would not be subject to degradation (29). The inhibition of Clk/Sty and Akt2 blocked alternative exon inclusion and not merely splicing, because SD-SA splicing (vector splice sites) was not affected.
Figure 2.
Clk/Sty and Akt2 siRNA block splicing of heterologous minigene. A, Heterologous splicing vector and siRNA. L6 myotubes were cotransfected with pSPL3-17 splicing vector (18), Clk/Sty, and/or Akt2 siRNA (Silencer siRNA 161218 and 200808 or the control siRNA, 15 nm for 48 h) with Lipofectamine. After 6 h serum starvation, cells were treated with insulin (10 nm, 30 min). Total RNA was extracted, and PKCβII exon inclusion was analyzed using primers to the splice site donor and acceptor (18) as shown. SSI, Utilization of PKCβII exon 5′ splice site I; SSII, utilization of PKCβII exon 5′ splice site II; SD (splice donor)-SA (splice acceptor), constitutive splicing of pSPL3 splicing vector, which also serves as an internal control. The effectiveness of the siRNA (series 1) in lowering protein levels is shown in Fig. 4. The densitometric scan of the image indicates that the combined Clk/Sty and Akt2 siRNA were additive in blocking splice site 1. B, A second set of Clk/Sty siRNA and Akt2 siRNA also blocked splicing. L6 myotubes were cotransfected with pSPL3-17 splicing vector (18), Clk/Sty, and/or Akt2 siRNA (Silencer siRNA 161219 and 100807 or the control siRNA, 15 nm for 48 h) with Lipofectamine and treated as described above. The effectiveness of the siRNA in lowering protein levels is shown on the top, and the splicing of the minigene in the presence of both siRNA is shown on the bottom.
Phosphorylation of Clk/Sty by Akt
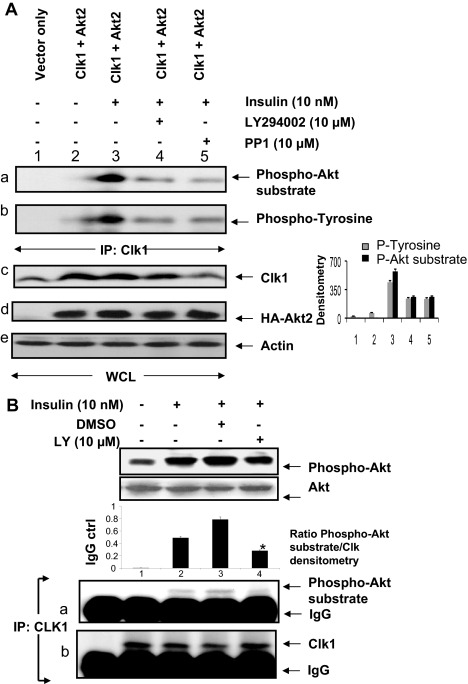
The possibility of Akt2 regulating Clk/Sty was examined because LY294002 blocked Clk-induced βII exon inclusion in Fig. 1B. Both serine and tyrosine phosphorylation of Clk/Sty are required for its full activation (11,12,13). However, the kinases regulating its activity have not been identified. The possible phosphorylation and activation of Clk/Sty by PI3K/Akt2 and Src tyrosine kinases was examined in COS7 cells. COS7 cells are easily transfected, and insulin signaling pathways have been demonstrated for them (30). COS7 cells were cotransfected with HA-tagged Akt2 and HA-tagged Clk/Sty before treatment with LY294002 or PP1 (an inhibitor of Src kinases), and then stimulated with insulin. Clk/Sty immunoprecipitates were analyzed by immunoblot using an antibody that specifically detects phosphorylation of serines in the Akt substrate motif and with another antibody that detects phosphorylated tyrosine residues. Insulin stimulated both Akt-motif serine and tyrosine phosphorylation of Clk/Sty (Fig. 3A, a and b), and the insulin-stimulated phosphorylation was suppressed by the PI3K inhibitor LY294002 and by the Src kinase inhibitor PP1 (lanes 4 and 5), respectively. Insulin was required to activate transfected Akt2 because in the absence of insulin, there was no phosphorylation of Clk (Fig. 3A, a and b, lane 2). It was also noted that there may be cross talk between Akt and Src kinases because the Src inhibitor PP1 blocked phosphorylation on Akt motifs and LY294002 blocked phosphotyrosine phosphorylation. The densitometric scan of the blot indicated the significance of the inhibition by PP1 and LY294002. Insulin is known to activate Src in skeletal muscle (31). Hence, Clk/Sty was a substrate for Akt in vivo, and insulin coordinated both serine and tyrosine phosphorylation of Clk/Sty.
Figure 3.
Akt2 involvement in Clk/Sty activation and Clk/Sty-mediated SR protein phosphorylation. A, Transiently expressed Clk/Sty was phosphorylated by Akt2. COS7 cells were transiently transfected with Clk/Sty, Akt2, or pcDNA3 control vector constructs for 36 h with Lipofectamine. After serum starvation for 6 h, cells were stimulated by insulin (10 nm, 30 min), and whole-cell lysates were immunoprecipitated with anti-Clk/Sty (IP: Clk1). The phosphorylation of tyrosine residues and Akt-substrate serine residues on Clk/Sty was analyzed by Western blot using anti-phosphotyrosine or anti-phospho-Akt substrate antibodies (top panels a and b). Whole-cell lysates run on gels were probed for Clk1, Ha-Akt2, and actin (lower panels c, d, and e). The graph to the right shows a densitometric scan of the Clk1 IP probed with anti-phospho-Akt substrate (black bars) and anti phosphotyrosine (gray bars). (For this and subsequent figures, the P value was determined using a two-tailed unpaired Student’s t test.) Lanes 4 and 5 were significantly (P < 0.005; n = 3) inhibited compared with lane 3. This experiment was repeated on three occasions with similar results. B, Endogenous Clk/Sty was phosphorylated by Akt2 during insulin signaling. L6 myotubes were serum starved, insulin stimulated, and lysed (WCL); Clk/Sty was immunoprecipitated and analyzed for phosphorylation of Akt-substrate serine residues as above (lower panels). Due to the difference in antibody source (polyclonal vs. HA-tagged), IgG is detected. The graph above the blot represents the densitometric scans for Clk1 IP probed with phospho-Akt substrate antibody. Inhibition of Clk phosphorylation in lane 4 was significant (P < 0.005; n = 3) compared with lane 3. Phosphorylation of Akt induced by insulin was confirmed with phospho-Akt-473 antibody (top panel).
Because the Clk antibody in Fig. 3A immunoprecipitated both endogenous Clk/Sty as well as overexpressed Clk, we then examined whether endogenous Clk/Sty was phosphorylated on its Akt substrate motifs in L6 myotubes. Increased phosphorylation of endogenous Clk/Sty immunoprecipitates was detected by the Akt substrate antibody, and phosphorylation was blocked by LY294002 (Fig. 3B, a). The IgG band was evident because a polyclonal antibody was used. It is evident that Clk/Sty was a substrate for insulin-activated Akt signaling in both transfected COS7 cells and in cultured L6 myotubes.
SR proteins as Clk/Sty substrates
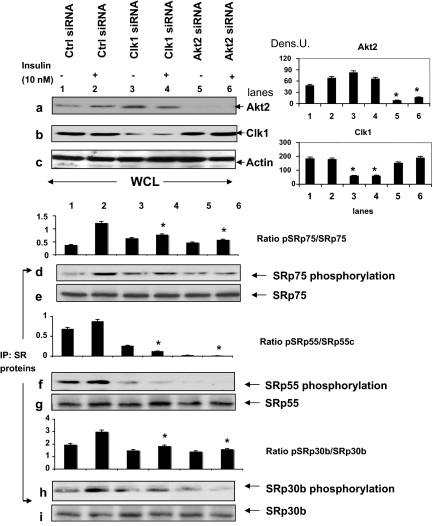
We hypothesized that both Akt2 and Clk/Sty participated in the phosphorylation of SR splicing factors. To determine whether insulin-activated Akt2 modulated Clk/Sty-mediated SR protein phosphorylation, we examined the effects of Clk/Sty- and Akt2-specific siRNA on SR protein phosphorylation (Fig. 4). Because insulin increased the intensity of phosphorylated SR proteins when detected with mAb104, we reasoned that the motif recognized by this antibody, Arg/Ser-rich regions, would be specific to Clk/Sty and Akt2. Clk/Sty- and Akt2-specific siRNA suppressed protein levels of Clk/Sty or Akt2, respectively, in transfected L6 myotubes (top panels a–c using whole-cell lysates). SRp75, SRp55, and SRp30b/SC35, identified previously as substrates for insulin-induced phosphorylation (15), were immunoprecipitated from L6 cell lysates after insulin stimulation and then separated by SDS-PAGE. Clk/Sty- or Akt2-specific siRNA-induced depletion (series 1 siRNA shown and data from series 2 not shown but corroborating results) markedly suppressed insulin-induced phosphorylation of SRp75, SRp55, and, to a lesser extent, SRp30b/SC35 (Fig. 4, d, f, and h). The identities of these SR proteins were confirmed by reprobing the same membranes with the corresponding SR protein antibodies, namely SRp75, SRp55, and SRp30b/SC35 (Fig. 4, e, g, and i). These results indicated that both Akt2 and Clk/Sty participate in SR protein phosphorylation. Because pre-mRNA splicing of PKCβII was also altered by Akt2 and Clk/Sty as shown earlier (Figs. 1 and 2), SR protein phosphorylation is likely to be the next step in the Akt2/Clk cascade.
Figure 4.
Akt2 and Clk/Sty siRNA suppress insulin-induced SR protein phosphorylation on RS domains. L6 cells were transfected with Clk/Sty, Akt2, or control (Ctrl) siRNA for 48 h as indicated in Fig. 2 (series 1), serum starved (6 h), insulin stimulated, and then lysed. Effectiveness of the siRNA was examined on protein levels with anti-Clk/Sty and anti-Akt2 (top panels). The graphs to the right are densitometric scans of the blots indicated and demonstrate significant (P < 0.05) reduction of Akt2 and Clk1 by the appropriate siRNA. SR proteins were individually immunoprecipitated and immunoprecipitates (IP: SR proteins) examined for phosphorylation of RS domains by mAb104 (lower panels). The identities of SR proteins in the immunoprecipitates were confirmed by stripping the blot and reprobing with appropriate SR protein antibodies. Graphs represent phospho-SR/SR protein (pSRp/SRp) ratio specified. The experiment was repeated twice. The inhibition of SR protein phosphorylation by Clk1 siRNA and Akt2 siRNA was significant (P < 0.05) for each SR protein compared with the insulin-stimulated sample (lane 2). A second set of siRNA to Clk/Sty and Akt2 also blocked SR protein phosphorylation (data not shown). Dens. U, Arbitrary densitometry unit.
Akt2 modulates Clk/Sty-mediated SR protein phosphorylation
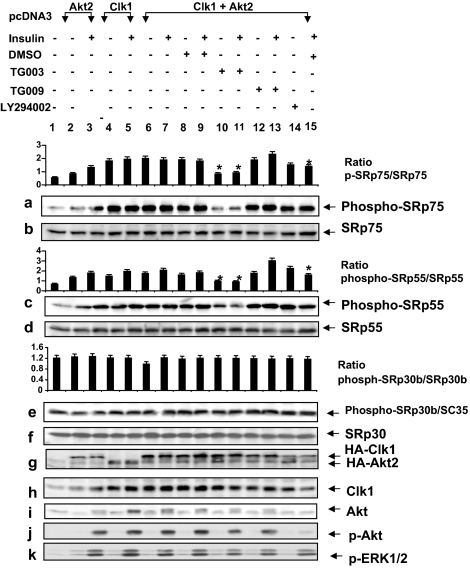
Akt2 phosphorylated Clk/Sty, and it activated Clk/Sty-mediated SR protein phosphorylation and βII exon inclusion in response to insulin stimulation (Figs. 1, 3, and 4). To distinguish between the effects of PI3K/Akt2 on Clk/Sty activation and SR protein phosphorylation, a Clk inhibitor was used. Clk/Sty and Akt2 were cotransfected into L6 myotubes and the cells treated with DMSO, a control, a PI3K inhibitor (LY294002), the Clk inhibitor (TG003) (28), or its control compound, TG009, before insulin stimulation. Akt2 rather than the constitutively active Akt2 was used so that insulin activation of phosphorylation could be demonstrated. Both HA-tagged Akt2 and HA-tagged Clk/Sty were overexpressed at detectable levels, demonstrated by anti-HA, anti-Akt, and anti-Clk/Sty immunoblotting (Fig. 5, g–i). Insulin induced the phosphorylation of Akt in transfected L6 myotubes (Fig. 5j) and serine phosphorylation was blocked by the PI3K inhibitor LY294002 (Fig. 5j, lane 15). The role of Akt2 in promoting phosphorylation that was detected by mAb104 antibody here was likely mediated through Clk, although other kinases cannot be ruled out.
Figure 5.
Akt2 regulates Clk/Sty-mediated SR protein phosphorylation in insulin signaling. L6 myotubes were transfected with pcDNA3 vector, Akt2, Clk/Sty, or Akt2 plus Clk/Sty constructs as indicated for 36 h, serum starved for 6 h, and then treated with Clk/Sty-specific inhibitor TG003 (10 μm) or control compound TG009, LY294002 (10 μm), or DMSO for 60 min before insulin stimulation (10 nm, 30 min), lysed, and analyzed for SR protein phosphorylation with mAb104 in addition to evaluation of the expression of the transfected genes (g), phospho-Akt (p-Akt) and phospho-ERK1/2 (p-ERK1/2; j and k). Identities of the SR proteins were confirmed by reprobing the stripped blots with the appropriate antibodies. The graph above each SR protein is the ratio of phospho-SR/SR protein (p-SRp/SRp). *, Inhibition of phosphorylation by TG003 or LY294992 is significant (P < 0.05; n = 3) using a two-tailed unpaired t test compared with lanes 7 and 9 with insulin.
Phosphorylation of ERK1/2 (a MAPK pathway also activated by insulin) was similarly induced by insulin, but its phosphorylation was not affected by either the Clk inhibitor TG003 or the PI3K inhibitor LY294002 (Fig. 5k), confirming that both inhibitors were specific for their target kinases. TG009, a control compound for TG003, had no effect (Fig. 5, a, c, and e, lanes 12–13). Importantly, when transfected alone, both Akt2 and Clk/Sty increased the serine phosphorylation of SRp75 and SRp55 (Fig. 5, a and c, lanes 3 and 5), as detected by mAb104, which recognizes phosphoserines in Arg/Ser regions.
Clk/Sty appears to be the stronger kinase, enhancing serine phosphorylation, because a marked reduction in phosphorylation occurred in the presence of its specific inhibitor. Cotransfection of Clk/Sty and Akt2 increased the serine phosphorylation of SRp75 and SRp55, which was suppressed best by TG003 (Fig. 5, a and c, lanes 10–11). The inhibition of phosphorylation was less dramatic with LY294002 (Fig. 5, a and c, lane 14) but significant in the presence of insulin (P < 0.05) (Fig. 5, a and c, lane 15). Increases in phosphorylation induced by Akt2 or Clk/Sty in SRp30b/SC35 were less dramatic (Fig. 5e), and the sensitivities to PI3K and Clk/Sty inhibitors were much weaker compared with that found for SRp75 and SRp55, indicating that SRp30b/SC35 was not a preferred substrate in the insulin pathway. Clk/Sty regulation induced by Akt2 (Fig. 5, a and c) is consistent with the data shown in Fig. 4 using Clk/Sty and Akt2 siRNA to specifically reduce protein levels. In addition, inhibition of SR protein phosphorylation by the PI3K inhibitor LY294002 was not as dramatic as that of the Clk inhibitor TG003 (Fig. 5, a and c), suggesting that there may be a basal activity of Clk/Sty or an alternate cascade for Clk/Sty activation. However, our data clearly indicate that Akt2 and Clk/Sty are both required for full insulin-induced phosphorylation of SRp75 and SRp55, and when viewed with the splicing data in Figs. 1B and 3, Clk/Sty appeared to be acting downstream of PI3K/Akt2 in modulating SR protein phosphorylation. These studies demonstrated that insulin also regulated SRp75 and SRp55 phosphorylation via Clk/Sty signaling.
PI3K/Akt2 regulates serum-induced, Clk/Sty-mediated SR protein phosphorylation
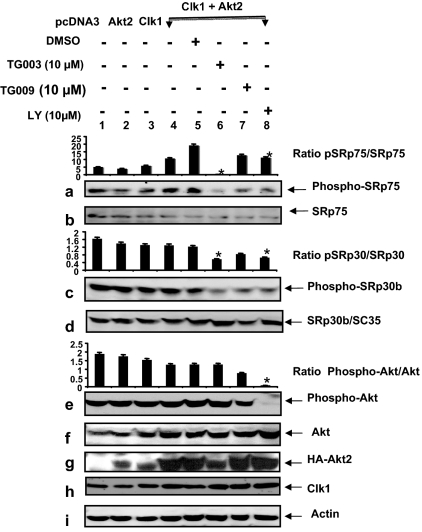
Insulin regulation of splicing is best demonstrated after serum starvation. As shown so far (Figs. 1–5), PKCβII exon inclusion in response to insulin stimulation was blocked by the Clk/Sty inhibitor and its siRNA, and by knocking down and inhibiting Akt2, a potential activator of Clk/Sty. The SR protein targets of Clk/Sty identified here were SRp75, SRp55, and SRp30b. To ascertain whether Akt2 and Clk/Sty regulation of phosphorylation and resulting activation of SR splicing factors was specific to insulin or represented a universal phenomenon, we examined whether SR proteins were substrates for Akt2-regulated Clk/Sty activation under culture conditions in the presence of 10% fetal calf serum, conditions that promote cell growth.
Clk/Sty and Akt2 were transfected into differentiated L6 cells before treatment with LY294002 or TG003 (32). In this case, we examined SRp75 and SRp30b. The cotransfection increased the serine phosphorylation of SRp75 as detected by mAb104 (Fig. 6a, lanes 4 and 5), and this was blocked by TG003 and LY294002 (lanes 6 and 8). The control, TG009, also had an inhibitory effect but was not as great as TG003 (Fig. 6a, lane 7). The possibility that TG009 may inhibit Akt or another upstream kinase under serum conditions was a possibility (Fig. 6e, lane 7). With serum present, SRp30b/SC35 appeared to be phosphorylated in a Clk/Sty- and Akt2-dependent manner with respect to TG003- and LY294002-mediated inhibition (Fig. 6c). Simultaneous analysis of Akt phosphorylation demonstrated an inhibition by LY294002 but no alteration by TG003 (Fig. 6e, lane 6). Importantly, cotransfected Akt2 enhanced Clk/Sty-mediated SRp75 phosphorylation, which was only partially blocked by LY294002, and especially by TG003, and demonstrated a requirement for Akt2 in Clk/Sty activation (Fig. 6a, lane 2 vs. 4, 6, and 8). Akt2 and Clk/Sty transfection caused no changes in SR protein levels (Fig. 6, b and d). Control blots for Akt2, Clk/Sty, HA, and actin supported the changes observed on protein phosphorylation and indicated that Clk/Sty was endogenously expressed in L6 cells (Fig. 6, f, h, and i). Hence, Akt2 enhanced the action of Clk/Sty on SRp75, suggesting a PI3K/Akt2→Clk/Sty connection was involved in the regulation of SRp75 phosphorylation induced by serum. These data demonstrated a requirement for PI3K/Akt2 in Clk/Sty phosphorylation of SRp75 and SRp30b/SC35 in response to serum. Compared with insulin, serum induced a much stronger phosphorylation on SRp30b/SC35 (Figs. 5e and 6c) because TG003 did not block insulin phosphorylation of SRp30b in Fig. 5e (lanes 10–11), suggesting that this serum-initiated, Akt2-mediated Clk/Sty pathway was distinct from the one stimulated by insulin, i.e. insulin and serum induce phosphorylation of different SR proteins. Thus, SRp30b/SC35 appears not to be a preferred substrate for Clk/Sty during insulin stimulation.
Figure 6.
Akt2 regulates SR phosphorylation both by itself and by stimulating Clk/Sty-mediated action in response to serum and insulin. L6 myotubes were transfected with pcDNA3 vector, Akt2, Clk/Sty plasmids, or Akt2 plus Clk/Sty plasmids as indicated for 36 h in 10% serum and treated with TG003, LY294002 (LY; 10 μm), or DMSO for 60 min. Cells were then lysed and analyzed with mAb104 by Western blot. Cell lysates were also evaluated for expression of transfected genes, phospho-Akt, SR protein levels, and β-actin. Specific SR proteins were identified by reprobing the blots with antisera after stripping mAb104 as indicated. The graphs are the ratio of the phosphorylated protein/protein for SRp75 (pSRp75/SRp75), SRp30b (pSRp30/SRp30), and phospho-Akt. *, Significant (P < 0.05; n = 3) inhibition of phosphorylation compared with the control lane 5.
SR proteins as direct substrates for Akt2
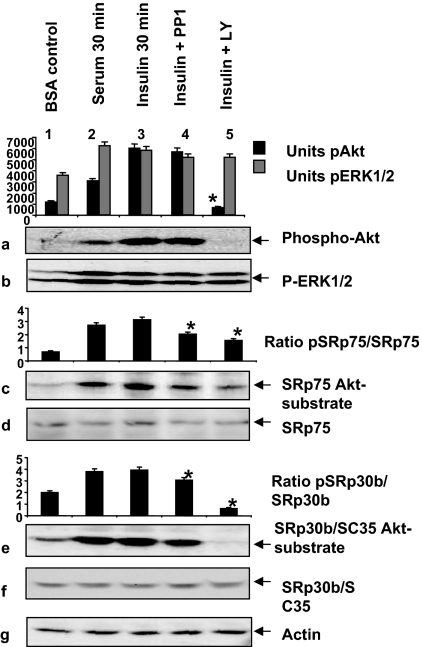
SR proteins have considerably more Akt phosphorylation motifs than Clk/Sty phosphorylation motifs. These motifs are all within the Arg/Ser domains. Thus, the potential for Akt2 to directly phosphorylate SR proteins on multiple sites is high. We examined Akt2-mediated phosphorylation of SR proteins in response to insulin stimulation, using an antibody for the phosphorylated Akt substrate motif. The antibody provides data similar to that of the conventional in vitro kinase assays for Akt2 as demonstrated by the effects of insulin on SRp40 phosphorylation (5). The antibody technique may even be preferable, because in vitro kinase assays cannot rule out the possibility of interacting kinases as we have described here. Insulin- and serum-stimulated serine phosphorylation on Akt-substrate motifs in SRp75 and SRp30b/SC35 (Fig. 7, c and e). Inhibition of PI3K/Akt signaling blocked this phosphorylation (lane 5); Src kinase inhibitor PP1 also reduced the intensity of phosphorylation, suggesting that there was further fine tuning of SR protein phosphorylation by Src family members (Fig. 7, c and e, lane 4). The results indicated that these SR proteins were also directly controlled by Akt2, as had been suggested by previous reports (5,21,33).
Figure 7.
Akt2 phosphorylates SR proteins in response to insulin and serum. L6 myotubes were serum starved, pretreated with 10 μm LY294002 or tyrosine kinase inhibitor PP1 for 60 min, and then stimulated with serum or insulin for 30 min. The cells were lysed and analyzed for Akt-mediated phosphorylation of SR proteins, Akt and ERK1/2 phosphorylation by Western blot similar to what was described in Fig. 6. The densitometric scan is plotted as a bar graph for units of phospho-Akt (pAkt) and phospho-ERK1/2 (P-ERK1/2). The inhibition of phospho-Akt by LY294992 is significant (P < 0.05) compared with the insulin-stimulated sample. Specific SR proteins were identified by reprobing the blots after mAb104 with antisera as indicated. Graphs for SRp75 and SRp55 represent ratios of phosphorylated SR protein/SR protein. The inhibition by LY294002 is significant (P < 0.05; n = 3) as determined by a two-tailed unpaired t test. β-Actin was detected as an internal control.
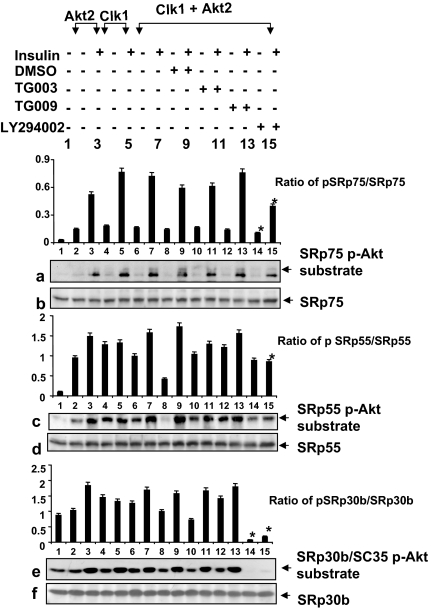
Because both Akt2 and Clk/Sty could phosphorylate the same Serine residues within Arg/Ser domains, and because there are more Akt substrate motifs than Clk/Sty motifs in SR proteins, we hypothesized that SR proteins phosphorylated by Akt2 should display different phosphorylation patterns from those effected by Clk/Sty. We examined the differences in phosphorylation patterns caused by inhibition of PI3K/Akt only or Clk/Sty only. For this purpose, we reprobed the same membranes described in Fig. 5 with phosphorylated Akt substrate antibody. The phosphorylation patterns revealed with the phospho-Akt substrate antibody were quite different from those revealed by mAb104, a widely used monoclonal antibody in SR protein studies, which appeared to detect predominately Clk/Sty-mediated phosphorylation (Fig. 5, a, c, and e, and Fig. 8, a, c, and e), indicating that Akt2 and Clk/Sty are working on different serine residues within the same Arg/Ser domains of SR proteins. For example, SRp30b/SC35, which was not significantly impacted by the Clk/Sty inhibitor (Fig. 5e), had a dramatically different insulin-dependent phosphorylation pattern that was blocked by the Akt signaling inhibitor LY294002 (Fig. 8e, lanes 14–15). These data suggest that SRp30b/SC35 is a preferred Akt2, but not Clk/Sty, substrate in insulin signaling and that Akt2 phosphorylates SR proteins directly in addition to Clk/Sty. The partial inhibition of SRp75 phosphorylation by LY294002 also suggests that another kinase may be phosphorylating some of the serine sites. The results implicated both Akt2 and Clk/Sty in the control of SR proteins and further suggested that the actions of Akt2 and Clk/Sty were distinctly different on each protein.
Figure 8.
Akt2 phosphorylates SRp75, SRp55, and SRp30b in Clk1- and Akt2-transfected cells. L6 myotubes were transfected with pcDNA3, Akt2, Clk/Sty, or Akt2 plus Clk/Sty plasmids as indicated for 36 h, serum starved for 6 h, and then treated with TG003, TG009, LY294002, or DMSO for 30 min before insulin stimulation (10 nm, 30 min; see Fig. 3), lysed, and analyzed for SR protein phosphorylation with phospho-Akt (p-Akt) substrate antibody in addition to evaluation for expression of the transfected genes and Akt phosphorylation by Western blot (shown in Fig. 3). Identities of these SR proteins were confirmed by reprobing the stripped blots with the corresponding antibodies (also shown in Fig. 3). The graphs are ratios of phospho-SR protein/SR protein (pSRp/SRp). *, Inhibition of phosphorylation was significant (P < 0.05; n = 3).
Akt2 is linked to Clk/Sty regulation in vivo
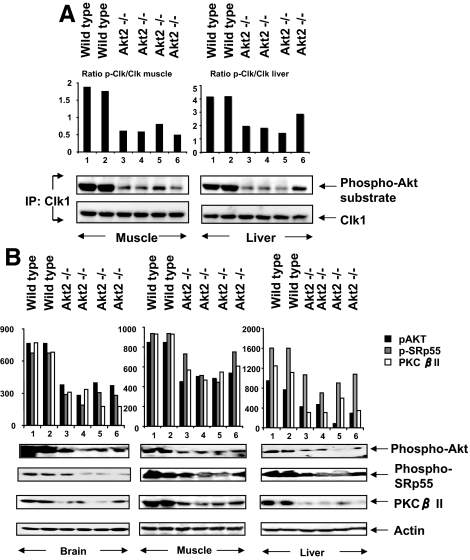
Clk/Sty kinases may be linked to the genetic susceptibility to type 2 diabetes in mice (34). To verify that the activation pathway observed in differentiated L6 cultures was related to that in the intact animal, we examined Clk/Sty phosphorylation on phospho-Akt substrate sites in tissues obtained from Akt2-null mice with insulin resistance and from wild-type mice (25). Tissues from skeletal muscle and liver were homogenized, Clk/Sty protein was immunoprecipitated, and the immunoprecipitates were analyzed by immunoblot using phosphorylated Akt substrate antibody or anti-Clk (Fig. 9). Samples from each of four Akt2-null mice had substantially decreased phosphorylation of Akt-substrate sites in Clk/Sty, compared with samples from two wild-type mice, indicating that Akt2 mediated the phosphorylation of Clk/Sty in the wild-type pathway and that absence of Akt2 led to reduced phosphorylation of Clk/Sty. The animal study further corroborated our observation that Akt2 is an upstream regulator of Clk/Sty in vivo.
Figure 9.
Reduced PKCβII expression and impaired Clk/Sty and SRp55 phosphorylation in Akt2-null mouse tissue. A, Impaired Clk/Sty phosphorylation in Akt2-null mouse tissues. Gastrocnemius and liver tissue samples were obtained from wild-type and Akt2-null mice. Tissue homogenates were immunoprecipitated with anti-Clk/Sty, and immunoprecipitates after SDS-PAGE were probed with Clk/Sty and phospho-Akt (p-Akt) substrate antibody. The graph of densitometric scans is a ratio of phospho-Clk (p-Clk)/Clk. For muscle, the wild-type ratio is 1.82 ± 0.06 (n = 2) and for Akt2−/− 0.62 ± 0.06 (n = 4); P < 0.003. For liver, the wild-type ratio was 4.18 ± 0.02 (n = 2) and for Akt2−/− 2.05 ± 0.28 (n = 4); P < 0.007. B, Impaired SRp55 phosphorylation and suppressed PKCβII expression in Akt2-null mouse tissues. Brain, gastrocnemius, and liver tissue samples after SDS-PAGE were analyzed for Akt phosphorylation at serine 473, phosphorylation of SRp55 recognized by mAb104, PKCβII, and actin levels. The graph above each tissue blot is the densitometric scan for the phosphorylated protein identified on the right. In each case, the phosphorylation or protein level was lower in the tissue from the Akt2−/− tissue compared with the wild type as summarized in Table 1.
Akt2 participated in maintaining normal phosphorylation of SRp55 and PKCβII levels
Western blot analysis of brain, muscle, and liver tissue lysates from Akt2-null mice showed that Akt phosphorylation of SRp55 was dramatically decreased in tissues from the null mice compared with wild-type mice (Fig. 9). The decrease in Akt phosphorylation/activation, we believe, was due to the absence of Akt2 protein, but the presence of Akt1 and Akt3 (Fig. 9a). All three phospho-Akt isoforms are recognized by the Akt-substrate antibody. Nevertheless, analysis of SRp55 from the Akt2-null mice revealed a corresponding and significant suppression of phosphorylation, compared with wild-type mice (Fig. 9b). SRp55 was the SR protein with the most dramatic decrease in phosphorylation with Clk siRNA; hence, it was thought to best represent Clk effects on the RS domain. Previously, we showed that PKCβII mRNA levels, but not PKCβI mRNA, were decreased in these animals (5); the Western blot analysis revealed that PKCβII protein levels were dramatically decreased (>75%) in Akt2-null mice (Fig. 9B), which demonstrated a functional requirement for Akt2 and Clk/Sty in the expression of PKCβII. The statistical analysis of densitometric scans of phospho-Akt, phospho-SRp55, and PKCβII from Fig. 9 and presented in Table 1 indicated that the Akt2−/− tissues contained significantly lower levels of phosphoproteins compared with corresponding wild-type tissues.
Table 1.
Statistical analysis of densitometric scans for wild-type and Akt2−/− tissue Western blots in Fig. 9
| Immunoblots | Wild type (n = 2) | Akt2−/− (n = 4) | P value |
|---|---|---|---|
| Brain | |||
| pAkt | 765 ± 0.5 | 357 ± 26 | 0.0005 |
| pSRp55 | 675 ± .5 | 267 ± 25 | 0.0005 |
| PKCβII | 727 ± 45 | 251 ± 42 | 0.002 |
| Muscle | |||
| pAkt | 846 ± 0.5 | 493 ± 18 | 0.0002 |
| pSRp55 | 936 ± 0.5 | 610 ± 76 | 0.047 |
| PKCβII | 930 ± 0.05 | 547 ± 30 | 0.001 |
| Liver | |||
| pAKT | 867 ± 90 | 330 ± 87 | 0.02 |
| pSRp55 | 1606 ± 1 | 938 ± 86 | 0.007 |
| PKCβII | 1192 ± 60 | 398 ± 69 | 0.002 |
Western immunoblots shown in Fig. 9B were scanned as JPEG files, and areas of interest were digitized using UN-SCAN-IT gel digitizing software by Silk Scientific. Densitometric values were imported into Excel (Microsoft Office 2004) and plotted. The values (arbitrary densitometric units) were then analyzed for significance using an unpaired t test (Prism4 version 4.0a; GraphPad Software). pAKT, phospho-Akt.
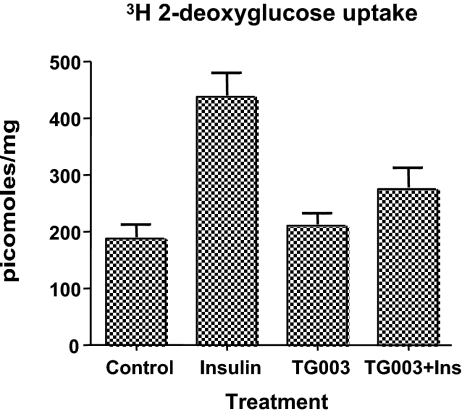
TG003 blocked insulin-stimulated glucose uptake
The importance of PKCβII for insulin-stimulated glucose uptake in L6 cells was previously demonstrated (19). To test whether Clk/Sty mediated this, we preincubated L6 cells with TG003 for 2 h before insulin treatment (30 min) and 2-deoxyglucose uptake. In Fig. 10, the Clk inhibitor TG003 significantly blocked insulin effects of glucose uptake under these treatment conditions.
Figure 10.
Inhibition of insulin-stimulated glucose uptake by TG003. L6 myocytes were serum starved for 4 h in Dulbecco’s PBS with or without 10 μm TG003 for 2 h. Insulin (50 nm) was added for 30 min before [3H]2-deoxyglucose for 6 min at 37 C. Uptake was stopped by aspirating the media and rinsing cells with ice-cold Dulbecco’s PBS. An aliquot of solubilized cell lysate was counted by scintillation counting for cellular uptake. Cytochalasin B-inhibitable counts were determined routinely and found to be less than 25% of control counts per minute. The data (mean ± sem) are from the averages of four separate experiments performed in triplicate for each point. Data were converted to specific activity and analyzed by Prism4 software using a one-way ANOVA with Tukey’s multiple comparison test. Control vs. insulin, P < 0.001; insulin vs. TG003 plus insulin (Ins), P < 0.005.
Discussion
Clk/Sty is a dual-specificity kinase shown to phosphorylate SR protein splicing factors predominately on serine residues (7,8,11,12,13,35). Because Clk/Sty phosphorylation of SR proteins subsequently alters their activity, subcellular localization, and ubiquitination (11,12,13), we sought to understand the mechanism by which Clk/Sty itself is regulated. Analysis of Clk/Sty protein revealed prominent phosphorylation on both serine/threonine and tyrosine residues (7), leading us to question whether it was regulated by any other serine/threonine kinase in addition to autophosphorylation. In this study, we determined Clk/Sty was regulated by a PI3K/Akt2 kinase pathway in vivo based on a number of criteria.
First we determined that Akt2 and Clk/Sty kinases were essential for insulin-mediated PKCβII splicing and expression. Our earlier work indicated that overexpression of Clk/Sty mimicked insulin signaling by promoting PKCβII exon inclusion (17). In the present study we found that insulin-regulated PKCβII mRNA alternative splicing was inhibited by Clk/Sty and Akt2 siRNA as well as by TG003, a specific Clk/Sty inhibitor, which further indicated that Clk/Sty was involved in insulin signaling. Additionally, Clk1-stimulated splicing of PKCβII mRNA was blocked by LY294002, suggesting that an upstream kinase was involved in the process.
We identified SRp75, SRp55, and SRp30b/SC35 as Clk/Sty substrates using various criteria including siRNA in addition to a specific Clk inhibitor to differentiate Clk phosphorylation from Akt phosphorylation in immunoprecipitated SR proteins. Furthermore, we found that insulin-induced Clk/Sty-mediated regulation of SR proteins was distinct from serum-induced Clk/Sty regulation. To ascertain whether Akt2 and Clk/Sty regulation of phosphorylation and resulting activation of SR splicing factors was a universal phenomenon, we showed that SR proteins were also substrates for Akt2-regulated Clk/Sty activation. A PI3K/Akt2→Clk/Sty connection was also involved in the regulation of SRp75 phosphorylation induced by serum, and there was also a requirement for PI3K/Akt2 in Clk/Sty phosphorylation of SRp30b/ SC35. SRp30b/SC35 appeared to be a less-preferred substrate for Clk/Sty during insulin activation but a more robust substrate with serum conditions. However, insulin regulation of splicing is best demonstrated after serum starvation. These results suggested that this serum-initiated PI3K/Akt2→Clk/Sty pathway was distinct from the one used by insulin.
We concluded that Akt2 phosphorylated Clk/Sty and activated Clk/Sty-mediated SR protein phosphorylation and PKCβII exon inclusion in response to insulin. Furthermore, our observations that SR protein phosphorylation depended on PI3K were supported by a recent finding that SRp55, SRp40, and SC35 phosphorylation was decreased by wortmannin, another inhibitor of PI3K, in anti-IgM receptor-induced splicing of BPV-1 pre-mRNAs in the ASF/SF2-depleted B cell line DT40 (36). It was also conceivable that the PI3K/Akt2 pathway regulated different kinases involved in SR protein phosphorylation and splicing complexes such as PKC or SRPK, because the insulin-initiated pathway was distinct from the one used by serum in regulating SR proteins.
Akt modulates gene expression via regulation of gene transcription by phosphorylating CREB and forkhead transcription factors (37,38). However, the role and the control of signaling by Akt in splicing regulation had not been clearly defined because Akt becomes nuclear after it is activated. Multiple Akt-substrate consensus motifs exist in the C-terminal Arg/Ser domains of all the SR proteins (5,21,33). We found that Akt phosphorylated these consensus sequences in multiple SR proteins (SRp75, SRp55, and SRp30b/SC35). A recent report provides both in vivo and in vitro evidence for Akt-mediated modification of SR proteins SF2/ASF and 9G8 by direct phosphorylation (33). Akt associates with and phosphorylates YB-1, another RNA-binding protein involved in regulation of mRNA transcription, splicing, and translation (24). The possibility exists that Akt2 phosphorylation directs SR proteins to the nucleus before Clk phosphorylation.
In this study, cellular and genetic evidence showed that Akt2 phosphorylated Clk/Sty. A new role played by Akt2 was also identified: regulation of alternative splicing and gene expression via simultaneously modulating Clk/Sty kinases and specific SR splicing factors. Tissues from Akt2-null mice with early stages of type 2diabetes had impaired Clk/Sty activation, reduced SR protein phosphorylation, and reduced PKCβII protein. To demonstrate a more physiological effect, the Clk inhibitor TG003, which also blocked SR protein phosphorylation and PKCβII splicing, blocked the effect of insulin on 2-deoxyglucose uptake in L6 cells, further suggesting the role of these kinases in insulin action.
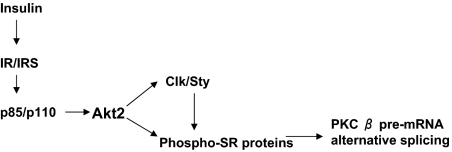
Taken together, the results of this study establish the requirement for PI3K/Akt2 signaling in SR protein regulation in response to multiple stimuli. As summarized in Fig. 11, the signaling linkage implied that PI3K/Akt2 was pivotal in mediating pre-mRNA splicing and gene expression through inducing a sophisticated phosphorylation network that acted on Clk/Sty and SR proteins. Whether Akt2 regulation of kinases such as SRPK2 (26,39) function similarly is under investigation. The hierarchy of SR protein phosphorylation sites is also questioned. Previous work demonstrated that PKCβII is necessary for full insulin action as measured by alterations in glucose uptake in multiple systems (18,19,20,40,41). The PKCβ splice variants differ in their binding to F-actin and implicate PKCβII in the process of insulin-stimulated actin rearrangements (42). The current experiments identifying a PI3K/Akt2/Clk/Sty/SR protein/PKCβII linkage place Clk/Sty in the signaling pathways of insulin action. Identification of the dual regulation demonstrated for Akt on SR protein phosphorylation introduces a new category of controlling pre-mRNA splicing and gene expression initiated by extracellular signaling.
Figure 11.
Signaling linkage between PI3K, Akt2, Clk/Sty, SR proteins, and PKCβ pre-mRNA alternative splicing. The diagram depicts the proposed dual role of Akt2 and Clk/Sty in mediating insulin-induced pre-mRNA splicing and gene expression via the networked phosphorylation of SR proteins.
Acknowledgments
We thank Dr. Morris J. Birnbaum (Howard Hughes Medical Institute, Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania) for providing Akt2-null mouse tissues and Dr. James Manley for providing Clk/Sty antibody and the HA-Clk/Sty construct (Department of Biological Sciences, Columbia University, New York, NY). We also thank Karen D. Corbin for technical assistance and Dr. Stefan Stamm for critical reading and useful comments.
Footnotes
The National Institutes of Health Grant DK54393 (D.R.C.), and Department of Veterans Affairs (tp D.R.C. and N.A.P.) supported this work.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 30, 2008
Abbreviations: Clk, CDC2-like kinase; DMSO, dimethylsulfoxide; FBS, fetal bovine serum; HA, hemagglutinin; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; siRNA, small interfering RNA; SR, serine/arginine-rich; SRPK, SR protein-specific kinase.
References
- Bourgeois CF, Lejeune F, Stevenin J 2004 Broad specificity of SR (serine/arginine) proteins in the regulation of alternative splicing of pre-messenger RNA. Prog Nucleic Acids Res Mol Biol 78:37–88 [DOI] [PubMed] [Google Scholar]
- Stamm S, Ben-Ari S, Rafalska I, Tang Y, Zhang Z, Toiber D, Thanaraj TA, Soreq H 2005 Function of alternative splicing. Gene 344:1–20 [DOI] [PubMed] [Google Scholar]
- Schwerk C, Schulze-Osthoff K 2005 Regulation of apoptosis by alternative pre-mRNA splicing. Mol Cell 19:1–13 [DOI] [PubMed] [Google Scholar]
- Staal SP, Hartley JW 1988 Thymic lymphoma induction by the AKT8 murine retrovirus. J Exp Med 167:1259–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NA, Kaneko S, Apostolatos HS, Bae SS, Watson JE, Davidowitz K, Chappell DS, Birnbaum MJ, Cheng JQ, Cooper DR 2005 Molecular and genetic studies imply Akt-mediated signaling promotes protein kinase CβII alternative splicing via phosphorylation of serine/arginine-rich splicing factor SRp40. J Biol Chem 280:14302–14309 [DOI] [PubMed] [Google Scholar]
- Gui JF, Lane WS, Fu XD 1994 A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature 369:678–682 [DOI] [PubMed] [Google Scholar]
- Lee K, Du C, Horn M, Rabinow L 1996 Activity and autophosphorylation of LAMMER protein kinases. J Biol Chem 271:27299–27303 [DOI] [PubMed] [Google Scholar]
- Nayler O, Stamm S, Ullrich A 1997 Characterization and comparison of four serine- and arginine-rich (SR) protein kinases. Biochem J 326(Pt 3):693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill K, Pawson T, Andrews B, Prasad J, Manley JL, Bell JC, Duncan PI 1996 The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J 15:265–275 [PMC free article] [PubMed] [Google Scholar]
- Hanes J, von der Kammer H, Klaudiny J, Scheit KH 1994 Characterization by cDNA cloning of two new human protein kinases. Evidence by sequence comparison of a new family of mammalian protein kinases. J Mol Biol 244:665–672 [DOI] [PubMed] [Google Scholar]
- Prasad J, Manley JL 2003 Regulation and substrate specificity of the SR protein kinase Clk/Sty. Mol Cell Biol 23:4139–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lin RI, Tarn WY 2003 Differential effects of hyperphosphorylation on splicing factor SRp55. Biochem J 371:937–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad J, Colwill K, Pawson T, Manley JL 1999 The protein kinase Clk/Sty directly modulates SR protein activity: both hyper- and hypophosphorylation inhibit splicing. Mol Cell Biol 19:6991–7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfant CE, Watson JE, Bisnauth LD, Kang JB, Patel N, Obeid LM, Eichler DC, Cooper DR 1998 Insulin regulates protein kinase CβII expression through enhanced exon inclusion in L6 skeletal muscle cells. A novel mechanism of insulin- and insulin-like growth factor-i-induced 5′ splice site selection. J Biol Chem 273:910–916 [DOI] [PubMed] [Google Scholar]
- Patel NA, Chalfant CE, Watson JE, Wyatt JR, Dean NM, Eichler DC, Cooper DR 2001 Insulin regulates alternative splicing of protein kinase CβII through a phosphatidylinositol 3-kinase-dependent pathway involving the nuclear serine/arginine-rich splicing factor, SRp40, in skeletal muscle cells. J Biol Chem 276:22648–22654 [DOI] [PubMed] [Google Scholar]
- Patel NA, Yamamoto M, Illingworth P, Mancu D, Mebert K, Chappell DS, Watson JE, Cooper DR 2002 Phosphoinositide 3-kinase mediates protein kinase CβII mRNA destabilization in rat A10 smooth muscle cell cultures exposed to high glucose. Arch Biochem Biophys 403:111–120 [DOI] [PubMed] [Google Scholar]
- Patel NA, Apostolatos HS, Mebert K, Chalfant CE, Watson JE, Pillay TS, Sparks J, Cooper DR 2004 Insulin regulates protein kinase CβII alternative splicing in multiple target tissues: development of a hormonally responsive heterologous minigene. Mol Endocrinol 18:899–911 [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Mischak H, Watson JE, Winkler BC, Goodnight J, Farese RV, Cooper DR 1995 Regulation of alternative splicing of protein kinase Cβ by insulin. J Biol Chem 270:13326–13332 [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Ohno S, Konno Y, Fisher AA, Bisnauth LD, Watson JE, Cooper DR 1996 A carboxy-terminal deletion mutant of protein kinase CβII inhibits insulin-stimulated 2-deoxyglucose uptake in L6 rat skeletal muscle cells. Mol Endocrinol 10:1273–1281 [DOI] [PubMed] [Google Scholar]
- Cooper DR, Watson JE, Patel N, Illingworth P, Acevedo-Duncan M, Goodnight J, Chalfant CE, Mischak H 1999 Ectopic expression of protein kinase CβII, -δ, and -ε, but not -βI or -ζ, provide for insulin stimulation of glucose uptake in NIH-3T3 cells. Arch Biochem Biophys 372:69–79 [DOI] [PubMed] [Google Scholar]
- Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley LC 2000 Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J Biol Chem 275:36108–36115 [DOI] [PubMed] [Google Scholar]
- Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M 1997 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol 7:776–789 [DOI] [PubMed] [Google Scholar]
- Mora A, Komander D, van Aalten DM, Alessi DR 2004 PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 15:161–170 [DOI] [PubMed] [Google Scholar]
- Evdokimova V, Ruzanov P, Anglesio MS, Sorokin AV, Ovchinnikov LP, Buckley J, Triche TJ, Sonenberg N, Sorensen PH 2006 Akt-mediated YB-1 phosphorylation activates translation of silent mRNA species. Mol Cell Biol 26:277–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw 3rd EB, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ 2001 Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292:1728–1731 [DOI] [PubMed] [Google Scholar]
- Umehara H, Nishii Y, Morishima M, Kakehi Y, Kioka N, Amachi T, Koizumi J, Hagiwara M, Ueda K 2003 Effect of cisplatin treatment on speckled distribution of a serine/arginine-rich nuclear protein CROP/Luc7A. Biochem Biophys Res Commun 301:324–329 [DOI] [PubMed] [Google Scholar]
- Roth MB, Murphy C, Gall JG 1990 A monoclonal antibody that recognizes a phosphorylated epitope stains lampbrush chromosome loops and small granules in the amphibian germinal vesicle. J Cell Biol 111:2217–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M 2005 Alternative splicing: a new drug target of the post-genome era. Biochim Biophys Acta 1754:324–331 [DOI] [PubMed] [Google Scholar]
- Patel NA, Cooper DR 2005 The signal to splice: insulin regulation of alternative splicing via the phosphoinositide 3-kinase pathway. J Clin Ligand Assay 28:75–81 [Google Scholar]
- Perrotti N, He RA, Phillips SA, Haft CR, Taylor SI 2001 Activation of serum- and glucocorticoid-induced protein kinase (Sgk) by cyclic AMP and insulin. J Biol Chem 276:9406–9412 [DOI] [PubMed] [Google Scholar]
- Rosenzweig T, Aga-Mizrachi S, Bak A, Sampson SR 2004 Src tyrosine kinase regulates insulin-induced activation of protein kinase C (PKC)δ in skeletal muscle. Cell Signal 16:1299–1308 [DOI] [PubMed] [Google Scholar]
- Muraki M, Ohkawara B, Hosoya T, Onogi H, Koizumi J, Koizumi T, Sumi K, Yomoda J, Murray MV, Kimura H, Furuichi K, Shibuya H, Krainer AR, Suzuki M, Hagiwara M 2004 Manipulation of alternative splicing by a newly developed inhibitor of Clks. J Biol Chem 279:24246–24254 [DOI] [PubMed] [Google Scholar]
- Blaustein M, Pelisch F, Tanos T, Muñoz MJ, Wengier D, Quadrana L, Sanford JR, Muschietti JP, Kornblihtt AR, Cáceres JF, Coso OA, Srebrow A 2005 Concerted regulation of nuclear and cytoplasmic activities of SR proteins by AKT. Nat Struct Mol Biol 12:1037–1044 [DOI] [PubMed] [Google Scholar]
- Mastaitis JW, Wurmbach E, Cheng H, Sealfon SC, Mobbs CV 2005 Acute induction of gene expression in brain and liver by insulin-induced hypoglycemia. Diabetes 54:952–958 [DOI] [PubMed] [Google Scholar]
- Howell BW, Afar DE, Lew J, Douville EM, Icely PL, Gray DA, Bell JC 1991 STY, a tyrosine-phosphorylating enzyme with sequence homology to serine/threonine kinases. Mol Cell Biol 11:568–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Mayeda A, Tao M, Zheng ZM 2003 Exonic splicing enhancer-dependent selection of the bovine papillomavirus type 1 nucleotide 3225 3′ splice site can be rescued in a cell lacking splicing factor ASF/SF2 through activation of the phosphatidylinositol 3-kinase/Akt pathway. J Virol 77:2105–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G 1998 Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev 12:2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Montminy M 1998 CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273:32377–32379 [DOI] [PubMed] [Google Scholar]
- Wang HY, Lin W, Dyck JA, Yeakley JM, Songyang Z, Cantley LC, Fu XD 1998 SRPK2: a differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells. J Cell Biol 140:737–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Rincon J, Soler A, Ryder JW, Nolte LA, Zierath JR, Wallberg-Henriksson H 1999 Changes in glucose transport and protein kinase Cβ(2) in rat skeletal muscle induced by hyperglycaemia. Diabetologia 42:1071–1079 [DOI] [PubMed] [Google Scholar]
- Cortright RN, Azevedo Jr JL, Zhou Q, Sinha M, Pories WJ, Itani SI, Dohm GL 2000 Protein kinase C modulates insulin action in human skeletal muscle. Am J Physiol Endocrinol Metab 278:E553–E562 [DOI] [PubMed] [Google Scholar]
- Blobe GC, Stribling DS, Fabbro D, Stabel S, Hannun YA 1996 Protein kinase CβII specifically binds to and is activated by F-actin. J Biol Chem 271:15823–15830 [DOI] [PubMed] [Google Scholar]