Abstract
Microtubules (MTs) are central to the organisation of the eukaryotic intracellular space and are involved in the control of cell morphology. For these purposes, MT polymerisation dynamics are tightly regulated. Using automated image analysis software, we investigate the spatial dependence of MT dynamics in interphase fission yeast cells with unprecedented statistical accuracy. We find that MT catastrophe frequencies (switches from polymerisation to depolymerisation) strongly depend on intracellular position. We provide evidence that compressive forces generated by MTs growing against the cell pole locally reduce MT growth velocities and enhance catastrophe frequencies. Furthermore, we find evidence for an MT length-dependent increase in the catastrophe frequency that is mediated by kinesin-8 proteins (Klp5/6). Given the intrinsic susceptibility of MT dynamics to compressive forces and the widespread importance of kinesin-8 proteins, we propose that similar spatial regulation of MT dynamics plays a role in other cell types as well. In addition, our systematic and quantitative data should provide valuable input for (mathematical) models of MT organisation in living cells.
Keywords: catastrophes, fission yeast, forces, kinesin-8, microtubules
Introduction
Microtubules (MTs) are dynamic protein polymers that change their length by switching between growing and shrinking states in a process termed ‘dynamic instability' (Mitchison and Kirschner, 1984; Desai and Mitchison, 1997). It is important to understand how dynamic instability is regulated, because this affects MT length (Verde et al, 1992; Dogterom and Leibler, 1993) and intracellular organisation (Kirschner and Mitchison, 1986; Hayles and Nurse, 2001), as well as the ability of MTs to exert pushing and pulling forces (Inoue and Salmon, 1995; Dogterom et al, 2005). Several proteins have been characterised that globally affect MT dynamics and catastrophe rates (Howard and Hyman, 2007), but it is a largely open question how such regulation is achieved locally, in response to spatially varying biochemical cues and/or mechanical effects induced by the shape and size of cells. In fact, in addition to global regulation, it has been reported that catastrophe frequencies can be locally enhanced, for example close to the periphery of animal cells (Komarova et al, 2002; Mimori-Kiyosue et al, 2005). There are several ways by which such a local catastrophe enhancement could be accomplished: (activity) gradients of destabilising MT-associated proteins (Niethammer et al, 2004) and/or MT length-dependent mechanisms (Dogterom et al, 1996) would lead to a gradual catastrophe enhancement when approaching the cell boundary. On the other hand, force-induced effects that may result from growing MTs pushing against the cell boundary (Dogterom and Yurke, 1997; Janson et al, 2003; Janson and Dogterom, 2004) would lead to an abrupt catastrophe enhancement once contact with the cell boundary has been made. A similar abrupt change would be expected in case there is an MT-destabilising factor associated locally with the membrane (Mimori-Kiyosue et al, 2005). To distinguish between long- and short-range mechanisms requires quantitative and spatially resolved measurements of the catastrophe frequency inside living cells. In mammalian cells, this is often a challenging task because MT networks are quite dense and reference points such as the cell boundary in interphase or chromosomes in mitosis are dynamic structures themselves. Another challenge is that catastrophes appear to be stochastic events that are governed by an average rate (Odde, 1995; Howell et al, 1997). This has two consequences: statistical accuracy is a serious issue when investigating catastrophe frequencies, and, the stochastic nature of the process makes it very difficult to avoid picking a subset of events when examining data by visual inspection.
In this paper, we present quantitative investigations of spatial MT catastrophe regulation in interphase fission yeast (Schizosaccharomyces pombe) cells with high statistical accuracy (Hayles and Nurse, 2001). Fission yeast is an excellent model system, because MTs are well organised and the rigid cylindrical cell wall makes it possible to accurately assign catastrophes to specific locations within the cell (Figure 1A and B). In interphase cells, MTs are organised into 3–6 bundles, consisting of 2–6 antiparallel MTs per bundle (Hoog et al, 2007; Hagan, 1998), which are usually (but not always) connected to the nucleus (Carazo-Salas and Nurse, 2006; Zheng et al, 2006; Daga et al, 2006a). MT minus ends are generally found close to the nucleus within the central overlap zone of the MTs, whereas dynamic plus tips grow and shrink between the nucleus and the cell poles (Drummond and Cross, 2000; Tran et al, 2001). Catastrophe events are mainly restricted to the regions of the two cell poles by an unknown mechanism. This local regulation of MT catastrophes is, however, crucial for the maintenance of correct fission yeast morphology and intracellular organisation (Beinhauer et al, 1997; Mata and Nurse, 1997; Browning et al, 2000; Brunner and Nurse, 2000; Hayles and Nurse, 2001; Tran et al, 2001; Sawin and Snaith, 2004; Tolic-Norrelykke et al, 2005; Daga et al, 2006b). To obtain good statistics and to ensure unbiased observations, we developed fully automated image analysis software that generates spatially resolved maps of MT dynamics from movies of GFP-labelled MTs in fission yeast (see Supplementary information). Our spatially resolved measurements show that there is both local enhancement of the catastrophe frequency specifically at cell poles as well as long-range modulation before the cell pole is reached. We find several indications that the local regulation at the cell pole is (at least in part) due to compressive forces that build up when bundle tips hit the cell pole. In addition, we find evidence that the long-range catastrophe regulation is a MT length-dependent effect mediated by the kinesin-8 proteins Klp5/6. Since physical boundaries as well as kinesin-8 proteins are also present in other eukaryotic systems, we think that the relevance of our findings reaches beyond the fission yeast model system.
Figure 1.
Spatially resolved catastrophe frequency measurements in fission yeast. (A) z-maximum projection of a confocal stack of images of an interphase fission yeast cell expressing GFP-tubulin (N=nuclear region; C=cytoplasmic region; P=cell pole region; x=distance from cell centre). (B) Scheme of MT organisation, including definitions of parameters and intracellular regions. Numbers are lengths in micrometres (bt=MT bundle tip; L=cell half length). (C) Number of catastrophes Ncat(x) and (D) bundle-tip dwell times Tdwell(x) as observed in 160 cells with L±ΔL=5±0.5 μm. (E) Local catastrophe frequency fcat(x)=Ncat(x)/Tdwell(x). Here and in other figures, due to the averaging over a certain cell size range, the x coordinate comprises values within the interval [x−ΔL, x+ΔL]; errors for fcat are computed as  assuming a Poisson process.
assuming a Poisson process.
Results
Spatial dependence of MT dynamics
We started by monitoring MT dynamics only in cells of similar size (with a half length of 5±0.5 μm). Our software was designed to specifically monitor catastrophes of the longest MTs in each bundle (the ‘bundle tips'; see Figure 1B), which we called ‘bundle tip catastrophes'. The dynamics of the longest MTs in a bundle are particularly relevant as they are the ones that make contact with the cell walls, where they can deliver proteins (Mata and Nurse, 1997; Feierbach et al, 2004) and generate pushing forces that mediate nuclear centring (Tran et al, 2001; Tolic-Norrelykke et al, 2005; Daga et al, 2006b).
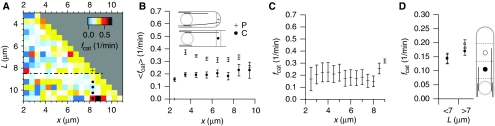
In agreement with earlier results obtained by visual inspection (Brunner and Nurse, 2000), the software reported that the great majority of bundle tip catastrophes (Ncat) occur at the cell poles (Figure 1C). This is sometimes interpreted as an indicator of spatial catastrophe regulation. However, observing a large number of catastrophes could simply be the consequence of the fact that the majority of bundle tips is found at the cell poles. To distinguish between these possibilities, our software also performed spatially resolved measurements of the time bundle tips were observed growing in a certain region, which we termed the dwell time Tdwell (Figure 1D; Supplementary Figure S1). This allowed us to compute a spatially resolved catastrophe frequency fcat(x)=Ncat(x)/Tdwell(x), where x is the distance from the cell centre. fcat(x) is the probability per unit time that individual MTs switch from a growing to a shrinking state at position x (Walker et al, 1988). Note that global (spatially averaged) values of fcat have been measured in fission yeast before (Tran et al, 2001; Busch and Brunner, 2004). Using this methodology, we found a strong enhancement of not only the number of catastrophes but also fcat itself at the cell pole (Figure 1E). To investigate whether this effect really depended on cell pole proximity rather than on the distance to the cell centre, we measured fcat in cells of different lengths. The half length (L) of fission yeast under laboratory conditions ranges from about 3 to 7 μm (Sveiczer et al, 1996), where the longer length regime is difficult to study, because cells are entering mitosis. To facilitate the investigation of a possible long-range regulation of MT dynamics, we administered low doses of the drug hydroxyurea (HU), which significantly increased cell length without altering MT catastrophe, growth or shrinkage dynamics (see Supplementary information). In Figure 2A, we show the results for fcat as a function of cell half length L and distance from the cell centre x (we obtained similar results with cells that were elongated using mutations in cell cycle regulatory genes instead of HU treatment, see Supplementary Figure S8). If we focus on a given distance from the cell centre, we find that fcat at the poles is generally larger than the fcat of MTs that roughly have the same length but that are not yet at the cell pole (Figure 2B). This indicates that there indeed exists a cell pole-specific fcat enhancement. Note however that the abrupt increase of fcat at the poles that is observed in short cells (Figure 1E) is less obvious in longer cells (Figure 2B and C). Interestingly, the data also show a gradual increase of fcat for MTs still growing in the cytoplasmic region (Figure 2A–C) (note: we use the terminology ‘cytoplasmic region' to classify MTs that are more than 1 μm away from the cell poles; we do not distinguish whether these MTs are in contact with cell walls or not). To further establish this spatial dependence of fcat in the cytoplasmic region (and exclude that this is a unique feature of overly long cells), we defined a proximal and distal cellular region, where the distal region is excluding the last 2 μm towards the cell pole (Figure 2D). For both cells of normal sizes (L<7 μm) and for elongated cells (L>7 μm), we find that fcat is significantly higher in the distal region. We in addition devised software to automatically measure the velocity at which the tips of bundles moved through the cytoplasmic region (see Supplementary Figure S4B). In contrast to the fcat measurements, we found no evidence for a strong spatial variation of cytoplasmic growth and shrinkage velocities (Figure 3A–C).
Figure 2.
Spatially resolved fcat measurements in wild-type cells as a function of distance to the cell centre (x) in cells with different half lengths L. (A) fcat(x,L). The solid horizontal line indicates the L at which cells normally undergo mitosis under laboratory conditions. Longer cells were obtained by delaying mitosis through hydroxyurea treatment. The cross and circles refer for one specific x to the presentation of the data in (B). Dash and dash-dot horizontal lines indicate data shown in Figures 1E and 2C, respectively. Statistics: Ncat=10878; Ncells=533; here and in other figures, data for large L are more noisy because of less statistics; see also Supplementary Figure S2. (B) Comparison of fcat at the pole (crosses) in cells of different lengths (L=x) to fcat in the cytoplasmic region at same position x in longer cells (circles; averaged over all cells with L>x+1 μm). (C) fcat(x) for cells with L=9±0.5 μm. (D) Average fcat in cytoplasmic regions proximal (x1<x<x2) and distal (x2<x<x3) to the cell centre, for cells with normal (L<7 μm) and elongated (L>7 μm) sizes. x1=1.5 μm; x3=L−2 μm; x2=(x1+x3)/2. Statistics: L<7 μm: Ncat=492; Ncells=339; L>7 μm; Ncat=1826; Ncells=286.
Figure 3.
Spatially resolved bundle tip velocity measurements in wild-type cells. (A, B) Bundle tip velocities for growing (vg) and shrinking (vs) microtubules. (C) Growth and shrinkage velocities for cells with L=9±0.5 μm, corresponding to dash-dot horizontal lines in (A, B).
Detailed analysis of MT dynamics at cell poles
In search for a mechanistic explanation for the cell pole specific fcat enhancement, visual inspection of movies suggested to us that the probability for catastrophes was higher when both tips of a bundle were simultaneously in contact with both cell poles. Figure 4A shows an example where the arrival of the opposite bundle tip at the opposite cell pole seemed to trigger catastrophes of both bundle tips. Given the stochastic nature of MT catastrophes, this sequence of events could be coincidental. To rigorously test whether there was an effect, we measured fcat in the cell pole region while keeping automatically track of whether the other bundle tip was close to the opposite cell pole or not (PP versus PC, Figure 4B; Supplementary Figure S4A). The quantification revealed that fcat was indeed significantly enhanced in the PP as compared with the PC situation (Figure 4C; Supplementary Figure S7B). In contrast, the fcat of MTs growing through the cytoplasmic region was similar no matter whether the other bundle tip was at the cell pole or not (CP versus CC). Note that in a recent study a higher fcat was found in the CP (compared to CC) situation in cells where the nucleus was displaced to one side of the cell by centrifugation (Daga et al, 2006b). We do not find such an effect in our cells. The difference could, however, simply be a statistical issue as the total observation time of cytoplasmic MTs in the study by Daga et al was rather short (33 min versus 1204 min in this study). To further investigate the difference between the PP and PC situation, we monitored MT growth velocities in both cases. This poses some technical difficulty because MT growth against the cell wall does not necessarily result in MT tip displacement (Drummond and Cross, 2000; Tran et al, 2001; Grallert et al, 2006). However, quantification of GFP-tubulin speckle translocation (Tran et al, 2001) revealed that the MT growth velocity at the pole was significantly reduced in the PP as compared with the PC situation (Figure 4D; Supplementary Figure S3C). When we monitored the velocity at which bundle tips moved through the cytoplasmic region we found the opposite effect: tip movement through the cytoplasmic region was faster in the presence of opposite pole contact (Figure 4E). We in addition found that the angles at which the bundle tips at the cell poles were growing with respect to the long axis of the cell were larger in the PP than in the PC case (and lowest for MTs that were growing in the cytoplasmic region) (Figure 4F). These large angles correlated with both a strong enhancement of fcat and a strong reduction of MT growth velocity (Figure 4G and H; see also Supplementary Figure S7C). We interpret the reduction in velocity at the cell poles as the result of force-induced effects (see Discussion below). However, an alternative explanation for the reduced velocity in the PP case may be that the overall polymer mass in the cell is higher, leading to a lower tubulin concentration and a global reduction in the growth velocity. When we measured the growth velocities of cytoplasmic bundle tips in the CC situation, depending on whether there was a neighbouring PP or PC bundle, we found however no significant difference (PP: vg=2.28±0.05 μm/min, N=469; PC: vg=2.29±0.06 μm/min, N=449).
Figure 4.
Microtubule dynamics at the poles of untreated cells with 3 μm⩽L⩽7 μm. (A) Sequence of GFP-tubulin z-maximum projections (Δt=32 s); white dots indicate the MT bundle tips referred to in the text. (B, C) Catastrophe frequency depending on bundle tip position (P=pole, C=cytoplasm) and depending on position of the opposite bundle tip (subscript P or C). Statistics: Ncat: PP 1566; PC 346; CP 192; CC 97. (D) Left panel: sequence of GFP-tubulin z-maximum projections (Δt=8 s). Middle panel: intensity profiles following the bundle axis in 3D (see Supplementary information). Solid and dashed lines correspond to images in (D). Right panel: growth velocity of bundle tips at cell poles as measured by 3D GFP-tubulin speckle motion, depending on opposite bundle tip position (mean±s.e.m. from 6455 PP and 3234 PC events in 272 cells). (E) Left panel: sequence of GFP-tubulin z-maximum projections (Δt=24 s); white dots mark a growing bundle tip. Right panel: velocities at which growing bundle tips move through the cytoplasmic region, depending on the position of the opposite bundle tip (mean±s.e.m. from 4089 CP and 1763 CC events in 272 cells). (F) Histogram of angles between bundle tips and the long axis of the cell (statistics: CP and CC 6410 events; PC 7532 events; PP 18485 events). 3D angles were computed using the (x,y,z) positions of the bundle tip and of a point about 0.8 μm behind the bundle tip (see inset and Supplementary information). (G, H) fcat and bundle tip growth velocity as a function of angle.
Spatial dependence of MT dynamics in Klp5/6 deletion strains
Next, we investigated whether the observed long-range increase of fcat depended on the presence of the fission yeast kinesin-8 proteins Klp5 and Klp6. This was motivated by recent in vitro experiments on the depolymerisation activity of the budding yeast kinesin-8 protein Kip3 (Varga et al, 2006) on GMPCPP-stabilised MTs. These experiments led to speculations that kinesin-8 proteins could have the capability to increase fcat of non-stabilised MTs in an MT length-dependent manner (Gardner et al, 2008; Stumpff et al, 2008). Corroborating observations by West et al (2001), bundles in klp5Δklp6Δ cells seemed on average longer than in wild-type cells (Supplementary Figure S2A and D). Quantification of bundle tip dynamics in klp5Δklp6Δ cells showed that fcat was overall about 40% reduced as compared with wild-type cells (Figure 5A). More interestingly, we found that the long-range fcat increase in the cytoplasmic region was strongly reduced in klp5Δklp6Δ cells (Figure 5A–D). Also the cell length dependence of fcat at cell poles was strongly reduced as compared with wild-type cells (Figure 5B). There was however still a clear difference in fcat for MTs at the pole and MTs not yet at the pole for a given value of x (Figure 5B). We also measured the spatial dependence of the growth and shrinkage velocities in these cells (Supplementary Figure S3). We found that the average cytoplasmic growth velocities of growing bundle tips were overall about 15% reduced in klp5Δklp6Δ cells (vgwt=2.42±0.01 μm/min (N=11578); vgKlp5/6Δ=2.05±0.02 μm/min (N=3677)), whereas shrinkage velocities were not significantly different (vswt=8.7±0.2 μm/min (N=613); vsKlp5/6Δ=8.7±0.3 μm/min (N=173)).
Figure 5.
Spatially resolved fcat measurements in klp5Δklp6Δ cells as a function of distance to the cell centre (x) in cells with different half lengths L. (A) fcat(x,L). The cross and circles refer for one specific x to the presentation of the data in (B). Statistics: Ncat=4797; Ncells=377; see also Supplementary Figure S2. (B) Crosses: fcat at the poles of cells of different lengths (x=L). Circles: fcat in the cytoplasm at position x, averaged over all cells with L>x+1 μm. (C) fcat(x) for cells with L=9±0.5 μm, corresponding to the dashed horizontal line in (B). (D) Average fcat in cytoplasmic regions proximal and distal to the cell centre, for cells with normal (L<7 μm) and elongated (L>7 μm) sizes; see also Figure 2D. Statistics: L<7 μm: Ncat=117; Ncells=241; L>7 μm: Ncat=245; Ncells=173.
Analysis of Klp5/6 distribution along MTs
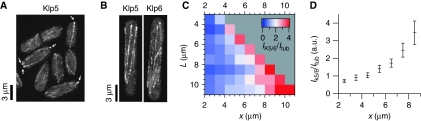
The data presented above indicate that Klp5/Klp6 proteins preferentially promote catastrophes at positions far from the cell centre, consistent with the idea that these proteins induce an MT length-dependent effect. To gain more insight into the mechanism, we investigated the localisation of Klp5/Klp6 along MTs. Although it has been shown that Klp5 and Klp6 colocalise on interphase MTs (West et al, 2001; Garcia et al, 2002), presumably due to heterodimerisation (Garcia et al, 2002), a detailed analysis of their spatial distribution is missing. We revisited Klp5/Klp6 localisation by monitoring Klp5-GFP and Klp6-GFP expressed under control of the respective endogenous promoter (West et al, 2001). Visual inspection of z-maximum projections of Klp5/6-GFP indicated that the intensity of Klp5/6-GFP was higher on the tips of MTs that were at the cell poles (Figure 6A). In addition, in elongated cells, we also observed high Klp5/6-GFP intensities on the tips of MTs that were not yet at cell poles (Figure 6B). To quantify this and to normalise for the fact that the tubulin polymer density (i.e. potential Klp5/6-GFP-binding sites) is higher in the cell centre, we measured ratios of average local Klp5/6-GFP intensities and average local GFP-bundle intensities (Figure 6C and D; see also Supplementary Figure S5). Corroborating the impression of visual inspections, this analysis indicated that there is more Klp5/6-GFP on MTs that are further away from the cell centre, with the highest intensity for MTs closest to the cell poles.
Figure 6.
Spatially resolved measurements of the distribution of Klp5/6 on MT bundles. (A) z-maximum projections of untreated cells expressing Klp5-GFP. Arrows point out instances of high intensities within the cell pole regions. (B) z-maximum projections of elongated (HU-treated) cells expressing Klp5-GFP (left panel), or Klp6-GFP (right panel). (C) Average Klp5-GFP and Klp6-GFP intensity (IK5/6) divided by average GFP-tubulin intensity (Itub) as a function of intracellular position for different cell lengths (see also Supplementary Figure S5). (D) Same data as in (C) shown for L=9±0.5 μm (error bars are computed using the s.e.m. for IKlp5/6 (Ncells=52) and for Itub (Ncells=37) and the laws of error propagation for the ratio).
Discussion
Force generation and the modulation of MT dynamics at cell poles
On performing quantitative in vivo measurements, we find with high statistical significance that both the catastrophe frequency fcat and the growth velocity vg of MT bundle tips near the pole of interphase fission yeast cells are different from the dynamics in the cytoplasmic region in a way that depends on whether the opposite bundle tip resides near the other cell pole or not (Figure 4A–E). We think the easiest explanation for these observations would be the following: the growth of a bundle tip against the cell pole generates pushing forces, which lead to compressive forces on the MT, which in turn cause a reduction in growth velocity because addition of new tubulin subunits to the MT tip is physically hindered (Peskin et al, 1993; Mogilner and Oster, 1999; Doorn et al, 2000). In vitro experiments have shown that compressive polymerisation forces that build up when MTs grow against micro-fabricated walls reduce MT growth velocities by similar factors as we observe here in vivo (Dogterom and Yurke, 1997; Janson and Dogterom, 2004). In vitro measurements have also shown that low growth velocities tend to correspond to high values of fcat, again in a manner that is quantitatively similar to our observations (Walker et al, 1988; Janson et al, 2003).
The build-up of compressive forces is expected to be strongest when bundle tips are simultaneously in contact with opposite cell poles (the PP situation), and is expected to be stronger for short MTs than for long MTs (Dogterom and Yurke, 1997; Janson and Dogterom, 2004). This last effect would explain why the increase in fcat at the cell poles is stronger in short cells than in long cells (Figures 2B and 5B). The presence of pushing forces that lead to motion of the central MT overlap zone away from the cell pole (Tran et al, 2001; Daga et al, 2006b) would also explain the increase in the velocity at which the opposite bundle tip moves through the cytoplasmic region in the CP versus CC situation (Figure 4E; note that the increase in velocity need not be identical to the MT growth velocity at the pole due to bending effects). In addition, the observed angle dependence of MT growth velocities and catastrophe rates appears to fit into this mechanical picture (Figure 4F–H). MTs growing through the cytoplasmic region do not experience any growth opposing forces (and therefore remain mostly straight) as long as they are able to elongate towards the cell pole. This is independent of whether there is contact with the cell membrane or not, as long as MT tips are able to freely slide along the cortex. Freely sliding tips may slightly bend due to lateral forces on the MT tip, but these forces are directed perpendicular to the growth direction and are therefore not expected to affect the growth velocity. Only when further sliding is prevented upon reaching the (extreme) cell pole, compressive forces build up and MTs start to seriously bend. Given the shape of the fission yeast cell, strong MT bending correlates on average with large contact angles (see e.g. Figure 4A). Already in the PC situation, the nuclear anchoring of MTs can be sufficient to support the build-up of significant forces (see also Supplementary Figure S9). However, the observation that angles are on average larger in the PP than in the PC situation is consistent with the idea that compressive forces are largest in the former case.
Although force-induced effects may explain the pole-specific fcat enhancement we observe in our experiments, it is important to note that this does not rule out a possible additional role for specific MT interacting proteins. General differences in MT growth dynamics between the cell pole and the cytoplasmic region, as have been observed by others before (Drummond and Cross, 2000; Tran et al, 2001; Grallert et al, 2006), may also be explained by (yet unknown) biochemical factors that are selectively located to either of these regions.
The role of Klp5/6 in MT length-dependent fcat regulation
Our data indicate that there also exists a long-range regulation of fcat in the cytoplasmic region, several micrometres away from the cell pole. This long-range regulation is diminished in strains where the kinesin-8 proteins Klp5/6 are deleted (Figures 2 and 5). We provide evidence that the density of Klp5/6 on MTs is highest near the plus tips of long MTs (Figure 6). As Klp5/6 are members of the kinesin-8 family, this non-uniform distribution is likely to be caused by plus-tip-directed motility (Pereira et al, 1997; Rischitor et al, 2004; Gupta et al, 2006; Varga et al, 2006; Mayr et al, 2007) (see also Supplementary Figure S5C and D where we show, for individual cases, motion of Klp5/6-GFP molecules along the MT lattice as well as the gradual increase in Klp5/6 intensity at a growing MT tip). These observations are in line with recent in vitro measurements on the budding yeast kinesin-8 protein Kip3, showing an increased density near the plus tips of long MTs (Varga et al, 2006). In vitro, it has also been shown that the kinesin-8 proteins Kip3 (Saccharomyces cerevisiae) and Kif18A (Homo sapiens) depolymerise longer GMPCPP-stabilised MTs faster than shorter ones (Varga et al, 2006; Mayr et al, 2007). On first glance, this may seem different to our findings, because we find no evidence that Klp5/6 would increase the MT depolymerisation velocity in vivo. However, dynamic MTs have an intrinsic tendency to depolymerise after a catastrophe event, which is different from the slow protein-driven depolymerisation observed for GMPCPP-stabilised MTs (Varga et al, 2006; Mayr et al, 2007). The effect of kinesin-8 proteins on dynamic MTs in vivo could thus be to promote catastrophes preferentially of long MTs, possibly by driving depolymerisation of a stabilising structure at the MT tip (Desai and Mitchison, 1997).
Interestingly, Daga et al (2006b) observed in fission yeast cells with an artificially displaced nucleus that MTs that were in the longer cell half underwent catastrophes more frequently than in the shorter cell half. We think these observations may be explained by an increase in fcat with MT length. An increase in fcat with MT length, but no length dependence of growth and shrinkage velocities also matches findings in mitotic Xenopus extracts (Dogterom et al, 1996), suggesting that there may be a similar mechanism in place.
In addition to the Klp5/6-dependent long-range regulation of fcat in the cytoplasmic region, we also observe a strong decrease in fcat at cell poles upon Klp5/6 deletion (compare upper traces in Figures 2B and 5B). This could (partly) be explained by the fact that MTs at cell poles are in fact the longest in a given cell. This is consistent with the fact that the decrease in fcat at cell poles upon Klp5/6 deletion is more pronounced in longer cells (compare Figures 2B and 5B). However, we also find evidence that the density of Klp5/6 on MT tips is particularly high at cell poles even when compared with MTs of the same length in longer cells (see Figure 6A–C). We therefore think it is possible that Klp5/6 additionally accumulate at MTs that are at the cell pole and thus specifically contribute to a fcat enhancement at cell poles. Interestingly, Gupta et al (2006) similarly reported that deletion of the Klp5/Klp6 homologue Kip3 in budding yeast lowered fcat at the cell periphery more than in the cell centre.
Finally, we would like to point out that changes in MT dynamics in Klp5/6 deletion cells may in addition be caused by indirect (secondary) system responses. For instance, due to the reduced fcat one expects more tubulin to be bound in polymer form and it could thus be that the slight (∼15%) reduction in MT growth velocity in Klp5/6 deletion cells is a response to a lower concentration of free tubulin. Also, as fcat is generally lower, there could on average be more MTs at cell poles. This could lead to lowering of the (shared) compressive forces on individual MTs, for example in the PC situation, leading to a diminished force-induced increase in fcat at cell poles.
Relevance of spatial regulation of fcat in interphase fission yeast cells
We present evidence for two kinds of spatial regulation of MT dynamics in interphase fission yeast: (i) a local decrease in the growth velocity and a local enhancement of fcat at cell poles and (ii) a long-range increase in fcat with distance to the cell centre. The local regulation at cell poles provides clear functional advantages for MT-mediated transport of cell growth-promoting factors such as Tea1 (Mata and Nurse, 1997). Such transport requires that MTs reliably reach and undergo catastrophes at the cell poles; when MTs either undergo premature catastrophes or do not cease growth at cell poles, Tea1 transport and cell shape control are perturbed (Beinhauer et al, 1997; Mata and Nurse, 1997; Browning et al, 2000; Brunner and Nurse, 2000; Hayles and Nurse, 2001; Feierbach et al, 2004; Sawin and Snaith, 2004; Castagnetti et al, 2007). In addition, there is evidence that a second function of MTs in interphase fission yeast is to dynamically centre the nucleus by creating pushing forces at the cell poles (Tran et al, 2001; Tolic-Norrelykke et al, 2005). This mechanism benefits from long-range catastrophe regulation where long MTs undergo more catastrophes, because, in cells with displaced nucleus, fewer MTs reach the distal pole (due to premature catastrophes) and spend less time at the distal pole (due to the relatively high fcat). Thus, the asymmetry of the pushing forces is enhanced and nuclear centring is accelerated (see accompanying paper by Föthke et al, 2009).
Relevance of our measurements to other systems
In addition to the situation in the fission yeast system, it is interesting to note that fcat is also enhanced at the boundaries of animal cells (Komarova et al, 2002; Mimori-Kiyosue et al, 2005) by a yet unknown mechanism. Our findings in fission yeast, taken together with earlier in vitro observations (Dogterom and Yurke, 1997; Janson et al, 2003; Janson and Dogterom, 2004), suggest that there are intrinsic relations between polymerisation force, vg, and fcat that help terminate MT growth at physical boundaries.
In a living cell, the spatial extent of the cell and the length of its MT cytoskeleton must be well adapted to each other. There has been evidence that MT dynamics play a role in establishing cell shape (Kirschner and Mitchison, 1986; Hayles and Nurse, 2001). Our data indicate that, vice versa, the shape of a cell also influences MT dynamics. Thus, cell shape and MT organisation may not be separable components but should be viewed as one system.
Klp5/Klp6 are part of the kinesin-8 family, comprising Kip3 (S. cerevisiae), KLP67A (Drosophila melanogaster), Kif18A (H. sapiens) and KipB (Aspergillus nidulans), which have in common that mutants show defects in mitosis (Garcia et al, 2002; West et al, 2002; Rischitor et al, 2004; Tytell and Sorger, 2006; Mayr et al, 2007; Stumpff et al, 2008). In this context, it has been speculated that a kinesin-8-mediated increase in fcat with MT length could contribute to proper chromosome centring and spindle length regulation (Gardner et al, 2008; Stumpff et al, 2008). As discussed above, our data provide good experimental evidence that kinesin-8 proteins indeed specifically enhance fcat of long MTs.
Conclusions
Our data suggest that MT polymerisation forces produced at fission yeast cell poles reduce MT growth velocities and enhance the probability that MTs undergo catastrophes. Moreover, we present evidence that kinesin-8 proteins enhance the fcat of long MTs. Both mechanisms contribute to a robust termination of MT growth at fission yeast cell poles without compromising growth through the cytoplasm. These discoveries were strongly facilitated by the development of automated image analysis procedures, which made it possible to obtain the statistics that are necessary to study the apparently stochastic processes of MT catastrophes. We would like to stress that regulation of MT dynamics in fission yeast is probably not only mediated by mechanical forces and kinesin-8 proteins but also by other regulatory proteins (e.g. Tip1, Tea1, Mal3 or Peg1; Mata and Nurse, 1997; Brunner and Nurse, 2000; Busch and Brunner, 2004; Grallert et al, 2006). Further systematic and quantitative studies will be necessary to fully uncover the complex interplay between mechanical and biochemical regulation of MT dynamics in fission yeast and other eukaryotic systems.
Materials and methods
Cell culturing
S. pombe cells were grown using standard conditions (Moreno et al, 1991). Cells expressing GFP-α2-tubulin from a single, exogenously integrated locus under the control of the nmt1 promoter, were grown in Edinburgh minimal medium supplemented with 15 μM thiamine. For klp5/6 deletion experiments we constructed a strain where both the klp5 and klp6 genes were deleted and GFP-α2-tubulin was expressed as above. These cells were also grown in Edinburgh minimal medium supplemented with 15 μM thiamine. Cells expressing Klp5-GFP or Klp6-GFP were grown in yeast extract medium.
Strains used in this study
YY105: leu1-32 ura4-d18 lys1+∷nmt1-GFP-α2tub, h90 (Yamamoto et al, 1999)
DB1767: klp5Δ∷ura4+klp6Δ∷ura4+lys1+∷nmt1-GFP-α2tub ade6-M216 his3-D1 leu1-32 ura4-D18, h90 (this study)
McI 485: klp5∷GFP∷ura4 ade6-M210 his3-D1 leu1-32 ura4-D18, h+ (West et al, 2001)
McI 486: klp6∷GFP∷ura4 ade6-M210 his3-D1 leu1-32 ura4-D18, h− (West et al, 2001).
Microscopy
Cells were mounted on agarose pads following protocols by Tran et al (2004) and imaged at 24–25°C with a confocal spinning disc microscope, comprising a confocal scanner unit (CSU22; Yokogawa Electric Corp.) attached to an inverted microscope (DMIRB; Leica) equipped with a × 100/1.3 NA oil immersion lens (PL FLUOTAR; Leica) and a built-in × 1.5 magnification changer lens. The sample was illuminated using a 488 nm laser (Sapphire 488-30; Coherent Inc.). Images were captured by an EM-CCD (C9100; Hamamatsu Photonics) controlled by software from VisiTech International. z-maximum projections were computed from stacks of about 20 images acquired with 125 ms exposure time at 0.3 μm z-spacing. For measurement of MT dynamics, 90 z-stacks were acquired with 8 s time delay between subsequent stacks, corresponding to 12 min observation time.
Image analysis
Note added in proof
We would like to point out two recently published articles investigating the effect of Klp5/6 on MT dynamics both in vivo (Unsworth et al, 2008) and in vitro (Grissom et al, 2008). In the first article, measurements consistent with our observations are presented showing that the catastrophe frequency of MTs is suppressed in Klp5/6 deletion mutants. In the second article, the authors show that unlike kinesin-8 proteins such as Kip3, Klp5/6 proteins do not possess an MT depolymerisation activity in vitro. This suggests that Klp5/6 may induce catastrophes by a mechanism that is distinct from kinesin-8 proteins in other species.
Supplementary Material
Supplemental information on image analysis, Supplemental information on hydroxyurea treatment, Supplemental references, Figures S1-9
Supplementary Figure S2B
Supplementary Figure S2C
Supplementary Figure S2E
Supplementary Figure S2F
Supplementary Figure S5A
Supplementary Figure S5B
Acknowledgments
This study is part of the research programme of the Stichting voor Fundamenteel Onderzoek der Materie (FOM), which is financially supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO). We thank Dietrich Foethke and Francois Nedelec for many fruitful discussions. We thank Sander Tans and Bela Mulder for carefully reading the paper and Takashi Toda for kindly providing klp5 and klp6 deletion strains as well as Dick McIntosh for sharing Klp5/6-GFP strains. We gratefully acknowledge support from HSFP Research Grant RPG11/2005. CT was supported by a Marie Curie Fellowship.
Footnotes
The authors declare that they have no conflict of interest.
References
- Beinhauer JD, Hagan IM, Hegemann JH, Fleig U (1997) Mal3, the fission yeast homologue of the human APC-interacting protein EB-1 is required for microtubule integrity and the maintenance of cell form. J Cell Biol 139: 717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning H, Hayles J, Mata J, Aveline L, Nurse P, McIntosh JR (2000) Tea2p is a kinesin-like protein required to generate polarized growth in fission yeast. J Cell Biol 151: 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D, Nurse P (2000) CLIP170-like tip1p spatially organizes microtubular dynamics in fission yeast. Cell 102: 695–704 [DOI] [PubMed] [Google Scholar]
- Busch KE, Brunner D (2004) The microtubule plus end-tracking proteins mal3p and tip1p cooperate for cell-end targeting of interphase microtubules. Curr Biol 14: 548–559 [DOI] [PubMed] [Google Scholar]
- Carazo-Salas RE, Nurse P (2006) Self-organization of interphase microtubule arrays in fission yeast. Nat Cell Biol 8: 1102–1107 [DOI] [PubMed] [Google Scholar]
- Castagnetti S, Novak B, Nurse P (2007) Microtubules offset growth site from the cell centre in fission yeast. J Cell Sci 120: 2205–2213 [DOI] [PubMed] [Google Scholar]
- Daga RR, Lee KG, Bratman S, Salas-Pino S, Chang F (2006a) Self-organization of microtubule bundles in anucleate fission yeast cells. Nat Cell Biol 8: 1108–1113 [DOI] [PubMed] [Google Scholar]
- Daga RR, Yonetani A, Chang F (2006b) Asymmetric microtubule pushing forces in nuclear centering. Curr Biol 16: 1544–1550 [DOI] [PubMed] [Google Scholar]
- Desai A, Mitchison TJ (1997) Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 13: 83–117 [DOI] [PubMed] [Google Scholar]
- Dogterom M, Felix M-A, Guet CC, Leibler S (1996) Influence of M-phase chromatin on the anisotropy of microtubule asters. J Cell Biol 133: 125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogterom M, Kerssemakers JW, Romet-Lemonne G, Janson ME (2005) Force generation by dynamic microtubules. Curr Op Cell Biol 17: 67–74 [DOI] [PubMed] [Google Scholar]
- Dogterom M, Leibler S (1993) Physical aspects of the growth and regulation of microtubule structures. Phys Rev Lett 70: 1347–1350 [DOI] [PubMed] [Google Scholar]
- Dogterom M, Yurke B (1997) Measurement of the force–velocity relation for growing microtubules. Science 278: 856–860 [DOI] [PubMed] [Google Scholar]
- Doorn GSv, Tanase C, Mulder BM, Dogterom M (2000) On the stall force for growing microtubules. Eur Biophys J 29: 2–6 [DOI] [PubMed] [Google Scholar]
- Drummond DR, Cross RA (2000) Dynamics of interphase microtubules in Schizosaccharomyces pombe. Curr Biol 10: 766–775 [DOI] [PubMed] [Google Scholar]
- Feierbach B, Verde F, Chang F (2004) Regulation of a formin complex by the microtubule plus end protein tea1p. J Cell Biol 165: 697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Föthke D, Makushok T, Brunner D, Nédélec F (2009) Force- and length-dependent catastrophe activities explain interphase microtubule organization in fission yeast. Mol Syst Biol 5: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MA, Koonrugsa N, Toda T (2002) Two kinesin-like Kin I family proteins in fission yeast regulate the establishment of metaphase and the onset of anaphase A. Curr Biol 12: 610–621 [DOI] [PubMed] [Google Scholar]
- Gardner MK, Odde DJ, Bloom K (2008) Kinesin-8 molecular motors: putting the brakes on chromosome oscillations. Trends Cell Biol 18: 307–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grallert A, Beuter C, Craven RA, Bagley S, Wilks D, Fleig U, Hagan IM (2006) S. pombe CLASP needs dynein, not EB1 or CLIP170, to induce microtubule instability and slows polymerization rates at cell tips in a dynein-dependent manner. Genes Dev 20: 2421–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom PM, Fiedler TA, Grishchuk EL, Nicastro D, West RR, McIntosh JR (2008) Kinesin-8 from fission yeast: a heterodimeric, plus end-directed motor that can couple microtubule depolymerization to cargo movement. Mol Biol Cell (e-pub ahead of print, November 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta ML, Carvalho P, Roof DM, Pellman D (2006) Plus end-specific depolymerase activity of Kip3, a kinesin-8 protein, explains its role in positioning the yeast mitotic spindle. Nat Cell Biol 8: 913–923 [DOI] [PubMed] [Google Scholar]
- Hagan IM (1998) The fission yeast microtubule cytoskeleton. J Cell Sci 111: 1603–1612 [DOI] [PubMed] [Google Scholar]
- Hayles J, Nurse P (2001) A journey into space. Nat Rev Mol Cell Biol 2: 647–656 [DOI] [PubMed] [Google Scholar]
- Hoog JL, Schwartz C, Noon AT, O'Toole ET, Mastronarde DN, McIntosh JR, Antony C (2007) Organization of interphase microtubules in fission yeast analyzed by electron tomography. Dev Cell 12: 349–361 [DOI] [PubMed] [Google Scholar]
- Howard J, Hyman AA (2007) Microtubule polymerases and depolymerases. Curr Opin Cell Biol 19: 31–35 [DOI] [PubMed] [Google Scholar]
- Howell B, Odde DJ, Cassimeris L (1997) Kinase and phosphatase inhibitors cause rapid alterations in microtubule dynamic instability in living cells. Cell Mot Cytoskeleton 38: 201–214 [DOI] [PubMed] [Google Scholar]
- Inoue S, Salmon ED (1995) Force generation by microtubule assembly disassembly in mitosis and related movements. Mol Biol Cell 6: 1619–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson ME, Dogterom M (2004) Scaling of microtubule force–velocity curves obtained at different tubulin concentrations. Phys Rev Lett 92: 248101–248104 [DOI] [PubMed] [Google Scholar]
- Janson ME, Dood MEd, Dogterom M (2003) Dynamic instability of microtubules is regulated by force. J Cell Biol 161: 1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M, Mitchison T (1986) Beyond self-assembly: from microtubules to morphogenesis. Cell 45: 329–342 [DOI] [PubMed] [Google Scholar]
- Komarova YA, Vorobjev IA, Borisy GG (2002) Life cycle of MTs: persistent growth in the cell interior, asymmetric transition frequencies and effects of the cell boundary. J Cell Sci 115: 3527–3539 [DOI] [PubMed] [Google Scholar]
- Mata J, Nurse P (1997) Tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell 89: 939–949 [DOI] [PubMed] [Google Scholar]
- Mayr MI, Hummer S, Bormann J, Gruner T, Adio S, Woehlke G, Mayer TU (2007) The human kinesin Kif18A is a motile microtubule depolymerase essential for chromosome congression. Curr Biol 17: 488–498 [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, Galjart N, Grosveld F, Vorobjev I, Tsukita S, Akhmanova A (2005) CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol 168: 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison J, Kirschner M (1984) Dynamic instability of microtubule growth. Nature 312: 237–242 [DOI] [PubMed] [Google Scholar]
- Mogilner A, Oster G (1999) The polymerization ratchet model explains the force–velocity relation for growing microtubules. Eur Biophys J 28: 235–242 [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Niethammer P, Bastiaens P, Karsenti E (2004) Stathmin–tubulin interaction gradients in motile and mitotic cells. Science 303: 1862–1866 [DOI] [PubMed] [Google Scholar]
- Odde DJ (1995) Kinetics of microtubule catastrophe assessed by probabilistic analysis. Biophys J 69: 796–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AJ, Dalby B, Stewart RJ, Doxsey SJ, Goldstein LSB (1997) Mitochondrial association of a plus end-directed microtubule motor expressed during mitosis in Drosophila. J Cell Biol 136: 1081–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin CS, Odell GM, Oster GF (1993) Cellular motions and thermal fluctuations—the Brownian ratchet. Biophys J 65: 316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rischitor PE, Konzack S, Fischer R (2004) The Kip3-like kinesin KipB moves along microtubules and determines spindle position during synchronized mitoses in Aspergillus nidulans hyphae. Eukaryot Cell 3: 632–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, Snaith HA (2004) Role of microtubules and tea1p in establishment and maintenance of fission yeast cell polarity. J Cell Sci 117: 689–700 [DOI] [PubMed] [Google Scholar]
- Stumpff J, von Dassow G, Wagenbach M, Asbury C, Wordeman L (2008) The kinesin-8 motor Kif18A suppresses kinetochore movements to control mitotic chromosome alignment. Dev Cell 14: 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveiczer A, Novak B, Mitchison JM (1996) The size control of fission yeast revisited. J Cell Sci 109: 2947–2957 [DOI] [PubMed] [Google Scholar]
- Tolic-Norrelykke IM, Sacconi L, Stringari C, Raabe I, Pavone FS (2005) Nuclear and division-plane positioning revealed by optical micromanipulation. Curr Biol 15: 1212–1216 [DOI] [PubMed] [Google Scholar]
- Tran PT, Marsh L, Doye V, Inoue S, Chang F (2001) A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J Cell Biol 153: 397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PT, Paoletti A, Chang F (2004) Imaging green fluorescent protein fusions in living fission yeast cells. Methods 33: 220–225 [DOI] [PubMed] [Google Scholar]
- Tytell JD, Sorger PK (2006) Analysis of kinesin motor function at budding yeast kinetochores. J Cell Biol 172: 861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth A, Masuda H, Dhut S, Toda T (2008) Fission yeast kinesin-8 Klp5 and Klp6 are interdependent for mitotic nuclear retention and required for proper microtubule dynamics. Mol Biol Cell 19: 5104–5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V, Helenius J, Tanaka K, Hyman AA, Tanaka TU, Howard J (2006) Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nat Cell Biol 8: 957–962 [DOI] [PubMed] [Google Scholar]
- Verde F, Dogterom M, Stelzer E, Karsenti E, Leibler S (1992) Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J Cell Biol 118: 1097–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RA, Obrien ET, Pryer NK, Soboeiro MF, Voter WA, Erickson HP, Salmon ED (1988) Dynamic instability of individual microtubules analyzed by video light-microscopy—rate constants and transition frequencies. J Cell Biol 107: 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RR, Malmstrom T, McIntosh JR (2002) Kinesins klp5(+) and klp6(+) are required for normal chromosome movement in mitosis. J Cell Sci 115: 931–940 [DOI] [PubMed] [Google Scholar]
- West RR, Malmstrom T, Troxell CL, McIntosh JR (2001) Two related kinesins, klp5(+) and klp6(+), foster microtubule disassembly and are required for meiosis in fission yeast. Mol Biol Cell 12: 3919–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, West RR, McIntosh JR, Hiraoka Y (1999) A cytoplasmic dynein heavy chain is required for oscillatory nuclear movement of meiotic prophase and efficient meiotic recombination in fission yeast. J Cell Biol 145: 1233–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LF, Schwartz C, Wee LM, Oliferenko S (2006) The fission yeast transforming acidic coiled coil-related protein Mia1p/Alp7p is required for formation and maintenance of persistent microtubule-organizing centers at the nuclear envelope. Mol Biol Cell 17: 2212–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental information on image analysis, Supplemental information on hydroxyurea treatment, Supplemental references, Figures S1-9
Supplementary Figure S2B
Supplementary Figure S2C
Supplementary Figure S2E
Supplementary Figure S2F
Supplementary Figure S5A
Supplementary Figure S5B