Summary
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited human renal disease and is caused by mutations in two genes, PKD1 (85%) and PKD2 (15%). Cyst epithelial cells are characterised by a complex cellular phenotype including changes in proliferation, apoptosis, basement membrane composition and apicobasal polarity. Since polycystin 1 (PC1), the PKD1 protein, has been located in the basolateral membrane of kidney epithelial cells, we hypothesised that it might have a key role in mediating or stabilising cell-cell interactions. In non-ciliated L929 cells, stable or transient surface expression of the PC1 extracellular domain was sufficient to confer an adhesive phenotype and stimulate junction formation. In MDCK cells, we found that PC1 was recruited to the lateral membranes coincident with E-cadherin within 30 minutes after a `calcium switch'. Recruitment of both proteins was significantly delayed when cells were treated with a PC1 blocking antibody raised to the PKD domains. Finally, PC1 and E-cadherin could be coimmunoprecipitated together from MDCK cells. We conclude that PC1 has a key role in initiating junction formation via initial homophilic interactions and facilitates junction assembly and the establishment of apicobasal polarity by E-cadherin recruitment.
Keywords: Polycystin 1, E-cadherin, Cell adhesion, MDCK, ADPKD
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited human renal disease (incidence 1 in 1000 live births) and is caused by mutations in two genes, PKD1 (85%) and PKD2 (15%) (Ong and Harris, 2005; Torres and Harris, 2006). ADPKD is an important cause of end-stage renal failure, accounting for ∼10% of patients on renal replacement therapy. The disease is characterised by the formation of fluid filled cysts in both kidneys of affected individuals, which ultimately result in end-stage renal failure. Other extrarenal manifestations of the disease include hypertension, cardiac valve abnormalities and cerebral aneurysms (Calvet and Grantham, 2001; Wilson, 2004).
The two proteins involved in ADPKD, polycystin 1 (PC1; also known as PKD1) and polycystin 2 (PC2; also known as PKD2) have been shown to function as a heterodimeric complex (Hanaoka et al., 2000; Newby et al., 2002), activating a number of key signalling pathways, which in turn regulate diverse cellular functions including proliferation, apoptosis, tubulogenesis and fluid secretion. This is consistent with the largely overlapping renal and extrarenal phenotypes of PKD1 and PKD2 patients. PC1 and PC2 are likely to function together in many systems but there is evidence to suggest they can also function independently (Ong and Harris, 2005). Both proteins have been located in several subcellular structures including primary cilia and the basolateral membrane. Functional evidence that the polycystins can transduce a mechanosensitive Ca2+ current and mediate cell adhesion has been reported (Ibraghimov-Beskrovnaya et al., 2000; Nauli et al., 2003; Streets et al., 2003).
PC1 is a large (>460 kDa) heavily glycosylated integral membrane protein with a predicted large N-terminal extracellular domain (∼2500 aa), 11 transmembrane domains and a short C-terminal cytoplasmic tail (Hughes et al., 1995). The extracellular region appears to have a modular structure suggesting the presence of potential functional motifs. These include two leucine-rich repeats (LRR), a C-type lectin, a LDL-A receptor motif and a large region (∼1000 residues) with strong homology to the sea urchin receptor for egg jelly (REJ) protein. The major part of the N-terminal region, however, consists of 16 novel repeats (80-90 aa) with low sequence homology to immunoglobulin domains. These so called PKD domains or repeats are arranged in tandem (II-XVI) except for domain I, which is present between the LRR and lectin modules. The extracellular domain of PC1 has been shown to be cleaved at a conserved G-protein-coupled receptor (GPCR) proteolytic site (GPS) (position T3049) resulting in N-terminal and C-terminal fragments tethered to each other at the cell surface (Qian et al., 2002) This proteolytic event is thought to be essential for the function of PC1 in the mature kidney (Yu et al., 2007).
Antibodies to the PKD domains of PC1 have been shown to disrupt cell-cell adhesion in subconfluent canine, murine and human kidney epithelial cells (Ibraghimov-Beskrovnaya et al., 2000; Streets et al., 2003). These results support a key role for PC1 in the regulation of cell adhesion. However, it is possible that the role of PC1 is dependent on the function of other adhesion molecules such as E-cadherin or desmosomal cadherins. In this regard, a role for PC1 in E-cadherin recruitment has also been reported (Charron et al., 2000). PC1 has also been shown to colocalise and coimmunoprecipitate with E-cadherin and the catenins in human pancreatic adenocarcinoma cells (Huan and van Adelsberg, 1999). In human cystic cells, the absence of surface PC1 is associated with concomitant loss of surface E-cadherin expression and its replacement by N-cadherin (Streets et al., 2003; Roitbak et al., 2004; Russo et al., 2005). A putative role for PC1 in desmosome function has also been postulated because of its immunolocalisation to desmosomal junctions, the mislocalisation of desmosome junction proteins in cystic cells and the reported interaction of the PC1 C-terminus with intermediate filaments (Scheffers et al., 2000; Xu et al., 2001; Russo et al., 2005; Silberberg et al., 2005). However, two studies reporting the timing of PC1 and desmoplakin recruitment after a `calcium switch' conflict with one study showing that PC1 precedes desmoplakin and another showing that it follows desmoplakin (Scheffers et al., 2000; Silberberg et al., 2005).
In this study, we present data demonstrating that expression of the extracellular domain of PC1 is sufficient to induce cell aggregation and junction formation in L929 cells and that it is involved in E-cadherin recruitment during MDCK junction reassembly. Our data support a proximal role for PC1 in the establishment of cell junctions and the subsequent acquisition of apicobasal polarity.
Results
Cell surface expression of the extracellular domain of PC1 as a GPI fusion protein (NT1-GPI)
To investigate whether PC1 is sufficient to mediate cell-cell adhesion, we expressed PC1 in L929 mouse fibroblasts which lack classical cell adhesion molecules. Previous studies have utilised this non-adhesive cell line to demonstrate the function of exogenous cell adhesion molecules including E-cadherin (Nagafuchi et al., 1987), and desmosome proteins (Marcozzi et al., 1998). In addition, we confirmed that these cells do not express primary cilia or detectable PC1 (not shown). Therefore we reasoned that this model system would also enable us to determine whether PC1 could function as a cell adhesion molecule independently of ciliary expression.
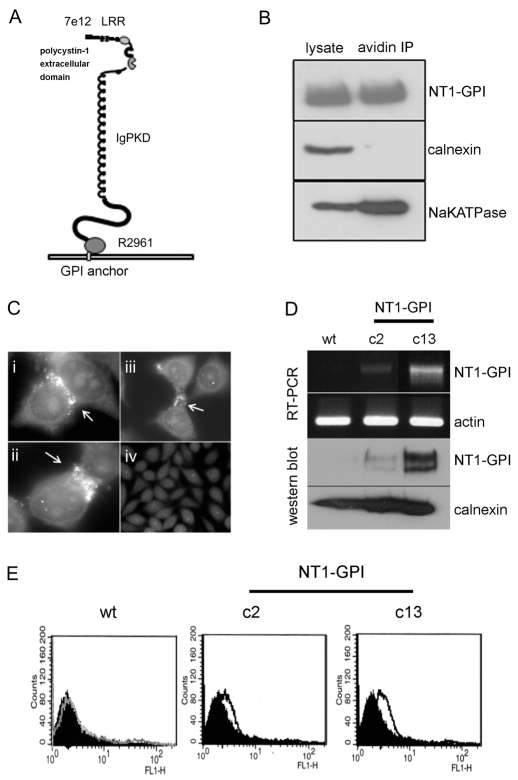
Because our initial attempts to generate stable L929 clones expressing full length PC1 were unsuccessful, an alternative strategy utilising a GPI anchor signal sequence (Bernasconi et al., 1996) to direct the expression of the extracellular domain of PC1 (aa M1-R2961) to the cell surface was devised (Fig. 1A). This site is N-terminal of the GPS cleavage site (located at T3049) and was chosen so as to exclude the possibility of proteolytic cleavage of the ectodomain (Qian et al., 2002). Surface expression of the NT1-GPI fusion protein was easily detectable in transiently transfected HEK293 cells by cell surface biotinylation (Fig. 1B) and immunofluorescence (not shown). Of note, transient expression of NT1-GPI was sufficient to induce the formation of cell-cell contacts in L929 cell with the protein concentrated at these regions (Fig. 1C). By contrast, L929 cells transfected with the empty vector showed no cell-cell contacts and no PC1 staining (Fig. 1C). We proceeded to generate a total of 20 stable L929 clones expressing NT1-GPI or the empty vector by screening G418-resistant cells by RT-PCR. Examples of two such clones are shown in Fig. 1D: c2 (a low expressor) and c13 (a high expressor). Using a previously characterised N-terminal PC1 antibody (7e12), we confirmed the expression of NT1 in both clones by immunoblotting (Fig. 1D) and surface expression by FACS analysis (Fig. 1E). Of interest, NT1-GPI was expressed as a doublet band possibly because of post-translational modification such as glycosylation. The absence of the GPS cleavage site excludes this as a possible cause.
Fig. 1.
A PC1 N-terminus-GPI anchor fusion protein is expressed at the cell surface in transfected HEK293 and L929 cells. (A) The extracellular domain of PC1 (M1-R2961) was fused in frame with a Thy-1 GPI anchor signal sequence (NT1-GPI) and cloned into a modified PCR-3 vector. The regions to which the different antibodies used in this study (7e12, LRR, IgPKD) bind, are indicated. (B) Surface expression of the N-terminus of PC1 was shown by cell surface biotinylation in transiently transfected HEK cells. Western blotting with a specific anti-PC1 N-terminus antibody (7e12) was carried out on biotinylated protein bound to and eluted from streptavidin beads. The blots show that NT1 is present at the cell surface. Immunoblotting with antibodies directed to an ER resident protein, calnexin and a cell surface marker, Na+/K+ATPase show that only cell surface proteins have been biotinylated. (C) Immunofluoresence staining was carried out on L929 cells transiently transfected with either (i-iii) NT-1/GPI or control empty vector (iv). NT1 was detected with 7e12 at points of cell-cell contact (arrows). These areas of cell-cell contact were absent in mock-transfected control L929 cells. (D) L929 cells were stably transfected with the NT1-GPI construct. Following G418 selection, a number of resistant clones were tested for expression of the NT1-GPI construct by RT-PCR using primers specific to the GPI signal sequence as well as NT1. Clones with high expression (c13) and low expression (c2) as well as mock-transfected wild-type clones were identified. Actin was used as a loading control. Western blotting with 7e12 shows that c13 expresses the NT1-GPI fusion protein. The protein is weakly expressed in c2 and is not detectable in mock-transfected control cells. Equal loading is shown by re-probing the same blot with an antibody to calnexin. (E) FACS was carried out on non-permeabilised L929 clones. Cells were stained with 7e12. There was a significant shift in fluorescence intensity in c13 compared with the negative control. c13 had the highest expression (fivefold increase over wild type) of NT1 at the cell surface, confirming the results seen by RT-PCR and western blotting.
Stable expression of NT1-GPI in L929 cells causes cell aggregation inhibitable by an anti-PC1 antibody
To extend the findings seen in transiently transfected L929 cells, we carried out aggregation assays on c13, the highest NT1-GPI-expressing L929 clone. After a 4 hour incubation, cells expressing the NT1-GPI fusion protein formed visible large aggregates in suspension. By contrast, control mock-transfected cells remained as a single cell suspension after the same treatment (Fig. 2A). To confirm that PC1 was directly responsible for the increase in cell aggregation, we incubated the cells simultaneously with anti-IgPKD, a previously characterised PC1 inhibitory antibody. As shown in Fig. 2A, this PC1 antibody completely inhibited cell aggregation whereas non-immune serum had no effect at the same dilution. These results confirm that PC1 can specifically confer a cell-cell adhesive phenotype mediated by homophilic interactions of the IgPKD domains in the absence of cell-matrix cues. In addition, we found no difference between c13 and mock-transfected L929 clones in adhesion, spreading or migration assays (not shown), confirming that expression of NT1-GPI confers an increase in cell-cell but not cell-matrix adhesion.
Fig. 2.
Expression of NT1-GPI in L929 cells causes cell aggregation, which is inhibited by an anti-PC1 antibody. (A) Cell aggregation assays were carried out on the highest expressing clone (c13) and mock-transfected control cells. Images were taken and particle numbers quantified from six random fields using ImageJ (NIH). Results were expressed as Nt/N0 (where Nt is the number of particles after 4 hours incubation and N0 is the number of particles at the start) and are representative of three separate experiments. Each bar represent the mean ± s.e.m. *A significant difference (P<0.05) between c13 and mock transfectant. Expression of NT1-GPI resulted in significant cell aggregation after 4 hour with an Nt/N0 ratio of 0.14. By contrast, mock-transfected cells showed no aggregation after the 4-hour incubation and remained as a single cell suspension with an Nt/N0 ratio of 1.04. To confirm that cell aggregation was as a direct result of the expression of NT1 at the cell surface, cells were incubated with a blocking antibody (IgPKD) raised to the IgPKD domains (residues 843-2145) of the N-terminus of PC1 or non-immune rabbit serum. The antibody significantly disrupted cell aggregation in the NT1-GPI-expressing L929 cells (Nt/N0 ratio of 0.24) compared with non-immune serum (Nt/N0 ratio 1.01). (B) The NT1-GPI construct was sub-cloned into a pEBTetD-inducible vector and transiently expressed in L929 cells. Protein expression was induced by addition of 2 μg/ml tetracycline to the culture media 16 hours prior to the cell aggregation assay. Mock-transfected cells and uninduced NT1-GPI-expressing cells remained as a single cell suspension after a 4-hour incubation. Tetracycline induced NT1-GPI-expressing cells showed significant cell aggregation (*P<0.05 versus no tetracycline). Western blotting with 7e12 confirmed expression of NT1-GPI in the tetracycline-induced cells alone. Equal loading was confirmed by probing the same blot with an anti-calnexin antibody. (C) Electron microscopy shows cell-cell junction formation in L929 c13 cells stably expressing the NT1-GPI fusion protein. Sites of cell-cell contact are visible (i, arrows). Higher magnification of an area of cell-cell contact (ii) shows the appearance of cell adhesion plaques characteristic of clustering of cell adhesion molecules (arrows).
Inducible expression of NT1-GPI in L929 cells causes cell aggregation
To further demonstrate that PC1 can directly mediate cell adhesion, we subcloned the NT1-GPI fusion protein into a tetracycline-inducible vector, pEBTetD (Bach et al., 2007) and expressed it transiently in L929 cells. Following a 16 hour induction with tetracycline, but not in its absence, expression of the NT1-GPI fusion protein was detectable by immunoblotting (Fig. 2B). As shown for the c13 line, induced cells showed significant cell aggregation after 4 hours compared with non-induced or wild-type cells (Fig. 2B).
Formation of cell junctions in NT1-GPI expressing L929 cells by electron microscopy
The structural nature of the cell-cell contacts formed in C13 NT1-GPI-expressing L929 cells following cell aggregation was investigated further by electron microscopy. Under lower magnification, cells can be seen to adhere to each other (Fig. 2Ci). With higher magnification, adhesion plaques, visible as regions of increased membrane density, could be seen (Fig. 2Cii). By contrast, wild-type L929 cells did not adhere and showed no signs of plaque formation (not shown).
PC1 regulates E-cadherin recruitment to intercellular junctions
The finding that NT1-GPI could induce structural junctions led us to study the role of PC1 during junction reassembly in polarised epithelial cells. To do this, we exploited the well-established `calcium switch' assay in MDCK cells (Nigam et al., 1992). In fully polarised cells grown to confluence, typical cell junctional proteins, E-cadherin (adherens junctions), desmoplakin (desmosomes) and ZO-1 (tight junctions) were appropriately located by immunofluorescence (Fig. 3A). In addition, endogenous PC1 detected with a previously well characterised antibody (anti-LRR) (Ibraghimov-Beskrovnaya et al., 1997; Ibraghimov-Beskrovnaya et al., 2000) could be clearly seen at primary cilia (not shown) and the lateral plasma membrane where it colocalised with E-cadherin and desmoplakin.
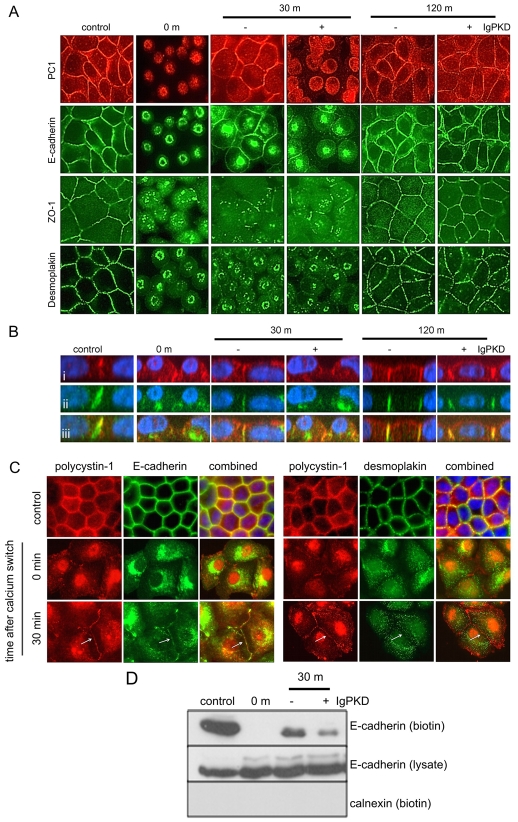
Fig. 3.
PC1 is involved in E-cadherin recruitment during junction reassembly following a calcium switch. (A) MDCK II cells were grown to confluence and subjected to a calcium switch assay. Data is representative of four separate experiments. Cells were fixed at various time points after restoring normal Ca2+ levels to the growth media. Cells were incubated either with a blocking antibody (IgPKD) or non-immune rabbit serum for the period of the calcium switch experiment. Cells were stained for E-cadherin, PC1, desmoplakin and ZO-1 expression. (B) Z section analysis of PC1 (i) and E-cadherin (ii) showing that the two proteins colocalised (iii) during junction reassembly. The presence of the IgPKD blocking antibody resulted in delayed recruitment of PC1 and E-cadherin compared with untreated cells. This effect is transient, as 2 hour after normal Ca2+ is restored, both proteins have relocalised to the lateral membrane. (C) Colocalisation and recruitment of PC1, E-cadherin and desmoplakin following a calcium switch was confirmed using a previously described antibody raised to the C-terminal of PC1 (BD3) in paraformaldehyde-fixed MDCK cells. PC1 and E-cadherin were recruited to the cell membrane after 30 minutes. At this time point, no surface recruitment of desmoplakin was observed. (D) Recruitment of E-cadherin to the cell surface following a calcium switch was detected by cell surface biotinylation. E-cadherin is absent from the cell surface at the moment when Ca2+ is restored to the culture (time 0). 30 minutes after the calcium switch, there is reduced E-cadherin cell surface expression in cells treated with IgPKD. Total E-cadherin levels in the cell lysate remain unchanged. Immunoblotting for calnexin show that only cell surface proteins have been biotinylated.
Following overnight incubation in low Ca2+ medium (<5 μM Ca2+), cell-cell contacts were lost and cells appeared to round up. Immunofluorescence staining revealed that E-cadherin, desmoplakin, ZO-1 and PC1 were all redistributed from the cell surface into cytoplasmic vesicles (Fig. 3A). When normal Ca2+ levels (1.8 mM Ca2+) were restored, intercellular junctions reformed over a period of 2 hours. Of the junctional proteins, E-cadherin was recruited earliest to the lateral membrane by 30 minutes as shown by immunofluorescence (Fig. 3A) and cell surface biotinylation (Fig. 3D). Lateral staining of ZO-1 and desmoplakin was only detectable after 2 hours (Fig. 3A). Interestingly PC1 was also recruited to the lateral membrane within 30 minutes of the calcium switch where analysis of Z-sections showed that it colocalised with E-cadherin for the 2-hour duration of the assay. The temporal and spatial coexpression of PC1 with E-cadherin indicated that PC1 is likely to be crucial to E-cadherin recruitment and the formation of adherens junctions (Fig. 3B). These findings were confirmed in paraformaldehyde-fixed MDCK cells using a second PC1 antibody (BD3) raised to the PC1 C-terminus (Fig. 3C). PC1 and E-cadherin were recruited to the cell membrane after 30 minutes. At this timepoint, no recruitment of desmoplakin was observed.
To investigate the possibility that polycystin 1 was crucial for E-cadherin recruitment to the plasma membrane and formation of adherens junctions, after the calcium switch we treated MDCK cells with the PC1 antibody, anti-IgPKD. As shown in Fig. 3B, antibody treatment following calcium switch resulted in a delay in the recruitment of both PC1 and E-cadherin to the lateral membrane at 30 minutes. This was confirmed by cell surface biotinylation experiments showing a reduction in surface E-cadherin expression in antibody-treated cells compared with cells treated with non-immune serum (Fig. 3D). The effect was, however, transient since by 120 minutes after the reintroduction of Ca2+, PC1 and E-cadherin could be seen at the lateral membranes in both antibody treated and untreated cells. Cilia expression was unchanged by antibody treatment (data not shown). These findings support the idea that PC1 regulates E-cadherin surface expression during junctional assembly.
Mapping the interaction domains between PC1 and E-cadherin
We next investigated the possibility that PC1 and E-cadherin form a complex by studying their association in native and heterologous cells. In MDCK cells, endogenous PC1 can be coimmunoprecipitated with E-cadherin (Fig. 4A). We confirmed this interaction by reciprocal immunoprecipitation of transiently transfected heterologous PC1 and E-cadherin in HEK293 cells (Fig. 4C). Surprisingly we found that a naturally occurring PKD1 mutant, R4227X, in which the coiled-coil domain (responsible for interacting with PC2) has been deleted, could still bind to E-cadherin in this assay. Transfected E-cadherin was detectable as a prominent doublet band as previous reported (Lickert et al., 2000). The upper band represents an immature precursor form which is also detectable for the endogenous protein (Fig. 3D).
Fig. 4.
The C terminus of PC1 associates with the C-terminus of E-cadherin to form a protein complex independent of PC2 and β-catenin. (A) Reciprocal immunoprecipitation of native PC1 and E-cadherin from MDCK cell lysates show that the two proteins form a complex. Non-immune rabbit IgG and mouse IgG2a control antibodies show that the IP is specific. Data shown is from a representative experiment of two. (B) Diagrams showing (i) E-cadherin with the serine-rich domain sequence highlighted at which HA-tagged E-cadherin truncations were made at amino acids S840X and S855X; (ii) full length Myc-tagged PC1; (iii) HA-tagged PC1-R4227X; and (iv) the Myc-tagged CT1-CD8 fusion. (C) Myc-tagged PC1 (>500 kDa) coimmunoprecipitated with E-cadherin (120 kDa) from HEK cell lysates. In addition, PC1-R4227X (>500 kDa) could still be coimmunoprecipitated with E-cadherin. Data from a representative experiment of three. (D) Wild-type E-cadherin and the S855X mutant were still able to form a complex with endogenous β-catenin but the E-cadherin S840X mutant showed almost no binding. (E) Full length HA-tagged E-cadherin as well as HA-tagged C-terminal truncations are still able to form a complex with 45 kDa Myc-tagged CT1-CD8-Myc but the E-cadherin S840X mutant showed reduced binding. (F) In vitro binding assay of purified MBP-CT1 with His-Thio-tagged E-cadherin C-terminus (CT) demonstrated the absence of a direct interaction between the two proteins. Bacterially expressed MBP (43kDa), MBP-CT1 (65 kDa) or MBP-CT1R4227X (56 kDa) protein were incubated with amylose beads and mixed with His-Thio-tagged E-cadherin CT (50 kDa) or His-Thio-tagged PC2 C-terminus (50 kDa). Bead-associated proteins were eluted by boiling and immunoblotted with antibodies to MBP or thioredoxin. As a positive control, a direct interaction between MBP-CT1 and His-Thio-CT2 (CT2) was clearly detected.
To further define the interacting domains between the two proteins, we expressed the entire PC1 C-terminus as an epitope-tagged fusion with CD8 (CT1-CD8-Myc) and found that it was sufficient to pull-down E-cadherin. Previous studies had shown that phosphorylation of a serine-rich domain (840-855) in the C-terminus of E-cadherin is important for β-catenin binding (Lickert et al., 2000). Indeed, an E-cadherin mutant with a deletion in this region (S840X) lost the ability to bind endogenous β-catenin (Fig. 4E) but was still capable of binding CT1, albeit with a weaker signal than a mutant (S855X) retaining this domain (Fig. 4D). These findings indicate that the binding domain for PC1 in the C-terminus of E-cadherin must lie upstream of S840. Nevertheless, it is possible that the serine-rich domain might act as a regulatory site for binding affinity, probably through phosphorylation at specific residues. It is apparent however that PC1 can bind to E-cadherin independently of PC2 or β-catenin.
In vitro binding assays of purified bacterial fusion proteins containing the C-termini of PC1 (MBP-CT1) and E-cadherin were performed to investigate whether the two proteins could interact directly. Using this assay, we were unable to demonstrate a direct interaction between PC1 and E-cadherin (Fig. 4F). By contrast, a direct interaction between the C-termini of PC1 and PC2 was clearly seen. Our findings indicate that PC1-E-cadherin interaction is likely to be indirect and mediated through other, yet to be identified, adapter proteins. Alternatively, their direct interaction may require an essential post-translational modification absent in the bacterial proteins.
Discussion
In this paper, we provide the first direct evidence that PC1 can act as a cell adhesion molecule by expressing the entire extracellular region of PC1 as a GPI-anchored protein (NT1-GPI) in L929 cells. Using a specific inhibitory antibody, we confirm that this is dependent on trans-homophilic interactions of the PKD extracellular domains. Previous studies had demonstrated the importance of this region for PC1 mediated cell-cell adhesion but were unable to distinguish between its homophilic and heterophilic interactions (with other cadherins) in renal epithelial cells (Ibraghimov-Beskrovnaya et al., 2000; Streets et al., 2003). In L929 cells, NT1-GPI also induced the formation of structural junctions when the cells were induced to aggregate in suspension implying that PC1 might play a crucial role in junction formation.
Our results also confirm that PC1 localisation is dynamic in MDCK cells and alters temporally with junctional assembly and the reacquisition of cell polarity. Using the well established calcium switch assay, we demonstrate that PC1 is localised best with desmoplakin in fully polarised cells but overlaps more with E-cadherin during repolarisation. This may explain some of the differences observed in previous work in which PC1 expression was found to colocalise either to adherens junctions or desmosomes (Scheffers et al., 2000; van Adelsberg, 2000). Differences in confluency, differentiation, polarity or cell type could have contributed to some of the observed differences (Ong, 2000).
The formation of a polarised kidney tubule relies on a series of sequential events including cell-cell association, the formation of epithelial junctions, the acquisition of apicobasal polarity and finally the imposition of planar polarity. A large body of work has shown that the assembly of epithelial junctions is a hierarchical process in which, E-cadherin plays a primary role (Adams and Nelson, 1998; Braga, 2002; Jamora and Fuchs, 2002). For instance, MDCK cells depleted of E-cadherin by siRNA show a marked delay in re-establishing cell polarity in a calcium switch assay (Capaldo and Macara, 2007). However, once formed, cell junctions are maintained even when E-cadherin was subsequently depleted, indicating that the role of E-cadherin is likely to be in the initial phase of junction assembly but is not essential for their maintenance. During junction assembly, E-cadherin is recruited early to cell-cell contact sites, forming a scaffold for other proteins such as the α-catenin and β-catenin, other actin-binding proteins and signalling molecules (Vasioukhin and Fuchs, 2001; Perez-Moreno et al., 2003; Bershadsky, 2004). The ectodomains of E-cadherin also mediate Ca2+-dependent homophilic interactions between adjacent cells thus strengthening cell-cell associations. Tight junction (TJ) proteins are initially recruited to cadherin contacts possibly through α-catenin-mediated recruitment of ZO-1 and only later move away to form the TJ (Muller et al., 2005). The desmosomal cadherin complex is then recruited to complete the formation of the intercellular junctions and the establishment of apicobasal polarity (Gumbiner et al., 1988; Yin and Green, 2004).
Our results suggest that PC1 plays a proximal role in the process of junction assembly in MDCK cells both by mediating initial cell-cell association and by regulating E-cadherin recruitment. In previous studies, we and others have shown that cell-cell association in a number of renal epithelial cells including MDCK can be disrupted by an antibody to the PKD domains (IgPKD) or soluble GST recombinant protein (Ibraghimov-Beskrovnaya et al., 2000; Streets et al., 2003). Of interest, the cell-cell dissociating effect of the antibody was reduced by the transgenic expression of PC1 and was absent in the PKD1 null cystic line (Streets et al., 2003). Taken together with our data from L929 cells, it seems highly probable that PC1 mediates cell-cell associations in the earliest phase of junction assembly.
During the calcium switch, PC1 and E-cadherin membrane localisation overlap temporally and spatially and can be delayed by pre-incubation with an antibody to PC1. The delay is, however, transient and can be overcome 2 hours after the reintroduction of Ca2+. These findings are corroborated by a recent study in which the rate of E-cadherin recruitment was reported to be enhanced in MDCK cells over-expressing PC1 following calcium switch (Markoff et al., 2007). Our results are very similar to those described for E-cadherin-depleted MDCK cells where junction formation was delayed but not prevented (Capaldo and Macara, 2007). These data indicate that PC1 and E-cadherin are important in the establishment of junctions but dispensable for their maintenance. Human cystic epithelial cells have been reported with variable levels of E-cadherin but with neo-expression of N-cadherin (Streets et al., 2003; Roitbak et al., 2004; Russo et al., 2005). Since these cells are primarily derived from large surface cortical cysts, we suggest that the loss of E-cadherin expression (rather than its early mislocalisation) and concomitant N-cadherin transcription are likely to occur as later events in cystogenesis.
We extended these findings by first showing that PC1 and E-cadherin can be coimmunoprecipitated from MDCK cells confirming previous findings in human pancreatic adenocarcinoma (HPAC) cells and normal human kidney cells (Huan and van Adelsberg, 1999; Roitbak et al., 2004). We show for the first time that both proteins can associate via their C-termini independently of previously described binding domains. In particular the PC1 mutant R4227X, which lacks a coiled-coil domain essential for PC2 binding (Qian et al., 1997) was still able to coimmunoprecipitate E-cadherin. For E-cadherin, it had been shown that a serine-rich domain between amino acids 840 and 855 is essential for β-catenin binding (Lickert et al., 2000). Significantly, binding affinity between E-cadherin and β-catenin could be altered by casein kinase II (CKII) and glycogen synthase kinase-3 β (GSK-3β) phosphorylation at specific serine residues (Lickert et al., 2000). Although deletion of this domain abolished the binding of endogenous β-catenin, PC1 association was still present, albeit reduced. We found no evidence of direct binding between PC1 and E-cadherin using in vitro binding assays. This could indicate the importance of key mediatory adapter proteins or the crucial role of post-translational modifications (including phosphorylation) in mediating and regulating this process. Future work will seek to address these questions.
In summary, we have demonstrated the ability of PC1 to promote strong aggregating ability in non-adherent L929 cells via homophilic interactions of the PKD domains. Moreover, its temporal, spatial and biochemical association with E-cadherin in MDCK cells indicates a probable crucial role in junction initiation rather than maintenance. This model could explain why the mislocalisation of some basolateral proteins such as Na+-K+-ATPase and epidermal growth factor receptor (EGFR) are often but not universally observed in cysts (Wilson et al., 1991; Du and Wilson, 1995) and why the deletion of Pkd1 in adult kidney tubules has a mild phenotype compared with that in the foetus (Lantinga-van Leeuwen et al., 2007). If the major role of PC1 is in junction initiation, a disturbance in its function (leading to cyst initiation) is most likely to be manifest under conditions of high junctional turnover such as during tubular elongation in the developing kidney or during tissue repair following injury in the mature kidney.
Materials and Methods
Materials
All chemicals were purchased from Sigma (Poole, UK) unless otherwise stated. MDCK II cells were a gift from N. Simmons (University of Newcastle, Newcastle, UK) and L929 cells were purchased from the ECACC (Salisbury, UK). Antibodies to PC1 (IgPKD, LRR and BD3) and desmoplakin were obtained from Oxana Ibraghimov-Beskrovnaya (Genzyme) and D. Garrod (University of Manchester, Manchester, UK), respectively. A rat monoclonal antibody to ZO-1 was obtained from the Developmental Studies Hybridoma Bank (University of Iowa, USA) and antibodies to E-cadherin and calnexin were purchased from BD Transduction Labs (Cowley, Oxford, UK). The following cDNA expression constructs were kind gifts from the following: PCMUIV-CD8α (S. Ponnambalam, University of Leeds, Leeds, UK), pBAT-EM2 (D. Garrod), PCR-3/GPI (N. Fasel, University of Lausanne, Lausanne, Switzerland) and pEBTetD (D. Grundemann, University of Cologne, Cologne, Germany).
Plasmid construction
A full-length PKD1 FLAG-tagged cDNA was generated by exon-linking (C.J.W., unpublished data). We modified this cDNA by replacing the C-terminus FLAG epitope with a Myc epitope (PKD1-Myc) using PCR. Similarly, an epitope-tagged PKD1 truncation mutant (PKD1-R4227X-HA) was generated by introducing a premature stop codon preceded by an HA-epitope tag sequence. To create NT1-GPI, the entire extracellular domain of PC1 (M1-R2961) was removed from full-length PKD1 cDNA by restriction digestion with EcoRI and BamHI and ligated in frame with a Thy-1 GPI anchor signal sequence in PCR-3/GPI. A tetracycline inducible NT1-GPI construct was generated by subcloning the NT1-GPI insert into the pEBTetD vector (Bach et al., 2007). Full length HA-tagged E-cadherin was generated in pcDNA3 by PCR from the plasmid pBATEM2, which contains the entire coding region of E-cadherin (Nose et al., 1988). HA-tagged E-cadherin truncation mutants were similarly constructed with stop codons replacing S840 and S855, respectively. The PKD1 C-terminus (aa 4107-4303) containing a Myc epitope tag replacing the CD8 cytoplasmic tail was cloned in frame with the preceding CD8α sequence to create the CT1-CD8-Myc fusion construct (Ponnambalam et al., 1994). All constructs were verified by sequencing.
Cell culture and transfection
HEK293, MDCK and L929 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. Transient transfection was carried out on cells cultured to 90% confluency using Lipofectamine 2000 (Invitrogen, Paisley, UK) according to the manufacturer's instructions. L929 cells stably expressing NT1-GPI were generated by selecting resistant clones with 800 μg/ml G418 for a period of 3 weeks. Protein expression in cells expressing a pEBTetD NT1-GPI inducible vector was induced by addition of 2 μg/ml tetracycline to the culture media for 16 hours.
RT-PCR
RNA was extracted from stable clones using Trizol reagent, cDNA was synthesised using a reverse transcriptase kit (Ambion, Applied Biosystems, UK) and expression of the NT1-GPI construct was determined by RT-PCR. A forward primer specific to the N-terminus of PC1 and a reverse primer specific to the Thy-1 signal sequence were used.
Immunoprecipitation and immunoblotting
Total cell lysates were prepared and processed for immunoprecipitation and western blotting as previously described (Newby et al., 2002).
Immunofluorescence
Cells were grown on filters and fixed with acid-ethanol at 4°C for 1 hour or 4% paraformaldehyde for 5 minutes followed by permeabilisation with PBS, 0.1% Triton X-100. Blocking was carried out for 1 hour with 5% milk powder in PBS, and primary antibodies were incubated overnight at 4°C in 3% BSA-PBS. Controls included cells stained without the primary antibody, an irrelevant IgG1 mAb (Serotec, Kidlington, UK) or a non-immune rabbit IgG fraction (Dako, Ely, UK). Antibody binding was visualised using FITC-conjugated goat ant-mouse IgG and Alexa-Fluor-568-labelled goat anti-rabbit secondary antibodies. Slides were viewed using an Olympus Imaging Systems inverted IX71 microscope configured for multi-fluorescence image capture. Images were acquired and analysed using SimplePCI imaging software (Compix). Z sections were acquired and analysed using a Zeiss LSM 510 META Axiovert 200M confocal microscope and Zeiss LSM image analysis software.
Cell surface biotinylation
Cell surface biotinylation was performed as previously described (Streets et al., 2006). Samples were analysed by SDS-PAGE and western blotting using the antibodies described.
Fluorescence-activated cell sorting (FACS)
Cells cultured to confluency in 10 cm culture dishes were detached using cell dissociation buffer (Invitrogen) and resuspended in ice cold 3% BSA-PBS at 1×107 cells/ml. They were incubated with 1/50 dilution of anti-PC1 7e12 antibody or mIgG1 negative control antibody (Serotec, Kidlington, UK) for 30 minutes and washed three times by centrifugation at 400 g for 5 minutes in PBS. Secondary antibody incubation was with a 1/300 dilution of goat anti-mouse FITC for 30 minutes. After washing cells were resuspended in PBS and analysed by flow cytometry.
Cell aggregation assay
Cells grown to confluence were detached using cell dissociation buffer (Invitrogen, UK). A single cell suspension was generated by gently passing the detached cells through a 21 gauge needle and this was verified by light microscopy. DNaseI (1 μg/ml) was added to prevent clumping as a result of release of DNA from dead cells. Cells were gently mixed at 100 r.p.m. in 12-well dishes pre-coated with 1.5% BSA for 4 hours. To test the effect of the anti-PC1 blocking antibody IgPKD on cell aggregation, IgPKD or non-immune rabbit serum was added at 1/50 for the same period of time. Images were taken and particle numbers quantified from six random fields using ImageJ (NIH). Results were expressed as Nt/N0 where Nt is the number of particles at 4 hours and N0 is the number of particles at the start (Hordijk et al., 1997).
Calcium switch assay
Calcium switch assay was carried out essentially as described previously (Nigam et al., 1992) with the following modifications. Cells were grown on filters to full confluence in complete medium. They were rinsed three times and then incubated overnight in Spinner's modified Eagle's medium (SMEM; low Ca2+ media: <5 μM Ca2+) with 5% dialysed FCS. The cells were then switched to complete medium for different lengths of time ranging from 0 minutes to 2 hours either in the presence of non-immune rabbit sera or 1/50 IgPKD antibody, after which cells were fixed as described above.
Electron microscopy
Following an aggregation assay, cells in suspension were fixed with an equal volume of 3% phosphate-buffered glutaraldehyde in PBS for 1 hour and then gently sedimented by centrifugation at 1000 g for 5 minutes. The supernatant was then removed and the cell pellet stored in 3% glutaraldehyde for a further 24 hours. The cells were then resuspended in molten 2% agarose. On setting, the agarose block was chopped into 1 mm cubes and further fixed in 3% phosphate-buffered glutaraldehyde. This was followed by further fixation in 1% aqueous osmium tetroxide, processing through graded alcohols, propylene oxide and into medium grade epoxy resin. Following heat polymerisation, resin blocks were sectioned using a Reichert ultracut E ultramicrotome at 0.6 μm, stained using 1% Toluidine blue in 1% sodium tetraborate and the sections examined by light microscopy. The most appropriate block was selected and thin-sectioned using a Diatome diamond knife at 85 nm. Thin sections were then stained in saturated uranyl acetate in 99% ethanol and Reynold's lead citrate. Sections were examined on a Philips 400 transmission electron microscope and photographed using large format Kodak EM negatives.
In vitro binding assays
Constructs encoding the PC1 C-terminal domain and the truncated PC1 C-terminal domain (CT1R4227X) as maltose-binding protein (MBP) fusions or MBP alone were made in pMAL-c2 (New England Biolabs). The C-terminus of E-cadherin or PC2 (CT2) were cloned in-frame with histidine (His) and thioredoxin (Thio) in the pet32a+ vector (Novagen) and the constructs verified by sequencing. Fusion proteins were induced in BL21-DE3 with 1 mM isopropyl β-D-thiogalactopyranoside for 2 hours and purified from cleared bacterial lysates using amylose (New England Biolabs) or Nickel His-Select beads (Sigma) as described by the manufacturer. Affinity-purified recombinant proteins were visualized on Coomassie blue stained gels and immunoblotting with MBP and thioredoxin (Invitrogen) antibodies. Binding assays were performed by mixing 2 μg of MBP, MBP-CT1, MBP-CT1R4227X fusion protein attached to amylose beads with either 2 μg of His-Thio E-cadherin CT or His-Thio CT2 in binding buffer (5 mM Hepes, 150 mM NaCl, 1.5 mM MgCl2, 0.1 mM EDTA, 0.1% NP-40). Samples were rotated for 1.5 hours at 4°C, pelleted by centrifugation and washed three times with bead-binding buffer prior to elution.
This work was funded by the Wellcome Trust (GR071201). We thank O. Ibraghimov-Beskrovnaya, D. Garrod, N. Simmons, S. Ponnambalam, N. Fasel and D. Grundemann for kind gifts of reagents, and G. Howell (Leeds Bioimaging Facility) for technical assistance with confocal imaging. Portions of this work were presented at the American Society of Nephrology 2007 meeting, in San Francisco and the British Society for Cell Biology meeting on `Epithelial Morphogenesis and Diseases' (London, 8-10 October 2008). A.J.S. is a Research Councils UK Academic Fellow and A.C.M.O. is a Wellcome Trust Research Leave Senior Fellow. Deposited in PMC for release after 6 months.
References
- Adams, C. L. and Nelson, W. J. (1998). Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 10, 572-577. [DOI] [PubMed] [Google Scholar]
- Bach, M., Grigat, S., Pawlik, B., Fork, C., Utermohlen, O., Pal, S., Banczyk, D., Lazar, A., Schomig, E. and Grundemann, D. (2007). Fast set-up of doxycycline-inducible protein expression in human cell lines with a single plasmid based on Epstein-Barr virus replication and the simple tetracycline repressor. FEBS J. 274, 783-790. [DOI] [PubMed] [Google Scholar]
- Bernasconi, E., Fasel, N. and Wittek, R. (1996). Cell surface expression of a functional rubella virus E1 glycoprotein by addition of a GPI anchor. J. Cell Sci. 109, 1195-1201. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A. (2004). Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 14, 589-593. [DOI] [PubMed] [Google Scholar]
- Braga, V. M. (2002). Cell-cell adhesion and signalling. Curr. Opin. Cell Biol. 14, 546-556. [DOI] [PubMed] [Google Scholar]
- Calvet, J. P. and Grantham, J. J. (2001). The genetics and physiology of polycystic kidney disease. Semin. Nephrol. 21, 107-123. [DOI] [PubMed] [Google Scholar]
- Capaldo, C. T. and Macara, I. G. (2007). Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 18, 189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron, A. J., Nakamura, S., Bacallao, R. and Wandinger-Ness, A. (2000). Compromised cytoarchitecture and polarized trafficking in autosomal dominant polycystic kidney disease cells. J. Cell Biol. 149, 111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, J. and Wilson, P. D. (1995). Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am. J. Physiol. 269, C487-C495. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B., Stevenson, B. and Grimaldi, A. (1988). The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 107, 1575-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka, K., Qian, F., Boletta, A., Bhunia, A. K., Piontek, K., Tsiokas, L., Sukhatme, V. P., Guggino, W. B. and Germino, G. G. (2000). Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408, 990-994. [DOI] [PubMed] [Google Scholar]
- Hordijk, P. L., ten Klooster, J. P., van der Kammen, R. A., Michiels, F., Oomen, L. C. and Collard, J. G. (1997). Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science 278, 1464-1466. [DOI] [PubMed] [Google Scholar]
- Huan, Y. and van Adelsberg, J. (1999). Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J. Clin. Invest. 104, 1459-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, J., Ward, C. J., Peral, B., Aspinwall, R., Clark, K., San Millan, J. L., Gamble, V. and Harris, P. C. (1995). The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 10, 151-160. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya, O., Dackowski, W. R., Foggensteiner, L., Coleman, N., Thiru, S., Petry, L. R., Burn, T. C., Connors, T. D., Van Raay, T., Bradley, J. et al. (1997). Polycystin: in vitro synthesis, in vivo tissue expression, and subcellular localization identifies a large membrane-associated protein. Proc. Natl. Acad. Sci. USA 94, 6397-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya, O., Bukanov, N. O., Donohue, L. C., Dackowski, W. R., Klinger, K. W. and Landes, G. M. (2000). Strong homophilic interactions of the Ig-like domains of polycystin-1, the protein product of an autosomal dominant polycystic kidney disease gene, PKD1. Hum. Mol. Genet. 9, 1641-1649. [DOI] [PubMed] [Google Scholar]
- Jamora, C. and Fuchs, E. (2002). Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell Biol. 4, E101-E108. [DOI] [PubMed] [Google Scholar]
- Lantinga-van Leeuwen, I. S., Leonhard, W. N., van der Wal, A., Breuning, M. H., de Heer, E. and Peters, D. J. (2007). Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum. Mol. Genet. 16, 3188-3196. [DOI] [PubMed] [Google Scholar]
- Lickert, H., Bauer, A., Kemler, R. and Stappert, J. (2000). Casein kinase II phosphorylation of E-cadherin increases E-cadherin/beta-catenin interaction and strengthens cell-cell adhesion. J. Biol. Chem. 275, 5090-5095. [DOI] [PubMed] [Google Scholar]
- Marcozzi, C., Burdett, I. D., Buxton, R. S. and Magee, A. I. (1998). Coexpression of both types of desmosomal cadherin and plakoglobin confers strong intercellular adhesion. J. Cell Sci. 111, 495-509. [DOI] [PubMed] [Google Scholar]
- Markoff, A., Bogdanova, N., Knop, M., Ruffer, C., Kenis, H., Lux, P., Reutelingsperger, C., Todorov, V., Dworniczak, B., Horst, J. et al. (2007). Annexin A5 interacts with polycystin-1 and interferes with the polycystin-1 stimulated recruitment of E-cadherin into adherens junctions. J. Mol. Biol. 369, 954-966. [DOI] [PubMed] [Google Scholar]
- Muller, S. L., Portwich, M., Schmidt, A., Utepbergenov, D. I., Huber, O., Blasig, I. E. and Krause, G. (2005). The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J. Biol. Chem. 280, 3747-3756. [DOI] [PubMed] [Google Scholar]
- Nagafuchi, A., Shirayoshi, Y., Okazaki, K., Yasuda, K. and Takeichi, M. (1987). Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature 329, 341-343. [DOI] [PubMed] [Google Scholar]
- Nauli, S. M., Alenghat, F. J., Luo, Y., Williams, E., Vassilev, P., Li, X., Elia, A. E., Lu, W., Brown, E. M., Quinn, S. J. et al. (2003). Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129-137. [DOI] [PubMed] [Google Scholar]
- Newby, L. J., Streets, A. J., Zhao, Y., Harris, P. C., Ward, C. J. and Ong, A. C. (2002). Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex. J. Biol. Chem. 277, 20763-20773. [DOI] [PubMed] [Google Scholar]
- Nigam, S. K., Rodriguez-Boulan, E. and Silver, R. B. (1992). Changes in intracellular calcium during the development of epithelial polarity and junctions. Proc. Natl. Acad. Sci. USA 89, 6162-6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose, A., Nagafuchi, A. and Takeichi, M. (1988). Expressed recombinant cadherins mediate cell sorting in model systems. Cell 54, 993-1001. [DOI] [PubMed] [Google Scholar]
- Ong, A. C. (2000). Polycystin expression in the kidney and other tissues: complexity, consensus and controversy. Exp. Nephrol. 8, 208-214. [DOI] [PubMed] [Google Scholar]
- Ong, A. C. and Harris, P. C. (2005). Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney Int. 67, 1234-1247. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno, M., Jamora, C. and Fuchs, E. (2003). Sticky business: orchestrating cellular signals at adherens junctions. Cell 112, 535-548. [DOI] [PubMed] [Google Scholar]
- Ponnambalam, S., Rabouille, C., Luzio, J. P., Nilsson, T. and Warren, G. (1994). The TGN38 glycoprotein contains two non-overlapping signals that mediate localization to the trans-Golgi network. J. Cell Biol. 125, 253-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, F., Germino, F. J., Cai, Y., Zhang, X., Somlo, S. and Germino, G. G. (1997). PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 16, 179-183. [DOI] [PubMed] [Google Scholar]
- Qian, F., Boletta, A., Bhunia, A. K., Xu, H., Liu, L., Ahrabi, A. K., Watnick, T. J., Zhou, F. and Germino, G. G. (2002). Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc. Natl. Acad. Sci. USA 99, 16981-16986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitbak, T., Ward, C. J., Harris, P. C., Bacallao, R., Ness, S. A. and Wandinger-Ness, A. (2004). A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol. Biol. Cell 15, 1334-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, R. J., Husson, H., Joly, D., Bukanov, N. O., Patey, N., Knebelmann, B. and Ibraghimov-Beskrovnaya, O. (2005). Impaired formation of desmosomal junctions in ADPKD epithelia. Histochem. Cell Biol. 124, 487-497. [DOI] [PubMed] [Google Scholar]
- Scheffers, M. S., van der Bent, P., Prins, F., Spruit, L., Breuning, M. H., Litvinov, S. V., de Heer, E. and Peters, D. J. (2000). Polycystin-1, the product of the polycystic kidney disease 1 gene, co-localizes with desmosomes in MDCK cells. Hum. Mol. Genet. 9, 2743-2750. [DOI] [PubMed] [Google Scholar]
- Silberberg, M., Charron, A. J., Bacallao, R. and Wandinger-Ness, A. (2005). Mispolarization of desmosomal proteins and altered intercellular adhesion in autosomal dominant polycystic kidney disease. Am. J. Physiol. Renal Physiol. 288, F1153-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streets, A. J., Newby, L. J., O'Hare, M. J., Bukanov, N. O., Ibraghimov-Beskrovnaya, O. and Ong, A. C. (2003). Functional analysis of PKD1 transgenic lines reveals a direct role for polycystin-1 in mediating cell-cell adhesion. J. Am. Soc. Nephrol. 14, 1804-1815. [DOI] [PubMed] [Google Scholar]
- Streets, A. J., Moon, D. J., Kane, M. E., Obara, T. and Ong, A. C. (2006). Identification of an N-terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin-2 in vivo and in vitro. Hum. Mol. Genet. 15, 1465-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, V. E. and Harris, P. C. (2006). Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat. Clin. Pract. Nephrol. 2, 40-55; quiz 55. [DOI] [PubMed] [Google Scholar]
- van Adelsberg, J. (2000). Polycystin-1 interacts with E-cadherin and the catenins-clues to the pathogenesis of cyst formation in ADPKD? Nephrol. Dial. Transplant. 15, 1-2. [DOI] [PubMed] [Google Scholar]
- Vasioukhin, V. and Fuchs, E. (2001). Actin dynamics and cell-cell adhesion in epithelia. Curr. Opin. Cell Biol. 13, 76-84. [DOI] [PubMed] [Google Scholar]
- Wilson, P. D. (2004). Polycystic kidney disease: new understanding in the pathogenesis. Int. J. Biochem. Cell Biol. 36, 1868-1873. [DOI] [PubMed] [Google Scholar]
- Wilson, P. D., Sherwood, A. C., Palla, K., Du, J., Watson, R. and Norman, J. T. (1991). Reversed polarity of Na(+) -K(+) -ATPase: mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am. J. Physiol. 260, F420-F430. [DOI] [PubMed] [Google Scholar]
- Xu, G. M., Sikaneta, T., Sullivan, B. M., Zhang, Q., Andreucci, M., Stehle, T., Drummond, I. and Arnaout, M. A. (2001). Polycystin-1 interacts with intermediate filaments. J. Biol. Chem. 276, 46544-46552. [DOI] [PubMed] [Google Scholar]
- Yin, T. and Green, K. J. (2004). Regulation of desmosome assembly and adhesion. Semin. Cell Dev. Biol. 15, 665-677. [DOI] [PubMed] [Google Scholar]
- Yu, S., Hackmann, K., Gao, J., He, X., Piontek, K., Garcia Gonzalez, M. A., Menezes, L. F., Xu, H., Germino, G. G., Zuo, J. et al. (2007). Essential role of cleavage of Polycystin-1 at G protein-coupled receptor proteolytic site for kidney tubular structure. Proc. Natl. Acad. Sci. USA 104, 18688-18693. [DOI] [PMC free article] [PubMed] [Google Scholar]