Abstract
LH and FSH play critical roles in mammalian reproduction by mediating steroidogenesis and gametogenesis in the gonad. Gonadal steroid hormone feedback to the hypothalamus and pituitary influences production of the gonadotropins. We previously demonstrated that progesterone differentially regulates the expression of the LH and FSH β-subunits at the level of the gonadotrope: FSHβ transcription is induced, whereas LHβ is repressed. In this study, we investigated the mechanism of progesterone repression of LHβ gene expression using immortalized gonadotrope-derived LβT2 cells. The progesterone suppression of both basal and GnRH-induced LHβ gene expression occurs in a hormone- and receptor-dependent manner. Chromatin immunoprecipitation demonstrates that the hormone-bound progesterone receptor (PR) is recruited to the endogenous mouse LHβ promoter. In addition, suppression requires both the amino-terminal and DNA-binding regions of PR. Furthermore, progesterone suppression does not require direct PR binding to the promoter, and, thus, PR is likely recruited to the promoter via indirect binding through other transcription factors. These data demonstrate that the molecular mechanism for progesterone action on the LHβ promoter is distinct from FSHβ, which involves direct PR binding to the promoter to produce activation. It also differs from androgen repression of LHβ gene expression in that, rather than Sp1 or steroidogenic factor-1 elements, it requires elements within −300/−250 and −200/−150 that also contribute to basal expression of the LHβ promoter. Altogether, our data indicate that progesterone feedback at the level of the pituitary gonadotrope is likely to play a key role in differential production of the gonadotropin genes.
Negative feedback in the pituitary gonadotrope cell by the ovarian steroid hormone, progesterone, results in inhibition of luteinizing hormone β-subunit gene expression.
The mammalian hypothalamic-pituitary-gonadal axis controls reproduction, including sexual development, puberty, the menstrual cycle, pregnancy, and menopause. GnRH is secreted in a pulsatile manner directly into the hypophyseal portal system by neurons in the hypothalamus (1). GnRH then activates its receptor on the surface of gonadotrope cells within the anterior pituitary. Activation of the GnRH receptor leads to synthesis and secretion of LH and FSH, consisting of a common α-subunit and a unique β-subunit (2). These glycoprotein hormones are secreted into the bloodstream to regulate gametogenesis; folliculogenesis; and production of testosterone, estrogen, and progesterone in the gonads (3,4,5). Subsequently these steroids feed back to regulate expression and secretion of GnRH, LH, and FSH in the hypothalamus and the anterior pituitary.
Because synthesis of LHβ and FSHβ is the rate-limiting step in gonadotropin production (6,7), transcriptional regulation is a key focus. The conserved proximal promoter of LHβ binds several transcriptional regulators that modulate cell-specific expression including steroidogenic factor (SF)-1 (8,9), early growth response protein (Egr)-1, and pituitary homeobox 1/orthodenticle homeobox 1 (10,11,12). These proteins, as well as those binding to more distal elements, interact to elicit basal promoter activity. GnRH induction of the rat LHβ promoter has been shown to involve a tripartite GnRH response element, composed of two Sp1 sites in the distal promoter, and proximal pairs of SF-1 and Egr-1 binding elements (10).
In addition to GnRH, steroid hormones play a pivotal role in LH synthesis. Many studies have shown that LHβ mRNA levels increase after gonadectomy and subsequently decrease with reintroduction of gonadal steroid hormones (13,14). Steroid hormone receptors are expressed in gonadotrope cells (15,16,17), indicating that regulation by gonadal steroids likely occurs directly at the level of the pituitary, in addition to the hypothalamus. Estrogens suppress LHβ synthesis due to negative feedback at the hypothalamus (18,19), but they also exert a direct effect on the pituitary by inducing LHβ gene expression in gonadotropes (20,21). In addition, many studies indicate that androgens repress LHβ mRNA directly in the pituitary. Androgens decrease LHβ mRNA levels in castrated, GnRH antagonist-treated rats (22) and primary pituitary cells (23). Furthermore, androgens suppress LHβ gene expression in immortalized gonadotrope cells (24,25).
Despite evidence for progesterone enhancement of GnRH-induced LH secretion (26), it is unclear whether progesterone regulates LHβ gene expression directly in the pituitary gonadotrope. Because levels of LHβ mRNA are lowest during the luteal phase of the ovulatory cycle when circulating progesterone levels are at their highest, progesterone is an excellent candidate for countering the stimulatory effects of GnRH on LHβ gene expression. Many studies have shown that, in the presence of estrogen, progesterone can suppress LHβ mRNA levels in rodents (27,28,29), although these experiments did not differentiate between hypothalamic and pituitary sites of action. Contrary to other studies that did not report an effect of progesterone on LHβ (30,31), we recently demonstrated that progesterone can suppress LHβ gene expression in gonadotrope cells, in contrast to its stimulatory effect on the FSHβ promoter (32), indicating that progesterone may serve as a differential regulatory influence on the two gonadotropins during the menstrual cycle.
In mammals, gonadotropes comprise approximately 10% of the anterior pituitary cell population, making it difficult to conduct mechanistic studies of their function (33). Development of the immortalized LβT2 cells provided the opportunity to analyze mechanisms of steroid hormonal regulation of the gonadotropin genes in the context of a pure population of gonadotrope cells (34). The immortalized LβT2 cell line expresses many markers of a mature gonadotrope including FSHβ, LHβ, activin, follistatin, and inhibin as well as activin, inhibin, and steroid receptors (32,35,36,37,38). In this study, we used transient transfection experiments in LβT2 cells to demonstrate that progesterone suppresses both basal transcription and GnRH-induced LHβ gene expression in gonadotrope cells. Because estrogen is necessary for the induction of progesterone receptor (PR) but may mask the repressive effects of progesterone due to its stimulatory effect on LHβ gene expression, we enhanced PR levels directly by overexpressing the receptor in LβT2 cells. We defined and characterized a progesterone-responsive region in the proximal LHβ promoter necessary and sufficient for the suppressive effect. We determined that progesterone suppression is dependent on the presence of PR and that PR is recruited to the endogenous LHβ promoter after hormone treatment in LβT2 cells. Interestingly, direct binding of PR to the LHβ promoter does not appear to be necessary for the suppression, although the DNA-binding domain (DBD) of PR is required. These results suggest that the progesterone suppression of LHβ gene expression is through indirect binding of PR to the DNA via other transcription factors.
Materials and Methods
Cell culture and transient transfection
Cell culture and transient transfections were performed as described previously (32). LβT2 cells were plated in 12-well plates at 3 × 105 cells/well. Wells were transfected with 400 ng of reporter plasmid and 100 ng of a PR expression vector, unless otherwise noted. Cells were also transfected with 200 ng of a thymidine kinase (TK)-β-gal reporter as a control for transfection efficiency. Six hours after transfection, cells were switched to serum-free DMEM. Eighteen hours later, cells were treated for 6 h (unless otherwise noted) with one of the following treatments: 0.1% ethanol and 0.1% BSA (vehicle control); 10−7 m promegestone (R5020) and 0.1% BSA; 0.1% ethanol and 10−8 m GnRH; or both 10−7 m R5020 and 10−8 m GnRH. R5020 was purchased from NEN Life Science Products Life Sciences (Boston, MA) and progesterone, mifepristone (RU486) and GnRH from Sigma (St. Louis, MO). Luciferase and β-galactosidase activity were measured as previously described (32). Transfection data were analyzed by one-way ANOVA, followed by post hoc comparisons with the Tukey-Kramer honestly significant difference test or two-way ANOVA using the statistical package JMP 7.0 (SAS, Cary, NC).
Subcloning and mutagenesis
PCR was performed using the appropriate primers (supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) to create 5′ truncations of the −1800-bp LHβ promoter at −500, −300, −150, and −87. Fragments were inserted between KpnI and HindIII in pGL3. A similar strategy was used to insert −300/−150 of the LHβ promoter into a −81-TK-luc reporter in forward or reverse orientation.
The PRB C577A DBD mutant unable to bind DNA was described previously (32). The QuikChange kit (Stratagene, La Jolla, CA) was used to generate the 5′ Sp1/CArG, 3′ Sp1, triple Sp1/CArG, 5′ SF-1, 3′ SF-1, 5′ Egr-1, and 3′ Egr-1 mutations in the −1800-bp LHβ promoter (oligonucleotides in supplemental Table 1). The progesterone response element (PRE) mutation was made in the −500 LHβ promoter. The −300/−150, −300/−250, −250/−200, and −200/−150 deletions were made in the −1800- or −500-bp LHβ promoters. Dideoxynucleotide sequencing confirmed mutagenesis.
EMSA
Full-length human PRB was overexpressed in Sf9 cells via a baculovirus expression system. The cell lysate was centrifuged at 40,000 rpm for 30 min, and the supernatant was taken as a soluble whole-cell extract or purified as described previously (39) and used in EMSA as described (32). The 1294 PR mouse monoclonal antibody was used to supershift PR and mouse IgG was used as a control. Oligonucleotides used for EMSA are shown in supplemental Table 1.
Chromatin immunoprecipitation (ChIP)
Confluent LβT2 cells in 15-cm plates were treated with vehicle or 10−7 m R5020 and cross-linked with 1% formaldehyde. The nuclear fraction was obtained and chromatin was sonicated. Protein-DNA complexes were incubated overnight with 1294 PR antibody or IgG control and precipitated with protein A/G beads. Beads were washed, the protein-DNA complexes eluted, the cross-links reversed, and the DNA precipitated, as described (32). PCR primers for the LHβ promoter spanned the 220-bp sequence in the mouse LHβ gene from −180 to +40 (supplemental Table 1).
Results
LHβ gene expression is suppressed in immortalized gonadotropes by progesterone
Recently we demonstrated that both testosterone and progesterone can differentially modulate gonadotropin synthesis in pituitary gonadotrope cells by enhancing FSHβ and repressing LHβ gene expression (32). Because testosterone has been shown to suppress GnRH induction of LHβ gene transcription (24,25), we hypothesized that progesterone might have a similar effect. Progesterone treatment suppressed both basal promoter activity and GnRH-induced LHβ gene transcription (Fig. 1A). Progesterone repressed basal gene expression by 10%. Treatment of the LβT2 cells with GnRH alone resulted in a 4.8-fold induction, which was reduced 65% by progesterone. This suppression was maintained for over 24 h (data not shown). Treatment of the cells with the synthetic progestin, R5020, elicited a similar suppression on basal and GnRH-induced LHβ gene expression (Fig. 1A), indicating that R5020 acts in an analogous manner to progesterone.
Figure 1.
Progesterone suppresses basal and GnRH-induced LHβ gene expression in immortalized gonadotrope cells. A, The −1800-bp LHβ-luc reporter plasmid was transiently transfected into LβT2 cells with 100 ng of rat PRB expression vector. After overnight starvation in serum-free media, the cells were treated for 6 h with vehicle, 100 nm progesterone, 100 nm R5020, 10 nm GnRH, or both GnRH and the relevant progestin as indicated. B, The −1800-bp LHβ-luc reporter plasmid was transiently transfected into LβT2 cells with 100 ng of rat PRB expression vector. After overnight starvation in serum-free media, cells were treated with vehicle, 100 nm R5020, 10 nm GnRH, or GnRH with increasing amounts of R5020 (10−12 to 10−7 m). Inset illustrates the decrease in basal due to R5020 alone on an expanded scale. C, The −1800-bp LHβ-luc reporter plasmid was transiently transfected into LβT2 cells along with 0, 100, or 400 ng of PRB expression vector. After overnight starvation in serum-free media, the cells were treated for 6 h with vehicle, 100 nm R5020, 10 nm GnRH, or both GnRH and R5020 as indicated. D, The −1800-bp LHβ-luc reporter plasmid was transiently transfected into LβT2 cells with 100 ng of PRB expression vector. After overnight starvation in serum-free media, the cells were treated for 6 h with vehicle, 100 nm R5020, 10 nm GnRH, or both GnRH and R5020 as well as either vehicle or 1 μm RU486. The data were normalized for transfection efficiency by expressing luciferase (luc) activity relative to β-galactosidase activity and relative to the empty pGL3 plasmid to control for hormone effects on the vector DNA. Results represent the mean ± sem of at least three independent experiments performed in triplicate and are presented as fold induction of hormone treatment relative to the vehicle control. *, Significant differences from the vehicle-treated control; †, interaction as defined by a two-way ANOVA (P < 0.05) (45).
To test whether suppression of GnRH induction was dose-dependent, cells were treated with vehicle, 10−7 m R5020, 10−8 m GnRH, or 10−8 m GnRH and increasing concentrations of R5020 from 10−12 to 10−7 m (Fig. 1B). In these experiments, the progestin alone repressed basal LHβ gene expression by 30% (shown on an expanded scale as an inset in Fig. 1B). GnRH treatment resulted in a 4.2-fold induction. As the R5020 concentration was increased, the suppression of GnRH induction increased in concert until saturation at 10−9 m (approximately 70% reduction by 10−9 to 10−7 m). All subsequent experiments used 10−7 m R5020 to ensure saturation of the receptor. Together these experiments demonstrate that the progesterone regulation of LHβ occurs in a saturable, dose-dependent manner.
Ligand-bound PR is necessary for maximal suppression of LHβ gene expression
We next examined whether the suppression of LHβ by progesterone required the presence of its classical nuclear receptor. LβT2 cells were transfected with increasing concentrations of the PRB expression vector to mimic estrogen induction of PR levels without the complication of estrogen action on LHβ gene expression. Basal LHβ gene expression was not suppressed by R5020 unless PR was transfected into the cells (Fig. 1C). With 100 and 400 ng of PR expression vector, R5020 repressed basal gene expression by 25 and 33%, respectively. As increasing amounts of PR were transfected into the cells, GnRH induction (in the absence of R5020) decreased from 3.8-fold (when no receptor was added) to 3.5-fold with 100 ng PR and then down to 2.5-fold with 400 ng PR, indicating that the unliganded PR may slightly repress GnRH-induced LHβ gene expression (Fig. 1C), although it had no effect on basal LHβ levels (data not shown). Whereas GnRH induction decreased due to higher levels of exogenous receptor, the suppression of the GnRH induction by PR liganded with R5020 remained relatively constant. Thus, although low levels of PR are expressed endogenously (32,40), transfection of exogenous receptor was necessary to observe significant suppression of both basal transcription and GnRH induction. Because suppression did not increase with greater amounts of exogenous receptor, all subsequent experiments used 100 ng of PR expression vector.
Once we demonstrated that the ligand-bound PR was required for suppression of LHβ gene expression by progesterone, we tested whether this effect could be blocked by the PR antagonist, RU486. Treatment with 100-fold higher levels of RU486 abrogated the suppressive effects of R5020 on basal and GnRH-induced LHβ gene expression (Fig. 1D). Interestingly, the GnRH induction was also suppressed 30% by the RU486 antagonist in the absence of the R5020 agonist, probably due to its known actions as a partial agonist when bound to PR (41,42).
PR DNA-binding domain is essential for progesterone suppression of LHβ gene expression
To determine whether PR can specifically associate with the endogenous mouse LHβ promoter in LβT2 cells, ChIP analysis with an antibody specific to PR was used. Figure 2A demonstrates that PR localizes to the endogenous mouse LHβ promoter; weakly in the absence of hormone and more strongly in the presence of R5020 (Fig. 2A, lanes 4 and 6). In contrast, there was no precipitation of LHβ promoter DNA with a nonspecific IgG control (lanes 3 and 5). The LHβ promoter was amplified from input chromatin (lanes 1 and 2) as a positive control for genomic DNA preparation and PCR conditions. This experiment demonstrated that PR is recruited to the endogenous proximal LHβ promoter, suggesting that the mechanism of progesterone suppression involves a direct action of the agonist-bound receptor on the LHβ promoter.
Figure 2.
PR binds to the endogenous LHβ promoter in LβT2 cells, and the DNA-binding domain is necessary for progesterone suppression of LHβ gene expression. A, ChIP was performed using cross-linked protein/chromatin from LβT2 cells treated with vehicle (Veh) or R5020 and antibodies directed against PR or nonspecific IgG as a negative control. PCR primers encompassing the proximal promoter of LHβ were used to detect precipitation of genomic DNA. PCR amplification was performed on 0.2% chromatin input (lanes 1 and 2), and chromatin was precipitated with either mouse IgG (lanes 3 and 5) or PR antibody (lanes 4 and 6). B, The −1800-bp LHβ-luc reporter plasmid was cotransfected with wild-type PRB or PRB DBD mutant (PRC577A). C, The −1800-bp LHβ-luc reporter plasmid was cotransfected with mouse PRB or PRA. After overnight starvation in serum-free media, the cells were treated with vehicle, 100 nm R5020, 10 nm GnRH, or both hormones as indicated. *, Significant differences from the vehicle-treated control; †, interaction as defined by a two-way ANOVA (P < 0.05). luc, Luciferase.
PR has two isoforms: PRB and PRA. The PRA isoform lacks the amino-terminal transactivation domain of PRB. Both isoforms are expressed in pituitary gonadotropes from the same gene and modulation of their ratio is thought to contribute to the degree of progesterone augmentation of GnRH-induced secretion (40). To determine which regions of PR play a critical role in the suppression of LHβ gene expression, transient transfection assays were performed using the PRA isoform and a PRB C557A DBD mutant receptor unable to bind to DNA. As observed previously, PRB suppressed basal LHβ promoter activity by 46% and the GnRH induction by 56%. The PRB-DBD mutant resulted in a complete disruption of R5020 repression in the absence or presence of GnRH induction (Fig. 2B). Similarly, when an expression vector for the mouse PRA isoform was transfected into LβT2 cells, there was no significant suppression of basal gene expression by progesterone (Fig. 2C). In the presence of PRA, suppression of GnRH induction was reduced by 19%, but the trend did not approach statistical significance. These data suggest that the PR DBD plays a critical role and that the unique amino-terminal region of PRB is involved in suppression of basal transcription and GnRH induction of the LHβ subunit.
Mutation of Sp1, SF-1, or Egr-1 elements in the distal or proximal LHβ promoter does not alleviate progesterone suppression of LHβ transcription
One potential mechanism for progesterone suppression of LHβ transcription is through an interaction between ligand-bound PR and the transcription factor Sp1 similar to the interaction reported between androgen receptor (AR) and Sp1 on the rat LHβ promoter (24). Alternatively, ligand-bound PR may interact with proximal promoter binding transcription factors, like the AR/SF-1 interaction observed on the bovine LHβ promoter (25).
To investigate the hypothesis that Sp1 binding sites are critical for PR suppression of LHβ transcription, specific mutations in Sp1 elements were created within the −1800-bp rat LHβ promoter. Figure 3A illustrates the location of the transcription factor binding sites on the LHβ promoter. The 5′ Sp1/CArG double mutation and the 3′ Sp1 mutation increased basal transcription compared with wild type (1.5- and 1.8-fold, respectively), whereas the triple mutation decreased basal activity by 93% (data not shown). In contrast, GnRH induction was not significantly altered by these mutations (Fig. 3B). There was no discernible difference in basal gene expression or GnRH induction of LHβ among the wild-type LHβ or these mutants after R5020 treatment. These data suggest that the Sp1-binding elements do not play a critical role in suppression of GnRH induction by progesterone.
Figure 3.
Mutation of Sp1, SF-1, and Egr-1 binding elements on the LHβ promoter does not alleviate progesterone suppression of LHβ transcription. A, A schematic of the rat LHβ promoter illustrating the known transcription factor binding sites involved in basal and GnRH-induction of LHβ gene expression. The rat LHβ promoter contains two Sp1 sites at −451/−442 and −398/−386. The most distal Sp1 element overlaps with a CArG box (−443/−434). The proximal promoter includes two SF-1 binding elements at −127/−119 and −58/−51 and two Egr-1 binding sites at −112/−104 and −49/−41. B and C, LβT2 cells were transiently transfected with either the −1800-bp LHβ-luc reporter plasmid or reporter plasmids containing mutations in the Sp1, SF-1, or Egr-1 binding elements as indicated. After overnight starvation in serum-free media, the cells were treated with vehicle, 100 nm R5020, 10 nm GnRH, or both hormones as indicated. *, Significant differences from the vehicle-treated control; †, interaction as defined by a two-way ANOVA (P < 0.05). luc, Luciferase.
Site-specific mutations were also generated in the 5′ SF-1, 3′ SF-1, 5′ Egr-1, and 3′ Egr-1 binding elements within the −1800-bp rat LHβ promoter. Each of these four mutations resulted in a reduction of basal promoter activity by approximately 40% (data not shown). GnRH treatment produced a 3.7-fold induction of the wild-type promoter (−1800 LHβ, Fig. 3C). Mutation of the 5′ SF-1 site led to a greater stimulation by GnRH, whereas mutation of the other proximal promoter binding sites resulted in a diminished response to GnRH: 3-fold with the 3′ SF-1 mutant, 1.6-fold with the 5′ Egr-1 mutant, and 1.5-fold with the 3′ Egr-1 mutant, similar to previous reports (8,9,43). Regardless of the mutation, R5020 still repressed basal gene expression and suppressed the remaining GnRH induction, indicating that these proximal promoter elements are not essential for the suppression of LHβ gene expression by progestins.
Progesterone suppression of LHβ transcription maps to a region located −300/−150 upstream of the transcription start site of the LHβ promoter
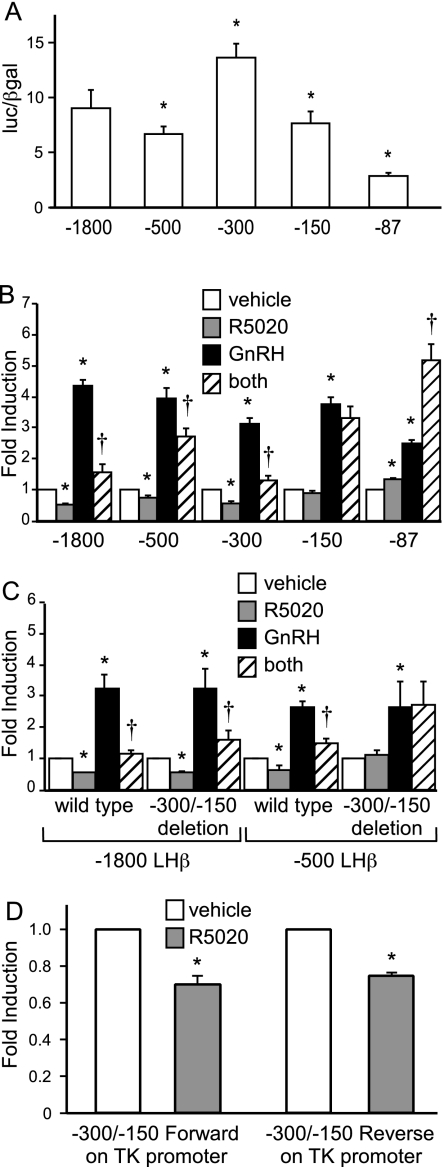
Because the suppression of LHβ gene expression by progesterone did not appear to require the known transcription factor binding elements, we used truncation analysis to identify the necessary regions. Truncation to −500, −150, or −87 resulted in significant reductions in basal activity in the absence of any hormones compared with the −1800-bp LHβ-luc reporter, whereas the −300 truncation increased basal gene expression (Fig. 4A). These results suggest that transcription factors important for basal gene expression also bind the −300/−150 region, in addition to the more distal and proximal regions of the LHβ promoter.
Figure 4.
Progesterone suppression of basal and GnRH-induced LHβ gene expression maps to the −300 to −150 region of the LHβ promoter. A, LβT2 cells were transiently transfected with either the −1800-bp LHβ-luc reporter plasmid or 5′ promoter truncations to compare basal transcriptional activity in the absence of hormone treatment. luc, Luciferase. B, LβT2 cells were transiently transfected with either the −1800-bp LHβ-luc reporter plasmid or 5′ promoter truncations to compare the effects of hormone treatments. C, LβT2 cells were transiently transfected with either −1800-bp or −500 LHβ-luc reporter plasmids or −1800-bp or −500 LHβ-luc reporter plasmids containing a 150-bp deletion of the region between −300/−150 of the LHβ promoter. D, Cells were transfected with a TK-luc reporter plasmid containing the −300/−150 repressive element in the forward or reverse orientation. After overnight starvation in serum-free media, the cells were treated with vehicle, 100 nm R5020, 10 nm GnRH, or both hormones as indicated (B–D). Luciferase activity was normalized to β-galactosidase activity and set relative to the empty reporter vector (A–C, pGL3; and D, TK-luc). Results represent the mean ± sem of at least three independent experiments performed in triplicate and are presented as luc/β-gal for basal gene expression (A) or fold induction of hormone treatment relative to the vehicle control (B–D). *, Significant differences from the vehicle-treated control; †, interaction as defined by a two-way ANOVA (P < 0.05).
GnRH induction of the −500 LHβ truncation was comparable with that seen with the −1800-bp LHβ-luc reporter (Fig. 4B). However, the −500 LHβ truncation exhibited only a partial reduction by progesterone of basal promoter activity and GnRH induction compared with the −1800-bp LHβ-luc reporter, suggesting the presence of an upstream region involved in the suppression of GnRH induction of LHβ by progesterone. The −300 LHβ-luc reporter showed a significant repression of basal activity and GnRH induction after progesterone treatment (Fig. 4B). In contrast, the −150 LHβ truncation lost the suppression of basal gene expression and GnRH induction by progesterone, suggesting that the −300/−150 region of the LHβ promoter is critical for the progesterone suppression. Interestingly, when the −87 LHβ promoter was tested, the effects were reversed. R5020 treatment resulted in a significant increase in −87 LHβ transcription (23.5%), and cells treated with both GnRH and R5020 showed a synergistic induction compared with the individual hormones.
The repressive element at −300/−150 of the LHβ promoter is necessary and sufficient for progesterone suppression
Because the suppression mapped to the −300/−150 region using 5′ truncations of the LHβ promoter, we tested whether this region was necessary for progesterone repression of both basal LHβ gene expression and GnRH induction. Deletion of this region within the context of the −1800-bp LHβ promoter still resulted in progesterone suppression (Fig. 4C). Given the results from the truncation analysis, we hypothesized that an upstream region may compensate for the repressive element at −300/−150, so we created a 150-bp deletion within the −500 LHβ promoter. Basal gene expression was reduced by 40% (data not shown), indicating that this region has a role in LHβ basal transcriptional regulation. Interestingly, there was no suppression of basal LHβ gene expression or the GnRH induction by R5020 with the repressive element deleted in the context of the −500 LHβ promoter (Fig. 4C), indicating that this 150-bp region (−300/−150) is necessary for the progesterone suppression in the context of the proximal promoter.
To assess whether the −300/−150 repressive element in the LHβ promoter is sufficient for suppression by progesterone, we inserted the 150-bp element upstream of a heterologous TK-luc reporter in the forward or reverse orientation. Basal TK promoter activity was suppressed more than 50% by the addition of this element (data not shown). Progesterone further suppressed promoter activity by 30% when the −300/−150 repressive element was in the forward or reverse orientation (Fig. 4D), indicating that this 150-bp element from the proximal LHβ promoter is sufficient to elicit the suppression by progesterone.
PR binding to the repressive element at −300/−150 is not critical for LHβ suppression
Given that the −300/−150 region of the LHβ promoter was essential for progesterone suppression, EMSA was performed to assess whether PR can bind to the proximal rat LHβ promoter in vitro. Six 35-mer oligonucleotide probes were designed to span the −300/−150 region. Purified PRB bound the −225/−191 oligonucleotide probe (Fig. 5A, lane 1), whereas no binding to the other probes in this region was observed (data not shown). To further demonstrate that PR bound specifically to the −225/−191 probe, the resulting complex was supershifted by a PR-specific antibody (lane 3) but not by IgG (lane 4). This complex also showed evidence of competition with a consensus PRE (data not shown). To further define where PR binds to the proximal LHβ promoter, EMSA with the wild-type −225/−191 probe and 12 3-bp scanning mutations were used as probes. The mutated probes that could not bind PR (Fig. 5B, lanes 2, 3, 5, and 6) encompassed a putative PRE at −221/−207.
Figure 5.
PR binds to a putative PRE at −221/−207, but mutation of the PRE does not alter progesterone suppression. A, Purified PRB was incubated with a wild-type (lane 1) or PRE mutant (lane 2) −225/−191 probe (supplemental Table 1) and tested for complex formation in EMSA. The addition of a PR antibody (PR Ab, lane 3) or nonspecific IgG control antibody (IgG, lane 4) to the binding reaction, are indicated. PR binding and the antibody supershift are also indicated. B, EMSA was performed with PR whole-cell extract using either wild-type (WT) −225/−191-labeled probe or oligonucleotides containing 3-bp scanning mutations as indicated, each with three adjacent A substitutions in the 3-bp sequence shown above each of the lanes. C, LβT2 cells were transfected with either the −500 LHβ-luc reporter plasmid or a reporter containing a PRE cis mutation in which the G and C residues important for high-affinity DNA binding were mutated (supplemental Table 1). Luciferase (Luc) activity was normalized to β-galactosidase activity and set relative to the empty reporter vector. Results represent the mean ± sem of at least three independent experiments performed in triplicate and are presented as fold induction of hormone treatment relative to the vehicle control. *, Significant differences from the vehicle-treated control; †, interaction as defined by a two-way ANOVA (P < 0.05).
Once we had ascertained that PR could bind a putative PRE at −221/−207, transient transfection assays were used to determine whether direct PR binding to the PRE played a functional role in the progesterone suppression of LHβ gene expression. For this experiment, LβT2 cells were transiently transfected with the −500 LHβ-luc reporter or a PRE cis mutation in which the G and C residues important for high-affinity DNA binding were mutated (supplemental Table 1). This PRE mutant does not bind PR in EMSA (Fig. 5A, lane 2). R5020 suppressed the basal promoter activity and the GnRH-induction of the wild-type LHβ promoter and the PRE mutant (Fig. 5C), indicating that direct PR binding to the putative −221/−207 PRE is not necessary for the suppressive effect of progesterone.
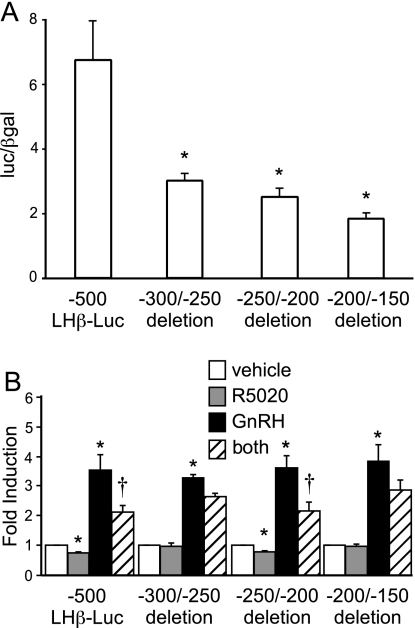
Two 50-bp regions of the repressive element are critical for LHβ suppression
Because direct binding of PR to the repressive region did not appear to be required for progesterone suppression, we created three sequential 50-bp deletions within the −500 LHβ promoter. Each of these three deletions resulted in greater than 50% reduction in basal gene expression (Fig. 6A), indicating that these regions must contribute to basal gene expression, most likely by binding specific transcriptional activators. The promoter containing the −250/−200 deletion was suppressed by progesterone for both basal LHβ expression and GnRH induction (Fig. 6B). In contrast, there was no significant progesterone suppression after either the −300/−250 or −200/−150 regions were deleted, indicating that more than one region of the 300-bp element is necessary for suppression by progesterone. In silico analysis of these 50-bp regions revealed a number of putative transcription factor binding elements. However, 10-bp scanning deletions through the two important regions did not prevent progesterone suppression of GnRH induction of the LHβ promoter (data not shown), suggesting that multiple elements within each region may be important.
Figure 6.
Multiple elements are required for suppression. A, LβT2 cells were transiently transfected with either the −500 bp LHβ-luc reporter plasmid or −500 LHβ-luc reporter plasmids containing specific 50-bp deletions, as indicated, to compare basal transcriptional activity in the absence of hormone treatment. B, LβT2 cells were transfected with either the −500 LHβ-luc reporter plasmid or reporter plasmids containing specific 50-bp deletions as indicated. After overnight starvation in serum-free media, the cells were treated with vehicle, 100 nm R5020, 10 nm GnRH, or both hormones as indicated. Luciferase (Luc) activity was normalized to β-galactosidase activity and set relative to the empty reporter vector. Results represent the mean ± sem of at least three independent experiments performed in triplicate and are presented as luc/β-gal for basal gene expression (A) or fold induction of hormone treatment relative to the vehicle control (B). *, Significant differences from the vehicle-treated control; †, interaction as defined by a two-way ANOVA P < 0.05).
Discussion
Our study demonstrates that progestins can suppress both basal transcription and GnRH induction of the LHβ gene in gonadotrope cells. Similar to androgens, progesterone suppression occurs in a hormone- and receptor-dependent manner, indicating that the actions of progesterone are through the classical PR. Moreover, the fact that PR is recruited to the endogenous LHβ promoter after hormone treatment and that the suppression occurred in as little as 6 h, indicate that progesterone mediates suppression of LHβ gene expression through a mechanism directly involving the LHβ promoter.
Maximal suppression of LHβ transcription by progesterone requires the hormone-bound PRB complex containing an intact DBD. Disruption of the PR DBD abrogated the repression of basal transcription as well as the suppression of GnRH induction by progesterone. One possible role for the PR DBD is for direct binding of PR to the LHβ promoter. To investigate this possibility, we searched for PREs within the −300/−150 repressive element necessary for progesterone suppression. We identified a putative PRE at −225/−191 that bound PR in EMSA. However, mutation of this site or deletion of a 50-bp region from −250/−200 that encompassed this element did not relieve progesterone suppression of LHβ transcription, suggesting that direct DNA binding by PR is not necessary for this effect, in contrast to progesterone induction of the FSHβ gene that requires direct binding of PR to the proximal promoter. Another possibility for the role of the PR DBD in the suppression of LHβ transcription by progesterone is through a protein-protein interaction with another transcription factor involved in LHβ expression, rather than direct DNA binding. For instance, Curtin et al. (24) demonstrated that the AR DBD interacts directly with Sp1 and as a result reduces Sp1 binding to the rat LHβ promoter, thereby decreasing the GnRH response. However, it is unlikely that Sp1 is involved in progesterone suppression because mutation of both of the Sp1 elements in the distal LHβ promoter had no effect and overexpression of Sp1 did not relieve repression (data not shown).
In addition to the PR DBD, we also demonstrated that the unique amino-terminal region of the PRB isoform is necessary for the full suppression of LHβ by progesterone. This region has been shown to contain a transactivation function that is thought to be the reason that PRB is generally a stronger activator than PRA. As a result of this additional region, PRB likely forms a more stable secondary and/or tertiary structure than PRA [recently reviewed by Bain et al. (44)] that favors the interaction of factor(s) necessary for progesterone suppression of LHβ gene expression.
Initially, we had hypothesized that the mechanism of action for progesterone suppression would be similar to the reported androgen suppression on either the rat (24) or bovine (25) promoter. Similarities between the two mechanisms include the necessity for liganded receptor and an intact DBD. The most significant difference is that the site of action for PR on the LHβ promoter is distinct from that of AR. Mutations in the distal Sp1 sites failed to relieve progesterone suppression of both basal and GnRH-induced LHβ gene expression, indicating that these elements are not critical for the suppression by progesterone. Mutation of the SF-1 or Egr-1 binding sites in the proximal promoter also did not relieve suppression by progesterone, suggesting that these elements do not play a critical role in progesterone suppression of LHβ mRNA levels. Our experiments with the 5′ truncations of the LHβ promoter provided supporting evidence for these conclusions. Specifically, a −300 LHβ-luc reporter lacking the distal Sp1 elements was sufficient for suppression by progesterone, and conversely, a −150 LHβ truncation (containing both Egr-1 and SF-1 binding sites) was not suppressed by progesterone.
In addition to highlighting the different mechanisms of repression by progestins vs. androgens, our analysis also revealed that several regions in the LHβ promoter contribute to the suppression by progesterone. Truncation analysis identified a repressive element between −300/−150 that was both necessary and sufficient to elicit progesterone suppression. Because this region is less conserved among mammalian species than the proximal LHβ promoter, it remains to be determined whether progesterone suppression of LHβ transcription occurs in a species-specific manner. The fact that deletion of the repressive element resulted in loss of suppression in the context of the −500 LHβ promoter but not the −1800-bp promoter suggests that a region upstream of −500 also contributes to progesterone suppression of both basal and GnRH-induced LHβ gene expression by progesterone.
To further characterize the critical region necessary for suppression of LHβ transcription in the proximal promoter, we created and tested three 50-bp deletions in the −300/−150 region. All three deletions reduced basal activity of the LHβ promoter by about 50%, indicating that they likely bind factors important for LHβ gene expression. Deletion of either the region from −300/−250 or −200/−150 resulted in a lack of suppression by progesterone, indicating that multiple elements may be required but that spacing is less critical because the middle 50 bp can be deleted without effects on progesterone regulation. Also supporting the idea that multiple elements in the proximal promoter may be responsible for the progesterone suppression is the fact that 10-bp scanning deletions through these two regions did not prevent progesterone suppression of GnRH induction. This situation is reminiscent of the mechanism of androgen suppression on the bovine LHβ promoter, in which cis mutations or block replacements affecting Egr-1, SF-1, or pituitary homeobox 1 binding elements had no effect, but the proximal promoter clearly mediated the androgen suppression (25). Furthermore, the data thus far support the conclusion that the repression of basal activity and GnRH induction of the LHβ gene by PR occur through modulation of factor(s) in common between the two processes.
In summary, our results demonstrate that progesterone can suppress both basal transcription and GnRH induction of LHβ gene expression in a hormone- and receptor-dependent manner in gonadotrope cells. We determined that the full suppressive effect of progesterone on LHβ gene expression requires the unique amino-terminal region of the PRB isoform and an intact DBD. However, we did not find any evidence that the progesterone suppression involves direct binding of PR to the LHβ promoter, although it is recruited to the endogenous promoter in live cells. Rather, our data suggest that these domains are necessary for tethering or binding to other transcription factor(s). Furthermore, we identified a repressive element in the proximal LHβ promoter that is both necessary and sufficient to elicit suppression by progesterone. Multiple regions at −300/−200 and −200/−150 appear to be involved in both basal transcription of the LHβ gene and suppression by progesterone, further supporting the concept that PR may act through other transcription factors bound to these regions. We also demonstrated that there is a region upstream of −500 in the rat LHβ promoter that may also be involved in the suppression of LHβ transcription by progesterone. Additional experiments will be necessary to define the cis-regulatory elements and transcription factors that play a role in the regulation of LHβ gene expression by ligand-bound PR. Altogether this work has revealed new insights into the pituitary action of progesterone. In particular, it has highlighted the role that progesterone may play in limiting preovulatory GnRH-induced LH secretion via progesterone suppression of GnRH-induced LHβ transcription.
Supplementary Material
Acknowledgments
The authors thank Djurdjica Coss and other members of the Mellon lab for helpful discussions and comments. We also thank Scott Kelley for his suggestions and critical reading of the manuscript. We are grateful to Mark Lawson, Benita Katzenellenbogen, and Dean Edwards for providing reagents. The −1800-bp rat LHβ luciferase-reporter plasmid (in pGL3) was donated by Mark Lawson. Rat PRB (in pCMV5) was provided by Benita Katzenellenbogen; the 1294 PR antibody and mouse PRB and PRA plasmids (in pcDNA-I) were donated by Dean Edwards. We also acknowledge the University of California, San Diego, Cancer Center DNA Sequencing Shared Resource for sequencing and the University of Colorado Cancer Center Tissue Culture Core Facility for baculovirus production.
Footnotes
This work was supported by National Institute of Child Health and Human Development/National Institutes of Health (NIH) through a cooperative agreement (U54 HD12303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to P.L.M.). This work was also supported by NIH Grant R01 HD20377 (to P.L.M.). V.G.T. was supported by NIH Grants F32 DK065437 and T32 HD07203.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 23, 2008
Abbreviations: AR, Androgen receptor; ChIP, chromatin Immunoprecipitation; DBD, DNA-binding domain; Egr, early growth response proteín; PR, progesterone receptor; PRE, progesterone response element; SF, steroidogenic factor; TK, thymidine kinase.
References
- Levine JE, Ramirez VD 1982 Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology 111:1439–1448 [DOI] [PubMed] [Google Scholar]
- Pierce JG, Parsons TF 1981 Glycoprotein hormones: structure and function. Ann Rev Biochem 50:465–495 [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM 1997 Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15:201–204 [DOI] [PubMed] [Google Scholar]
- Ma X, Dong Y, Matzuk MM, Kumar TR 2004 Targeted disruption of luteinizing hormone β-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA 101:17294–17299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KH, Matzuk MM 2002 Minireview: genetic models for the study of gonadotropin actions. Endocrinology 143:2823–2835 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Jakubowiak A, Steinberger A, Chin WW 1997 Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 138:1224–1231 [DOI] [PubMed] [Google Scholar]
- Papavasiliou SS, Zmeili S, Khoury S, Landefeld TD, Chin WW, Marshall JC 1986 Gonadotropin-releasing hormone differentially regulates expression of the genes for luteinizing hormone α and β subunits in male rats. Proc Natl Acad Sci USA 83:4026–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri RA, Nilson JH 1996 A steroidogenic factor-1 binding site is required for activity of the luteinizing hormone β subunit promoter in gonadotropes of transgenic mice. J Biol Chem 271:10782–10785 [DOI] [PubMed] [Google Scholar]
- Halvorson LM, Kaiser UB, Chin WW 1996 Stimulation of luteinizing hormone β gene promoter activity by the orphan nuclear receptor, steroidogenic factor-1. J Biol Chem 271:6645–6650 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Halvorson LM, Chen MT 2000 Sp1, steroidogenic factor 1 (SF-1), and early growth response protein 1 (egr-1) binding sites form a tripartite gonadotropin-releasing hormone response element in the rat luteinizing hormone-β gene promoter: an integral role for SF-1. Mol Endocrinol 14:1235–1245 [DOI] [PubMed] [Google Scholar]
- Weck J, Anderson AC, Jenkins S, Fallest PC, Shupnik MA 2000 Divergent and composite gonadotropin-releasing hormone-responsive elements in the rat luteinizing hormone subunit genes. Mol Endocrinol 14:472–485 [DOI] [PubMed] [Google Scholar]
- Rosenberg SB, Mellon PL 2002 An Otx-related homeodomain protein binds an LHβ promoter element important for activation during gonadotrope maturation. Mol Endocrinol 16:1280–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC 2004 Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol 33:559–584 [DOI] [PubMed] [Google Scholar]
- Jorgensen JS, Quirk CC, Nilson JH 2004 Multiple and overlapping combinatorial codes orchestrate hormonal responsiveness and dictate cell-specific expression of the genes encoding luteinizing hormone. Endocr Rev 25:521–542 [DOI] [PubMed] [Google Scholar]
- Stefaneanu L 1997 Pituitary sex steroid receptors: localization and function. Endocr Pathol 8:91–108 [DOI] [PubMed] [Google Scholar]
- Pelletier G, Labrie C, Labrie F 2000 Localization of oestrogen receptor α, oestrogen receptor β and androgen receptors in the rat reproductive organs. J Endocrinol 165:359–370 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Waring DW 2000 Progesterone regulation of the progesterone receptor in rat gonadotropes. Endocrinology 141:3422–3429 [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Gharib SD, Chin WW 1988 Estrogen suppresses rat gonadotropin gene transcription in vivo. Endocrinology 122:1842–1846 [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Fallest PC 1994 Pulsatile GnRH regulation of gonadotropin subunit gene transcription. Neurosci Biobehav Rev 18:597–599 [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Gharib SD, Chin WW 1989 Divergent effects of estradiol on gonadotropin gene transcription in pituitary fragments. Mol Endocrinol 3:474–480 [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Rosenzweig BA 1991 Identification of an estrogen-responsive element in the rat LH β gene. DNA-estrogen receptor interactions and functional analysis. J Biol Chem 266:17084–17091 [PubMed] [Google Scholar]
- Wierman ME, Wang C 1990 Androgen selectively stimulates follicle-stimulating hormone-beta mRNA levels after gonadotropin-releasing hormone antagonist administration. Biol Reprod 42:563–571 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Ishizaka K, Kitahara S, Troen P, Attardi B 1992 Effects of testosterone on gonadotropin subunit messenger ribonucleic acids in the presence or absence of gonadotropin-releasing hormone. Endocrinology 130:726–734 [DOI] [PubMed] [Google Scholar]
- Curtin D, Jenkins S, Farmer N, Anderson AC, Haisenleder DJ, Rissman E, Wilson EM, Shupnik MA 2001 Androgen suppression of GnRH-stimulated rat LHβ gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Mol Endocrinol 15:1906–1917 [DOI] [PubMed] [Google Scholar]
- Jorgensen JS, Nilson JH 2001 AR suppresses transcription of the LHβ subunit by interacting with steroidogenic factor-1. Mol Endocrinol 15:1505–1516 [DOI] [PubMed] [Google Scholar]
- Levine JE, Chappell PE, Schneider JS, Sleiter NC, Szabo M 2001 Progesterone receptors as neuroendocrine integrators. Front Neuroendocrinol 22:69–106 [DOI] [PubMed] [Google Scholar]
- Simard J, Labrie C, Hubert J-F, Labrie F 1988 Modulation by sex steroids and [d-TRP6, des-gly-NH210] luteinizing hormone (LH)-releasing hormone ethylamide of α-subunit and LHβ messenger ribonucleic acid levels in the rat anterior pituitary gland. Mol Endocrinol 2:775–784 [DOI] [PubMed] [Google Scholar]
- Abbot SD, Docherty K, Clayton RN 1988 Regulation of LH subunit mRNA levels by gonadal hormones in female rats. J Mol Endocrinol 1:49–60 [DOI] [PubMed] [Google Scholar]
- Corbani M, Counis R, Wolinska-Witort E, d'Angelo-Bernard G, Moumni M, Jutisz M 1990 Synergistic effects of progesterone and oestradiol on rat LH subunit mRNA. J Mol Endocrinol 4:119–125 [DOI] [PubMed] [Google Scholar]
- Kerrigan JR, Dalkin AC, Haisenleder DJ, Yasin M, Marshall JC 1993 Failure of gonadotropin-releasing hormone (GnRH) pulses to increase luteinizing hormone β messenger ribonucleic acid in GnRH-deficient female rats. Endocrinology 133:2071–2079 [DOI] [PubMed] [Google Scholar]
- Park D, Cheon M, Kim C, Kim K, Ryu K 1996 Progesterone together with estradiol promotes luteinizing hormone β-subunit mRNA stability in rat pituitary cells cultured in vitro. Eur J Endocrinol 134:236–242 [DOI] [PubMed] [Google Scholar]
- Thackray VG, McGillivray SM, Mellon PL 2006 Androgens, progestins and glucocorticoids induce follicle-stimulating hormone β-subunit gene expression at the level of the gonadotrope. Mol Endocrinol 20:2062–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SN, Moussa SM, Childs GV 1986 Morphometric studies of rat anterior pituitary cells after gonadectomy: correlation of changes in gonadotropes with the serum levels of gonadotropins. Endocrinology 119:629–637 [DOI] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL 1996 Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang H-J, Miller WL, Mellon PL 2001 Cell-specific transcriptional regulation of FSHb by activin and GnRH in the LbT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 [DOI] [PubMed] [Google Scholar]
- Graham KE, Nusser KD, Low MJ 1999 LβT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to activin A. J Endocrinol 162:R1–R5 [DOI] [PubMed] [Google Scholar]
- Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W 2000 β-Glycan binds inhibin and can mediate functional antagonism of activin signalling. Nature 404:411–414 [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Stoler MH, Shupnik MA 2000 Differential expression and regulation of estrogen receptors (ERs) in rat pituitary and cell lines: estrogen decreases ERα protein and estrogen responsiveness. Endocrinology 141:2174–2184 [DOI] [PubMed] [Google Scholar]
- Thackray VG, Toft DO, Nordeen SK 2003 Novel activation step required for transcriptional competence of progesterone receptor on chromatin templates. Mol Endocrinol 17:2543–2553 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Waring DW 2006 Differential expression and regulation of progesterone receptor isoforms in rat and mouse pituitary cells and LβT2 gonadotropes. J Endocrinol 190:837–846 [DOI] [PubMed] [Google Scholar]
- Meyer ME, Pornon A, Ji JW, Bocquel MT, Chambon P, Gronemeyer H 1990 Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J 9:3923–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Auboeuf D, Wong J, Chen JD, Tsai SY, Tsai MJ, O'Malley BW 2002 Coactivator/corepressor ratios modulate PR-mediated transcription by the selective receptor modulator RU486. Proc Natl Acad Sci USA 99:7940–7944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorson LM, Ito M, Jameson JL, Chin WW 1998 Steroidogenic factor-1 and early growth response protein 1 act through two composite DNA binding sites to regulate luteinizing hormone β-subunit gene expression. J Biol Chem 273:14712–14720 [DOI] [PubMed] [Google Scholar]
- Bain DL, Heneghan AF, Connaghan-Jones KD, Miura MT 2007 Nuclear receptor structure: implications for function. Annu Rev Physiol 69:201–220 [DOI] [PubMed] [Google Scholar]
- Slinker BK 1998 The statistics of synergism. J Mol Cell Cardiol 30:723–731 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.