Summary
Cue1p is an N-terminally anchored endoplasmic reticulum (ER) protein essential for the activity of the two major yeast RING finger ubiquitin ligases (E3s) implicated in ER-associated degradation (ERAD). Cue1p contains a CUE domain, which for several proteins is known to bind ubiquitin. We now establish that the CUE domain is dispensable for ERAD of substrates of both Hrd1p and Doa10p and that the Cue1p transmembrane domain is similarly not required for degradation of the Hrd1p substrate CPY*. Cue1p interacts with the ERAD E2 Ubc7p in vivo. We show that a discrete C-terminal Ubc7p binding region (U7BR) of Cue1p is required for ERAD and for Ubc7p-dependent ubiquitylation by Hrd1p in vitro. Strikingly, when Ubc7p is stabilized by direct anchoring to the ER membrane, the U7BR is sufficient to restore ERAD in cells lacking Cue1p. Thus, discrete E2 binding sites independent of ubiquitin ligase domains have the potential to activate ubiquitylation.
Keywords: ER-associated degradation, Proteasome, Ubiquitin, S. cerevisiae, CUE1, UBC7
Introduction
Endoplasmic reticulum (ER)-associated degradation (ERAD) represents the primary means by which proteins that are misfolded, unassembled or whose levels require regulation are targeted for degradation from the secretory pathway. ERAD is a highly complex and tightly regulated multi-step process with luminal, transmembrane and cytosolic components. Ubiquitylation and proteasomal degradation are critical features of ERAD. When either is inhibited substrates accumulate, often within the ER lumen or membrane (Kostova et al., 2007; Meusser et al., 2005; Nakatsukasa and Brodsky, 2008). In mammals at least five different transmembrane ubiquitin ligases (E3s) play roles in ERAD of various proteins, with implications for arthritis, renal carcinoma, regulation of cholesterol metabolism and in enhancing the metastatic potential of tumors (Brauweiler et al., 2007; Gao et al., 2008; Kostova et al., 2007; Lee et al., 2006; Song et al., 2005; Tsai et al., 2007). Among these E3s, the most extensively studied is gp78 or the tumor autocrine motility factor receptor (AMFR) (Chen et al., 2006; Fang et al., 2001). This polytopic RING finger E3 has a complex domain structure that, in addition to the RING finger, includes a ubiquitin-binding CUE domain and a discrete and specific binding region for the ERAD E2 Ube2g2, known as the G2BR (Chen et al., 2006); all of these domains are required for gp78 to function in cells.
In yeast the closest ortholog of gp78 is Hrd1p/Der3p. This archetypal ERAD E3 exists in a complex (HRD1 ligase) with a number of other proteins essential for its function in targeting misfolded proteins for degradation (Carvalho et al., 2006). This ligase also senses sterol levels and consequently regulates proteolysis of the ER resident enzyme, HMGCoA reductase (Gardner et al., 2001; Kostova et al., 2007; Meusser et al., 2005). Although Hrd1p shares with gp78 a polytopic membrane-spanning domain and a RING finger, it lacks both an intrinsic ubiquitin binding domain and evidence of an E2 binding domain distinct from its RING finger. However, both the HRD1 ligase and the other known yeast ERAD E3, the DOA10 complex, include a type I transmembrane protein known as Cue1p, which is required for their function (Carvalho et al., 2006). This protein includes a CUE domain, which binds ubiquitin much more weakly than CUE domains of yeast Vps9p and Cue2p, and mammalian Tollip and gp78 (Chen et al., 2006; Kang et al., 2003; Shih et al., 2003). Cue1p associates with the yeast ERAD E2 and Ube2g2 ortholog, Ubc7p. Expression of Cue1p is required to prevent the rapid degradation of Ubc7p and presumably also recruits this E2 to the ER membrane (Biederer et al., 1997). However, neither the region of Cue1p responsible for Ubc7p binding nor a relationship between E2 binding by Cue1p and Cue1p-dependent ERAD has been established.
We now report that unlike the CUE domain of gp78, the CUE domain of Cue1p is dispensable for ERAD and that Cue1p lacking its transmembrane anchor is sufficient to restore ERAD in Δcue1 cells. Furthermore, when Ubc7p is stabilized independently of Cue1p, by direct anchoring to the ER membrane, a discrete E2 binding region at the C terminus of Cue1p is sufficient to restore ERAD. Consistent with this activating role, this same region greatly enhances the ubiquitylation mediated by Hrd1p and Ubc7p in vitro.
Results
The CUE domain is dispensable for ERAD
To determine the domains within Cue1p required for ERAD we first focused on its only characterized region, the CUE domain. CUE domains were identified as a consequence of their similarity to a region of yeast Cue1p (Ponting, 2000). For several proteins this ∼40 amino acid domain is known to bind ubiquitin. In the case of gp78 it is required for its ligase function in vivo (Chen et al., 2006). Structural analyses of CUE domains from Cue2p and Vps9p (Kang et al., 2003; Prag et al., 2003) reveal a three alpha helical structure with overall similarity to the well-characterized UBA domain.
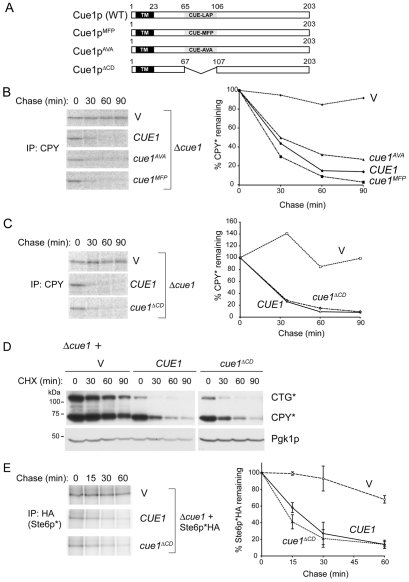
Unlike other CUE-domain proteins the ability of Cue1p to bind ubiquitin is extremely weak. This is contributed to by the lack of a Met-Phe-Pro (MFP) tripeptide at the boundary between the A and B alpha helices in ubiquitin-binding CUE domains. Mutation of the Leu-Ala-Pro (LAP) tripeptide in the corresponding position (amino acids 76-78) in Cue1p to MFP was reported to increase ubiquitin binding (Kang et al., 2003). We mutated the region encoding LAP in Cue1p to either MFP (Cue1pMFP) or altered it to potentially further disrupt the CUE domain structure by removing the conserved proline (Cue1pAVA; Fig. 1A). These proteins were expressed in a Δcue1 background from low copy CEN plasmids. When degradation of the commonly used Hrd1p ERAD substrate CPY* was assessed by metabolic labeling, neither mutation had an impact on Cue1p-dependent CPY* degradation (Fig. 1B).
Fig. 1.
The CUE domain of Cue1p is not necessary for in vivo ERAD. (A) Schematic representation of wild-type and CUE domain mutants of Cue1p with transmembrane (TM) and CUE domains indicated. The LAP sequence within the Cue1p CUE domain (residues 76-78) was mutated as indicated or the entire CUE domain was deleted. (B) 35S pulse-chase analysis of CPY* degradation in Δcue1 cells bearing vector (V), wild-type CUE1, cue1AVA or cue1MFP expressed from low copy plasmids. The graph on the right is quantification of pulse-chase data. (C) Pulse-chase analysis of CPY* in Δcue1 cells expressing vector containing no insert (V), CUE1 or cue1ΔCD. The graph on the right is quantification of pulse-chase data. (D) The Δcue1 strain bearing the chromosomal CPY* allele was co-transformed with a plasmid encoding membrane bound CTG* and the indicated wild-type or mutant CUE1-containing vectors. Degradation of CTG* and CPY* was assessed by cycloheximide (CHX) chase and immunoblotting using anti-CPY. Pgk1p (phosphoglycerate kinase) levels were assessed to determine relative protein loading. (E) Degradation of the DOA10 ligase substrate Ste6p*HA in Δcue1 with vector (V), CUE1 or cue1ΔCD plasmids was assessed by 35S pulse-chase labeling. Immunoprecipitation was with anti-HA antibody. Graphical representation of the Ste6p*HA degradation is shown on the right. Data are the means and standard deviations of two independent experiments.
Based on these findings we deleted the entire CUE domain (Cue1pΔCD). As with the point mutations, this was similarly without effect on CPY* degradation (Fig. 1C). Equivalent results were obtained at 23°C where degradation proceeds more slowly (not shown). A similar lack of requirement for the Cue1p CUE domain was found when CTG*, a transmembrane form of CPY* (Taxis et al., 2003), was expressed in a Δcue1 strain expressing the CPY* allele from its chromosomal locus (Fig. 1D). To evaluate whether the CUE domain was also dispensable for Doa10p-mediated ERAD we evaluated the degradation of Ste6p*, a primarily DOA10 ligase substrate (Huyer et al., 2004). Again, deletion of the CUE domain had no effect on Ste6p* degradation (Fig. 1E). Thus, the Cue1p CUE domain has no discernable role in the function of either ERAD E3. One possible explanation is that this domain is playing a subtle role in ERAD not evident with these substrates. However, no difference was observed between Cue1p and Cue1pΔCD re-expression when ER stress was induced either pharmacologically in a Δcue1 strain by tunicamycin or genetically by deletion of the ER stress response gene IRE1 (not shown).
A C-terminal Ubc7p binding region (U7BR) in Cue1p required for ERAD
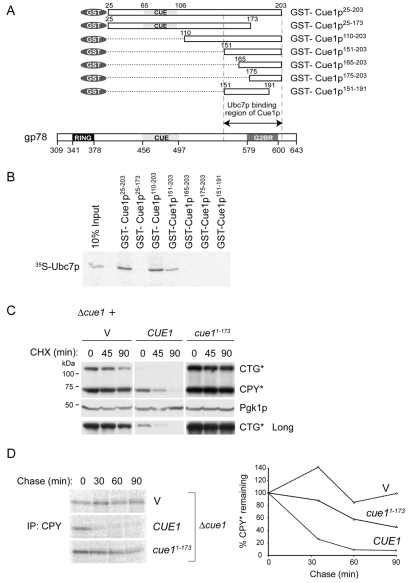
We next evaluated the requirements for Cue1p binding to Ubc7p in vitro using N-terminal GST fusions (Fig. 2A). A truncation that removed the membrane-proximal region of Cue1p, as well as the CUE domain (Cue1p110-203), bound Ubc7p as effectively as the full cytoplasmic domain (Cue1p25-203; Fig. 2B). Moreover, N-terminal deletions up to amino acid 151 retained Ubc7p binding in vitro whereas deletions beyond that point, as well as C-terminal truncations, abrogated binding. This establishes a Ubc7p binding region (U7BR) between amino acids 151 and 203 of Cue1p. To address whether Ubc7p binding is required in vivo, Cue1p1-173 was expressed in a Δcue1 strain. This protein is stable and membrane anchored (not shown). Cue1p1-173 was unable to restore ERAD of either CPY* or CTG* as determined both by cycloheximide chase and pulse-chase metabolic labeling (Fig. 2C,D). As expression of CTG* was lower than CPY* under wild-type ERAD conditions, a longer exposure of CTG* is shown to allow for the visualization of this protein (Fig. 2C, lower panel). Similar results were obtained with the DOA10 substrate Ste6p* (not shown). Importantly however, as with the Δcue1 strain, yeast reconstituted with Cue1p1-173 exhibited marked instability of Ubc7p (Fig. 5A, lower right panel; supplementary material Fig. S2), consistent with its inability to bind the E2 in vitro (Fig. 2B).
Fig. 2.
The C-terminal region of Cue1p binds Ubc7p and is necessary for ERAD. (A) Schematic representation of GST fusions of Cue1p tested for Ubc7p binding. Shown below for comparison is the C-terminal cytoplasmic domain of gp78 showing the position of the RING finger, CUE domain and Ube2G2-binding region (G2BR). For both, numbering corresponds to positions in the full-length proteins. (B) 35S-labeled in vitro translated Ubc7p was incubated with similar amounts of the indicated GST-Cue1p fusion proteins prebound to glutathione-Sepharose beads (supplementary material Fig. S1). After washing, bound material was resolved by SDS-PAGE. Ten percent of the input used in binding assays is shown. (C) The Δcue1 strain bearing the chromosomal CPY* allele was co-transformed with a plasmid encoding membrane bound CTG* and vectors expressing either wild-type CUE1 or the C-terminal truncation, cue11-173. Degradation of CTG* and CPY* was assessed by cycloheximide (CHX) chase and immunoblotting using anti-CPY. Pgk1p was used as a loading control. A longer exposure of the CTG* immunoblot is shown to show expression and degradation of CTG* in the wild-type strain. (D) 35S pulse-chase analysis of degradation of CPY* in Δcue1 cells expressing vector (V), CUE1 or cue11-173 (vector and CUE1 controls are identical to those in Fig. 1C).
Fig. 5.
The U7BR of Cue1p is necessary and sufficient for ERAD. (A) Δcue1 cells were co-transformed with plasmids encoding the indicated proteins and CPY* degradation monitored by cycloheximide (CHX) chase. Expression of Ubc7pHA and its rapid degradation in cells expressing cue11-173 is apparent on longer exposures (supplementary material Fig. S2). (B) Degradation of CPY* and stability of Ubc7pHA in the double null Δcue1Δubc7 strain expressing ™Ubc7pHA alone or together with the indicated regions of Cue1p. (C) Graph of 35S pulse-chase analysis of CPY* degradation in Δcue1Δubc7 cells expressing ™Ubc7pHA and the indicated forms of Cue1p. Data are the means and s.d. of two independent experiments.
The Cue1p transmembrane domain is not required for ERAD of CPY*
To address the requirements for stabilization of Ubc7p and how this correlates with ERAD, a Δcue1Δubc7 strain was generated and reconstituted with C-terminal HA-tagged Ubc7p (Ubc7pHA) and various forms of Cue1p, all of which lack a membrane anchor. Consistent with previous findings (Ravid and Hochstrasser, 2007), Cue1p24-203, which fractionated into the cytosolic fraction (not shown), stabilized Ubc7pHA (Fig. 3A, bottom panel). In sharp contrast, Cue1p151-203, which includes only the U7BR, resulted in a markedly lower initial level and rapid degradation of Ubc7p whereas Cue1p110-203 substantially stabilized Ubc7p. Unexpectedly Cue1p24-203 not only stabilized Ubc7p but also reconstituted CPY* degradation (Fig. 3A, upper panel). This was confirmed in a Δcue1 strain where expression of cytoplasmic Cue1p24-203 or of full length Cue1p provided similar results (Fig. 3B). By contrast, Cue1p151-203 did not reconstitute CPY* degradation, as expected because of loss of Ubc7p (Fig. 3A). Variable results were found with Cue1p110-203: CPY* either remained relatively stable or was degraded with delayed kinetics, perhaps reflecting the incomplete stabilization of Ubc7p (Fig. 3A).
Fig. 3.
Soluble Cue1p can restore in vivo ERAD and stabilize Ubc7p. (A) Cycloheximide (CHX) chase assays were performed to determine degradation of CPY* and stability of Ubc7pHA in Δcue1Δubc7 expressing Ubc7pHA together with the indicated regions of Cue1p. A longer exposure of the HA immunoblot is also shown to demonstrate that Ubc7pHA is expressed under all conditions. (B) Δcue1 cells were transformed with plasmids containing no CUE1 gene (V), CUE1 or cue124-203. Degradation of CPY* was assayed by cycloheximide chase analysis at the indicated times. Pgk1p was used as loading control.
U7BR alone can activate ERAD
To dissociate issues of E2 stabilization from ERAD and to explore the significance of membrane recruitment of Ubc7p we directly anchored Ubc7p to the ER membrane (™Ubc7pHA) by fusing C-terminally HA-tagged Ubc7p to amino acids 1-44 of Cue1p (Fig. 4A). ™Ubc7pHA was stabilized when expressed in a Δcue1 strain, in contrast to Ubc7pHA, which is known to be degraded in the absence of Cue1p (Fig. 4B). ™Ubc7pHA encoded a functional E2 as degradation of CPY* proceeded at comparable rates in Δubc7 cells expressing either Ubc7pHA or ™Ubc7pHA (Fig. 4C). Thus, direct membrane anchoring does not interfere with the function of Ubc7p. However, when assessed in a Δcue1Δubc7 strain, ™Ubc7pHA was insufficient to restore CPY* degradation in the absence of Cue1p (Fig. 4D, left side). These findings suggest that the function of Cue1p cannot be explained simply by membrane recruitment and stabilization of Ubc7p.
Fig. 4.
Membrane tethered Ubc7p requires Cue1p to restore ERAD. (A) Schematic representation of soluble (Ubc7pHA) and membrane tethered (™Ubc7pHA) Ubc7p. Ubc7p was C-terminally HA-tagged (black box). Residues 1-44 of Cue1p, containing the N-terminal transmembrane (TM) domain plus ∼20 cytosolic residues, were N-terminally fused to Ubc7pHA to generate ™Ubc7pHA. (B) Δcue1 cells were transformed with plasmids expressing ™Ubc7pHA or Ubc7pHA. Stability of ™Ubc7pHA was determined by cycloheximide (CHX) chase. (C) Δubc7 cells expressing CPY* from the endogenous locus were transformed with empty vector (V) or vectors encoding Ubc7pHA or ™Ubc7pHA and degradation of CPY* assessed by 35S pulse-chase. Graphical representation of CPY* degradation is shown on right. Data are the means and s.d. of two independent experiments. (D) Δcue1Δubc7 cells transformed with plasmids encoding ™Ubc7pHA or Ubc7pHA and Cue1p were assessed for CPY* degradation by cycloheximide chase.
Having now generated a stable, membrane anchored, active Ubc7p we re-evaluated the capacity of Cue1p1-173 to restore ERAD. Even when Ubc7p is membrane bound and stably expressed, Cue1p lacking an intact U7BR failed to reconstitute ERAD (Fig. 5A). This demonstrates the importance of Ubc7p binding to Cue1p, independent of both membrane recruitment and stabilization of this E2. Given the apparent dispensability of the CUE domain and the necessity of the E2 binding site for the function of membrane-anchored Cue1p, we asked whether truncated forms of Cue1p, lacking the transmembrane domain, could restore ERAD in cells expressing stable membrane anchored ™Ubc7pHA. Both Cue1p151-203 and Cue1p110-203 as well as Cue1p24-203 reconstituted ERAD of CPY* in the Δcue1Δubc7 strain, as assessed by both cycloheximide chase (Fig. 5B) and pulse-chase metabolic labeling (Fig. 5C). This establishes that at least when the E2 is stabilized by tethering to the ER membrane, the C-terminal U7BR of Cue1p is sufficient to reconstitute Ubc7p-dependent ERAD.
U7BR activates ubiquitylation of Ubc7p-Hrd1pC
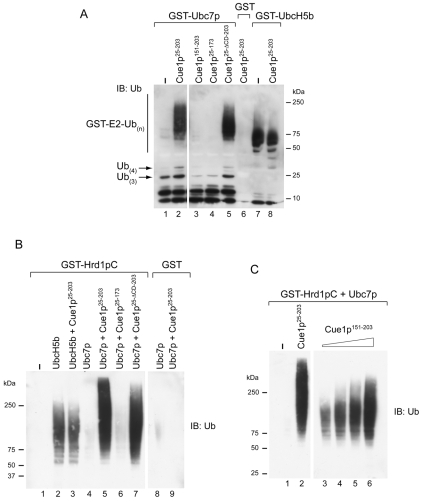
Bazirgan and Hampton have recently shown (Bazirgan and Hampton, 2008) that the complete cytoplasmic domain of Cue1p can activate Ubc7p in vitro, in the absence of a known ligase domain, enhancing the known potential of this E2 to form polyubiquitin chains that can be thiolester linked to the active site cysteine of Ubc7p (Ravid and Hochstrasser, 2007). To corroborate and extend these findings, in vitro ubiquitylation assays were employed using components that were independently expressed in bacteria and purified. In Fig. 6A various fragments of Cue1p were added to glutathione-Sepharose-immobilized GST-Ubc7p and ubiquitylation reactions carried out without added E3. Ubiquitin that remained bound to immobilized GST-Ubc7p after washing of beads was analyzed following elution of bead-bound material by heating in SDS-PAGE sample buffer containing reducing agent to disrupt thiolester bonds. As reported (Bazirgan and Hampton, 2008), Cue1p25-203 resulted in an increase in chains of three or four ubiquitins (Fig. 6A, compare lower molecular mass regions of lanes 1 and 2). Most striking, however, was a dramatic increase in ubiquitin conjugated to GST-Ubc7p in a stable, non-reducible form when Cue1p25-203 was added to the reaction. Importantly, no substantial Cue1p25-203-induced activation was seen with GST-UbcH5b, a close relative of the yeast E2s Ubc4p and Ubc5p (Fig. 6A, lanes 7 and 8) (Hatakeyama et al., 1997).
Fig. 6.
Cue1p151-203 is sufficient to enhance Ubc7p activity in vitro. (A) Equimolar amounts of GST, GST-Ubc7p or GST-UbcH5b were pre-bound to glutathione-Sepharose, and the indicated purified fragments of Cue1p were added to the reaction mix containing ubiquitin and recombinant human E1. For Cue1p25-203, Cue1p25-173 and Cue1p25-ΔCD-203 30 pmol was used in each reaction, whereas for Cue1p151-203 240 pmol was used. Following incubation at 30°C, beads were extensively washed and material bound to glutathione-Sepharose was eluted by heating in SDS-PAGE buffer containing β-mercaptoethanol to remove thiolester-linked ubiquitin from the GST fusions, resolved by SDS-PAGE and immunoblotted with anti-ubiquitin. (B) E3-dependent ubiquitylation reactions were performed using GST-Hrd1pC (cytoplasmic domain) pre-bound to glutathione-Sepharose, purified Ubc7p or UbcH5b and 30 pmol of the Cue1p fragments. Samples were treated and analyzed as in A. (C) Reactions were performed essentially as in B, but with increasing amounts of Cue1p151-203. The amount of Cue1p25-203 used was 30 pmols, whereas the amount of Cue1p151-203 ranged from ∼60 pmol (lane 3) to 360 pmols (lane 6).
Consistent with our in vivo findings, the ability to activate Ubc7p did not require the CUE domain (Cue1p25-ΔCD-203) but did require an intact U7BR (compare lanes 2, 4 and 5). Notably, despite the ability of Cue1p151-203 to restore ERAD in cells, the U7BR was insufficient to cause this dramatic in vitro activation of GST-Ubc7p even at eight-fold higher concentration than the other Cue1p fragments (Fig. 6A, lane 3). Qualitatively similar results were obtained when ubiquitylation was assessed using soluble Ubc7p and immobilized GST fusions of Cue1p (not shown).
E3-dependent ubiquitylation was then evaluated as described (Fang et al., 2001) using a GST fusion of the cytoplasmic RING-finger-containing domain of Hrd1p (Hrd1pC), soluble Ubc7p and forms of Cue1p that were expressed and purified separately. Consistent with previous findings (Bazirgan and Hampton, 2008), Cue1p25-203 activated Hrd1pC-mediated ubiquitylation (Fig. 6B). As with the E3-independent ubiquitylation, this was not observed with UbcH5b (lanes 2 and 3), was independent of the CUE domain and absolutely dependent on the U7BR (lanes 5-7). However, unlike the E3 independent reaction, the U7BR of Cue1p (Cue1p151-203) activated ubiquitylation in a dose-dependent manner, albeit to a lesser extent than the full cytoplasmic domain (Fig. 6C). This finding provides an in vitro correlate for the capacity of the U7BR (Cue1p151-203) to restore Ubc7p-dependent ERAD in vivo.
Discussion
In this study we explored the requirements for Cue1p to function in ERAD and to activate ubiquitylation in vitro. We found the CUE domain to be dispensable for degradation of substrates of the two major ERAD E3s. It was also unnecessary for Cue1p to rescue cells from ER stress and was not required for binding of Cue1p to Ubc7p. We have also evaluated binding of both Cue1p and its isolated CUE domain to four ubiquitin-like domains containing proteins implicated in ERAD, namely Rad23p, Dsk2p, Sel1p and Usa1p. By GST pulldown we did not see any evidence of binding (Z.K. and A.M.W., unpublished observations). Thus, our findings raise the possibility that the CUE domain is vestigial, redundant or required for processes other than ERAD.
We also assessed the requirements for binding of Ubc7p to Cue1p. Analogous to gp78, a discrete domain at the C terminus of the protein, the Ubc7p binding region (U7BR), between residues 151-203, was sufficient for binding. Consistent with this we found the U7BR necessary for stabilization of Ubc7p by Cue1p in vivo. However, at least when Cue1p is expressed without its membrane anchor more N-terminal regions of Cue1p are required to prevent Ubc7p from being degraded. Most important was the striking observation that the isolated U7BR could activate ERAD, as assessed by the degradation of CPY*, when Ubc7p is stabilized by tethering to the ER membrane.
The activating role played by the U7BR in vivo was corroborated by in vitro ubiquitylation assays in which the U7BR activated ubiquitylation, mediated by Hrd1pC and Ubc7p in a dose-dependent manner. It is also apparent, however, that including more N-terminal regions of the protein substantially increases ubiquitylation and is required for the E3-independent activation of Ubc7p by Cue1p, as demonstrated by Bazirgan and Hampton (Bazirgan and Hampton, 2008). As for in vivo activation, the CUE domain was completely dispensable for in vitro activation. Determination of how the U7BR facilitates ubiquitylation by Ubc7p will require further study. Similarly, whether the inclusion of more N-terminal sequences serves to conform this domain in a manner that optimizes activation, or if there are unappreciated additional interactions between Ubc7p and regions of Cue1p proximal to amino acid 151 remains to be determined. In this regard it is noteworthy that although the U7BR clearly reconstitutes ERAD with stable membrane-bound Ubc7p, the inclusion of amino acids 110-150 results in enhancement in the rate of CPY* degradation in some experiments (Fig. 5B).
As noted above, Bazirgan and Hampton recently reported both Hrd1p-independent and -dependent activation of Ubc7p in vitro and they also explored in vivo functions (Bazirgan and Hampton, 2008). Their in vivo studies also provided evidence that cytoplasmic Cue1p could activate a chimeric Ubc7p to function with a non-ERAD E3. Their study was, however, limited to an assessment of the functions of the complete cytoplasmic domain of Cue1p (analogous to our Cue1p24-203) and thus comparisons between the two studies are limited. For the most part our findings with this extended region of Cue1p, using a transmembrane form of Ubc7p with a short linker to the ER membrane and employing different assays systems and substrates (CPY* versus Hmg2-GFP), yielded qualitatively similar results to those of Bazirgan and Hampton. However, one major difference was our finding that ERAD of CPY* can be reconstituted when both Ubc7p and Cue1p are expressed from their endogenous promoters with neither protein having a transmembrane anchor. Bazirgan and Hampton reported no such reconstitution. Whether this reflects differences between substrates or other technical issues cannot be discerned. Regardless, our findings with soluble Cue1p and soluble Ubc7p underscore that activation of ERAD by Cue1p can occur independent of its role in recruitment of Ubc7p to the ER membrane by membrane-anchored Cue1p.
The findings presented herein provide new insights into a critical component of the ERAD machinery. Most importantly, we have now identified a discrete binding site for Ubc7p at the C terminus of Cue1p that is sufficient to reconstitute ERAD when Ubc7p is stabilized at the ER membrane and which, by itself, activates ubiquitylation mediated by Ubc7p-Hrd1p. Thus, in addition to stabilizing and possibly increasing the local concentration of Ubc7p at the ER membrane, Cue1p through the U7BR, plays a critical role in activating Ubc7p through as yet unknown mechanisms (Fig. 7). The exact means by which this occurs will require further structural analysis.
Fig. 7.
Schematic representation of Ubc7p-Cue1p interaction. Association with the C-terminal U7BR of Cue1p leads to activation of ubiquitin-bound Ubc7p, which, in the presence of an active RING finger domain on the E3 ligase Hrd1p, allows polyubiquitylation of substrates. The CUE domain of Cue1p is dispensable for activation of Ubc7p and ERAD and the transmembrane anchor of Cue1p is similarly not required, at least for the test substrate CPY*. The C-terminal 151-203 amino acids of Cue1p (U7BR), however, mediate the interaction with Ubc7p and are both necessary and sufficient for in vivo ERAD and in vitro polyubiquitylation. In the schematic the asterisk indicates Ubc7∼Ub activated by Cue1p. S∼Ub4 represents a polyubiquitylated substrate undergoing movement out of the ER through a putative `retrotranslocon'.
Beyond ERAD it is intriguing to consider our findings in the context of ubiquitylation in general. Proteins, including the E2-like protein Mms2p, can interact with specific E2s and stimulate the formation of specific polyubiquitin linkages (Christensen et al., 2007; Hofmann and Pickart, 1999). Additionally, E2 binding sites distinct from ligase domains exist in E3s including Ubr1p, gp78 and Nedd4 (Chen et al., 2006; Hatakeyama et al., 1997; Madura et al., 1993). How widespread these discrete binding sites for E2s are, the degree to which they exist in the context of multi-subunit ubiquitin ligase complexes and to what extent they serve roles in activation independent of E2 binding now become important issues for understanding ubiquitin ligase function.
Materials and Methods
Yeast strains
Yeast cells were cultured at 30°C in YPD or SD medium supplemented as necessary. The Saccharomyces cerevisiae strains Δcue1 (Plemper et al., 1999), Δubc7 (Hiller et al., 1996) and Δire1 (Taxis et al., 2002) are derived from W303-1C (MATa, ura3-1, leu2-3,112, his3-11,15, ade2-1, trp1-1, can1-100, prc1-1) and were a generous gift from Dieter H. Wolf. Mating of the respective haploid strains generated Δcue1Δubc7 and Δcue1Δire1. Yeast transformations were carried out using standard methods.
Plasmid constructs
A ∼2 kb fragment derived from pGEM5Z-27k (from Thomas Sommer) spanning the wild-type CUE1 gene and promoter was subcloned into the BamHI site of pRS314 and pRS316 to yield pRS314-CUE1 and pRS316-CUE1, respectively. The cue1AVA, cue1MFP, cue1ΔCD, cue11-173 and cue124-203 (superscripts indicate amino acids name or numbers) mutants were created using Stratagene's QuikChange® XL Site-Directed Mutagenesis Kit. A 1.2 kb SacII-ClaI fragment of UBC7 was PCR amplified from yeast genomic DNA and cloned into pRS314 and a C-terminal HA tag was subsequently introduced by PCR. pRS316-™UBC7HA was created by replacing the CUE1 sequence in pRS316-CUE1 starting from the Bsu36I site (corresponding to amino acid 44 of Cue1p) with PCR amplified UBC7HA. PCR-amplified DNA fragments corresponding to cue1110-203 and cue1151-203 were inserted behind the CUE1 promoter into NcoI-ClaI-digested pRS314CUE1, thus replacing the wild-type CUE1 sequence. To generate pRS424-GFPcue1110-203 and pRS424-GFPcue1151-203, the GFP gene was PCR amplified from pCTG*, inserted between the CUE1 promoter and the cue1110-203 or cue1151-203 coding regions of pRS314-GFPcue1110-203 or pRS314-GFPcue1151-203, respectively, then transferred into pRS424. pSM1911 (2 μ, URA3, PPGK::ste6-166::HA), encoding Ste6p*HA, was a gift from Susan Michaelis (Huyer et al., 2004). pCTG* (pRS316-PCUE1::prc1-1::pdr54332-4532::GFP), obtained from Dieter H. Wolf, encodes the membrane-bound and GFP-tagged CPY* variant CTG* (Taxis et al., 2003) expressed from the CUE1 promoter (Kostova and Wolf, 2005). Constructs for bacterial protein expression of the various Cue1p mutants, Hrd1pC, Ubc7p and UbcH5b were generated by PCR amplification of the respective genes and ligation into pGEX-4T-1 or pGEX-KG (Amersham Biosciences). The identity of all constructs was determined by sequencing of the individual clones. Further cloning details are available upon request. Plasmid constructs used in this study are summarized in Table 1.
Table 1.
Summary of the yeast and bacterial expression plasmids used in this study
| Protein product | Reference | |
|---|---|---|
| Yeast expression plasmids | ||
| pRS316 (CEN/URA3) | Sikorski and Hieter, 1989 | |
| pRS316-CUE1 | Cue1p | This study |
| pRS316-cue1AVA | Cue1pAVA | This study |
| pRS316-cue1MFP | Cue1pMFP | This study |
| pRS316-cue1ΔCD | Cue1pΔCD | This study |
| pRS316-cue11-173 | Cue1p1-173 | This study |
| pRS314 (CEN/TRP1) | Sikorski and Hieter, 1989 | |
| pRS314-CUE1 | Cue1p | This study |
| pRS314-cue1ΔCD | Cue1pΔCD | This study |
| pRS314-cue11-173 | Cue1p1-173 | This study |
| pRS314-cue124-203 | Cue1p24-203 | This study |
| pRS314-cue1110-203 | Cue1p110-203 | This study |
| pRS314-cue1151-203 | Cue1p151-203 | This study |
| pRS424 (2 μ/TRP1) | Sikorski and Hieter, 1989 | |
| pRS424-GFPcue1110-203 | GFP-Cue1p110-203 | This study |
| pRS424-GFPcue1151-203 | GFP-Cue1p151-203 | This study |
| pRS316-UBC7HA | Ubc7pHA | This study |
| pRS316-™ UBC7HA | ™Ubc7pHA | This study |
| pCTG* | CTG* | Kostova and Wolf, 2005 |
| pSM1911 | Ste6p*HA | Huyer et al., 2004 |
| Bacterial expression plasmids | ||
| pGEX-4T-1 | GST | Amersham Biosciences |
| pGEX-4T-1cue125-203 | GST-Cue1p25-203* | This study |
| pGEX-4T-1cue125-173 | GST-Cue1p25-173* | This study |
| pGEX-4T-1cue1110-203 | GST-Cue1p110-203 | This study |
| pGEX-4T-1cue1151-203 | GST-Cue1p151-203* | This study |
| pGEX-4T-1cue1165-203 | GST-Cue1p165-203 | This study |
| pGEX-4T-1cue1175-203 | GST-Cue1p175-203 | This study |
| pGEX-4T-1cue1151-191 | GST-Cue1p151-191 | This study |
| pGEX-4T-1cue125-ΔCD-203 | GST-Cue1p25-ΔCD-203* | This study |
| pGEX-4T-1hrd1207-551 | GST-Hrd1pC | This study |
| pGEX-4T-1UBC7 | GST-Ubc7p* | This study |
| pDEST47-UBC7 | Ubc7p | This study |
| pGEX-KG-UBCH5b | GST-UbcH5b | This study |
Letters and numbers shown in superscript, indicate amino acid name and positions in Cue1p. Protein products denoted with an asterisk were used both in their GST-fused and thrombin-cleaved forms.
Cycloheximide chase
Yeast cells were grown to late-log phase in selective media. Cycloheximide was added to a concentration of 50 μg/3 OD600 of cells and aliquots of 3 OD600 equivalents were removed into an equal volume of 30 mM NaN3 at the indicated time points. Cell extracts were prepared by alkaline lysis (1.85 M NaOH, 7.5% β-mercaptoethanol) on ice for 10 minutes with intermittent vortexing. Following TCA precipitation, the protein pellet was resuspended in 100 μl urea sample buffer (8 M urea, 5% SDS, 200 mM Tris-HCl pH 6.8, 0.1 mM EDTA, 0.03% Bromophenol blue, 1.5% β-ME) by shaking at 37°C. Proteins separated by SDS-PAGE were analyzed by immunoblotting with the appropriate antibodies. Pgk1p (phosphoglycerate kinase) was used to assess loading.
In vivo labeling and immunoprecipitation
Yeast strains grown in selective media to logarithmic phase were labeled for 20 minutes (CPY* and CTG*) or 10 minutes (Ste6p*) with 20 μCi/1 OD600 Easytag [35S]methionine (Perkin Elmer). The chase was initiated by addition of an excess of non-radioactive methionine and 2.5 OD600 equivalents were removed into ice-cold TCA at the indicated time points. Cells were broken by glass bead lysis in breaking buffer (6 M urea, 50 mM Tris-HCl pH 7.5, 1% SDS, 1 mM EDTA) then diluted tenfold with IP buffer (50 mM Tris-HCl pH 7.5, 190 mM NaCl, 6 mM EDTA, 1.25% Triton X-100). Following removal of cell debris, the supernatant was incubated with anti-CPY antibodies for 1 hour at 4°C and immunoprecipitated using Protein A-Sepharose 4B (Zymed) beads for 1 hour at 4°C. Immunoprecipitated material was washed with IP buffer, eluted in urea sample buffer and analyzed by autoradiography following SDS-PAGE. Labeling and immunoprecipitation of Ste6p*HA was done according to the procedure described by Huyer et al. (Huyer et al., 2004). Radioactive material was quantified using Storm Phosphorimager and ImageQuant software (GE Health Care Life Sciences).
In vitro binding assays
GST-Cue1p truncation mutants (schematized in Fig. 2A) were expressed in E. coli BL21 cells and quantified by Coomassie Blue staining (supplementary material Fig. S1). Ubc7p was in vitro translated from pDEST47-UBC7 in a rabbit reticulocyte lysate system (Promega) using [35S]methionine (GE Healthcare). Binding assays were carried out overnight at 4°C by incubating the GST-Cue1p proteins prebound to glutathione-Sepharose™ 4B beads (Amersham Pharmacia Biosciences) with 35S-labeled Ubc7p in binding buffer (25 mM Tris-HCl pH 7.4, 50 mM NaCl, 5 mM DTT, 0.5% NP-40). Beads were then washed with binding buffer, eluted from beads, resolved by SDS-PAGE and visualized using a Storm Phosphorimager.
In vitro ubiquitylation assays
For the E3-independent ubiquitylation assays GST-Ubc7p and GST-UbcH5b expressed in E. coli BL21 were bound to glutathione-Sepharose beads. Free Cue1 proteins were generated by thrombin cleavage of the respective GST-fusions. Protein concentrations were calculated by Coomassie staining and densitometry, by comparison to BSA standards. Approximately 80 pmol GST-Ubc7p or GST-UbcH5b and 150 pmol GST were incubated with the Cue1 proteins (30 pmol of Cue1p25-203, Cue1p25-173 or Cue1p25-ΔCD-203 and 240 pmol Cue1p151-203) for 90 minutes at 30°C in 50 μl reactions containing 50 nM human E1 (Boston Biochem) and 1 μg ubiquitin (Sigma) in 1× ubiquitylation buffer (50 mM Tris-HCl pH 7.4, 0.2 mM ATP, 0.5 mM MgCl2, 0.1 mM DTT, 1 mM phosphocreatine). For ubiquitylation reactions in the presence of E3, GST-Hrd1pC was expressed in bacteria and bound to glutathione-Sepharose beads. 100 μl reactions were assembled in 1× ubiquitylation buffer containing 5 pmol GST-Hrd1pC, thrombin-cleaved Ubc7p or UbcH5b (∼30 pmol) and Cue1 proteins (∼30 pmol except were indicated), human E1 (30 nM), and ubiquitin (1 μg). Following incubation, beads were washed in 1× TBS and bound material was eluted in SDS-reducing sample buffer. Reaction products were analyzed by SDS-PAGE and anti-ubiquitin immunoblotting.
Antibodies
Mouse monoclonal anti-CPY and anti-PGK (Molecular Probes), and rat monoclonal anti-HA-peroxidase (Roche) were used for immunoblotting according to the manufacturers' recommendations. Rabbit polyclonal anti-CPY (Rockland) and rat monoclonal anti-HA Affinity Matrix (Roche) were used for immunoprecipitation. Rabbit polyclonal anti-Cue1p and anti-ubiquitin antibodies were generated by immunizing rabbits with bacterially expressed cytosolic Cue1p25-203 or with ubiquitin, respectively.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/9/1374/DC1
We are grateful to Jeffrey L. Brodsky, Susan Michaelis, Thomas Sommer and Dieter H. Wolf for kindly supplying reagents. We thank Michael R. Kuehn, Michael H. Glickman, Mary E. Perry, Mickael M. Cohen and Yien Che Tsai for helpful discussions. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. Deposited in PMC for release after 12 months.
References
- Bazirgan, O. A. and Hampton, R. Y. (2008). Cue1p is an activator of Ubc7p E2 activity in vitro and in vivo. J. Biol. Chem. 283, 12797-12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer, T., Volkwein, C. and Sommer, T. (1997). Role of Cue1p in ubiquitination and degradation at the ER surface. Science 278, 1806-1809. [DOI] [PubMed] [Google Scholar]
- Brauweiler, A., Lorick, K. L., Lee, J. P., Tsai, Y. C., Chan, D., Weissman, A. M., Drabkin, H. A. and Gemmill, R. M. (2007). RING-dependent tumor suppression and G2/M arrest induced by the TRC8 hereditary kidney cancer gene. Oncogene 26, 2263-2271. [DOI] [PubMed] [Google Scholar]
- Carvalho, P., Goder, V. and Rapoport, T. A. (2006). Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126, 361-373. [DOI] [PubMed] [Google Scholar]
- Chen, B., Mariano, J., Tsai, Y. C., Chan, A. H., Cohen, M. and Weissman, A. M. (2006). The activity of a human endoplasmic reticulum-associated degradation E3, gp78, requires its Cue domain, RING finger, and an E2-binding site. Proc. Natl. Acad. Sci. USA 103, 341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, D. E., Brzovic, P. S. and Klevit, R. E. (2007). E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat. Struct. Mol. Biol. 14, 941-948. [DOI] [PubMed] [Google Scholar]
- Fang, S., Ferrone, M., Yang, C., Jensen, J. P., Tiwari, S. and Weissman, A. M. (2001). The tumor autocrine motility factor receptor, gp78, is a ubiquitin protein ligase implicated in degradation from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 98, 14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, B., Lee, S. M., Chen, A., Zhang, J., Zhang, D. D., Kannan, K., Ortmann, R. A. and Fang, D. (2008). Synoviolin promotes IRE1 ubiquitination and degradation in synovial fibroblasts from mice with collagen-induced arthritis. EMBO Rep. 9, 480-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, R. G., Shearer, A. G. and Hampton, R. Y. (2001). In vivo action of the HRD ubiquitin ligase complex: mechanisms of endoplasmic reticulum quality control and sterol regulation. Mol. Cell. Biol. 21, 4276-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama, S., Jensen, J. P. and Weissman, A. M. (1997). Subcellular localization and ubiquitin-conjugating enzyme (E2) interactions of mammalian HECT family ubiquitin protein ligases. J. Biol. Chem. 272, 15085-15092. [DOI] [PubMed] [Google Scholar]
- Hiller, M. M., Finger, A., Schweiger, M. and Wolf, D. H. (1996). ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science 273, 1725-1728. [DOI] [PubMed] [Google Scholar]
- Hofmann, R. M. and Pickart, C. M. (1999). Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96, 645-653. [DOI] [PubMed] [Google Scholar]
- Huyer, G., Piluek, W. F., Fansler, Z., Kreft, S. G., Hochstrasser, M., Brodsky, J. L. and Michaelis, S. (2004). Distinct machinery is required in Saccharomyces cerevisiae for the endoplasmic reticulum-associated degradation of a multispanning membrane protein and a soluble luminal protein. J. Biol. Chem. 279, 38369-38378. [DOI] [PubMed] [Google Scholar]
- Kang, R. S., Daniels, C. M., Francis, S. A., Shih, S. C., Salerno, W. J., Hicke, L. and Radhakrishnan, I. (2003). Solution structure of a CUE-ubiquitin complex reveals a conserved mode of ubiquitin binding. Cell 113, 621-630. [DOI] [PubMed] [Google Scholar]
- Kostova, Z. and Wolf, D. H. (2005). Importance of carbohydrate positioning in the recognition of mutated CPY for ER-associated degradation. J. Cell Sci. 118, 1485-1492. [DOI] [PubMed] [Google Scholar]
- Kostova, Z., Tsai, Y. C. and Weissman, A. M. (2007). Ubiquitin ligases, critical mediators of endoplasmic reticulum-associated degradation. Semin. Cell Dev. Biol. 18, 770-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. N., Song, B., DeBose-Boyd, R. A. and Ye, J. (2006). Sterol-regulated degradation of Insig-1 mediated by the membrane-bound ubiquitin ligase gp78. J. Biol. Chem. 281, 39308-39315. [DOI] [PubMed] [Google Scholar]
- Madura, K., Dohmen, R. J. and Varshavsky, A. (1993). N-recognin/Ubc2 interactions in the N-end rule pathway. J. Biol. Chem. 268, 12046-12054. [PubMed] [Google Scholar]
- Meusser, B., Hirsch, C., Jarosch, E. and Sommer, T. (2005). ERAD: the long road to destruction. Nat. Cell Biol. 7, 766-772. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa, K. and Brodsky, J. L. (2008). The recognition and retrotranslocation of misfolded proteins from the endoplasmic reticulum. Traffic 9, 861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemper, R. K., Deak, P. M., Otto, R. T. and Wolf, D. H. (1999). Re-entering the translocon from the lumenal side of the endoplasmic reticulum: studies on mutated carboxypeptidase yscY species. FEBS Lett. 443, 241-245. [DOI] [PubMed] [Google Scholar]
- Ponting, C. P. (2000). Proteins of the endoplasmic-reticulum-associated degradation pathway: domain detection and function prediction. Biochem. J. 351, 527-535. [PMC free article] [PubMed] [Google Scholar]
- Prag, G., Misra, S., Jones, E. A., Ghirlando, R., Davies, B. A., Horazdovsky, B. F. and Hurley, J. H. (2003). Mechanism of ubiquitin recognition by the CUE domain of Vps9p. Cell 113, 609-620. [DOI] [PubMed] [Google Scholar]
- Ravid, T. and Hochstrasser, M. (2007). Autoregulation of an E2 enzyme by ubiquitin-chain assembly on its catalytic residue. Nat. Cell Biol. 9, 422-427. [DOI] [PubMed] [Google Scholar]
- Shih, S. C., Prag, G., Francis, S. A., Sutanto, M. A., Hurley, J. H. and Hicke, L. (2003). A ubiquitin-binding motif required for intramolecular monoubiquitylation, the CUE domain. EMBO J. 22, 1273-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R. S. and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, B. L., Sever, N. and DeBose-Boyd, R. A. (2005). Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol. Cell 19, 829-840. [DOI] [PubMed] [Google Scholar]
- Taxis, C., Vogel, F. and Wolf, D. H. (2002). ER-golgi traffic is a prerequisite for efficient ER degradation. Mol. Biol. Cell 13, 1806-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxis, C., Hitt, R., Park, S. H., Deak, P. M., Kostova, Z. and Wolf, D. H. (2003). Use of modular substrates demonstrates mechanistic diversity and reveals differences in chaperone requirement of ERAD. J. Biol. Chem. 278, 35903-35913. [DOI] [PubMed] [Google Scholar]
- Tsai, Y. C., Mendoza, A., Mariano, J. M., Zhou, M., Kostova, Z., Chen, B., Veenstra, T., Hewitt, S. M., Helman, L. J., Khanna, C. et al. (2007). The ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nat. Med. 13, 1504-1509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.