Negative feedback is a ubiquitous regulatory motif in physiological systems, and signalling and genetic networks. The benefits of negative feedback in homoeostasis have been appreciated for a long time. It helps keep body temperature, and concentrations of ions and metabolites in a narrow range that is compatible with the proper functioning of the organism (Schmidt and Simon, 1982). A related phenomenon is noise suppression, whereby random changes in the concentration of a component in single cells become biased towards the population mean. In this way, negative feedback can narrow down the variability of gene expression in a cell population so that the cells can operate in concord, even when individually they experience fluctuating external or internal milieus.
Although noise suppression has been addressed by a few studies, little attention has been paid to how the mean value of the input signal is converted by negative feedback. A surprising aspect of this signal conversion was revealed by a recent work of Balázsi and his colleagues (Nevozhay et al, 2009). They demonstrate that negative autoregulation can act as a signal linearizer. A linearizer enables a signal to propagate faithfully without distorting its shape. This feature has the potential to greatly enhance the accurate functioning of cells.
Much of the prior research in cellular signalling has focused on how cells can amplify the signal and how they can increase the sensitivity of the response, so that a small change in the input is accompanied by a larger relative change in the output. Switch-like behaviour due to high sensitivity can facilitate decision-making during cell differentiation, and also enables a more efficient propagation of signals through a cascade (see e.g. Paliwal et al, 2007). However, amplification may entail undesired saturation of a response. For example, amplification can occur when a transcriptional activator produced from a weak promoter triggers a strong gene expression at its target promoter. To achieve this, the target promoter has to contain multiple binding sites for the activator, and the cooperative binding to these sites makes the response switch-like, whereby sensitivity is high but the response quickly saturates as the activator binding increases (Becskei et al, 2005). In this way, linearity is lost during amplification.
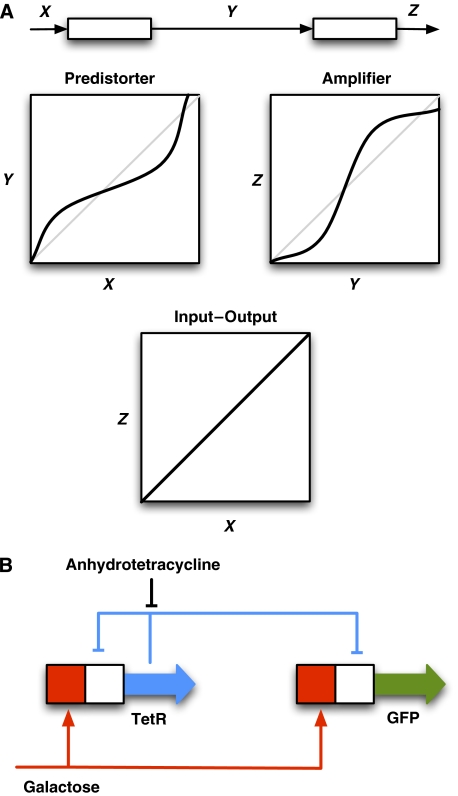
In wireless communication, a contrivance, named predistorter, has been employed to counteract the saturating behaviour of the amplifier. The predistorter has the inverse response characteristics of the amplifier. Thus, when a signal propagates through both the predistorter and the amplifier, the signal becomes linearly amplified (Figure 1A).
Figure 1.
The principles of linearization by a predistorter and a gene circuit where linearization was observed. (A) When the input signal propagates through the predistorter and the amplifier, the output signal is a linear function of the input, provided the predistorting function is the inverse function of the amplifier function. The perfect predistorter function can be obtained by reflecting the amplifier function across the diagonal between the axes. (B) The autoregulatory genetic construct used by Nevozhay et al. The promoters are activated by Gal4 when galactose was added, and they are inhibited by TetR. The binding of TetR to the promoters is inhibited by anhydrotetracycline.
Nevozhay et al made a surprising finding while exploring the mathematical model that describes a synthetic circuit containing a negative feedback loop. They showed that the autoregulatory loop coupled to a reporter gene behaves similarly to a pair of a predistorter and an amplifier. Their actual circuit consisted of two genes, expressing the bacterial repressor protein, TetR, and the reporter gene, GFP, in the eukaryotic model organism, the budding yeast (Figure 1B). For eukaryotic gene expression to be turned on, a transcriptional activator has to bind to the promoter. Thus, the promoters of the genes contain binding sites for the galactose-inducible Gal4 activator and binding sites for TetR, to introduce negative regulation. In this way, TetR inhibits its own expression and the expression of GFP, which was used to quantify the output. The drug anhydrotetracycline was used to modulate the binding of TetR to the promoter.
They observed a highly linear dependence of GFP expression on anhydrotetracycline concentration, up to a point close to the maximal expression. The promoters driving TetR and GFP expression are identical, so they have the same response function. However, the negative feedback inverts the response to anhydrotetracycline. In this way, the distorted function can be re-distorted at the level of the GFP readout, which results in a highly linear output. A corollary of this mechanism is that if the responses of the promoters are not identical, the linearity will be compromised, because the distorted function will not be the exact inverse function of the amplifier function. To prove this experimentally, they changed the number of TetR-binding sites in the promoter of the reporter gene, which alters the sensitivity of the response. Indeed, they found that linearity was compromised, when the response functions at the two promoters differed.
Subsequently, Balázsi and his colleagues explored noise suppression. Previous studies showed that autoregulated prokaryotic transcriptional repressors expressed from plasmids suppress noise in gene expression caused by fluctuations in plasmid copy number (Dublanche et al, 2006). Noise suppression occurs by shifting noise to higher frequencies where it may have a negligible effect on the behaviour of gene circuits that dampen out rapid fluctuations (Austin et al, 2006). However, negative feedback could, in principle, augment noise in some conditions (Paulsson, 2004). For example, when the feedback is mediated by multiple steps, the propagating signal feeds back after a longer delay, which can have a destabilizing effect. In such case, even oscillations can arise so that the concentration of the repressor would meander around the population mean (Kurosawa et al, 2002). A delay can arise due to nuclear transport in eukaryotes, as the transcription factor crosses the nuclear envelope. As the auto-repression construct used by Nevozhay et al was integrated into a yeast chromosome, it was unclear, a priori, whether noise unrelated to plasmid fluctuations could be suppressed. Furthermore, the delay due to nuclear transport could have resulted in increased noise levels. Therefore, it was a rather unexpected observation, that negative feedback resulted in a massive, sevenfold, noise reduction. When the feedback loop was eliminated, a broad bimodal distribution of gene expression was unmasked in the cell population.
An effect related to linearization has recently been identified in the pheromone response system of yeast (Yu et al, 2008). There, negative feedback aligns the dose–response of consecutive steps in a pathway, which then results in a highly linear input–output relation between the pheromone concentration and kinase activity. It will be of interest to see whether the two forms of linearization are based on shared mechanisms and what the important parameters are that enable the negative feedback to act as a linearizer. It remains also to be determined whether the predistortion by negative feedback contributes to noise suppression. This is not necessarily the case. For example, if a predistorter is linearly coupled to an amplifier, it can actually increase noise. On the other hand, when it is incorporated within a feedforward system, then noise is expected to be reduced (McNeilage et al, 1998). It will be certainly exciting to explore and to design circuits that not only amplify but also transmit signals faithfully, in a linear manner. This would not only boost our theoretical understanding but might also lead to the improvement of expression systems in biotechnological applications.
Footnotes
The authors declare that they have no conflict of interest.
References
- Austin DW, Allen MS, McCollum JM, Dar RD, Wilgus JR, Sayler GS, Samatova NF, Cox CD, Simpson ML (2006) Gene network shaping of inherent noise spectra. Nature 439: 608–611 [DOI] [PubMed] [Google Scholar]
- Becskei A, Kaufmann BB, van Oudenaarden A (2005) Contributions of low molecule number and chromosomal positioning to stochastic gene expression. Nat Genet 37: 937–944 [DOI] [PubMed] [Google Scholar]
- Dublanche Y, Michalodimitrakis K, Kummerer N, Foglierini M, Serrano L (2006) Noise in transcription negative feedback loops: simulation and experimental analysis. Mol Syst Biol 2: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa G, Mochizuki A, Iwasa Y (2002) Comparative study of circadian clock models, in search of processes promoting oscillation. J Theor Biol 216: 193–208 [DOI] [PubMed] [Google Scholar]
- McNeilage C, Ivanov EN, Stockwell PR, Searls JH (1998) Review of feedback and feedforward noise reduction techniques. In Proceedings of the 1998 IEEE International Frequency Control Symposium, pp 146–155 [Google Scholar]
- Nevozhay D, Adams RM, Murphy KF, Josic K, Balázsi G (2009) Negative autoregulation linearizes the dose–response and suppresses the heterogenity of gene expression. Proc Natl Acad Sci USA 106: 5123–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal S, Iglesias PA, Campbell K, Hilioti Z, Groisman A, Levchenko A (2007) MAPK-mediated bimodal gene expression and adaptive gradient sensing in yeast. Nature 446: 46–51 [DOI] [PubMed] [Google Scholar]
- Paulsson J (2004) Summing up the noise in gene networks. Nature 427: 415–418 [DOI] [PubMed] [Google Scholar]
- Schmidt I, Simon E (1982) Negative and positive feedback of central nervous system temperature in thermoregulation of pigeons. Am J Physiol 243: R363–R372 [DOI] [PubMed] [Google Scholar]
- Yu RC, Pesce CG, Colman-Lerner A, Lok L, Pincus D, Serra E, Holl M, Benjamin K, Gordon A, Brent R (2008) Negative feedback that improves information transmission in yeast signalling. Nature 456: 755–761 [DOI] [PMC free article] [PubMed] [Google Scholar]