Summary
Copper is an essential nutrient for a variety of biochemical processes; however, the redox properties of copper also make it potentially toxic in the free form. Consequently, the uptake and intracellular distribution of this metal is strictly regulated. This raises the issue of whether specific pathophysiological conditions can promote adaptive changes in intracellular copper distribution. In this study, we demonstrate that oxygen limitation promotes a series of striking alterations in copper homeostasis in RAW264.7 macrophage cells. Hypoxia was found to stimulate copper uptake and to increase the expression of the copper importer, CTR1. This resulted in increased copper delivery to the ATP7A copper transporter and copper-dependent trafficking of ATP7A to cytoplasmic vesicles. Significantly, the ATP7A protein was required to deliver copper into the secretory pathway to ceruloplasmin, a secreted copperdependent enzyme, the expression and activity of which were stimulated by hypoxia. However, the activities of the alternative targets of intracellular copper delivery, superoxide dismutase and cytochrome c oxidase, were markedly reduced in response to hypoxia. Collectively, these findings demonstrate that copper delivery into the biosynthetic secretory pathway is regulated by oxygen availability in macrophages by a selective increase in copper transport involving ATP7A.
Keywords: ATP7A, Copper, Hypoxia, Macrophage, Oxygen, Trafficking
Introduction
Copper is a trace element that is critical for aerobic life. Its ability to accept and donate electrons has been harnessed by a select group of enzymes that function in mitochondrial respiration, connective tissue formation, pigmentation, iron oxidation, neurotransmitter processing and antioxidant defense (La Fontaine and Mercer, 2007; Madsen and Gitlin, 2007; Prohaska and Gybina, 2004). However, this same redox property of copper and its ability to generate reactive oxygen species, also underscores its potential toxicity. For this reason, copper-handling proteins have evolved to deliver copper to specific sites of utilization, thereby preventing the formation of potentially damaging free ionic copper in the cytoplasm. Copper uptake in mammalian cells is mediated by CTR1 (SLC31A1), a ubiquitously expressed homotrimeric transporter (Zhou and Gitschier, 1997). Once in the cytoplasm, small cytoplasmic proteins known as copper chaperones deliver copper to distinct target enzymes by direct protein-protein interactions. The copper chaperones, CCS and COX17 are involved in copper delivery to Cu/Zn superoxide dismutase (SOD1) in the cytoplasm, and to cytochrome c oxidase (CCO) in the mitochondria (Amaravadi et al., 1997; Casareno et al., 1998). SCO1 and SCO2 are copper chaperones that are also involved in copper delivery to CCO via a poorly understood process (Leary et al., 2004). The third target for copper delivery is the ATP7A protein (or closely related ATP7B protein), a copper transporter located in the Golgi complex that receives copper from the ATOX1 copper chaperone in the cytoplasm (Larin et al., 1999; Petris et al., 1996; Yamaguchi et al., 1996). ATP7A transports copper into the Golgi lumen to supply copper to a select group of copper-dependent enzymes, which are either secreted from cells, or reside within vesicular compartments (Barnes et al., 2005; El Meskini et al., 2003; Petris et al., 2000; Qin et al., 2006). An additional function of ATP7A is the export of excess copper from cells. This export activity is associated with copper-stimulated trafficking of ATP7A to post-Golgi compartments, including cytoplasmic vesicles and the plasma membrane (Petris et al., 1996). The trafficking of ATP7A is triggered when cytoplasmic copper levels are elevated (Hamza et al., 2003), and this process requires both copper binding to cytoplasmic regions of the ATPase as well as its catalytic turnover (Petris et al., 2002; Strausak et al., 1999). The biological importance of the ATP7A protein is illustrated by Menkes disease, a lethal disorder of copper deficiency caused by ATP7A mutations (Kaler, 1998).
An area of copper metabolism of which we have little understanding is whether specific pathophysiological conditions lead to adaptive changes in intracellular copper homeostasis. Hypoxia is a stress that is inherent within the microenvironment of injured tissues, including dermal wounds and burns (Arnold, 1987), atherosclerotic plaques (Moreno et al., 2006) and avascular regions of solid tumors (Bristow and Hill, 2008). Cells of the myeloid lineage such a macrophages are specifically recruited to these hypoxic sites and are metabolically adapted to function within this hostile milieu. For example, it has been known for many years that neutrophils and macrophages are highly dependent on anaerobic glycolysis for ATP production, and suppress oxidative phosphorylation in the presence of hypoxia (Simon et al., 1977). In this study we investigated the impact of hypoxia on copper homeostasis in the murine macrophage cell line, RAW264.7. Hypoxia resulted in increased copper uptake and enhanced the expression of the CTR1 transporter. Copper delivery to the ATP7A protein was also enhanced as evidenced by trafficking from the Golgi and enhanced copper transport into the secretory pathway to ceruloplasmin. By contrast, hypoxia triggered a decrease in the levels of other intracellular copper targets including, CCS, SOD1, and the copper-binding subunit of CCO, COX1. These findings suggest that oxygen status can regulate copper allocation to the secretory pathway for hypoxia-induced cuproenzymes, and reveal hypoxia as a unique pathophysiological regulator of intracellular copper hierarchy.
Results
Hypoxia stimulates trafficking of the ATP7A protein in RAW264.7 macrophages
We began this study by investigating whether reduced oxygen tension might alter the localization of the ATP7A copper transporter in the murine macrophage cell line, RAW264.7. The relocalization of ATP7A from the trans-Golgi network is a key biological indicator of increased cytoplasmic copper availability and has been documented in several different cell types (Cobbold et al., 2002; La Fontaine et al., 1998; Petris et al., 1996). Using immunofluorescence microscopy, the ATP7A protein was found within the perinuclear region of RAW264.7 cells exposed to normoxic conditions (21% O2), consistent with its location in the trans-Golgi network (Fig. 1A). As expected, treatment of these cells with copper resulted in the trafficking of ATP7A from the perinuclear region to cytoplasmic vesicles (Fig. 1A). Significantly, when these cells were exposed to chronic hypoxia (4% O2 for 96 hours), the ATP7A protein was also dispersed to post-Golgi vesicles (Fig. 1A). This redistribution of the ATP7A protein required at least 48 hours of hypoxia and was not accelerated by lower levels of oxygen (data not shown). The return of hypoxic cells to normoxic conditions restored the location of the ATP7A protein to the perinuclear region, indicating that the effect of hypoxia on ATP7A was reversible (Fig. 1A). The intracellular location of the trans-Golgi marker protein, syntaxin 6 (STX6) and the Golgi matrix protein, GM130, were not altered by hypoxia in RAW264.7 cells (Fig. 1B,C), suggesting that the effects on ATP7A were not the result of a general effect on Golgi structure. The viability of RAW264.7 cells was not altered by these conditions and cells could be passaged continuously in 4% O2 (data not shown).
Fig. 1.
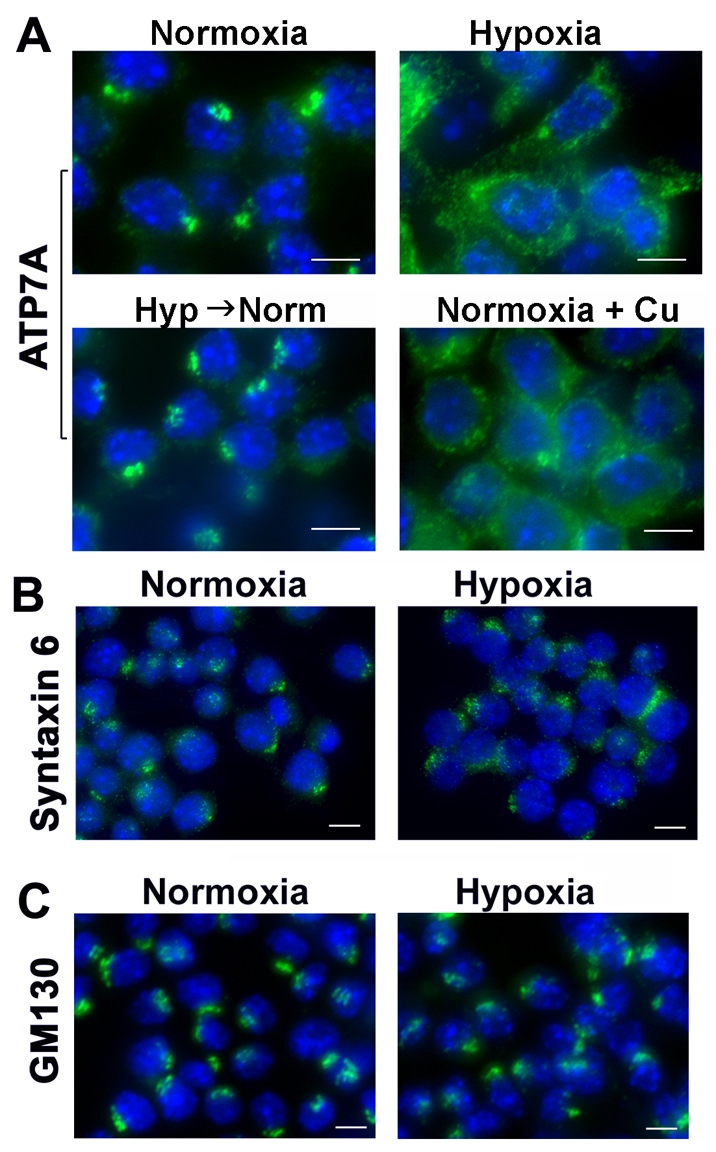
Hypoxia stimulates trafficking of the ATP7A protein. (A) Immunofluorescence analysis of ATP7A protein in RAW264.7 cells grown under normoxic (21% O2) or hypoxic (4% O2) conditions for 96 hours. Cells were fixed, permeabilized and probed with antibodies against for ATP7A and anti-rabbit antibodies conjugated to Alexa Fluor 488 (green). Nuclei were labeled with DAPI (blue). Note the trafficking of ATP7A protein from the perinuclear region in hypoxic cells and copper-treated normoxic cells. ATP7A was rapidly retrieved to the perinuclear compartment upon the transfer of hypoxic cells to normoxic media for 30 minutes (Hyp→Norm). (B,C) Analysis of Golgi marker proteins in hypoxic RAW264.7 cells. Cells were cultured under hypoxic or normoxic conditions as described in A and probed using antibodies against the trans-Golgi network marker protein syntaxin 6 (B), or the Golgi matrix protein, GM130 (C). Nuclei were labeled with DAPI (blue). Scale bars: 7 μm.
Oxygen limitation stimulates ATP7A expression and copperdependent trafficking of ATP7A
Since the trafficking of ATP7A from the trans-Golgi network is known to be triggered by increased copper delivery to this transporter (Petris et al., 1996), we tested whether a membrane-permeable copper chelator, tetrathiomolybdate (TTM), could suppress ATP7A trafficking in response to hypoxia in RAW264.7 cells. As shown in Fig. 2A, TTM inhibited ATP7A relocalization in response to hypoxia. These findings support the hypothesis that oxygen limitation increases copper binding to the ATP7A protein, resulting in its trafficking from the Golgi. We also explored the possibility that hypoxia may also increase the expression of ATP7A in RAW264.7 macrophages. Western blot analysis demonstrated that hypoxia increased ATP7A protein levels above normoxic controls in a time-dependent manner, beginning between 24 and 48 hours (Fig. 2B). A similar increase in ATP7A levels was observed in primary peritoneal macrophages isolated from C57BL mice and cultured under hypoxic conditions (Fig. 2C). These findings suggest that hypoxia stimulates copper-dependent trafficking and expression of the ATP7A protein.
Fig. 2.
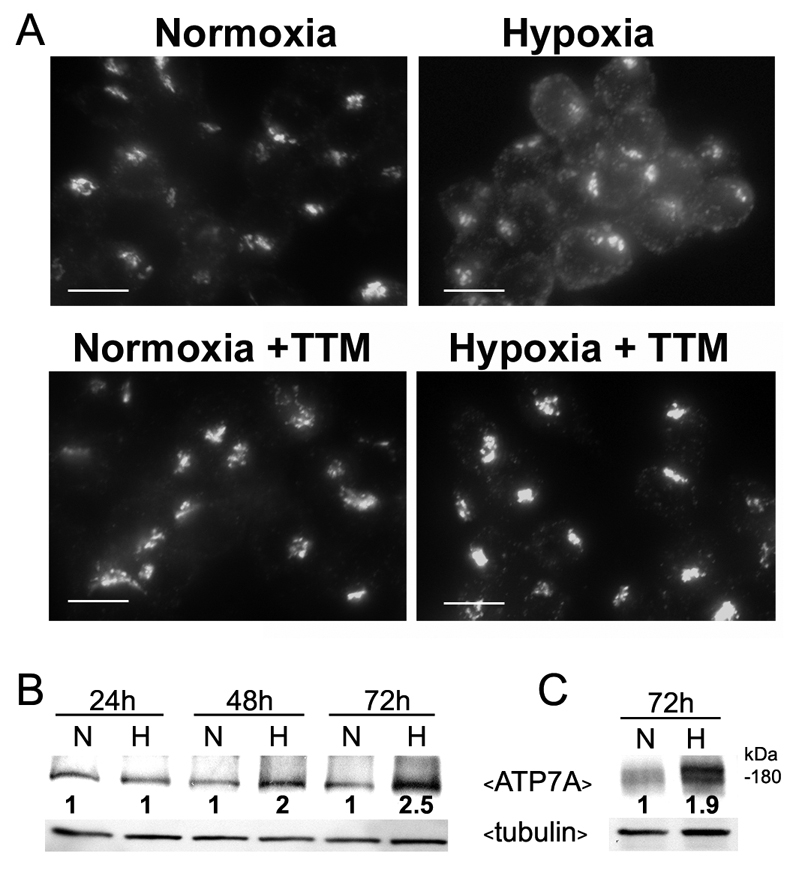
Hypoxia stimulates both copper-dependent trafficking and increased expression of ATP7A. (A) RAW264.7 cells were cultured under hypoxic or normoxic conditions as described in Fig. 1 in the presence or absence of the copper chelator, tetrathiomolybdate (TTM; 5 nM). ATP7A protein was detected using immunofluorescence as described in Fig. 1. Scale bars: 10 μm. (B,C) Immunoblot analysis of ATP7A protein in RAW264.7 cells (B) and primary peritoneal macrophages (C) cultured under normoxic (N; 21% O2) or hypoxic (H; 4% O2) conditions for the indicated times. Tubulin was detected as a loading control. Relative ATP7A band intensities at each time point normalized against tubulin are shown for each normoxic and hypoxic pair.
Hypoxia stimulates trafficking of the ATP7A protein in tumor-associated macrophages
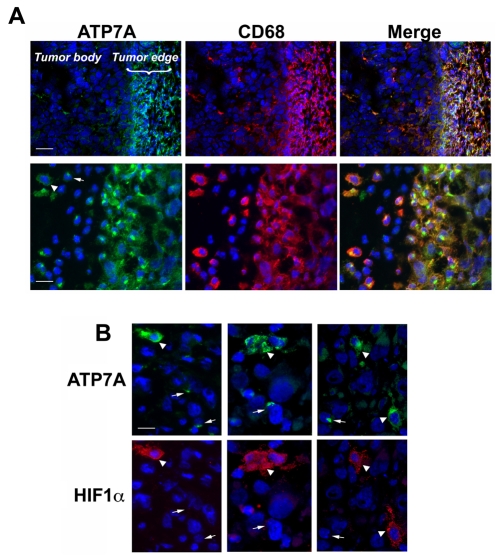
We then sought to confirm whether hypoxia could elicit similar changes in ATP7A localization in hypoxic macrophages in vivo. Previous studies have demonstrated that macrophages are recruited to the hypoxic regions of solid tumors where they play important roles in promoting angiogenesis (reviewed by Mantovani et al., 2006). A prostate tumor xenograft model provided an opportunity to investigate the intracellular distribution of ATP7A in these hypoxic tumor-associated macrophages. The tumorigenic human prostate cell line, PC-3, was chosen for these studies since ATP7A expression was very low in these cells, thus allowing for easy identification of ATP7A in macrophages recruited to the tumor. PC-3 tumors were grown in immunocompromised SCID mice for 5 weeks to a size of approximately 1 cm diameter (0.5 g), and then excised and cryosectioned for immunofluorescence analysis of ATP7A expression. This revealed abundant ATP7A protein expression in tumor-associated macrophages that were identified using the macrophage-specific marker CD-68 (Fig. 3A). As expected, there was little if any expression of ATP7A in the PC-3 tumor cells (Fig. 3A). Consistent with previous studies, the macrophages were typically concentrated at the tumor edges, with occasional infiltration into the tumor body (Biswas et al., 2006; Lewis and Pollard, 2006; Murdoch et al., 2004). It was noted that in some macrophages ATP7A was restricted to the perinuclear Golgi complex, whereas in other macrophages ATP7A was dispersed throughout the cell in a manner reminiscent of the trafficking seen earlier in cultured hypoxic RAW264.7 cells (Fig. 3A, lower left panel). Significantly, this dispersed localization of the ATP7A protein occurred only in macrophages that coexpressed the HIF1α protein (Fig. 3B). The HIF1α transcription factor is the master regulator of gene expression responses to low oxygen, and is upregulated in macrophages within hypoxic areas of tumors (Burke et al., 2002; Talks et al., 2000). These findings, together with our earlier observations in cultured RAW264.7 cells, suggest that hypoxia triggers copper-dependent trafficking of ATP7A in macrophages.
Fig. 3.
ATP7A trafficking in HIF1α-positive tumor-associated macrophages. (A) ATP7A is strongly expressed in tumor-associated macrophages. Human prostate cell PC-3 xenograft tumors from SCID mice were cryosectioned and probed with antibodies against ATP7A (green) or antibodies against the macrophage marker CD68 (red). Nuclei were stained using DAPI (blue). Upper panels show both the tumor mass and the tumor edge, with strong ATP7A expression in CD68-positive macrophages associated with the tumor edge Scale bar: 60 μm. The lower panels are higher magnifications of the tumor periphery and reveal extensive coexpression of ATP7A in CD68-positive macrophages, as indicated by yellow signal in the merged image. Scale bar: 30 μm. The arrow and arrowhead (lower left panel) indicate heterogeneous localization of the ATP7A in macrophages, in either a perinuclear or dispersed distribution. (B) The dispersed distribution of the ATP7A occurs in HIF1α-positive macrophages. Three different PC-3 tumor cryosections were immunostained for ATP7A (green) and HIF1α staining (red). ATP7A was restricted to the perinuclear region of macrophages negative for HIF1α expression (arrows), whereas a dispersed distribution of ATP7A occurred in HIF1α-positive macrophages (arrowheads) (original magnification, ×600). Scale bar: 10 μm.
Oxygen limitation stimulates the expression of CTR1 and copper uptake in macrophages
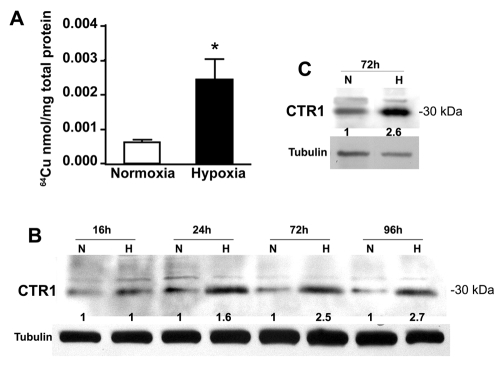
Based on our finding that the trafficking of ATP7A in response to hypoxia was dependent on copper, we investigated whether this might occur through increased copper uptake. Radioactive 64Cu uptake experiments were carried out using RAW264.7 macrophages that had been pre-exposed to hypoxic or normoxic conditions. A significant increase in copper uptake was found for RAW264.7 cells pre-exposed to hypoxia relative to normoxia (Fig. 4A). Interestingly, this increased copper uptake was associated with a time-dependent increase in the levels of the CTR1 copper importer (Fig. 4B). A similar increase in CTR1 expression was observed in murine primary peritoneal macrophages cultured under hypoxic conditions (Fig. 4C). Taken together with our earlier results, these findings suggest that the copper-dependent trafficking the ATP7A protein is associated with an increase in CTR1 expression and copper uptake.
Fig. 4.
Hypoxia stimulates copper uptake and CTR1 expression in RAW264.7 macrophages. (A) Copper uptake activity. RAW264.7 cells were pre-exposed to normoxia (21% O2) or hypoxia (4% O2) for 72 hours and 64Cu uptake was measured over 5 minutes. Values were normalized against total protein concentrations (mean + s.d.; n=3; *P<0.05). (B,C) The effect of hypoxia on CTR1 protein levels in RAW264.7 cells (B) and primary peritoneal macrophages (C) cultured under normoxia (N; 21% O2) or hypoxia (H; 4% O2) for the indicated times. Immunoblot analysis was used to detect CTR1 protein in lysates using anti-CTR1 antibodies. Tubulin was detected as a loading control. Relative CTR1 band intensities at each time point, normalized against tubulin, are shown for each normoxic and hypoxic pair.
Hypoxia stimulates copper transport to ceruloplasmin via ATP7A
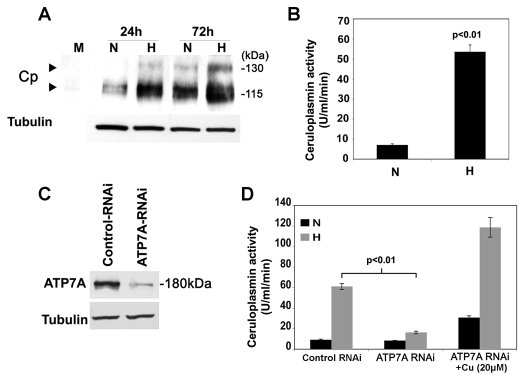
The major function of ATP7A is to pump copper into the secretory pathway to supply copper to secreted cuproenzymes. We hypothesized that a potential target for this copper delivery in response to hypoxia might be ceruloplasmin, a cuproenzyme that requires copper delivery into the secretory pathway. Ceruloplasmin is a ferroxidase secreted from macrophages and hepatocytes, the expression and activity of which are stimulated by hypoxia (Martin et al., 2005; Mukhopadhyay et al., 2000). Hypoxia was found to increase both the abundance and the activity of ceruloplasmin secreted into the culture medium of RAW264.7 cells relative to normoxia (Fig. 5A,B). To examine whether the increase in ceruloplasmin activity was dependent on ATP7A copper transport activity, we depleted ATP7A expression in RAW264.7 cells using RNAi-mediated gene silencing (ATP7A/RNAi cells; Fig. 5C). Control cells were transfected with a construct expressing an irrelevant RNAi against GFP (Fig. 5C). Compared with control cells, ceruloplasmin activity in ATP7A/RNAi cells was markedly reduced under hypoxic conditions, suggesting that ATP7A copper transport activity was required for copper delivery to ceruloplasmin (Fig. 5D). Consistent with this postulate, the addition of copper to the medium of these cells bypassed the requirement for ATP7A and restored ceruloplasmin activity, indicating that the effect of ATP7A gene silencing was due to a blockage of copper delivery to ceruloplasmin (Fig. 5D). Control experiments indicated that ATP7A silencing did not alter ceruloplasmin protein levels in the medium relative to control cells in either hypoxic or normoxic conditions (data not shown). Taken together with our earlier results, these findings suggest that hypoxia stimulates an increase in copper delivery to ceruloplasmin by increasing CTR1-mediated copper uptake as well as ATP7A-dependent copper delivery into secretory compartments.
Fig. 5.
ATP7A-dependent copper transport is required for hypoxia-stimulated ceruloplasmin activity. (A) Immunoblot analysis of ceruloplasmin (Cp) secreted from RAW264.7 cells grown under normoxic (N; 21% O2) or hypoxic (H; 4% O2) conditions for the indicated times. Conditioned medium was concentrated and subjected to non-denaturing SDS-PAGE and immunoblot analysis with anti-Cp antibodies. Tubulin levels from corresponding cell lysates are also shown. Lane 1 is a negative control of concentrated growth medium alone (M). (B) Hypoxia stimulates ceruloplasmin activity. Ceruloplasmin activity (p-phenylenediamine oxidase activity) was measured in the concentrated conditioned medium from RAW264.7 cells following exposure to normoxia (N; 21% O2) or hypoxia (H; 4% O2) for 72 hours. Activity was normalized against total protein content of the corresponding cell lysates (mean + s.d.; n=3). (C) RNAi-mediated silencing of the ATP7A protein. Immunoblot analysis of ATP7A protein levels in RAW264.7 cells stably transfected with either ATP7A-RNAi or control-RNAi. (D) Ceruloplasmin activity was measured in conditioned medium from the ATP7A-RNAi or control-RNAi cells exposed to hypoxia (N; 21% O2) or hypoxia (H; 4% O2) for 72 hours (mean + s.d.; n=3). Note the failure to activate ceruloplasmin in ATP7A-depleted cells, and the restoration by addition of copper to the growth medium (+Cu).
Hypoxia differentially affects intracellular copper pathways
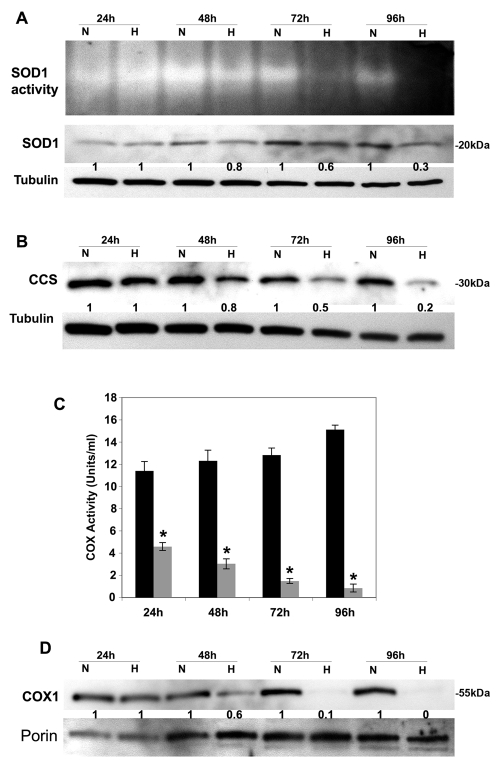
Having established that hypoxia increases the delivery of copper to ceruloplasmin via the ATP7A copper transporter, we explored the effect of hypoxia on two additional targets of intracellular copper pathways. These include Cu/Zn-superoxide dismutase (SOD1) in the cytoplasm and CCO in the mitochondria. Hypoxia resulted in a time-dependent decrease of SOD1 activity in RAW264.7 macrophages (Fig. 6A). Interestingly, this reduction in SOD1 activity was associated with a marked reduction in the level of CCS protein, which is the copper chaperone required for copper delivery to SOD1 (Fig. 6B). The activity of the cuproenzyme, CCO, was also markedly reduced in mitochondrial preparations isolated from hypoxic RAW264.7 macrophages (Fig. 6C), and this was accompanied by reduced levels of COX1 protein, a copper containing subunit of CCO (Fig. 6D). Hypoxia did not result in detectable changes in other copper chaperones COX17, SCO1 or SCO2, which are required for copper delivery to CCO (data not shown). Taken together, these findings suggest that hypoxia differentially impacts intracellular copper handling pathways by decreasing copper delivery to SOD1 and CCO, while up-regulating copper delivery to the secretory pathway via ATP7A.
Fig. 6.
Hypoxia downregulates alternative copper pathways in RAW264.7 macrophages. (A) Effect of hypoxia on SOD1 protein and activity. RAW264.7 cells were grown under normoxic (N; 21% O2) or hypoxic (H; 4% O2) conditions for the indicated times. Cell lysates were subjected to non-denaturing SDS-PAGE for the in-gel SOD1 activity assay (top panel). Immunoblots from the same lysates were probed with anti-SOD1 antibodies to detect SOD1 protein. Tubulin was detected as a loading control. Relative SOD1 band intensities at each time point, normalized against tubulin, are shown for each normoxic and hypoxic pair. (B) Effect of hypoxia on the abundance of CCS, the copper chaperone for SOD1. The same lysates as in A were subjected to SDS-PAGE and immunoblot analysis with anti-CCS antibodies and relative band intensities at each time point normalized against tubulin are shown for each normoxic and hypoxic pair. (C) Analysis of CCO activity in hypoxic (shaded bars) and normoxic (solid bars) conditions. Activity was measured in mitochondrial preparations isolated from RAW264.7 cells cultured as described in A. Values were normalized against total mitochondrial protein (mean + s.d.; n=3; *P<0.05). (D) Analysis of COX1 protein levels in RAW264.7 cells cultured under normoxic (N; 21% O2) or hypoxic (H; 4% O2) conditions for the indicated times. Mitochondrial preparations from B were subjected to SDS-PAGE and probed with antibodies against the copper-binding subunit COX1 of the CCO complex. Immunoblots were probed with an antibody against porin to indicate protein loading, and relative COX1 band intensities at each time point, normalized against porin, are shown for each normoxic and hypoxic pair.
Discussion
Hypoxia is a stress that is commonly encountered by macrophages as they migrate away from the vasculature. Accordingly, this cell type is uniquely adapted to function within this hostile microenvironment. In this study we demonstrate that reduced oxygen levels promote adaptive changes in intracellular copper homeostasis in macrophages by specifically enhancing copper delivery to the biosynthetic pathway via the ATP7A protein (illustrated in Fig. 7). Copper-dependent trafficking of the ATP7A protein from the trans-Golgi network is one of the clearest biological indicators of increased cytoplasmic copper concentrations. Previous studies have shown that copper-stimulated trafficking is dependent on ATP7A copper transport activity as well as copper binding to cysteines within its amino-terminal region (Strausak et al., 1999; Petris et al., 2002). Our finding that hypoxia stimulated the relocalization of ATP7A to post-Golgi vesicles in a copperdependent manner is consistent with increased copper binding to this protein, as well as an increase in its copper transport activity into the secretory pathway. This was supported by the finding that the increased activity of ceruloplasmin, an enzyme that acquires copper within secretory compartments, was dependent on ATP7A expression in hypoxic cells.
Fig. 7.
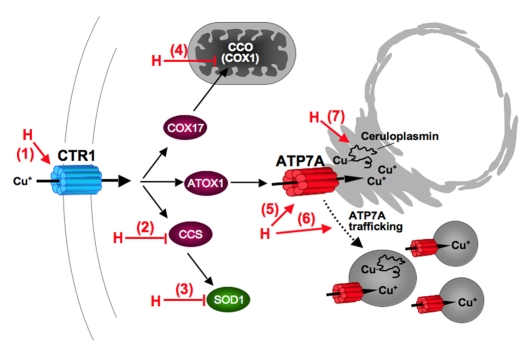
Schematic model of hypoxia-induced changes in copper homeostasis resulting in ATP7A-mediated copper transport to the secretory pathway. Effects of hypoxia (H) include: (1) increased expression of the CTR1 copper importer and increased copper uptake; (2) decreased CCS expression; (3) reduced activity of SOD1; (4) reduced CCO activity and reduced expression of COX1; (5) increased expression of ATP7A; (6) copper-dependent trafficking of ATP7A; and (7) ATP7A-dependent copper transport to the secretory pathway of ceruloplasmin.
In contrast to ceruloplasmin, the abundance and/or activity of CCS, SOD1 and CCO were reduced by hypoxia. These findings provide the first evidence that the pathways of intracellular copper distribution can be differentially regulated in response to an environmental stress. By reducing the flow of copper from CCS to SOD1, this may provide a mechanism to redirect copper to the ATP7A protein. Like SOD1, the activity of CCO was also diminished by hypoxia in RAW264.7 cells and this was associated with a decrease in levels of COX1 protein, the subunit of CCO containing the CuB site. Although a decrease in CCO activity has been previously reported in hypoxic macrophages to facilitate the metabolic shift from oxidative phosphorylation to glycolysis (Murphy et al., 1984; Simon et al., 1977), our findings highlight the possibility that COX1 depletion serves an additional purpose of diverting precious copper stores to secretory compartments via ATP7A.
The finding that ceruloplasmin was a recipient of increased copper delivery to the secretory pathway is in agreement with the function of this protein in iron homeostasis. Ceruloplasmin is a ferroxidase required for cellular iron export, which is a critical step in the loading of iron onto transferrin in the blood (Nittis and Gitlin, 2002). This process is an adaptive response to hypoxia to meet the increased iron demand of hematopoiesis (Sarkar et al., 2003). Thus, the prioritization of copper delivery to ceruloplasmin via ATP7A may ultimately function to regulate iron homeostasis in response to hypoxia. The finding that ATP7A trafficking in hypoxic cells occurred concurrently with ceruloplasmin activation raises the possibility that under hypoxic conditions, copper delivery to ceruloplasmin may occur in post-Golgi vesicles rather than in the trans-Golgi network where copper loading normally takes place. Thus, ATP7A trafficking may not simply reflect an increased flux of copper to this protein, but facilitate copper-loading of ceruloplasmin in post-Golgi compartments. Indeed, a recent study demonstrating a subset of ATP7A protein was required in post-Golgi melanosomes for the copper loading of tyrosinase is consistent with this model (Setty et al., 2008). A particularly intriguing finding of our study was the strong expression of ATP7A in tumor-associated macrophages. Copper has been shown to play an important role in angiogenesis, and copper chelation via TTM has proved to be an effective suppressor of tumor growth in animals (Alessandri et al., 1984; Camphausen et al., 2004; Cox et al., 2003; Cox et al., 2001; Pan et al., 2003a; Pan et al., 2003b; Pan et al., 2002; Redman et al., 2003; Teknos et al., 2005). It is therefore tempting to speculate that the adaptive changes in macrophage copper homeostasis described in this study may underlie the role of copper in tumor growth.
A notable finding of our study was that the changes in copper homeostasis in response to hypoxia appear to be restricted to macrophages. ATP7A trafficking and/or changes in SOD1 and CCO activity were not observed in our analysis of cultured cells from a variety of sources including HeLa (cervical carcinoma), HEK293 (human embryonic kidney), N2a (neuroblastoma), primary human aortic endothelial cells and primary rat smooth muscle cells (data not shown). The macrophage-specific effects of hypoxia on copper homeostasis may reflect inflammatory responses, since hypoxia is known to specifically activate inflammatory pathways in macrophages (Rius et al., 2008). Consistent with this postulate, our unpublished studies demonstrate that pro-inflammatory agents can stimulate copper-dependent ATP7A trafficking in macrophages under normoxic conditions. It will be of interest to determine whether other physiological conditions regulate changes in the intracellular distribution of copper in other mammalian cell types. For example, copper transport via ATP7A is required for melanin production via tyrosinase, norepinephrine synthesis via dopamine β-hydroxylase, and collagen cross-linking via lysyl oxidase, and each of these cuproenzymes is stimulated by particular physiological cues in specific cell types. The challenge of future studies will be to address whether this involves adaptive changes that promote an increase in ATP7A-dependent copper transport into the secretory pathway.
Materials and Methods
Reagents and antibodies
All reagents were from Sigma (St Louis, MO), unless otherwise indicated. The rabbit polyclonal CTR1 antibody (Nose et al., 2006) was kindly provided by Dennis Thiele (Duke University, Durham, NC). The rabbit polyclonal antibody against ATP7A was raised against the C-terminal portion of the protein and was a generous gift from Elizabeth Eipper (Steveson et al., 2003). Additional affinity purified anti-ATP7A antibodies were raised in rabbits against the synthetic peptide NH2-CDKHSLLVGDFREDDDTTL-COOH (Bethyl Laboratories, Mongomery, TX). Anti-tubulin antibody, and secondary HRP-conjugated antibodies were purchased from Roche Molecular Biochemicals. Antibodies against COXI, and fluorescent Alexa Fluor 488- and Alexa Fluor 594-conjugated antibodies were from Invitrogen (Carlsbad, CA). Antibodies against GM130 and syntaxin 6 were purchased from BD Transduction Laboratories (San Jose, CA). Antibodies against CD68 and HIF1α were purchased from Serotec (Raleigh, NC) and Novus Biologicals (Littleton, CO), respectively. Antibodies against Cu/Zn-SOD, CCS and ceruloplasmin antibodies were purchased from Stressgen (Ann Arbor, MI), Santa Cruz Biotechnology (Santa Cruz, CA), and Abcam (Cambridge, MA), respectively.
Cell culture
All cell lines were obtained from the American Type Culture Collection and were maintained in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum and 100 i.u./ml penicillin and streptomycin (Invitrogen) in 5% CO2 at 37°C. Primary macrophages were isolated by peritoneal lavage. C57BL/6J mice were injected with 2 ml of thioglycolate medium into the peritoneum to elicit macrophage infiltration. After 72 hours, macrophages were isolated by peritoneal lavage using ice-cold PBS. Cells were seeded in six-well plates for each experiment as described. Hypoxic atmospheres were generated by displacement with N2 and CO2 gas using a trigas hypoxic incubator. RNAi-mediated silencing of ATP7A in RAW264.7 cells was performed by stable transfection of a pRS vector expressing a 29 nucleotide short hairpin (sh) RNA against ATP7A (Origene, Rockville, MD) followed by selection in 25 μg/ml puromycin. Control cells were transfected with the same vector expressing shRNA against GFP. Lipofectamine 2000 (Invitrogen) was used in all transfections.
Copper uptake
Radioactive copper (64Cu) was purchased from the Mallinckrodt Institute of Radiology, Washington University (Saint Louis, MO). Cells were pre-cultured in triplicate for 72 hours in 6-well plates under either normoxic (21% O2) or hypoxic (4% O2) conditions, and then exposed to 1 μM 64Cu for 5 minutes, washed extensively in ice-cold PBS and radioactivity measured using a gamma counter. Counts were normalized against total protein.
Immunocytochemistry and PC-3 tumor xenografts
Immunofluorescence microscopy and western blot analysis were performed as described previously (Mao et al., 2007). PC-3 prostate carcinoma cells (5×106) were injected subcutaneously into one flank of anesthetized 4-week-old ICRSC-M SCID mice obtained from Taconic (Germantown, NY). Mice were maintained in an approved pathogen-free institutional housing and studies were conducted as outlined in the NIH Guidelines for the Care and Use of Laboratory Animals and the Policy and Procedures for Animal Research of the Harry S. Truman Veterans Memorial Hospital. After a period of 4 weeks solid tumors of appropriately 1 cm diameter were excised from anesthetized mice and flash frozen in isopentane. Frozen tumors were cryosectioned, fixed in acetone for 10 minutes, washed in phosphate-buffered saline (PBS) and blocked overnight in 1% casein in PBS. Immunostaining was performed using antibodies against ATP7A, CD68 or HIF1α, followed by staining with Alexa Fluor 488 anti-rabbit and Alexa Fluor 594 anti-mouse antibodies, as indicated in the figure legends. Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI).
Enzyme assays
CCO activity assays were performed using mitochondrial preparations from RAW264.7 cells obtained using the Cell Mitochondria Isolation Kit from Sigma. CCO activity was measured using a CCO assay kit (Sigma) according to the manufacturer's instructions and activity was normalized against mitochondrial protein content.
Superoxide dismutase assays were performed as described previously (Flohe and Otting, 1984). Briefly, RAW264.7 cell lysates were fractionated using nondenaturing 12% polyacrylamide gel electrophoresis and superoxide dismutase activity was detected by incubation of gels in nitro blue tetrazolium at room temperature. Ceruloplasmin activity in concentrated conditioned media was determined by its p-phenylenediamine oxidase activity as previously described (Sunderman and Nomoto, 1970). RAW264.7 cells were grown in 6-well plates and the conditioned medium was collected and concentrated using Amicon Ultra-4 filter tubes (Millipore). A low level of endogenous ceruloplasmin activity in concentrated medium alone was subtracted from that of conditioned medium for each experiment. Ceruloplasmin activity was normalized against total protein content in the cell pellets. Ceruloplasmin protein levels were detected in concentrated medium using immunoblot analysis.
We thank Dennis Thiele (Duke University, Durham, NC) for anti-CTR1 antiserum and Betty Eipper (University of Connecticut Health Center, Farmington, CT) for anti-ATP7A antiserum. We thank members of the Petris lab for their support. This work was supported by grants from the National Institutes of Health DK66333 and DK59893 to M.J.P. and DK79209 to J.L. The production of 64Cu at Washington University School of Medicine is supported by a grant from the NIH-NCI R24 CA86307. T.K. was supported, in part, by a fellowship from the Uehara Memorial Foundation and the Naito Foundation. Deposited in PMC for release after 12 months.
References
- Alessandri, G., Raju, K. and Gullino, P. M. (1984). Angiogenesis in vivo and selective mobilization of capillary endothelium in vitro by heparin-copper complex. Microcirc. Endothelium Lymphatics 1, 329-346. [PubMed] [Google Scholar]
- Amaravadi, R., Glerum, D. M. and Tzagoloff, A. (1997). Isolation of a cDNA encoding the human homolog of COX17, a yeast gene essential for mitochondrial copper recruitment. Hum. Genet. 99, 329-333. [DOI] [PubMed] [Google Scholar]
- Arnold, F., West, D. and Kumar, S. (1987). Wound healing: the effect of macrophage and tumour derived angiogenesis factors on skin graft vascularization. B. J. Exp. Pathol. 68, 569-574. [PMC free article] [PubMed] [Google Scholar]
- Barnes, N., Tsivkovskii, R., Tsivkovskaia, N. and Lutsenko, S. (2005). The copper-transporting ATPases, menkes and wilson disease proteins, have distinct roles in adult and developing cerebellum. J. Biol. Chem. 280, 9640-9645. [DOI] [PubMed] [Google Scholar]
- Biswas, S. K., Gangi, L., Paul, S., Schioppa, T., Saccani, A., Sironi, M., Bottazzi, B., Doni, A., Vincenzo, B., Pasqualini, F. et al. (2006). A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 107, 2112-2122. [DOI] [PubMed] [Google Scholar]
- Bristow, R. G. and Hill, R. P. (2008). Hypoxia and metabolism: hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 8, 180-192. [DOI] [PubMed] [Google Scholar]
- Burke, B., Tang, N., Corke, K. P., Tazzyman, D., Ameri, K., Wells, M. and Lewis, C. E. (2002). Expression of HIF-1alpha by human macrophages: implications for the use of macrophages in hypoxia-regulated cancer gene therapy. J. Pathol. 196, 204-212. [DOI] [PubMed] [Google Scholar]
- Camphausen, K., Sproull, M., Tantama, S., Venditto, V., Sankineni, S., Scott, T. and Brechbiel, M. W. (2004). Evaluation of chelating agents as anti-angiogenic therapy through copper chelation. Bioorg. Med. Chem. 12, 5133-5140. [DOI] [PubMed] [Google Scholar]
- Casareno, R. L., Waggoner, D. and Gitlin, J. D. (1998). The copper chaperone CCS directly interacts with copper/zinc superoxide dismutase. J. Biol. Chem. 273, 23625-23628. [DOI] [PubMed] [Google Scholar]
- Cobbold, C., Ponnambalam, S., Francis, M. J. and Monaco, A. P. (2002). Novel membrane traffic steps regulate the exocytosis of the Menkes disease ATPase. Hum. Mol. Genet. 11, 2855-2866. [DOI] [PubMed] [Google Scholar]
- Cox, C., Teknos, T. N., Barrios, M., Brewer, G. J., Dick, R. D. and Merajver, S. D. (2001). The role of copper suppression as an antiangiogenic strategy in head and neck squamous cell carcinoma. Laryngoscope 111, 696-701. [DOI] [PubMed] [Google Scholar]
- Cox, C., Merajver, S. D., Yoo, S., Dick, R. D., Brewer, G. J., Lee, J. S. and Teknos, T. N. (2003). Inhibition of the growth of squamous cell carcinoma by tetrathiomolybdate-induced copper suppression in a murine model. Arch. Otolaryngol. Head Neck Surg. 129, 781-785. [DOI] [PubMed] [Google Scholar]
- El Meskini, R., Culotta, V. C., Mains, R. E. and Eipper, B. A. (2003). Supplying copper to the cuproenzyme peptidylglycine alpha-amidating monooxygenase. J. Biol. Chem. 278, 12278-12284. [DOI] [PubMed] [Google Scholar]
- Flohe, L. and Otting, F. (1984). Superoxide dismutase assays. Methods Enzymol. 105, 93-104. [DOI] [PubMed] [Google Scholar]
- Hamza, I., Prohaska, J. and Gitlin, J. D. (2003). Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc. Natl. Acad. Sci. USA 100, 1215-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler, S. G. (1998). Metabolic and molecular bases of Menkes disease and occipital horn syndrome. Pediatr. Dev. Pathol. 1, 85-98. [DOI] [PubMed] [Google Scholar]
- La Fontaine, S. and Mercer, J. F. (2007). Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch. Biochem. Biophys. 463, 149-167. [DOI] [PubMed] [Google Scholar]
- La Fontaine, S., Firth, S. D., Camakaris, J., Engelzou, A., Theophilos, M. B., Petris, M. J., Lockhart, P., Greenough, M., Brooks, H., Reddel, R. R. et al. (1998). Correction of the copper transport defect of Menkes patient fibroblasts by expression of the Menkes and Wilson ATPases. J. Biol. Chem. 273, 31375-31380. [DOI] [PubMed] [Google Scholar]
- Larin, D., Mekios, C., Das, K., Ross, B., Yang, A. S. and Gilliam, T. C. (1999). Characterization of the interaction between the Wilson and Menkes disease proteins and the cytoplasmic copper chaperone, HAH1p. J. Biol. Chem. 274, 28497-28504. [DOI] [PubMed] [Google Scholar]
- Leary, S. C., Kaufman, B. A., Pellecchia, G., Guercin, G. H., Mattman, A., Jaksch, M. and Shoubridge, E. A. (2004). Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum. Mol. Genet. 13, 1839-1848. [DOI] [PubMed] [Google Scholar]
- Lewis, C. E. and Pollard, J. W. (2006). Distinct role of macrophages in different tumor microenvironments. Cancer Res. 66, 605-612. [DOI] [PubMed] [Google Scholar]
- Madsen, E. and Gitlin, J. D. (2007). Copper deficiency. Curr. Opin. Gastroenterol. 23, 187-192. [DOI] [PubMed] [Google Scholar]
- Mantovani, A., Schioppa, T., Porta, C., Allavena, P. and Sica, A. (2006). Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 25, 315-322. [DOI] [PubMed] [Google Scholar]
- Mao, X., Kim, B. E., Wang, F., Eide, D. J. and Petris, M. J. (2007). A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J. Biol. Chem. 282, 6992-7000. [DOI] [PubMed] [Google Scholar]
- Martin, F., Linden, T., Katschinski, D. M., Oehme, F., Flamme, I., Mukhopadhyay, C. K., Eckhardt, K., Troger, J., Barth, S., Camenisch, G. et al. (2005). Copperdependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood 105, 4613-4619. [DOI] [PubMed] [Google Scholar]
- Moreno, P. R., Purushothaman, K. R., Sirol, M., Levy, A. P. and Fuster, V. (2006). Neovascularization in human atherosclerosis. Circulation 113, 2245-2252. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, C. K., Mazumder, B. and Fox, P. L. (2000). Role of hypoxia-inducible factor-1 in transcriptional activation of ceruloplasmin by iron deficiency. J. Biol. Chem. 275, 21048-21054. [DOI] [PubMed] [Google Scholar]
- Murdoch, C., Giannoudis, A. and Lewis, C. E. (2004). Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 104, 2224-2234. [DOI] [PubMed] [Google Scholar]
- Murphy, B. J., Robin, E. D., Tapper, D. P., Wong, R. J. and Clayton, D. A. (1984). Hypoxic coordinate regulation of mitochondrial enzymes in mammalian cells. Science 223, 707-709. [DOI] [PubMed] [Google Scholar]
- Nittis, T. and Gitlin, J. D. (2002). The copper-iron connection: hereditary aceruloplasminemia. Semin. Hematol. 39, 282-289. [DOI] [PubMed] [Google Scholar]
- Nose, Y., Kim, B. E. and Thiele, D. J. (2006). Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 4, 235-244. [DOI] [PubMed] [Google Scholar]
- Pan, Q., Kleer, C. G., van Golen, K. L., Irani, J., Bottema, K. M., Bias, C., De Carvalho, M., Mesri, E. A., Robins, D. M., Dick, R. D. et al. (2002). Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 62, 4854-4859. [PubMed] [Google Scholar]
- Pan, Q., Bao, L. W., Kleer, C. G., Brewer, G. J. and Merajver, S. D. (2003a). Antiangiogenic tetrathiomolybdate enhances the efficacy of doxorubicin against breast carcinoma. Mol. Cancer Ther. 2, 617-622. [PubMed] [Google Scholar]
- Pan, Q., Bao, L. W. and Merajver, S. D. (2003b). Tetrathiomolybdate inhibits angiogenesis and metastasis through suppression of the NFkappaB signaling cascade. Mol. Cancer Res. 1, 701-706. [PubMed] [Google Scholar]
- Petris, M. J., Mercer, J. F., Culvenor, J. G., Lockhart, P., Gleeson, P. A. and Camakaris, J. (1996). Ligand-regulated transport of the Menkes copperP-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J. 15, 6084-6095. [PMC free article] [PubMed] [Google Scholar]
- Petris, M. J., Strausak, D. and Mercer, J. F. (2000). The Menkes copper transporter is required for the activation of tyrosinase. Hum. Mol. Genet. 9, 2845-2851. [DOI] [PubMed] [Google Scholar]
- Petris, M. J., Voskoboinik, I., Cater, M., Smith, K., Kim, B. E., Llanos, R. M., Strausak, D., Camakaris, J. and Mercer, J. F. (2002). Copper-regulated trafficking of the Menkes disease copper ATPase is associated with formation of a phosphorylated catalytic intermediate. J. Biol. Chem. 277, 46736-46742. [DOI] [PubMed] [Google Scholar]
- Prohaska, J. R. and Gybina, A. A. (2004). Intracellular copper transport in mammals. J. Nutr. 134, 1003-1006. [DOI] [PubMed] [Google Scholar]
- Qin, Z., Itoh, S., Jeney, V., Ushio-Fukai, M. and Fukai, T. (2006). Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. FASEB J. 20, 334-336. [DOI] [PubMed] [Google Scholar]
- Redman, B. G., Esper, P., Pan, Q., Dunn, R. L., Hussain, H. K., Chenevert, T., Brewer, G. J. and Merajver, S. D. (2003). Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin. Cancer Res. 9, 1666-1672. [PubMed] [Google Scholar]
- Rius, J., Guma, M., Schachtrup, C., Akassoglou, K., Zinkernagel, A. S., Nizet, V., Johnson, R. S., Haddad, G. G. and Karin, M. (2008). NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453, 807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, J., Seshadri, V., Tripoulas, N. A., Ketterer, M. E. and Fox, P. L. (2003). Role of ceruloplasmin in macrophage iron efflux during hypoxia. J. Biol. Chem. 278, 44018-44024. [DOI] [PubMed] [Google Scholar]
- Setty, S. R., Tenza, D., Sviderskaya, E. V., Bennett, D. C., Raposo, G. and Marks, M. S. (2008). Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature 454, 1142-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, L. M., Robin, E. D., Phillips, J. R., Acevedo, J., Axline, S. G. and Theodore, J. (1977). Enzymatic basis for bioenergetic differences of alveolar versus peritoneal macrophages and enzyme regulation by molecular O2. J. Clin. Invest. 59, 443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steveson, T. C., Ciccotosto, G. D., Ma, X. M., Mueller, G. P., Mains, R. E. and Eipper, B. A. (2003). Menkes protein contributes to the function of peptidylglycine alpha-amidating monooxygenase. Endocrinology 144, 188-200. [DOI] [PubMed] [Google Scholar]
- Strausak, D., La, Fontaine, S., Hill, J., Firth, S. D., Lockhart, P. J. and Mercer, J. F. (1999). The role of GMXCXXC metal binding sites in the copper-induced redistribution of the Menkes protein. J. Biol. Chem. 274, 11170-11177. [DOI] [PubMed] [Google Scholar]
- Sunderman, F. W., Jr and Nomoto, S. (1970). Measurement of human serum ceruloplasmin by its p-phenylenediamine oxidase activity. Clin. Chem. 16, 903-910. [PubMed] [Google Scholar]
- Talks, K. L., Turley, H., Gatter, K. C., Maxwell, P. H., Pugh, C. W., Ratcliffe, P. J. and Harris, A. L. (2000). The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 157, 411-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teknos, T. N., Islam, M., Arenberg, D. A., Pan, Q., Carskadon, S. L., Abarbanell, A. M., Marcus, B., Paul, S., Vandenberg, C. D., Carron, M. et al. (2005). The effect of tetrathiomolybdate on cytokine expression, angiogenesis, and tumor growth in squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Head Neck Surg. 131, 204-211. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, Y., Heiny, M. E., Suzuki, M. and Gitlin, J. D. (1996). Biochemical characterization and intracellular localization of the Menkes disease protein. Proc. Natl. Acad. Sci. USA 93, 14030-14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B. and Gitschier, J. (1997). hCTR1: A human gene for copper uptake identified by complementation in yeast. Proc. Natl. Acad. Sci. USA 94, 7481-7486. [DOI] [PMC free article] [PubMed] [Google Scholar]