Abstract
The control of reproductive function involves actions of sex steroids upon their nuclear receptors in the hypothalamus and preoptic area (POA). Whether hypothalamic hormone receptors change their expression in aging male mammals has not been extensively pursued, although such changes may underlie functional losses in reproductive physiology occurring with aging. We performed a stereological analysis of immunoreactive androgen receptor (AR) and estrogen receptor alpha (ERα) cells in three POA nuclei of male Sprague-Dawley rats [anteroventral periventricular nucleus (AVPV), median preoptic area (MePO), and medial preoptic nucleus (MPN)], at young (3 mo), middle-aged (12 mo) and old (20 mo) ages. Serum testosterone and estradiol levels were assayed. Testosterone concentrations decreased significantly and progressively with aging. Estradiol concentrations were significantly higher in middle-aged than either young or old rats. Stereologic analyses of the POA demonstrated that AR-immunoreactive cell numbers and density in the AVPV, MePO and MPN were significantly higher in old compared with young or middle-aged rats. No change in the total number or density of ERα immunoreactive cells was detected with age, although when cells were subdivided by intensity of immunolabeling, the most heavily-labeled ERα cells increased in number with aging in the AVPV and MePO, and in density in the AVPV. There are several interpretations to our finding of substantially increased AR cell numbers during aging, including a potential compensatory up-regulation of the AR under diminished testosterone concentrations. These results provide further information about how the neural targets of steroid hormones change with advancing age.
Keywords: androgen receptor, estrogen receptor, aging, anteroventral periventricular nucleus (AVPV), median preoptic area (MePO), medial preoptic nucleus (MPN)
Introduction
In mammals, the process of aging results in an increased probability of infertility or reproductive dysfunction (Wise et al, 2002; Mobbs, 2004). However, the mechanisms underlying these processes are not well-understood. Nevertheless, it is clear that the three levels of the hypothalamic-pituitary-gonadal (HPG) axis exhibit age-related changes. From the hypothalamic perspective, reproductive systems comprise the neural circuits regulating gonadotropin-releasing hormone (GnRH) neurons, together with the central nervous pathways that control reproductive physiology. Any of these neural systems may undergo age-associated alterations, changes to which may underlie some of the reproductive losses during aging.
Critical to reproductive function are actions of sex steroid hormones upon their receptors in the nervous system. Several groups have assayed concentrations of gonadal steroid concentrations during aging, although results differ depending upon species and even strain. Reports on testosterone in male mammals are relatively consistent, showing age-related declines in humans (Harman, 2001), rhesus monkeys (Downs and Urbanski, 2006) and rats [Sprague-Dawley (Roselli et al., 1986); Wistar (Bernardi et al., 1998; Taylor et al., 1996); Brown Norway (Chen et al., 1994; Gruenewald et al., 2000), Fischer 344 (Chambers et al., 1991; Luine et al., 2007)]. Results for estradiol and aging in males are conflicting. In Fischer 344 rats (Fujita et al. 1990; Luine et al., 2007) and Wistar rats (Herath et al., 2001) serum estradiol concentrations increase with aging, while for Sprague-Dawley rats (Goya et al., 1990) and Brown Norway rats (Gruenewald et al., 2000) serum estradiol concentrations do not change with aging. In primates, a significant age-related increase of plasma estradiol was reported in normal adult men (Drafta et al., 1982; Vermeulen et al., 2002) but not in rhesus macaques (Chambers et al., 1982, 1992). Thus, further information on whether and how serum estradiol levels change with aging is needed. Notably, in adult male rats (Sprague-Dawley) circulating estradiol concentrations are relatively high, being comparable to those in diestrous females (see data below, and Smith et al., 1975; Oliveira et al., 2004), consistent with important and physiologically-relevant roles of estrogens on reproductive functions (Larsson et al., 1973; Vagell and McGinnis, 1997).
Understanding the expression of steroid hormone receptors in the brain is crucial for elucidating the causes and consequences of reproductive aging. In young adults, brain nuclei that are abundant in nuclear steroid hormone receptors including, but not limited to, androgen receptor (AR; Simerly et al., 1990) and the estrogen receptors (ER), ERα and ERβ (Shughrue et al., 1997; Mitra et al., 2003)] mediate effects of testosterone and estradiol, respectively, and play key roles in the control of reproductive physiology and behavior. Observations that sociosexual behaviors decline in male rats with aging (Gray, 1978; Chambers et al., 1991; Smith et al., 1992; Taylor et al., 1996), and that these cannot be rescued by replacing steroid hormones to young levels (Hsu et al., 1986, Taylor et al., 1996), are consistent with both steroid-dependent and -independent neurobiological changes including potential alterations in numbers or responsiveness of steroid hormone receptors in the brain. However, with the exception of pathological disorders, the aging brain does not appear to undergo a wholesale loss of neurons (Finch, 2003). Rather, there appear to be changes to or losses in specific cell phenotypes that may underlie functional losses. This point can be applied to those neurons expressing nuclear steroid hormone receptors, although to date, there are few published reports on how AR and ER expression change with aging in male hypothalamus. Thus, studies on changes in hormones and neural receptors in aging mammals will contribute to better understanding these biological processes.
In the current study, we used immunohistochemistry and stereology to quantify the number of cells expressing AR and ERα in the hypothalamus of aging male rats. Three preoptic brain regions were chosen because of their robust expression of both ERα and AR: two, the anteroventral periventricular nucleus (AVPV) and medial preoptic nucleus (MPN), play functional roles in reproductive physiology and/or behavior (Wiegand and Terasawa, 1982; Simerly and Swanson, 1988). Less is known about the median preoptic nucleus (MePO) in reproduction, although it is involved in the control of vasopressin release (Xu et al., 2003), a hormone involved in pair bonding behaviors (Winslow et al., 1993), and which is anatomically interconnected to the AVPV (Thompson & Swanson, 2003; Gu & Simerly, 1997).
Methods
Animals
Male Sprague-Dawley rats were purchased at 3 months (young, N=7), 12 months (middle-aged, N=8) and 20 months (old, N=8), from the rat colony at the Animal Resource Center, University of Texas at Austin (UT). This rat colony originally derived from a Harlan Sprague-Dawley colony and is regularly introduced with new Harlan Sprague-Dawley rats. The middle-aged and old rats were retired breeders. Although exact sexual experience was not known, our animal colony allows a male to mate with two females periodically over the course of three to four months. It is not possible to match this exact life history of sexual experience in young rats, but for the young group, we placed each male rat in a cage with two receptive female rats (rotated among males) at least five nights in a row. Mating was confirmed by checking for sperm in the females’ vaginas. Rats were housed in an AAALAC-approved facility (two per cage, cage dimensions 47 × 20 × 25 cm) with Rat Sterilizable Diet (Harlan Teklad LM −485 7012, Madison, WI) and water available ad libitum. The light cycle was 12 h light, 12 h dark cycle (lights on 2300h), and temperature was 21 ± 1°C. All animal procedures performed herein were approved by the UT-Austin IACUC (Protocol number: 05031102) and studies were performed following the Guide for the Care and Use of Experimental Laboratory Animals.
Perfusion
Animals were deeply anesthetized with ketamine (100 mg/ml) and xylazine (20 mg/ml) (0.4 ml + 0.4 ml, respectively). Rats were perfused (48 ml/min) sequentially with 0.9% saline (24 ml), 0.9% saline with 10% heparin (24 ml), and 1% paraformaldehyde with 3.75% acrolein (48 ml), followed by 4% paraformaldehyde (480 ml; Chakraborty et al., 2003b). All fixatives were dissolved in PBSA (phosphate-buffered saline A: 0.08 M, PO4: 0.12 M, pH=7.3). The brains were removed from the skull and post-fixed for 3 hours in 4% paraformaldehyde and then transferred into PBSA with 0.05% sodium azide for storage at 4°C. Tissue sections (40 μm-thick) were cut on a vibrating microtome (Leica VT 1000S, Leica Microsystems, Nussloch, Germany) and stored in PBSA with sodium azide at 4°C.
Immunohistochemistry
For tissue processing and analyses, tissues were henceforward recoded so that the experimenter was blind to age group. Sections at the level of the POA were rinsed in TBST (Trizma-buffered saline/Triton X, Trizma base: 0.1 M, NaCl: 0.15 M, Triton X-100: 0.1%, pH = 7.3) at room temperature on a shaker. Although there were too many sections to process in a single run, animals from each age group were equally represented in every run. Sodium borohydride [1% in PBSB (phosphate-buffered saline B: saline: 0.16 M, PO4: 0.01M, pH=7.3)] was used to clear the acrolein for 20 minutes. Sections were washed until no bubbles were observed. Then, the sections were treated to eliminate any endogenous peroxide activity [3:1 methanol:3% H2O2, 20 minutes at room temperature]. For AR immunohistochemistry, sections were then washed, and incubated in the AR antibody PG21−36 (1:2000; generously provided by Dr. Gail S. Prins, University of Illinois-Chicaco). This antibody is a rabbit polyclonal raised against amino acids 1−21 of the rat AR and has been extensively characterized by Prins et al. (1991) and Zhou et al. (1994). These laboratories have demonstrated that preabsorption of this antibody with the antigen resulted in an abolition of immunoreactivity, and western blots showed strong immunoreactivity at the expected molecular weight of 110 kDa. Furthermore, application of the antibody to AR-negative tissue (rat spleen) resulted in no detectable immunoreactivity (Prins et al, 1991). In one of those published studies, castration resulted in a loss of nuclear ARir, a finding that could be attributable either to an up-regulation/maintenance of ARir by peripheral testosterone that is lost after castration, or to the possibility that the antibody only binds to liganded AR receptor (Zhou et al., 1994). However, in the current studies, all males were gonadally intact and had detectable concentrations of serum testosterone.
In our study, the primary antibody incubation was performed in 10% normal goat serum (NGS) and 0.1% Triton-X overnight at 4°C on a shaker. The sections were then washed and incubated in biotinylated anti-rabbit immunoglobulin G (IgG, 1:600, Vector Laboratories, Burlingame, CA) for 2 hours followed by rinsing in TBST. After rinsing, sections were incubated in ABC (Vector Laboratories) for 1 hour. They were rinsed in buffer and developed using 3,3-diaminobenzidine (DAB) as a chromogen. Sections were rinsed, dried at room temperature and then Nissl-stained (Toluidine blue; Chakraborty et al., 2003a; Salama et al., 2003), and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA). Controls were also run with the primary antibody omitted, and no specific binding was observed.
The procedure of ERα immunohistochemistry was identical to that described above for AR immunohistochemistry with the following exceptions. The assay buffer was PBSB. The ERα antibody was the rabbit polyclonal anti-estrogen receptor alpha antibody (1:20,000, C1355, Upstate Biotechnology, Waltham, MA) that was produced against the last 15 amino acids of ERα. This region has no homology to the corresponding region of ERβ, so detection of immunoreactivity should reflect the presence of ERα receptor only. In addition, Friend et al. (1997) showed using immunoblots that the antibody recognizes the recombinant ERα of the appropriate molecular weight, and recognizes both liganded and unliganded ERα (Friend et al., 1997). Specific binding was eliminated in that study by preabsorption with the antigen. Previously published work from our own laboratory, using ovariectomized female rats, showed the ability of the antibody to detect nuclear ERα both in estradiol- and vehicle-replaced animals, again suggesting that the antibody can bind to liganded and unliganded ERα (Chakraborty et al., 2003a, 2003b). In the present study, when either primary antibody, AR or ERα, was omitted as a negative control, no immunoreactivity was detected.
Stereological analysis
A stereological analysis was performed according to methods described in detail previously (Chakraborty et al., 2003a, 2005). For the AVPV and MePO, every other section was used to provide a 1:2 series for analysis of each nuclear receptor. For the MPN, every fourth section was used to provide a 1:4 series of each receptor. A wet-mount of fresh tissue showed that average tissue thickness was 40.9 μm. For each rat, 6−7 sections containing AVPV and MePO, and 8−9 sections containing MPN were chosen. The sections were carefully matched for rostral-caudal landmarks among all the animals, and the AVPV, MPN and MePO were identified in Nissl-stained sections by comparing anatomical landmarks to an atlas of the rat brain (Swanson, 1998). Quantitative analyses were done using a computer-assisted morphometry system that consisted of a MAC 5000 Manual joystick control (Ludl Electronic Products Ltd. Hawthorne, NY), MicroFire S99808 video camera (Optronics, Goleta, CA), Dell computer (Dimension 4550 series, Austin, TX), and Cintiq 15× screen (Wacom, Vancouver, WA) and Stereo Investigator® software. Using the Stereo Investigator® software (MicroBrightField, Williston, VT), closed contours were drawn to surround the region of interest (AVPV, MPN and MePO) at low magnification (4×) using the laboratory Olympus BX-61 microscope. Each section mount thickness was measured within the contour. A buffer zone at the top and bottom of the tissue, where the slice thickness is most likely to have been affected by tissue processing and is not representative of the tissue specimen from the volume, was set at 3 μm for all experimental stereology. For each rat, the volume of the regions of interest in each section was extrapolated based on the contours and tissue thickness (Volume = regional area × thickness). The Stereo Investigator software randomly placed 135 μm × 80 μm grids (“disector frames”) within each contour. Within these disector frames, the DAB-stained ERα or AR labeled nuclei were counted within a 40 μm × 40 μm counting frame (“optical disectors”). The counting criteria were: 1) only nuclei that came into focus as we focused down from the top of the counting frame were counted; 2) only nuclei falling entirely within the counting frame or touching/crossing the top or right lines of the counting frame were counted. Nuclei touching/crossing the bottom or left lines were excluded. These counting rules ensured that the same cells would not be counted twice. In addition, because the diameter of nuclei is approximately 10 μm, tissue thickness is 40 μm, and either a 1:2 series (or AVPV, MePO) or 1:4 series (MPN) was used, nuclei could not be double-counted. Based on these parameters, the number and density (# immunoreactive cells/volume of each nucleus) of AR-immunoreactive (ARir) nuclei or ERα-immunoreactive (ERαir) nuclei falling within the regions was quantified. The coefficients of error and variation of the estimates were calculated as per Schmitz and Hof (2000) and shown in Tables 1 and 2. CE's were low and never exceeded 0.09 (see Tables 1 and 2). Photomicrographs were taken to produce the figures, and images were subjected to only minor adjustments using Adobe Photoshop 7.0 (Adobe, San Jose, CA), with any adjustments applied equally to tissues from rats of different ages to avoid any bias, and so as not to alter the appearance from the original tissues.
Table 1.
Optical Fractionator Analysis of AR-Immunoreactive Cell Number and Volume
| AVPV | ||||||
|---|---|---|---|---|---|---|
| Young | CE | Middle-aged | CE | Old | CE | |
| Heavily Labeled AR-ir Cell Number | 329 ± 74 | 0.07 | 758 ± 350 | 0.07 | 2680 ± 556 | 0.09 |
| Lightly Labeled AR-ir Cell Number | 1249 ± 126 | 0.08 | 2098 ± 581 | 0.07 | 3830 ± 601 | 0.07 |
| Total AR-ir Cell Number | 1578 ± 149 | 0.08 | 2856 ± 881 | 0.09 | 6510 ± 1147 | 0.07 |
| Volume (μm3) × 107 | 6.95 ± 0.91 | 0.05 | 6.80 ± 0.64 | 0.07 | 7.02 ± 0.73 | 0.07 |
| MePO | ||||||
|---|---|---|---|---|---|---|
| Young | CE | Middle-aged | CE | Old | CE | |
| Heavily Labeled AR-ir Cell Number | 131 ± 73 | 0.09 | 637 ± 215 | 0.06 | 2175 ± 407 | 0.09 |
| Lightly Labeled AR-ir Cell Number | 844 ± 80 | 0.08 | 1351 ± 282 | 0.09 | 4126 ± 941 | 0.08 |
| Total AR-ir Cell Number | 975 ± 151 | 0.06 | 2096 ± 474 | 0.08 | 6300 ± 1346 | 0.07 |
| Volume (μm3) × 108 | 1.06 ± 0.13 | 0.09 | 0.95 ± 0.11 | 0.09 | 1.06 ± 0.10 | 0.09 |
| MPN | ||||||
|---|---|---|---|---|---|---|
| Young | CE | Middle-aged | CE | Old | CE | |
| Heavily Labeled AR-ir Cell Number | 7098 ± 1001 | 0.04 | 12732 ± 2378 | 0.03 | 34446 ± 3871 | 0.03 |
| Lightly Labeled AR-ir Cell Number | 19648 ± 1163 | 0.03 | 22299 ± 2581 | 0.03 | 32770 ± 693 | 0.04 |
| Total AR-ir Cell Number | 26747 ± 1333 | 0.03 | 35031 ± 4118 | 0.03 | 67216 ± 3992 | 0.07 |
| Volume (μm3) × 108 | 5.99 ± 0.18 | 0.03 | 6.26 ± 0.17 | 0.03 | 6.18 ± 0.11 | 0.03 |
Data shown are mean ± SEM. Abbreviations: AR: androgen receptor; AVPV: anteroventral periventricular nucleus; MePO: median preoptic nucleus; MPN: medial preoptic nucleus; CE: Coefficient of error.
Table 2.
Optical Fractionator Analysis of ERα-Immunoreactive Cell Number and Volume
| AVPV | ||||||
|---|---|---|---|---|---|---|
| Young | CE | Middle-aged | CE | Old | CE | |
| Heavily Labeled ERα-ir Cell Number | 1871 ± 327 | 0.09 | 4199 ± 526 | 0.08 | 4641 ± 1015 | 0.06 |
| Lightly Labeled ERα-ir Cell Number | 3715 ± 683 | 0.03 | 3717 ± 295 | 0.06 | 3952 ± 681 | 0.07 |
| Total ERα-ir Cell Number | 5586 ± 913 | 0.07 | 7916 ± 654 | 0.06 | 8593 ± 1380 | 0.06 |
| Volume (μm3) × 107 | 8.05 ± 0.97 | 0.05 | 8.24 ± 0.43 | 0.05 | 7.94 ± 0.39 | 0.05 |
| MePO | ||||||
|---|---|---|---|---|---|---|
| Young | CE | Middle-aged | CE | Old | CE | |
| Heavily Labeled ERα-ir Cell Number | 644 ± 202 | 0.08 | 1597 ± 312 | 0.07 | 3094 ± 1013 | 0.06 |
| Lightly Labeled ERα-ir Cell Number | 3383 ± 451 | 0.07 | 4999 ± 562 | 0.05 | 6730 ± 1476 | 0.03 |
| Total ERα-ir Cell Number | 4027 ± 605 | 0.06 | 6596 ± 826 | 0.05 | 9824 ± 2387 | 0.04 |
| Volume (μm3) × 108 | 1.49 ± 0.18 | 0.09 | 1.35 ± 0.11 | 0.09 | 1.58 ± 0.10 | 0.07 |
| MPN | ||||||
|---|---|---|---|---|---|---|
| Young | CE | Middle-aged | CE | Old | CE | |
| Heavily Labeled ERα-ir Cell Number | 4850 ± 1555 | 0.07 | 12488 ± 1795 | 0.05 | 13947 ± 3000 | 0.04 |
| Lightly Labeled ERα-ir Cell Number | 27471 ± 4300 | 0.04 | 35082 ± 4340 | 0.03 | 25715 ± 3907 | 0.03 |
| Total ERα-ir Cell Number | 32839 ± 5405 | 0.04 | 47570 ± 5545 | 0.04 | 39663 ± 6419 | 0.03 |
| Volume (μm3) × 108 | 6.66 ± 0.41 | 0.03 | 6.91 ± 0.29 | 0.04 | 6.95 ± 0.51 | 0.03 |
Data shown are mean ± SEM. Abbreviations: ERα: estrogen receptor α; AVPV: anteroventral periventricular nucleus; MePO: median preoptic nucleus; MPN: medial preoptic nucleus; CE: Coefficient of error.
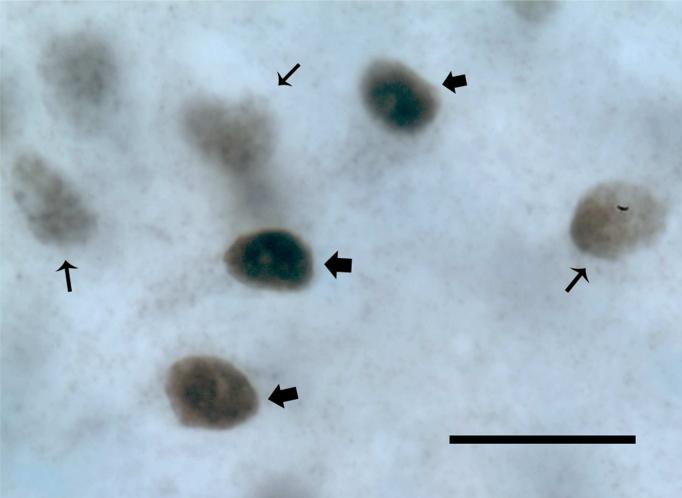
During the course of stereologic analyses we noted that ARir and ERαir cell nuclei fell into two qualitatively distinct classes that we have defined as “heavily labeled” and “lightly labeled” (Figure 1) similar to a recent report (Phillips-Farfán et al., 2007). Criteria for “heavily labeled” cells included a markedly more intense nuclear coloration (black) that was largely evenly distributed through the nucleus (nuclei indicated by thick arrows in Figure 1). Criteria for “lightly labeled” cells were a notably less intense coloration (gray) often characterized by a spotty appearance (nuclei indicated by thin arrows in Figure 1). We used an internal scale of cell density from 1−6, with 1=most lightly immunolabeled and 6=most heavily immunolabeled that we used in characterizing the cells during analysis. Cells that were in the range 1−3 were considered lightly-labeled and those 4−6 were heavily-labeled. There was virtually no ambiguity in the assignment of cells to one of the other of these two classes, as most cells fell into one of the two extremes. Therefore, in stereologic analyses we subdivided immunoreactive cells in these two classes separately, and also combined data for analysis and presentation.
Figure 1. Criteria for assignment of cells as heavily and lightly immunolabeled.
Data shown are for ERα, and similar criteria were applied to the AR analysis. The nuclei indicated by large arrows are identified as heavily labeled based on black coloration and relatively homogeneous distribution of label. Nuclei numbers indicated by small arrows are identified as lightly labeled, as they are gray in color and have a spotty, uneven appearance. Cells were easily categorized into one of the two classes using an internal scale of 1 to 3=lightly labeled, 4 to 6=heavily labeled. Scale bar = 50 μm.
Serum Hormone Concentrations
A terminal blood sample was collected from the anesthetized rat by cardiac puncture just prior to euthanasia by perfusion. The serum was separated by centrifugation (8000 rpm, 5 min) and stored at −80 °C for hormone assays.
Enzyme immunoassay (EIA) of serum testosterone
Testosterone concentrations were measured by a single testosterone enzyme immunoassay using the EIA kit DSL-10−4000 according to the method described by Diagnostic Systems Laboratories, Inc. (Webster, TX). Duplicate samples were run at a volume of 50 μl each. The minimum detectable level of testosterone was 0.04 ng/ml per tube. Intra-assay variability was 2.00%.
Radioimmunoassay (RIA) of serum estradiol
Estradiol concentrations were measured in a single ultra-sensitive estradiol RIA using the DSL-4800 RIA kit according to the method described by Diagnostic Systems Laboratories, Inc. (Webster, TX). Duplicate samples were run at a volume of 200 μl each. The minimum detectable level of estradiol was 2.2 pg/ml per tube. Intra-assay variability was 1.41%.
Statistical Analysis
Statistical analysis was done with each rat as the unit of analysis. Using SPSS statistical software (13.0) (SPSS Inc., Chicago, Illinois), effects of age were evaluated on the following endpoints: number of ARir or ERαir cell numbers (subdivided into heavily-labeled, lightly-labeled, and total, as described above), regional volume of each brain nucleus, ARir or ERαir density (calculated as cell numbers/volume), and serum hormone levels. First, datasets were tested for homogeneity of variance and normality. For datasets that met these criteria, comparisons were made by one-way ANOVA followed by Tukey post hoc analysis when indicated by a significant main effect. Otherwise the nonparametric Kruskal-Wallis H test was applied and followed by Mann-Whitney U test in the event of significant main effects. Correlations between serum testosterone with total AR- and ERα-immunoreactive cell numbers or density were evaluated by regression analysis using SPSS. In all cases, the criterion for statistical significance was p < 0.05.
Results
Stereology of AR in AVPV, MePO and MPN of aging male rats
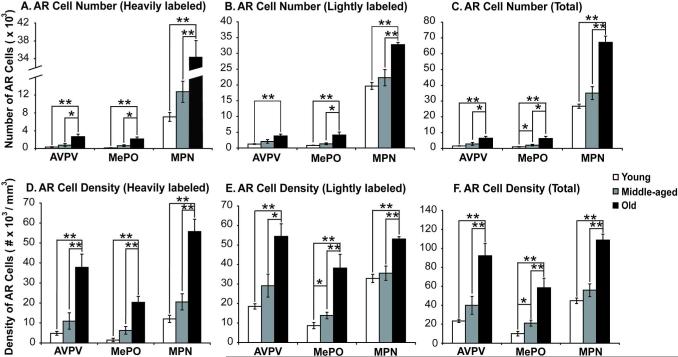
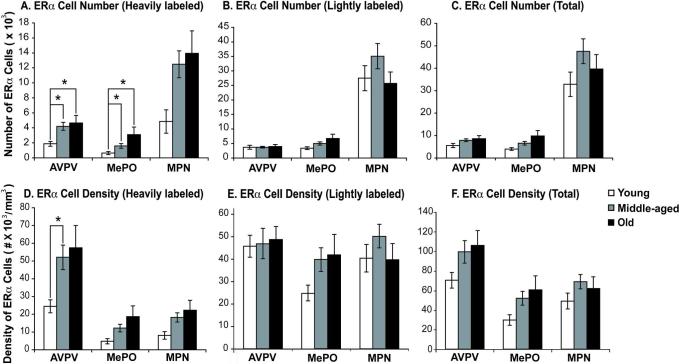
Representative photomicrographs of the expression and distribution of AR are shown in Figures 2 and 3, and demonstrate the robust expression of AR in the nucleus of cells in the AVPV, MePO and MPN. Stereologic analysis of ARir cell numbers was performed in the three regions, taking into consideration whether nuclei were lightly- or heavily-labeled. As shown in Figure 4, numbers of ARir cells increased significantly with age from young to middle-aged to old for heavily-labeled, lightly-labeled and total numbers of ARir cells. In all cases, numbers and densities of ARir cells were significantly higher in old than young male rats, and in most cases (with the sole exception of lightly labeled AR cell numbers in AVPV) old rats had higher AR cell numbers and densities than middle-aged rats. Middle-aged rats also had higher AR numbers and densities than young rats in MePO for total AR cell numbers and density, and for lightly labeled AR cell density in MPN (Figure 4).
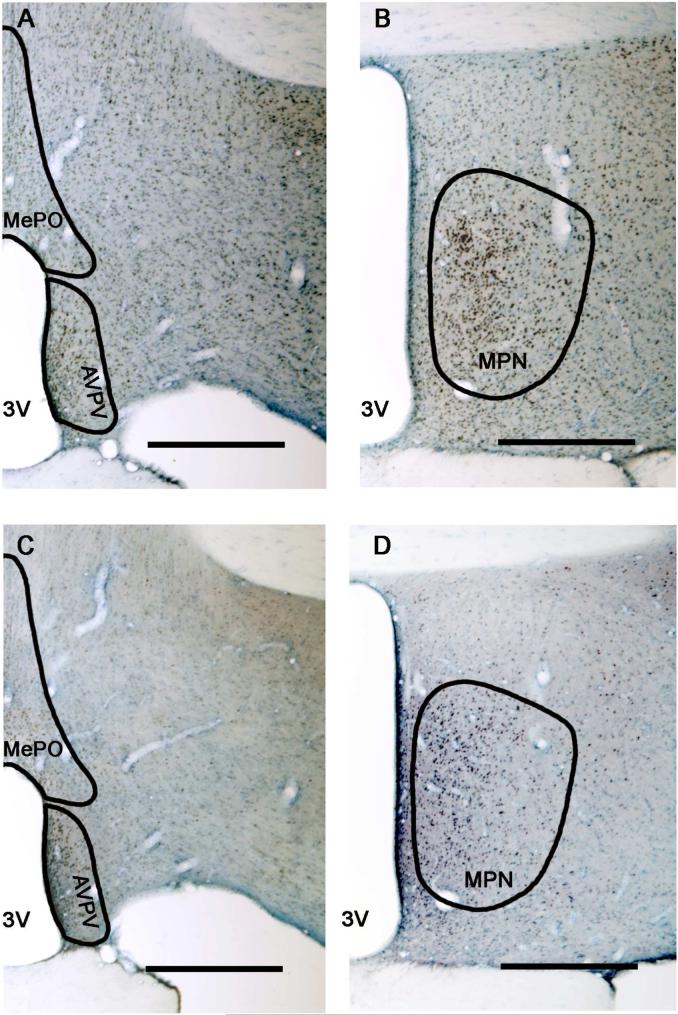
Figure 2. Photomicrographs of representative ERα and AR immunolabeled sections.
The POA is shown at 4× magnification at the level of the AVPV and MePO (panels A, C) and the MPN (panels B, D) of a representative old male rat. Sections shown in A and C were adjacent, as were B and D. Sections were labeled for AR immunoreactivity (top row) or ERα immunoreactivity (bottom row), and counterstained by Nissl labeling. The contours of the AVPV, MePO and MPN are drawn based on Nissl staining and in comparison to Swanson's rat brain atlas (1998). [Note that during the actual stereological analysis, contours were drawn according to the Nissl staining which is out of focus in the figure.] Scale bar = 500 μm.
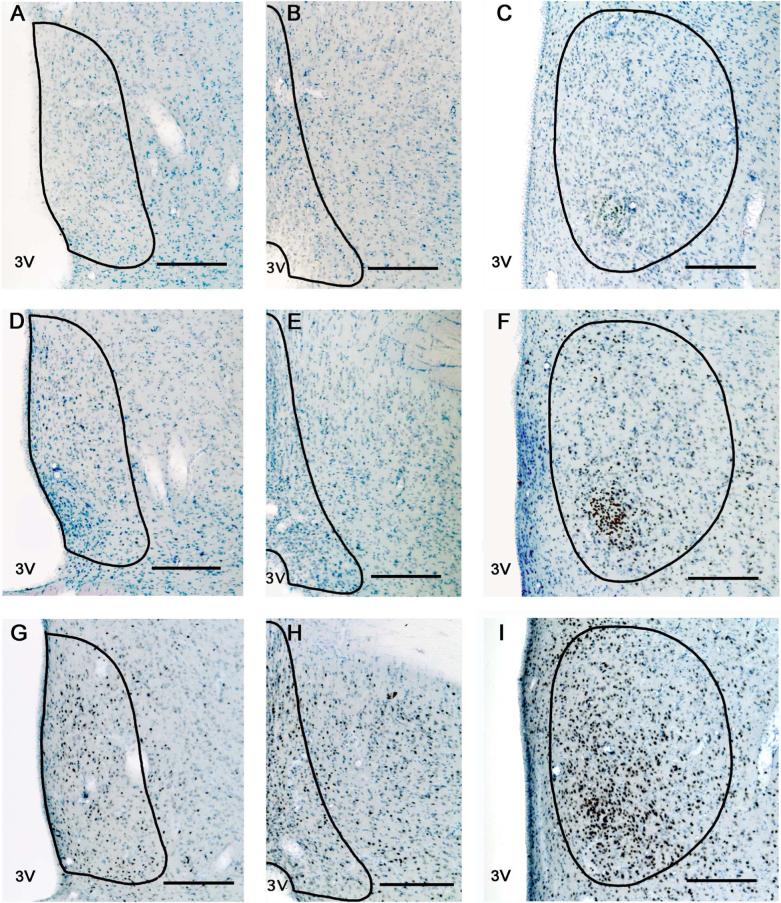
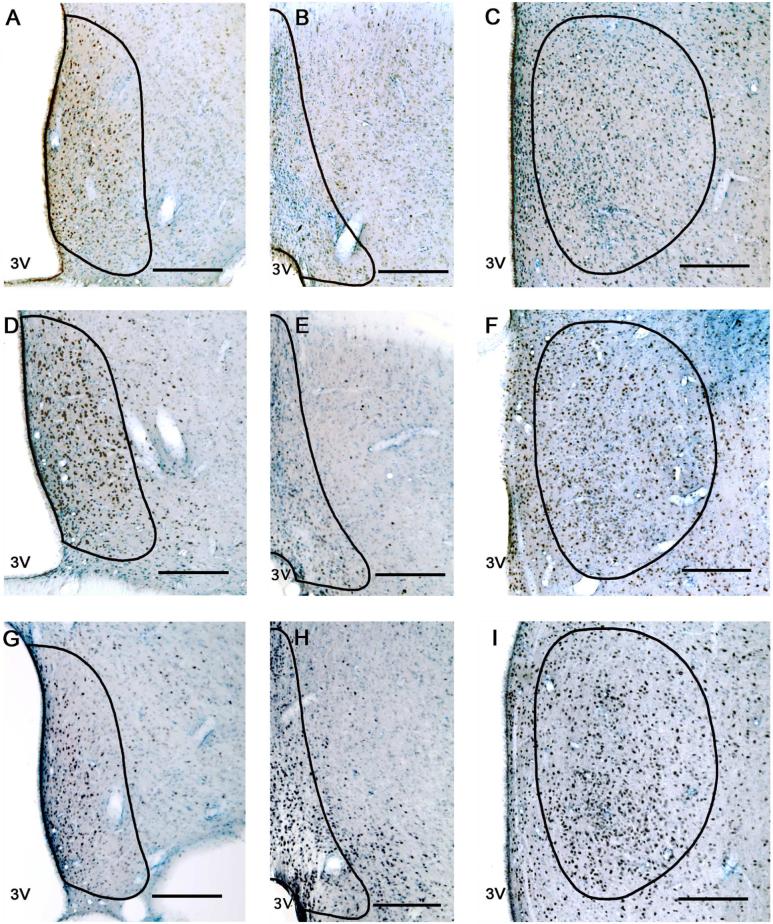
Figure 3. Photomicrographs of AR immunoreactivity in young, middle-aged and old rats.
Representative sections from a young (panels A, B, C) middle-aged (panels D, E, F) and old (panels G, H, I) male rat of the three brain regions [AVPV (left column), MePO (middle column) and MPN (right column)] are shown. The contours of the AVPV, MePO and MPN are drawn based on Nissl staining and according to Swanson's rat brain atlas (1998). Scale bar = 200 μm.
Figure 4. Stereologic analysis of AR immunoreactive cell numbers and density.
AR immunoreactive cell numbers were counted in young, middle-aged and old rats in AVPV, MePO and MPN. There were significant age-related increases detected for heavily labeled (A), lightly labeled (B) and total numbers (C) of AR cells. Similar observations were made for AR density in the three regions (panels D, E and F for heavily, lightly and total AR cell densities, respectively). *, p < 0.05; **, p < 0.01.
Stereology of ERα in AVPV, MePO and MPN of aging male rats
Representative photomicrographs of the AVPV, MePO and MPN are shown in Figures 2 and 5, showing the regions of analysis and the expression and distribution of ERα. The data demonstrate that ERαir is abundant in the selected hypothalamic areas. Stereologic analyses of ERαir cell numbers were performed, taking into consideration whether cell nuclei were lightly- or heavily-labeled (see Figure 1). No age-related change in the total number of ERαir cell numbers and densities were detected (Figure 6). When cells were subdivided into the lightly- or heavily-immunolabeled subgroups, there were no significant age differences on lightly-labeled cells (Figure 6). However, a significant effect of age on the heavily-labeled ERα cell numbers was detected in AVPV (p < 0.05) and MePO (p < 0.05), with a trend in the MPN (p = 0.065). Post-hoc analyses of significant main effects showed that in the AVPV and MePO (but not the MPN) numbers of heavily-labeled ERαir cells were significantly higher in middle-aged than in young rats and higher in old than young rats. An effect of age on the density of heavily labeled ERα immunoreactive cells was found only in AVPV (p < 0.05), with a trend in MePO (p = 0.086) and MPN (p = 0.065). Post-hoc analysis of the AVPV showed this effect was attributable to a difference between the young and middle-aged groups.
Figure 5. Photomicrographs of ERα immunoreactivity in young, middle-aged and old rats.
Representative sections from a young (panels A, B, C) middle-aged (panels D, E, F) and old (panels G, H, I) rat of the three brain regions [AVPV (left column), MePO (middle column) and MPN (right column)] are shown. The contours of the AVPV, MePO and MPN are drawn based on Nissl labeling and in comparison to Swanson's rat brain atlas (1998). Scale bar = 200 μm.
Figure 6. Stereologic analysis of ERα immunoreactive cell numbers and density.
ERα immunoreactive neuron numbers were quantified in young, middle-aged and old rats in the AVPV, MePO and MPN. Data are shown for heavily labeled (A), lightly labeled (B) and total (C) ERαir cell numbers. Although there were no differences with age in total or lightly labeled ERαir cells, there was a significant increase in heavily labeled cells with age from young to middle-aged in the AVPV and MePO. Panels D, E and F present similar data for ERα cell densities in aging rats. The only significant difference in density was found for heavily labeled ERαir cells, with an increase from young to middle-aged rats in the AVPV. *, p < 0.05; **, p < 0.01.
Serum testosterone and estradiol concentrations
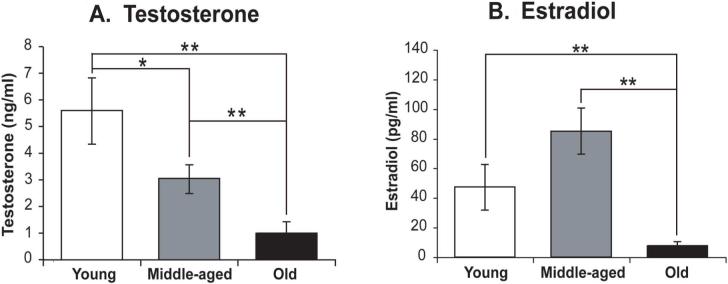
Serum testosterone levels declined significantly with age (p < 0.001). Testosterone concentrations were significantly lower in the old group than in the young or middle-aged rats (p < 0.001 for both; Figure 7A). There was also a significant difference between middle-aged and young rats (p < 0.05).
Figure 7. Serum testosterone and estradiol concentrations in aging male Sprague-Dawley rats.
Serum testosterone (A) and estradiol (B) concentrations are shown. Both hormones were affected significantly by age. *, p < 0.05; **, p < 0.01.
Serum estradiol levels varied significantly with age (p < 0.001) and were highest in middle-aged rats and lowest in the old rats (Figure 7B). A significant difference between middle-aged and old rats was detected (p < 0.001), as was a significant difference between old and young rats (p < 0.01).
Correlations between androgen receptor and serum testosterone levels
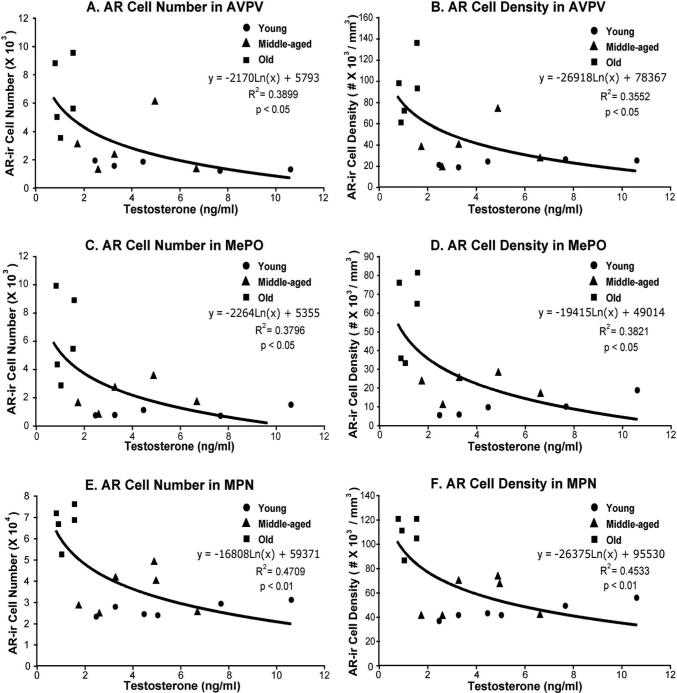
Based on observations for overall age-related increases in ARir cells, and decreases in serum testosterone concentrations, regression analyses were performed between ARir cells (total number and density) and serum testosterone levels (Figure 8) irrespective of age. Both ARir cell number and density in AVPV, MePO and MPN were negatively correlated with serum testosterone levels (p < 0.05 for all).
Figure 8. Correlations between total androgen receptor cell (number and density) and serum testosterone concentrations in aging male Sprague-Dawley rats.
Regression analyses were performed for ARir numbers and density in AVPV (panels A, B), MePO (panels C, D) and MPN (panels E, F). There were significant negative correlations between serum testosterone concentrations and ARir cells in AVPV (p < 0.05), MePO (p < 0.05) and MPN (p < 0.01).
Discussion
In this study, we demonstrated a 3 to 4-fold age-related increase in AR immunoreactive cells in three preoptic brain areas, the AVPV, MePO and MPN, in male Sprague Dawley rats. During this same period, total serum testosterone levels decreased profoundly. Thus, there is a significant inverse relationship between ARir cells and circulating testosterone. Although ERαir cells increased modestly in these same three regions with age, effects were only significant for the most heavily-labeled cells. Serum estradiol levels peaked in middle age, suggesting that the regulation of expression of ERα by estradiol in male rats of increasing age may be controlled by differing mechanisms, and at the very least, that there is a non-linear relationship between the serum hormone and its nuclear receptor. These results demonstrate the capacity of the aging male brain to continue to express nuclear hormone receptors, and even its ability to increase the numbers of cells that synthesize these proteins.
Roles of three preoptic regions in male reproduction
The regions of interest in the current study were chosen based on their reported roles in reproductive physiology and behavior, evidence for sexual dimorphisms, along with our own pilot analyses showing their abundant expression of both AR and ERα in aging male rats. The AVPV is a sexually dimorphic nucleus (Simerly, 1998) the function of which in male rats is not well-understood, although it appears to be necessary for female ovulatory function (Wiegand and Terasawa, 1982). The AVPV is smaller in male than female rats, and this effect is organized perinatally by sex differences in steroid hormones (Polston et al., 2004). Finally, the AVPV has abundant expression of both AR and ERα (Simerly et al., 1990; Chakraborty et al., 2003a, 2003b). The MPN is a central integrative site for the regulation of male sexual behavior. It receives inputs from sensory systems (Simerly and Swanson, 1986) and projects to structures that are involved in copulatory behavior (Simerly and Swanson, 1988). Like the AVPV, neonatal steroid hormones organize the size of the MPN, which is larger in males than females (Bloch and Gorski, 1986; Portillo et al., 2006), and both AR and ERα are expressed in this region (Chakraborty et al., 2003a, 2003b; Portillo et al., 2006). Although the MePO is not well studied in the context of reproduction, it is highly interconnected with the AVPV (Thompson and Swanson, 2003; Gu & Simerly, 1997) and it expresses ERα and AR (Yokosuka et al., 1997; Iqbal et al., 1995). Thus, these three regions were chosen from the many hypothalamic-preoptic regions that express AR and ERα as a first step in understanding how these receptors change during male aging.
Androgen receptor cell numbers increase robustly with aging in POA, while serum testosterone concentrations decrease
Quantitative stereologic analyses of numbers and densities of cells expressing AR immunoreactivity in AVPV, MPN and MePO demonstrated abundant labeling and similar densities in all three regions. Although there were some small differences between the regions, in general, AR cell numbers and density were highest in old rats, with this age group having roughly 3−4 times higher total AR cells compared to the young group, which had the lowest AR expression. During this same period, total serum testosterone decreased from the young to the old group, with young rats having approximately four times higher testosterone levels than old rats. Indeed, our regression analysis showed a significant inverse relationship between serum testosterone and expression of total ARir cell numbers and density in the AVPV, MePO and MPN. This result is surprising, as other studies evaluating the relationship between androgen receptor and circulating testosterone have reported the opposite, namely, that the presence or administration of androgen maintains or up-regulates the nuclear AR (Coolen and Wood, 1999), and castration down-regulates AR mRNA (Handa et al. 1996), or changes its localization from nuclear to cytoplasmic (Wood and Newman, 1993; Krey and McGinnis, 1990). These relationships do not appear to be the case in aging male rats.
There are several interpretations of our data that are not mutually exclusive. First, our testosterone assay measured total testosterone (both bound and free), leaving open the possibility that there may be differential age-related changes in free, as opposed to total testosterone. To our knowledge this issue has not been addressed in aging male rats, although human studies demonstrate that both total and free testosterone decline with age (Kang et al., 2003; Feldman et al., 2002). Ongoing studies are investigating this question in a new cohort of animals. A second and related possibility is that age-related differences in sex hormone binding globulin, which increases with age (Kang et al., 2003; Feldman et al., 2002), decreases the amount of bioavailable testosterone, and as a consequence, there is a compensatory up-regulation of the AR. A third possibility is related to the sexual experience of the rats. Fernandez-Guasti reported that sexual activity in male Wistar rats reduced hypothalamic AR immunoreactivity in MPN compared to naive rats, but had no effect on total androgens (Fernandez-Guasti et al., 2003). In the current study, although males were sexually experienced they were used at time points post-experience that differed considerably. Our young rats were perfused 1−2 weeks after they gained sexual experience while middle-aged and old rats were retired breeders that were used 4- or 12-months, respectively, after retirement. Fourth, although evidence in young male rodents discussed above indicates a positive correlation between serum androgen and AR expression in hypothalamus-POA, it is possible that this relationship becomes disconnected or dysregulated when rats reach middle age. In fact, our results are suggestive of a compensatory up-regulation of the AR in response to diminished testosterone concentrations. Fifth, testosterone may be synthesized within the brain (rat: Hojo et al., 2004; human: Stoffel-Wagner et al., 1999) and this may change with aging, or compensate for the age-related loss in peripheral steroids. Finally, the aging brain may increase its synthesis of the AR independent of influences by gonadal steroids. We are currently performing experiments on male rats that are castrated and given testosterone (or vehicle) replacement to test what role, if any, testosterone plays in the regulation of its receptor with aging, or if the age-related increase in AR is a function of aging that is independent of peripheral gonadal hormones.
Estrogen Receptor α immunoreactive cells undergo limited changes with age, whereas serum estradiol peaks at middle age
The results of our stereologic analyses showed small and primarily non-significant age-related increases in total ERα in the POA of male rats. This finding is consistent with the report of Madeira et al. (Madeira et al., 2000), who showed no difference in ERα cell numbers in the aging male MPN. In addition, Jarry's laboratory reported that ERα mRNA did not change significantly in the POA of aging Wistar male rats (Böttner et al., 2007), a result consistent with our relative lack of change of the ERα protein. We also quantified ERαir cells in the MePO and AVPV, in which we found significant increases in heavily-labeled ERα cells, but not lightly labeled or total numbers of cells, similar to the MPN. As discussed above for the AR, we do not know whether there is any functional difference between heavily- and lightly-labeled ERαir cells, although this observation is not unique to our laboratory, as the classification of ERα cells into lightly- and heavily-immunolabeled was published by another group (Phillips-Farfán et al., 2007). Heavily-immunolabeled cells may express more estrogen receptors, may have a different post-translational status (e.g., phosphorylation states), and/or may be differentially sensitive to estradiol. The most parsimonious interpretation of our results, however, is that total ERα immunoreactive cell numbers do not change significantly in the POA of aging male rats.
Despite our finding of little change in total ERα cell numbers, serum estradiol levels were about two-fold and eight-fold higher in middle-aged than young or old males rats, respectively, indicating that there is no obvious relationship between serum estradiol level and ERαir cell numbers. Thus, if ERαir cell numbers are negatively regulated in male rats, as they are in females (Lauber et al., 1990), there is a disconnection in this regulation with aging. Notably, our current results on estradiol are not consistent with previous reports showing either no change or an overall increase in estradiol with aging (Fujita et al. 1990; Luine et al., 2007; Herath et al., 2001; Goya et al., 1990; Gruenewald et al., 2000; Drafta et al., 1982; Vermeulen et al., 2002; Chambers et al., 1982, 1992). We do not know the reason for this difference but it may be attributable to species/strain, and/or sexual experience. Finally, in considering the regulation of nuclear receptors in the POA, it is necessary to understand the role of local aromatization of testosterone to estradiol. Roselli et al. (1986) reported that hypothalamic aromatase activity was unchanged in aging male rats, and there may be sufficient estrogen in the POA of aged males, even with their lower testosterone levels, to maintain ERα protein expression at a relatively similar level.
It should be noted that there is a second nuclear receptor for estrogens, the estrogen receptor beta (ERβ), which is expressed in the MPN and AVPV of male and female rats (Zhang et al., 2002; Chakraborty et al, 2003c; Bu & Lephart, 2007). In female rats, aging is associated with a decrease in ERβ immunoreactive cell numbers in the AVPV (Chakraborty et al, 2003c). ERβ mRNA in another hypothalamic region of female rats, the suprachiasmatic nucleus, declined with age, although it did not change in periventricular preoptic nucleus (roughly equivalent to AVPV), MPN or paraventricular nucleus (Wilson et al, 2001). To our knowledge, age-related changes in ERβ in male brains have not been reported. We were unable to quantify ERβ in the current study due to failure of available antibodies to work in our tissues, but this is an interesting future area of research, particularly considering the changes of serum estradiol in aging mammals discussed above.
Summary and Conclusions
Data from the present study add to previous observations showing that the AR and ERα are expressed abundantly in AVPV, MePO and MPN, and extend these findings to the aging male brain. ERα immunoreactivity increased modestly with age, an effect largely limited to the most heavily immunoreactive cells, as total ERαir cell numbers and density did not change with aging. Numbers and density of ARir cells in these three preoptic regions increased robustly with aging, a period during which circulating testosterone declines precipitously. Indeed, a significant negative correlation between serum testosterone concentrations and AR cell numbers was detected in the AVPV, MePO and MPN, regardless of chronological age. These findings provide quantitative evidence for differential expression of nuclear hormone receptors in the aging compared to the young adult male POA.
Acknowledgments
We thank Erin Kristobak for the pilot work on estrogen receptor and Deena Walker for running RIAs of steroid hormones. We are grateful to Dr. Gail Prins, University of Illinois-Chicago, for the generous gift of the androgen receptor antibody.
Footnotes
This work was supported by NIH AG16765 and AG028051 to ACG.
Literature Cited
- Bernardi F, Salvestroni C, Casarosa E, Nappi RE, Lanzone A, Luisi S, Purdy RH, Petraglia F, Genazzani AR. Aging is associated with changes in allopregnanolone concentrations in brain, endocrine glands and serum in male rats. Eur J Endocrinol. 1998;138(3):316–21. doi: 10.1530/eje.0.1380316. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Gorski RA. Estrogen/progesterone treatment in adulthood affects the size of several components of the medial preoptic area in the male rat. J Comp Neurol. 1988;275:613–622. doi: 10.1002/cne.902750409. [DOI] [PubMed] [Google Scholar]
- Böttner M, Leonhardt S, Wuttke W, Jarry H. Changes of expression of genes related to the activity of the gonadotrophin-releasing hormone pulse generator in young versus middle-aged male rats. J Neuroendocrinol. 2007;19(10):779–87. doi: 10.1111/j.1365-2826.2007.01589.x. [DOI] [PubMed] [Google Scholar]
- Bu L, Lephart ED. AVPV neurons containing estrogen receptor-beta in adult male rats are influenced by soy isoflavones. BMC Neurosci. 2007;8:13. doi: 10.1186/1471-2202-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty TR, Hof PR, Ng L, Gore AC. Stereologic analysis of estrogen receptor α (ER α) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol. 2003a;466(3):409–21. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Ng L, Gore AC. Colocalization and hormone regulation of estrogen receptor α and N-methyl-D-aspartate receptor in the hypothalamus of female rats. Endocrinology. 2003b;144(1):299–305. doi: 10.1210/en.2002-220749. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Ng L, Gore AC. Age-related changes in estrogen receptor beta in rat hypothalamus: a quantitative analysis. Endocrinology. 2003c;144(9):4164–4171. doi: 10.1210/en.2003-0052. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Rajendren G, Gore AC. Expression of estrogen receptor α in the anteroventral periventricular nucleus of hypogonadal mice. Exp Biol Med (Maywood) 2005;230(1):49–56. doi: 10.1177/153537020523000106. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Resko JA, Phoenix CH. Correlation of diurnal changes in hormones with sexual behavior and age in male rhesus macaques. Neurobiol Aging. 1982;3(1):37–42. doi: 10.1016/0197-4580(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Thornton JE, Roselli CE. Age-related deficits in brain androgen binding and metabolism, testosterone, and sexual behavior of male rats. Neurobiol Aging. 1991;12(2):123–30. doi: 10.1016/0197-4580(91)90050-t. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Phoenix CH. Sexual behavior and serum levels of prolactin, testosterone, and estradiol in young and old rhesus males. Physiol Behav. 1992;52(1):13–6. doi: 10.1016/0031-9384(92)90426-3. [DOI] [PubMed] [Google Scholar]
- Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl. 1994;15(6):551–7. [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Testosterone stimulation of the medial preoptic area and medial amygdala in the control of male hamster sexual behavior: redundancy without amplification. Behav Brain Res. 1999;98(1):143–53. doi: 10.1016/s0166-4328(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Aging-related sex-dependent loss of the circulating leptin 24-h rhythm in the rhesus monkey. J Endocrinol. 2006;190(1):117–27. doi: 10.1677/joe.1.06745. [DOI] [PubMed] [Google Scholar]
- Drafta D, Schindler AE, Stroe E, Neacsu E. Age-related changes of plasma steroids in normal adult males. J Steroid Biochem. 1982;17(6):683–7. doi: 10.1016/0022-4731(82)90571-4. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Swaab D, Rodríguez-Manzo G. Sexual behavior reduces hypothalamic androgen receptor immunoreactivity. Psychoneuroendocrinology. 2003;28(4):501–12. doi: 10.1016/s0306-4530(02)00036-7. [DOI] [PubMed] [Google Scholar]
- Finch CE. Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging. 2003;24(Suppl 1):S123–7. doi: 10.1016/s0197-4580(03)00051-4. [DOI] [PubMed] [Google Scholar]
- Friend KE, Resnick EM, Ang LW, Shupnik MA. Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steroid hormones. Mol Cell Endocrinol. 1997;131:147–155. doi: 10.1016/s0303-7207(97)00098-1. [DOI] [PubMed] [Google Scholar]
- Fujita S, Chiba M, Ohta M, Kitani K, Suzuki T. Alteration of plasma sex hormone levels associated with old age and its effect on hepatic drug metabolism in rats. J Pharmacol Exp Ther. 1990;253(1):369–74. [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193(2):529–39. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Goya RG, Lu JK, Meites J. Gonadal function in aging rats and its relation to pituitary and mammary pathology. Mech Ageing Dev. 1990;56(1):77–88. doi: 10.1016/0047-6374(90)90116-w. [DOI] [PubMed] [Google Scholar]
- Gray GD. Age-related changes in penile erections and circulating testosterone in middle-aged male rats. Adv Exp Med Biol. 1978;113:149–58. doi: 10.1007/978-1-4684-8893-7_9. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in hypothalamic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male Brown Norway rat. J Androl. 2000;21(1):72–84. [PubMed] [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol. 1997;385:142–164. [PubMed] [Google Scholar]
- Handa RJ, Kerr JE, DonCarlos LL, McGivern RF, Hejna G. Hormonal regulation of androgen receptor messenger RNA in the medial preoptic area of the male rat. Brain Res Mol Brain Res. 1996;39(1−2):57–67. doi: 10.1016/0169-328x(95)00353-t. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Herath CB, Watanabe G, Wanzhu J, Noguchi J, Akiyama K, Kuramoto K, Groome NP, Taya K. Elevated levels of inhibin-A and immunoreactive inhibin in aged male Wistar rats with testicular Leydig cell tumor. J Androl. 2001;22(5):838–46. [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P450−17alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101(3):865–70. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HK, Hsu C, Yu JY, Peng MT. Effects of long-term testosterone replacement on copulatory activity in old male rats. Gerontology. 1986;32(1):10–7. doi: 10.1159/000212760. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Swanson JJ, Prins GS, Jacobson CD. Androgen receptor-like immunoreactivity in the Brazilian opossum brain and pituitary: distribution and effects of castration and testosterone replacement in the adult male. Brain Res. 1995;703(1−2):1–18. doi: 10.1016/0006-8993(95)00983-3. [DOI] [PubMed] [Google Scholar]
- Kang YG, Bae CY, Kim S, Kim MJ, Lee YJ, Seo J, Kim YC. Age-related change in serum concentrations of testosterone in middle-aged Korean men. Aging Male. 2003;6(1):8–12. [PubMed] [Google Scholar]
- Krey LC, McGinnis MY. Time-courses of the appearance/disappearance of nuclear androgen + receptor complexes in the brain and adenohypophysis following testosterone administration/withdrawal to castrated male rats: relationships with gonadotropin secretion. J Steroid Biochem. 1990;35(3−4):403–8. doi: 10.1016/0022-4731(90)90247-p. [DOI] [PubMed] [Google Scholar]
- Larsson K, Södersten P, Beyer C. Induction of male sexual behaviour by oestradiol benzoate in combination with dihydrotestosterone. J Endocrinol. 1973;57(3):563–4. doi: 10.1677/joe.0.0570563. [DOI] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Mobbs CV, Pfaff DW. Estradiol regulation of estrogen receptor messenger ribonucleic acid in rat mediobasal hypothalamus: an in situ hybridization study. J Neuroendocrinol. 1990;2:605–611. doi: 10.1111/j.1365-2826.1990.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19(10):743–51. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Andrade JP, Paula-Barbosa MM. Hypertrophy of the ageing rat medial preoptic nucleus. J Neurocytol. 2000;29(3):173–97. doi: 10.1023/a:1026598906739. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, Vivas L, Wade JD, Oldfield BJ. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol. 2004;16(4):340–7. doi: 10.1111/j.0953-8194.2004.01184.x. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology. 2003;144(5):2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mobbs CV. Not wisely but too well: aging as a cost of neuroendocrine activity. Sci Aging Knowledge Environ. 2004;2004(35):33. doi: 10.1126/sageke.2004.35.pe33. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Mahecha GA, Carnes K, Prins GS, Saunders PT, França LR, Hess RA. Differential hormonal regulation of estrogen receptors ERα and ERβ and androgen receptor expression in rat efferent ductules. Reproduction. 2004;128(1):73–86. doi: 10.1530/rep.1.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Farfán BV, Lemus AE, Fernández-Guasti A. Increased estrogen receptor α immunoreactivity in the forebrain of sexually satiated rats. Horm Behav. 2007;51(3):328–34. doi: 10.1016/j.yhbeh.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Polston EK, Gu G, Simerly RB. Neurons in the principal nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic GABAergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience. 2004;123(3):793–803. doi: 10.1016/j.neuroscience.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Portillo W, Díaz NF, Cabrera EA, Fernández-Guasti A, Paredes RG. Comparative analysis of immunoreactive cells for androgen receptors and oestrogen receptor α in copulating and non-copulating male rats. J Neuroendocrinol. 2006;18(3):168–76. doi: 10.1111/j.1365-2826.2005.01401.x. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129(6):3187–99. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Kaler LW, Resko JA. Hypothalamic aromatase activity in young and old male rats. Neurobiol Aging. 1986;7(2):121–5. doi: 10.1016/0197-4580(86)90150-8. [DOI] [PubMed] [Google Scholar]
- Salama J, Chakraborty TR, Ng L, Gore AC. Effects of polychlorinated biphenyls on estrogen receptor- β expression in the anteroventral periventricular nucleus. Environ Health Perspect. 2003;111(10):1278–82. doi: 10.1289/ehp.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20(1):93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α (ER-α) and β (ER-β) mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246(3):312–42. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270(2):209–42. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res. 1998;92(2):195–203. doi: 10.1016/s0166-4328(97)00191-5. [DOI] [PubMed] [Google Scholar]
- Smith ER, Stefanick ML, Clark JT, Davidson JM. Hormones and sexual behavior in relationship to aging in male rats. Horm Behav. 1992;26(1):110–35. doi: 10.1016/0018-506x(92)90035-t. [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96(1):219–26. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, Steckelbroeck S, Schramm J, Bidlingmaier JF, Klingmüller D. Expression of 17beta-hydroxysteroid dehydrogenase types 1, 2, 3 and 4 in the human temporal lobe. J Endocrinol. 1999;160(1):119–26. doi: 10.1677/joe.0.1600119. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; 1998. [Google Scholar]
- Taylor G, Bardgett M, Farr S, Humphrey W, Womack S, Weiss J. Aging of the brain-testicular axis: reproductive systems of healthy old male rats with or without endocrine stimulation. Proc Soc Exp Biol Med. 1996;211(1):69–75. doi: 10.3181/00379727-211-43953. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev. 2003;41(2−3):153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Vagell ME, McGinnis MY. The role of aromatization in the restoration of male rat reproductive behavior. J Neuroendocrinol. 1997;9(6):415–21. doi: 10.1046/j.1365-2826.1997.00598.x. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. Estradiol in elderly men. Aging Male. 2002;5(2):98–102. [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34(6):395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) gene expression in specific regions of the rat brain. Mech Ageing Devel. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–8. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, Böttner M, Rosewell KL. Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res. 2002;57:235–56. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Intracellular partitioning of androgen receptor immunoreactivity in the brain of the male Syrian hamster: effects of castration and steroid replacement. J Neurobiol. 1993;24(7):925–38. doi: 10.1002/neu.480240706. [DOI] [PubMed] [Google Scholar]
- Xu Z, Torday J, Yao J. Functional and anatomic relationship between cholinergic neurons in the median preoptic nucleus and the supraoptic cells. Brain Res. 2003;964(2):171–8. doi: 10.1016/s0006-8993(02)03800-3. [DOI] [PubMed] [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-α immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389(1):81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cai W, Zhou D, Su B. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935(1−2):73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology. 1994;134(6):2622–7. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]