Abstract
Restless legs syndrome (RLS) is a common neurological disorder characterized by an irresistible urge to move the legs, usually accompanied or caused by uncomfortable sensations in the legs. Symptoms are worse in the evening or at night than during the day and generally begin or worsen during periods of rest or inactivity. The chronic sleep disturbance often associated with RLS is likely due, at least in part, to the circadian nature of the symptoms. The relationship between disturbed sleep and reduced daytime functioning is well known and thus the accurate diagnosis and effective management of RLS is imperative.
Keywords: restless legs syndrome, RLS, sleep disturbance, reduced daytime functioning, circadian
Introduction
Restless legs syndrome (RLS) is a chronic neurological disorder that is estimated to affect up to 10% of adults in the US (Phillips et al 2000; Ulfberg et al 2001a, 2001b). RLS occurs as two forms, a primary disorder independent of any other disease, and secondary, in which symptoms are triggered by another condition such as end-stage renal disease (ESRD), iron deficiency, and normal pregnancy (Allen and Earley 2001).
RLS is characterized by an irresistible urge to move the legs, usually accompanied or caused by uncomfortable sensations in the legs. The symptoms generally begin or worsen during periods of rest or inactivity, are partially or totally relieved by movement (at least as long as the activity continues) and are worse in the evening or at night than during the day. In addition to these symptoms that are required for diagnosis, approximately 80% of patients with RLS experience periodic leg movements (PLMs) during sleep or while awake (Montplaisir et al 1997).
Many studies have demonstrated that sleep disturbances are a key consequence, and often the most troublesome symptom, of RLS. Thus, sleep problems are commonly reported by patients with both primary and secondary RLS (Walker et al 1995; Suzuki et al 2003; Hening et al 2004; Rijsman et al 2004a). In addition, virtually all patients seeking treatment for RLS report that they have disturbed sleep (Allen et al 2003). Furthermore, in a large survey of the general population in Norway and Denmark, both insomnia and excessive daytime sleepiness were significant predictors of the presence of diagnostic symptoms of RLS (Bjorvatn et al 2005).
Sleep is a fundamental regulatory process of the nervous system, and the need for sleep is a powerful homeostatic drive. Lack of sleep, resulting from insomnia, has many consequences, including reduced quality of life and social functioning, impaired work performance, impaired memory and cognitive functioning, and increased healthcare utilization (Simon and VonKorff 1997; NIH 2005). Thus, it is important that sleep disturbances associated with RLS are identified and managed as effectively as possible.
Effects of RLS on patients’ sleep
Effects in patients with primary RLS
In a large (n=1485 respondents), single-center, population-based study in The Netherlands (conducted before the key diagnostic symptoms of RLS were published), a positive answer to the question “Is your sleep disturbed by leg movements?” was considered to indicate the presence of RLS. Overall, 7.1% of respondents answered “Often” or “Always” to this question, and were considered to have some symptoms of RLS (Rijsman et al 2004a). Those positive respondents reported more difficulties in initiating sleep, experienced more awakenings during the night and less recuperative sleep, were more tired, and took more naps during the day than those without RLS. However, because of the non-specific nature of the one diagnostic question used in this survey, these sleep disturbances might have resulted partly or wholly from PLMs rather than the symptoms of RLS in this population.
The recently published National Sleep Foundation poll (Phillips et al 2006) (1506 US adults) asked respondents how often, in the past year, they had experienced unpleasant feelings in their legs at night with an urge to move when lying down to sleep. A total of 9.7% of those answering reported having these symptoms at least a few nights per week and that the feelings were worse in the evening or at night than at other times of the day, thus meeting the authors’ criteria for RLS. This group was more likely to stay up longer than planned for more than a few nights per week, take more than 30 minutes to fall asleep, sleep for less than 6 hours per night, have body twitches–movements during the night and report symptoms of insomnia, than those not at risk of RLS (p<0.05 for all). Furthermore, those at risk of RLS were also more likely to report daytime fatigue (>55% of respondents) and problems of daytime functioning, including being late to or missing work, making errors at work, missing events, and driving while drowsy (p<0.05 for all).
Another large (n=23 052), multinational, study of patients in primary care described a group of patients who experienced all four diagnostic symptoms of RLS at least twice weekly, with these symptoms having some negative impact on their quality of life (n=551) (Hening et al 2004). Most of these patients (88%) reported at least one sleep-related symptom along with the symptoms of RLS, and 43% of patients rated a sleep-related symptom as their most troublesome. In addition, when these patients had symptoms of RLS, 69% of them took more than 30 minutes to fall asleep and 60% reported waking three or more times per night. A similar study (n=16202) identified a group of patients with the diagnostic symptoms of RLS from general-population surveys in six countries (Allen et al 2005). Of the patients with at least twice-weekly symptoms that caused moderate distress (n=416), 38% reported that problems related to sleep disturbance were their most troublesome symptoms. In addition, 61% reported disturbed or interrupted sleep, 48% reported an inability to fall asleep, and around 40% of patients reported that they had insufficient sleep.
Two population surveys conducted in Sweden have investigated the prevalence of RLS and its impact on other complaints including sleep disturbance. In a survey of men (n=4000), sleep complaints were more frequent among those who met the diagnostic criteria for RLS, than those who did not (Ulfberg et al 2001a). The odds ratio (OR) for not being refreshed at awakening was 3.8 (95% CI 2.8–5.3) for those with RLS. In addition, those men with RLS reported more problems in initiating and maintaining sleep, and more disturbed sleep. Similar problems of insomnia were also reported from a survey of women (n=200) from the same region (Ulfberg et al 2001b). The OR for not being refreshed at awakening was 4.7 (95% CI 1.5–14.8) for women with symptoms of RLS.
In addition to these surveys of people in the general population who have symptoms of RLS, polysomnographic (PSG) studies of patients with RLS have shown that patients with RLS demonstrated detrimental effects on sleep. These patients had a reduced total sleep time (mean 326.3 minutes) compared with controls (383.3 minutes); reduced sleep efficiency (mean 73.2% vs 86.6%); and showed increased numbers of awakenings (mean 12.2 vs 7.4). They also showed a reduction in subjective sleep quality and efficacy (measured using the Pittsburgh Sleep Quality Index), compared with those without RLS (Saletu et al 2002a).
Effects in patients with secondary RLS
Sleep disturbance and symptoms of RLS are common in patients with ESRD who are undergoing dialysis (Walker et al 1995). In a survey of 54 patients undergoing dialysis, 57% reported symptoms of RLS, and most of these patients reported symptoms of sleep disturbance (Walker et al 1995). Three quarters of these patients complained of daytime sleepiness, 48% believed that symptoms of RLS caused delayed sleep onset, and 42% stated that these symptoms caused frequent awakenings.
In a large (n=333) study of patients undergoing dialysis, that used questions based on the diagnostic symptoms of RLS, 14% of patients were identified as meeting these diagnostic criteria (Mucsi et al 2005). Compared with patients who did not have RLS, these patients were twice as likely to have clinically significant insomnia (defined as a score of at least 10 on the Athens Insomnia Scale) and more frequently reported specific sleep problems, such as problems with sleep initiation and sleep fragmentation. Another survey of patients undergoing dialysis showed that 70% of patients with ESRD reported the diagnostic symptoms of RLS (Hui et al 2002). Almost all of those patients also reported sleep disturbance, such as sleep-onset and sleep-maintenance insomnia, and daytime sleepiness.
Sleep problems are also common in pregnancy. A large (n=16528) survey of pregnant women was conducted in Japan to assess the prevalence of RLS in these women and its relationship to sleep problems (Suzuki et al 2003). RLS (assessed using one question about the presence of paresthesias) was present in 20% of women, and was associated with a reduction in sleep duration, difficulties in initiating and maintaining sleep, early-morning waking, and excessive daytime sleepiness.
The relationship between RLS and PLMs
A large proportion of patients with RLS also experience large numbers of PLMs (an incidence of more than five PLMs per hour of sleep – PLM index [PLM-I] – is considered greater than normal). However, the relationship between the presence of PLMs and sleep disturbances is unclear.
In a PSG study of 133 patients with RLS, 80% had a PLM-I that was greater than 5 (mean PLM-I was 28.9; the index of periodic leg movements during the wake time – PLMW-I – was similar at 29.3) (Montplaisir et al 1997). Most of these patients reported in a questionnaire that they had difficulty falling asleep and maintaining sleep because of RLS symptoms. However, there was no correlation between the PLM index and either the number of awakenings or sleep efficiency. These results suggest that PLMs are not a major cause of sleep disruption in RLS. This conclusion is supported by data from a large (n = 1124) retrospective review of patients undergoing PSG for assessment of sleep-disordered breathing (SDB, an umbrella term that covers a spectrum of common sleep-related respiratory disorders), which is often associated with PLMs (Chervin 2001). In that study, neither daytime sleepiness (assessed by multiple sleep latency tests) nor subjective sleep problems were associated with the presence of PLMs. In addition, in a study of patients with RLS, a reduction in subjective sleep quality was significantly correlated with the PLM-I on the first but not the second night of testing (Hornyak et al 2004). The authors account for this correlation in the first night of testing as an adaptation effect to the PSG protocol, probably resulting in more superficial sleep and heightened perception on the first night of testing.
On the other hand, other authors have reported that PLMs are associated with significant levels of sleep disturbance. EEG monitoring has shown that even when PLMs are not associated with microarousals or full awakening, they almost always produce EEG activation and changes in heart rate (Sforza et al 1999). Possible effects of PLMs were also reported in a PSG study of 33 patients with RLS (who had a mean PLM-I of 49) and 26 patients with PLM disorder (experienced as PLMs only, rather than the sensory and motor symptoms that are characteristic of RLS; PLM-I was 46). Patients with RLS showed reduced total sleep time and sleep efficiency and increased wakefulness during the sleep period, compared with healthy controls (Saletu et al 2002a). Patients with PLM disorder showed an increase in nocturnal awakenings, but no changes in other sleep parameters in comparison with controls.
Some of these differences in results may be accounted for by differences in the etiology of PLMs in different disorders. In SDB, PLMs may be associated with respiratory effort (Briellmann et al 1997; Baran et al 2003), whereas the etiology of PLMs in RLS may be, at least partly, a consequence of central dopaminergic dysfunction (Allen and Earley 2001). In RLS, the sensory and motor symptoms of RLS, combined with PLMs, may have more pronounced effects on patients’ sleep than either disorder alone. Thus, in patients with RLS secondary to renal disease, the presence of either RLS or PLMs alone produced detrimental effects on sleep parameters (Rijsman et al 2004b). Moreover, the combination of both RLS and PLMs produced greater effects in reducing sleep quality and efficacy, and more complaints of insomnia, than in patients without RLS or PLMs, or in those patients with only one of these conditions. Likewise, in a small study of patients with RLS and PLMs, subjective sleep efficacy was lower in patients with both disorders than in those with PLMs alone (Inoue et al 2002). These results indicate that it may be a combination of the symptoms of RLS and PLMs that result in greater detrimental effects on sleep parameters.
Treatment of the sleep problems associated with RLS
Dopaminergic therapies
Dopaminergic drugs are considered the first-line therapy for moderate-to-severe RLS (Silber et al 2004), and these treatments have also been shown to improve sleep disturbances in these patients.
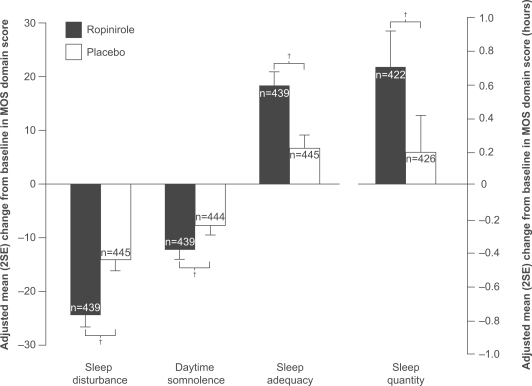
Ropinirole has recently been approved for the treatment of moderate-to-severe primary RLS in eight countries including the USA, France, Switzerland, Australia, and New Zealand. This approval followed a large clinical-trial program; three key clinical trials of this program – TREAT RLS 1 and 2 (Therapy with Ropinirole: Efficacy And Tolerability in RLS 1 and 2) and TREAT RLS US – investigated the efficacy of ropinirole in treating the diagnostic symptoms of primary RLS and its associated sleep disturbance (Trenkwalder et al 2004; Walters et al 2004; Bogan et al 2006). These three multicenter, randomized, double-blind, parallel-group, placebo-controlled trials involved a total of 932 patients with moderate-to-severe primary RLS determined clinically as well as measured by the International Restless Leg Scale (IRLS) scale. Patients were treated for 12 weeks with ropinirole, 0.25–4 mg/day, or placebo. Ropinirole treatment produced statistically significant improvements, in comparison with placebo, in the symptoms of RLS as measured by the overall IRLS total score and the proportion of responders on the Clinical Global Impression-Improvement (CGI–I) scale. In addition, in the TREAT RLS 1 and 2 trials, ropinirole treatment produced statistically significant improvements on each of the four domains assessed on the Medical Outcomes Study (MOS) Sleep Scale. Thus, ropinirole treatment produced reductions in sleep disturbance and daytime somnolence, and increases in sleep adequacy and sleep quantity. Similarly, in the TREAT RLS US study, improvements in ropiniroletreated patients were seen in each of the four domains assessed on the MOS Sleep Scale, although the improvement did not reach statistical significance compared with placebo for daytime somnolence. Figure 1 shows pooled MOS data results from all three trials. In another trial, the maintenance of these beneficial effects of ropinirole was demonstrated out to 9 months of treatment (Karrasch et al 2004). The effects of ropinirole on PLMs were assessed in 65 patients in the RESET PLM (Ropinirole Efficacy and SafEty in the Treatment of PLMs) study (Allen et al 2004). Ropinirole, compared with placebo, given for 12 weeks was associated with a statistically significant reduction in the PLM-I (from 48.5 to 11.8), in the number of PLMs producing arousal (PLMA-I; from 7.0 to 2.5), and in PLMW-I (from 56.5 to 23.6). Likewise, in the TREAT RLS US trial, ropinirole, compared with placebo, produced a statistically significant reduction in PLM-I (PLM per hour when supine throughout the night, measured using actigraphy over 3 nights) from 39.8 to 15.6 (Bogan et al 2006). The most common adverse event during these clinical trials of ropinirole was nausea; most adverse events were mild or moderate in severity, and their incidence declined over time.
Figure 1.
Adjusted mean change from baseline in domains of the Medical Outcomes Study Sleep Scale following treatment with ropinirole or placebo in patients with RLS, at week 12 last observation carried forward. Benefits are indicated by positive changes in sleep quantity and adequacy, and negative changes in sleep disturbance and somnolence. Pooled data are from three studies with similar design, TREAT RLS 1, 2 and US (Trenkwalder et al 2004; Walters et al 2004; Bogan et al 2006)a.
Adjusted mean treatment difference:
Sleep disturbance: −10.4; 95% CI: −13.4, −7.4; p<0.001
Daytime somnolence: −4.6; 95% CI: −7.0, −2.2; p<0.001
Sleep adequacy: 11.8; 95% CI: 8.3, 15.2; p<0.001
Sleep quantity: 0.5 hours; 95% CI: 0.3, 0.7; p<0.001
a In each individual study, adjusted mean treatment differences were in favor of ropinirole compared with placebo on all domains, except that statistical significance was not reached for daytime somnolence in the TREAT RLS US study.
†p<0.001.
Abbreviations: CI, confidence interval; MOS, Medical Outcomes Study Sleep Scale.
Small studies with the dopamine precursor levodopa (L-dopa) have indicated that this drug is effective in the management of both the symptoms of and sleep problems associated with RLS; and this drug is registered for use in the treatment of primary RLS or symptomatic RLS as a result of renal impairment requiring dialysis in Germany and Switzerland. L-dopa was shown to be effective in improving subjective sleep quality and efficacy (Montplaisir et al 1986; Brodeur et al 1988; Trenkwalder et al 1995; Benes et al 1999). In addition, L-dopa had some efficacy in reducing objective (PSG) sleep parameters. In a study of 32 patients, L-dopa was associated with a smaller PLM-I compared with placebo (45 vs 63) and greater total sleep time (316 minutes vs 281 minutes) (Trenkwalder et al 1995). In seven patients, L-dopa significantly improved sleep efficiency (from 88.9% to 94.4%) (Montplaisir et al 1986). In addition, in a study of six patients, L-dopa significantly reduced sleep latency from 9.2 minutes to 3.7 minutes (Brodeur et al 1988). However, one of the problems associated with L-dopa therapy in RLS is its relatively short duration of action, resulting in wearing-off of effects during the night (Trenkwalder et al 1995). This problem can be alleviated by treatment with both a regular-release and sustained-release formulation of L-dopa. Such a combination treatment has been shown to improve subjective sleep parameters – reducing nocturnal awakenings and daytime fatigue – and to reduce PLMs (Collado-Seidel et al 1999; Saletu et al 2003). Another potential problem during long-term L-dopa therapy is augmentation of symptoms (earlier onset with an increase in severity and the involvement of other limbs), which can limit the use of this therapy in a large proportion of patients (Allen and Earley 1996). Therefore, in a recent algorithm for the treatment of RLS, L-dopa was recommended only for the treatment of intermittent RLS, as this infrequent use of L-dopa may carry less risk for the development of augmentation (Silber et al 2004).
Some short-term, double-blind, placebo-controlled studies of pergolide have demonstrated its efficacy in improving the symptoms of RLS, and both subjective and objective (PSG) measures of sleep, and on PLMs in patients with RLS (Earley et al 1998; Wetter et al 1999). In a study of 30 patients, pergolide, in comparison with placebo, was associated with greater total sleep time (mean 373.6 minutes vs 261.9 minutes) and a reduced PLM-I (5.7 vs 55.0) (Wetter et al 1999). In addition, in 16 patients, pergolide produced improvements in sleep efficiency (from 61% to 79%) and reduced PLM-I (from 48.9 to 14.5) (Earley et al 1998). Long-term (median duration of about 1 year) follow-up of 22 patients from one of these trials, taking open-label per-golide, suggested that its positive treatment effects on PLMs and sleep parameters were maintained for this time (Stiasny et al 2001). However, pergolide is an ergot-related dopamine agonist, and is therefore subject to safety concerns typical of ergot derivatives, such as the development of cardiac-valve disease (Pritchett et al 2002; Baseman et al 2004). A large (n=302), 6-month, open-label trial of cabergoline has demonstrated improvements in patients’ symptoms of RLS and in their subjective sleep quality (Benes et al 2004), and a small study (n=9) showed a reduction in PLM-I (from 195.8 to 26.4; this measure is for all PLMs, not just those while asleep) and an increase in total sleep time (from 302.7 minutes to 379.0 minutes) (Stiasny et al 2000). However, cabergoline, as an ergot-related dopamine agonist, may be subject to the same safety concerns as pergolide.
There are a few reports of small studies (one open-label, two placebo-controlled) of the non-ergot dopamine agonist pramipexole. These have shown that pramipexole improved patients’ symptoms of RLS and their subjective ratings of sleep (Saletu et al 2002b; Stiasny-Kolster and Oertel 2004). In addition, these studies showed that pramipexole improved objective measures of sleep, and PLMs. In 10 patients, pramipexole was associated with a PLM-I of 1.7, compared with 68.0 for placebo (Montplaisir et al 1999). In a study of 11 patients, pramipexole, compared with placebo, reduced PLM-I (8.2 vs 35.0) and increased total sleep time (377.6 minutes vs 301.1 minutes) (Saletu et al 2002b). Similarly, in 17 patients, pramipexole reduced PLM-I (11.3 vs 73.3 for placebo), and increased total sleep time (374.5 minutes vs 215.4 minutes) and sleep efficiency (77.1% vs 44.5%) (Stiasny-Kolster and Oertel 2004). As expected for a dopamine agonist, the main adverse events related to pramipexole in these trials were gastrointestinal effects, particularly nausea.
Gabapentin therapy
Gabapentin, an analog of gamma-aminobutyric acid, was compared with L-dopa in an open-label study involving patients with RLS secondary to renal disease (Micozkadioglu et al 2004). After 4 weeks’ treatment, both therapies improved symptoms of RLS, and sleep quality, latency, and duration. In addition, gabapentin produced statistically significantly greater benefits than L-dopa on RLS symptoms, on sleep quality, latency, and disturbance. In a small (n=24) double-blind comparison with placebo, in a group of patients most of whom had primary RLS, gabapentin produced statistically significant reductions in the symptoms of RLS, in PLM-I (11.1 compared with 20.8 for placebo), and improvements in sleep time (6.0 hours vs 5.5 hours) and sleep efficiency (84.7% vs 79.4%) (Garcia-Borreguero et al 2002). The most common adverse event during gabapentin in this trial treatment was malaise.
Conclusions
Sleep problems are extremely common in patients with RLS. These sleep disturbances may result from a combination of the sensory symptoms of RLS and the presence of PLMs and PLMW. Subjective sleep disturbances, objective measures of sleep, and PLMs can all be improved by the standard treatment for primary RLS, dopamine-agonist therapy.
Footnotes
Disclosures
Dr Richard K Bogan has been a consultant for GSK, Cephalon, and Jazz Pharmaceuticals, and has participated in clinical research of RLS for Boehringer Ingelheim, GSK, Schwartz Pharmaceutical, and Xenoport. He has been on the speakers bureau for GSK, Takeda, Cephalon, Jazz, Sanofi Aventis, Wyeth, and Sepracor.
References
- Allen R, Becker PM, Bogan R, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep. 2004;27:907–14. doi: 10.1093/sleep/27.5.907. [DOI] [PubMed] [Google Scholar]
- Allen R, Picchietti D, Hening W, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Allen RP, Earley CJ. Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep. 1996;19:205–13. doi: 10.1093/sleep/19.3.205. [DOI] [PubMed] [Google Scholar]
- Allen RP, Earley CJ. Restless legs syndrome:a review of clinical and pathophysiologic features. J Clin Neurophysiol. 2001;18:128–47. doi: 10.1097/00004691-200103000-00004. [DOI] [PubMed] [Google Scholar]
- Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact:REST general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- Baran AS, Richert AC, Douglass AB, et al. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep. 2003;26:717–20. doi: 10.1093/sleep/26.6.717. [DOI] [PubMed] [Google Scholar]
- Baseman DG, O’Suilleabhain PE, Reimold SC, et al. Pergolide use in Parkinson disease is associated with cardiac valve regurgitation. Neurology. 2004;63:301–4. doi: 10.1212/01.wnl.0000129842.49926.07. [DOI] [PubMed] [Google Scholar]
- Benes H, Heinrich CR, Ueberall MA, et al. Long-term safety and efficacy of cabergoline for the treatment of idiopathic restless legs syndrome:results from an open-label 6-month clinical trial. Sleep. 2004;27:674–82. doi: 10.1093/sleep/27.4.674. [DOI] [PubMed] [Google Scholar]
- Benes H, Kurella B, Kummer J, et al. Rapid onset of action of levodopa in restless legs syndrome: a double-blind, randomized, multicenter, crossover trial. Sleep. 1999;22:1073–81. doi: 10.1093/sleep/22.8.1073. [DOI] [PubMed] [Google Scholar]
- Bjorvatn B, Leissner L, Ulfberg J, et al. Prevalence, severity and risk factors of restless legs syndrome in the general adult population in two Scandinavian countries. Sleep Med. 2005;6:307–12. doi: 10.1016/j.sleep.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Bogan RK, Fry JM, Schmidt MH, et al. Ropinirole in the treatment of patients with restless legs syndrome:a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 2006;81:17–27. doi: 10.4065/81.1.17. [DOI] [PubMed] [Google Scholar]
- Briellmann RS, Mathis J, Bassetti C, et al. Patterns of muscle activity in legs in sleep apnea patients before and during nCPAP therapy. Eur Neurol. 1997;38:113–18. doi: 10.1159/000113173. [DOI] [PubMed] [Google Scholar]
- Brodeur C, Montplaisir J, Godbout R, et al. Treatment of restless legs syndrome and periodic movements during sleep with L-dopa:a double-blind, controlled study. Neurology. 1988;38:1845–8. doi: 10.1212/wnl.38.12.1845. [DOI] [PubMed] [Google Scholar]
- Chervin RD. Periodic leg movements and sleepiness in patients evaluated for sleep-disordered breathing. Am J Respir Crit Care Med. 2001;164:1454–8. doi: 10.1164/ajrccm.164.8.2011062. [DOI] [PubMed] [Google Scholar]
- Collado-Seidel V, Kazenwadel J, Wetter TC, et al. A controlled study of additional sr-L-dopa in L-dopa-responsive restless legs syndrome with late-night symptoms. Neurology. 1999;52:285–90. doi: 10.1212/wnl.52.2.285. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Yaffee JB, Allen RP. Randomized, double-blind, placebo-controlled trial of pergolide in restless legs syndrome. Neurology. 1998;51:1599–602. doi: 10.1212/wnl.51.6.1599. [DOI] [PubMed] [Google Scholar]
- Garcia-Borreguero D, Larrosa O, de la Llave Y, et al. Treatment of restless legs syndrome with gabapentin:a double-blind, cross-over study. Neurology. 2002;59:1573–9. doi: 10.1212/wnl.59.10.1573. [DOI] [PubMed] [Google Scholar]
- Hening W, Walters AS, Allen RP, et al. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population:the REST (RLS Epidemiology, Symptoms, and Treatment) Primary Care study. Sleep Med. 2004;5:237–46. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hornyak M, Riemann D, Voderholzer U. Do periodic leg movements influence patients’ perception of sleep quality? Sleep Med. 2004;5:597–600. doi: 10.1016/j.sleep.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Hui DS, Wong TY, Li TS, et al. Prevalence of sleep disturbances in Chinese patients with end stage renal failure on maintenance hemodialysis. Med Sci Monit. 2002;8:CR331–6. [PubMed] [Google Scholar]
- Inoue Y, Nanba K, Honda Y, et al. Subjective sleep quality and suggested immobilization test in restless leg syndrome and periodic limb movement disorder. Psychiatry Clin Neurosci. 2002;56:293–4. doi: 10.1046/j.1440-1819.2002.00966.x. [DOI] [PubMed] [Google Scholar]
- Karrasch J, Haan J, Kruger AJ, et al. 2004Maintained efficacy with ropinirole:results of a multinational 36-week study of patients with RLS Sleep 27(Abstract suppl):A294:658 [Google Scholar]
- Micozkadioglu H, Ozdemir FN, Kut A, et al. Gabapentin versus levodopa for the treatment of Restless Legs Syndrome in hemodialysis patients:an open-label study. Ren Fail. 2004;26:393–7. doi: 10.1081/jdi-120039823. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Boucher S, Poirier G, et al. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome:a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Godbout R, Poirier G, et al. Restless legs syndrome and periodic movements in sleep:physiopathology and treatment with L-dopa. Clin Neuropharmacol. 1986;9:456–63. doi: 10.1097/00002826-198610000-00006. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Nicolas A, Denesle R, et al. Restless legs syndrome improved by pramipexole:a double-blind randomized trial. Neurology. 1999;52:938–43. doi: 10.1212/wnl.52.5.938. [DOI] [PubMed] [Google Scholar]
- Mucsi I, Molnar MZ, Ambrus C, et al. Restless legs syndrome, insomnia and quality of life in patients on maintenance dialysis. Nephrol Dial Transplant. 2005;20:571–7. doi: 10.1093/ndt/gfh654. [DOI] [PubMed] [Google Scholar]
- [NIH] National Institutes of Health 2005State-of-the-Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults National Institutes of Health State-of-the-Science Conference Statement6February2006[online]. Accessed 16 August 2006. URL: http://consensus.nih.gov/2005/2005InsomniaSOS026html.htm [Google Scholar]
- Phillips B, Hening W, Britz P, et al. Prevalence and correlates of restless legs syndrome:results from the 2005 National Sleep Foundation Poll. Chest. 2006;129:76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160:2137–41. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- Pritchett AM, Morrison JF, Edwards WD, et al. Valvular heart disease in patients taking pergolide. Mayo Clin Proc. 2002;77:1280–6. doi: 10.4065/77.12.1280. [DOI] [PubMed] [Google Scholar]
- Rijsman R, Neven AK, Graffelman W, et al. Epidemiology of restless legs in The Netherlands. Eur J Neurol. 2004a;11:607–11. doi: 10.1111/j.1468-1331.2004.00848.x. [DOI] [PubMed] [Google Scholar]
- Rijsman RM, de Weerd AW, Stam CJ, et al. Periodic limb movement disorder and restless legs syndrome in dialysis patients. Nephrology (Carlton) 2004b;9:353–61. doi: 10.1111/j.1440-1797.2004.00330.x. [DOI] [PubMed] [Google Scholar]
- Saletu B, Anderer P, Saletu M, et al. EEG mapping, psychometric, and polysomnographic studies in restless legs syndrome (RLS) and periodic limb movement disorder (PLMD) patients as compared with normal controls. Sleep Med. 2002a;3:S35–42. doi: 10.1016/s1389-9457(02)00147-8. [DOI] [PubMed] [Google Scholar]
- Saletu M, Anderer P, Hogl B, et al. Acute double-blind, placebo-controlled sleep laboratory and clinical follow-up studies with a combination treatment of rr-L-dopa and sr-L-dopa in restless legs syndrome. J Neural Transm. 2003;110:611–26. doi: 10.1007/s00702-003-0814-z. [DOI] [PubMed] [Google Scholar]
- Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Acute placebo-controlled sleep laboratory studies and clinical follow-up with pramipexole in restless legs syndrome. Eur Arch Psychiatry Clin Neurosci. 2002b;252:185–94. doi: 10.1007/s00406-002-0380-7. [DOI] [PubMed] [Google Scholar]
- Sforza E, Nicolas A, Lavigne G, et al. EEG and cardiac activation during periodic leg movements in sleep:support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- Silber MH, Ehrenberg BL, Allen RP, et al. An algorithm for the management of restless legs syndrome. Mayo Clin Proc. 2004;79:916–22. doi: 10.4065/79.7.916. [DOI] [PubMed] [Google Scholar]
- Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- Stiasny K, Robbecke J, Schuler P, et al. Treatment of idiopathic restless legs syndrome (RLS) with the D2-agonist cabergoline—an open clinical trial. Sleep. 2000;23:349–54. [PubMed] [Google Scholar]
- Stiasny K, Wetter TC, Winkelmann J, et al. Long-term effects of pergolide in the treatment of restless legs syndrome. Neurology. 2001;56:1399–402. doi: 10.1212/wnl.56.10.1399. [DOI] [PubMed] [Google Scholar]
- Stiasny-Kolster K, Oertel WH. Low-dose pramipexole in the management of restless legs syndrome. An open label trial. Neuropsychobiology. 2004;50:65–70. doi: 10.1159/000077943. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ohida T, Sone T, et al. The prevalence of restless legs syndrome among pregnant women in Japan and the relationship between restless legs syndrome and sleep problems. Sleep. 2003;26:673–7. doi: 10.1093/sleep/26.6.673. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Garcia-Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs syndrome:results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry. 2004;75:92–7. [PMC free article] [PubMed] [Google Scholar]
- Trenkwalder C, Stiasny K, Pollmacher T, et al. L-dopa therapy of uremic and idiopathic restless legs syndrome:a double-blind, crossover trial. Sleep. 1995;18:681–8. doi: 10.1093/sleep/18.8.681. [DOI] [PubMed] [Google Scholar]
- Ulfberg J, Nystrom B, Carter N, et al. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001a;16:1159–63. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- Ulfberg J, Nystrom B, Carter N, et al. Restless legs syndrome among working-aged women. Eur Neurol. 2001b;46:17–9. doi: 10.1159/000050750. [DOI] [PubMed] [Google Scholar]
- Walker S, Fine A, Kryger MH. Sleep complaints are common in a dialysis unit. Am J Kidney Dis. 1995;26:751–6. doi: 10.1016/0272-6386(95)90438-7. [DOI] [PubMed] [Google Scholar]
- Walters AS, Ondo WG, Dreykluft T, et al. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2:a 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord. 2004;19:1414–23. doi: 10.1002/mds.20257. [DOI] [PubMed] [Google Scholar]
- Wetter TC, Stiasny K, Winkelmann J, et al. A randomized controlled study of pergolide in patients with restless legs syndrome. Neurology. 1999;52:944–50. doi: 10.1212/wnl.52.5.944. [DOI] [PubMed] [Google Scholar]