Abstract
Transthyretin (TTR) accounts for a quarter of the protein content of ventricular cerebrospinal fluid (CSF) yet its exact role in the brain remains unknown. Patients with a diagnosis of depression have reduced CSF levels of TTR and the locus encoding the TTR gene has been implicated in a Danish pedigree of bipolar patients. Lithium, the major treatment for bipolar disorder in the UK, was subcutaneously infused into rats for 28 days in the form of lithium chloride using osmotic minipumps. In situ hybridizations using oligonucleotide probes targeted against the TTR transcript were performed on coronal brain sections. Lithium significantly reduced the level of transthyretin mRNA in the rat choroid plexus within the lateral and third ventricle. The down-regulation was confirmed using semi-quantitative reverse transcription PCR on dissected brain tissue. Recent studies in mice suggest that the TTR gene is implicated in depression-like behavior therefore this effect of lithium may be relevant to its use as a mood stabilizer or an adjuvant to antidepressant drugs.
Keywords: bipolar disorder, in-situ hybridization, mood stabilizer, minipump, (SQ)-RT-PCR
Introduction
Transthyretin (TTR) is a 55-kDa tetrameric protein that is synthesized both in the liver and in the choroid plexus of the brain and secreted into the plasma and cerebrospinal fluid (CSF) respectively. Although TTR can bind both thyroxin and retinol (via retinol binding protein) in the plasma, its precise role in the CNS has yet to be established. The regulation of TTR synthesis and secretion from the choroid plexus is independent from that in the liver (Dickson et al 1986) and it does not appear to be involved in the distribution of thyroid hormones throughout the brain, at least not in rodents (Blay et al 1993; Palha et al 2000). TTR has been implicated in both Alzheimer’s disease (Riisoen 1988) and affective disorder (Sullivan et al 1999) as patients suffering from these disorders show reduced levels of TTR in their CSF compared to controls. A recent behavioral study also suggests that TTR-null mice demonstrate behaviors consistent with those of subjects which have been administered antidepressants in some preclinical screens (Sousa et al 2004).
Evidence that TTR is implicated in the pathophysiology of bipolar disorder comes from a genetic study that demonstrated positive LOD scores for markers on Chromosome 18q12 (the same region as the TTR locus) with a Danish pedigree of BD patients (Ewald et al 1997). Lithium is the major treatment for bipolar disorder in the UK but the neural mechanisms underlying its therapeutic efficacy are not known. One of the effects lithium has at the molecular level is to modulate AP-1 binding to DNA (reviewed (Ikonomov et al 1999)) and the TTR promoter has been shown to contain a functional AP-1 binding site (Qian et al 1995). To investigate if in vivo lithium administration regulated brain TTR expression we examined the effects of 28 days continuous infusion in the rat.
Methods
Subjects and treatment
Experiments were conducted in accordance with the UK Animals (Scientific Procedures) Act 1986. Male Lister hooded rats (257 ± 3 g) were obtained from Harlan UK and housed in pairs in polycarbonate cages kept within a temperature-controlled room (21°C) on a 12-h light-dark cycle (lights on at 6 am). Food and water were available ad lib. Rats were anesthetized using halothane (5% for induction and 2% maintenance) and osmotic minipumps (Alzet) were implanted subcutaneously through a skin incision made at the level of the shoulder. Minipumps were preloaded with either vehicle (n = 6) or lithium chloride dissolved in sterile water (n = 6) at a concentration designed to provide a constant infusion of 75 mg/kg/day. A solution of NaCl was offered in water bottles as a precaution against lithium toxicity.
Twenty-eight days following minipump implantation the rats were killed and the brains removed and frozen in isopentane (−35°C). The mean plasma lithium concentration, measured using flame emission spectrophotometry (Instrument Laboratory Model 943), was 0.35 ± 0.04 mmol/L. Brains were stored at −80°C before being sectioned coronally (20 μm) between 4.7 mm anterior and 3.8 mm posterior to Bregma on a cryostat. Sections were thaw-mounted onto superfrost slides (BDH) and stored at −80°C for no more than 2 weeks prior to further processing.
In-situ hybridization
A synthetic oligonucleotide probe designed to specifically target rat mRNA for TTR was synthesized by Invitrogen (UK). Probes sequence: TTR, bases 38–82 (5′- AGCGAGGCAGAGGAGGAACAGGCGAAGGGAAGCCATCCTGTCAGG-3′). Probes for two housekeeping genes β-actin and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were synthesized by Life Technologies (GIBCO, BRL). Probe sequences: β-actin, bases 251–295 (5′- TGGACCGGGACGGAGGAGCTGCAAGGAGGTTGTACTCGCGGGTGG-3′) and G3PDH, bases 698–737 (5′-CACGGAAGGCCATGCCAGTGAGCTTCCCGT-3′). Oligonucleotides were labeled with 33P-dATP (3000 Ci/mmol) by tailing the 3′-end using terminal transferase (Promega) as described previously (Henry et al 1999). Probes were purified using Micro Bio-Spin chromatography columns (P-30 Tris RNase-free, Bio-Rad).
Sections were prepared, incubated with labeled probes for 18 hours at 42°C and washed as previously described (Henry et al 1999). After dehydration, the slides were placed in a cassette and exposed to X-ray film (BioMax MR-1, Kodak) and then developed using a Kodak automatic developer (M-35M X-OMAT Processor). Following development of autoradiographs, all slides were counter-stained with Haematoxylin and Eosin (H&E, DAKO). Analysis of H&E-stained sections indicated no overt deterioration in section quality or morphology. Densitometric analysis of autoradiographs was performed using a Microcomputer Imaging Device (MCID) system (Imaging Research Inc.). At a 2-hour exposure time the measured optical density remained on the linear part of the film response and was chosen for further analysis.
Optical density measurements were obtained for the lateral ventricles by taking 2 readings (left and right) from 3 sections at each of 4 regions of interest (level 5: AP + 0.2 mm, Level 6: AP −2.2 mm, Level 7: AP −2.8 mm and Level 8: AP −3.8 mm) for each rat. For the third ventricle a single reading was taken from 3 sections at 3 regions of interest (Level 6, 7, and 8) for each rat. The mean value for each level was calculated after background subtraction for all subjects. The data were analyzed using analysis of variance for repeated measures (ANOVAR) with GROUP as the between subject factor and LEVEL as the within subject factor.
Semi-quantitative (SQ)-RT-PCR
The hippocampus together with associated choroid plexus (Puskas et al 2003) was removed from another set of rats treated with lithium (n = 3) or vehicle (n = 3) in an identical manner. Total RNA was isolated from each before being pooled into single control and treated samples that were treated to remove contaminating DNA. 1 μg from each pool was reverse transcribed in the presence of 20 pmol oligo(dT)18 primer in a final reaction volume of 20 μL. After incubation at 42oC for 1 hour the enzyme was heat-inactivated and resulting cDNA was diluted to 10 ng/μL and integrity checked by PCR using β-actin or TTR gene primer pairs. Each 50 μl PCR reaction contained; 10 ng cDNA, 10 mM Tris-HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 200 μM each dNTP, 100 ng each primer, and 2.5 U AmpliTaq Gold. Reactions were initiated for 10 min at 95°C and then continued with 35 cycles at; 94°C for 1 min, 45°C for 1 min and 72°C for 2 min. Cycle number optimization was performed with 10 ng cDNA per reaction and was determined by graphical means by plotting cycle number, in 2 cycle increments ranging from 18 to 30 cycles, against total counts per PCR product. The optimal number of cycles was determined to be when the accumulation of product was linear in a dilution series. Following optimization (data not shown) radioactive SQ-RT-PCR was carried out by addition of 0.5 μCi [α-33P]dATP per reaction. Products were run on 6% non-denaturing acrylamide-TBE gels (Novex). Total counts of radioactivity per band were recorded on the Storm 840 phosphorimager and subject to imageQuant analysis. Counts for TTR from control and treated samples were normalized to respective β-actin counts at each dilution and the values obtained from the treated sample were recorded as a percentage of control levels.
Results
In-situ hybridization
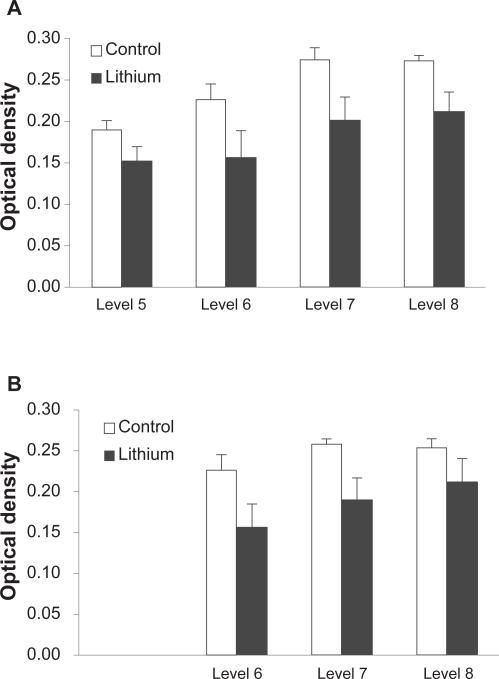
The TTR signal was confined to the lateral and third ventricles in areas of the brain that contain choroid plexus: (Figure 1A, D) consistent with previous studies (Herbert et al 1986; Stauder et al 1986). Treatment with lithium chloride reduced the mean optical density in both the lateral (Figure 2A) and third (Figure 2B) ventricles. There were significant effects of GROUP for both the lateral (F1,10= 5.8, p <0.05) and third ventricle (F1,10= 5.2, p <0.05) analyses confirming that lithium significantly reduced TTR expression. There were also significant effects of LEVEL in the expression of TTR for both lateral (F3,30= 18.7, p <0.001) and third (F2,20= 5.2, p <0.05) ventricles but no significant GROUP by LEVEL interactions suggesting that although there was significant variation in the expression of TTR rostrocaudally throughout the ventricular system, the decrease in TTR gene expression following lithium treatment occurred consistently across the regions of interest.
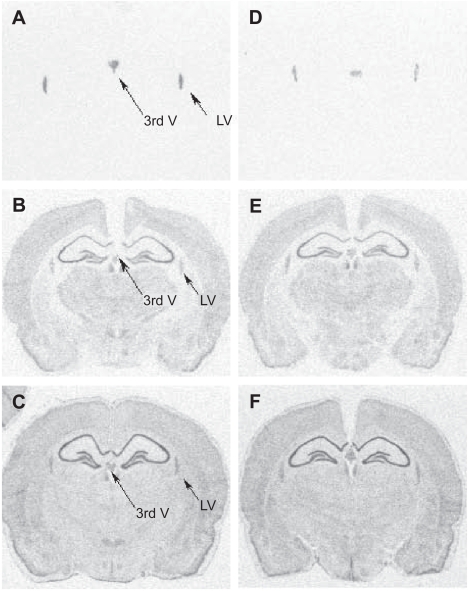
Figure 1.
Autoradiographs of coronal sections (AP −2.8 mm) from a typical vehicle-treated (panel A–C) or lithium-treated (panel D–F) animal hybridized with 3’ end 33P-labelled oligoprobes to TTR (A, D), glyceraldehyde-3-phosphate dehydrogenase (B,E), and β-actin (C,F) mRNA. Note that the TTR signal (indicated by arrows) is restricted to the choroid plexus of the 3rd ventricle (3rdV) and lateral ventricle (LV) and is weaker in the lithium-treated brain. The signal distribution for the house-keeping genes G3PDH or β-actin from adjacent sections was similar to that previously described and did not differ between the groups.
Figure 2.
Effect of lithium treatment on TTR mRNA expression throughout the brain. Semi-quantitative densitometric analysis revealed a significant decrease in optical density in both the lateral ventricle (A) and the third ventricle (B) in rats treated with lithium chloride (n = 6) compared with vehicle (n = 6) at each level. Data represent mean of 6 optical density readings for lateral ventricle and 3 optical density readings for third ventricle ± SEM.
Optical density readings for oligonucleotide probes targeted against the housekeeping genes β-actin (Figure 1B and E) or G3PDH (Fig 1C and F) obtained from the lateral and third ventricle of adjacent sections showed similar levels of expression in lithium-treated and vehicle treated groups (Table 1). Statistical analysis (ANOVA) confirmed no significant differences between the two groups in the overall levels of expression of either β-actin (p >0.5) or G3PDH (p >0.1).
Table 1.
Expression of mRNA for house-keeping genes in the choroid plexus region of the ventricles following infusion of vehicle or lithium chloride for 28 days
| β-actin
|
G3PDH
|
|||
|---|---|---|---|---|
| Lat ventricle | 3rd ventricle | Lat ventricle | 3rd ventricle | |
| Vehicle(n = 6) | 0.143 ± 0.005 | 0.136 ± 0.008 | 0.082 ± 0.019 | 0.077 ± 0.026 |
| Lithium(n = 6) | 0.141 ± 0.004 | 0.130 ± 0.005 | 0.072 ± 0.006 | 0.063 ± 0.012 |
Notes: Sections adjacent to those used for TTR mRNA expression (level 7) were hybridized with labeled probes for β-actin or G3PDH. For the lateral ventricle the mean value (±SE) was calculated from 2 optical density readings (left and right) in 3 sections and for the third ventricle from a single reading in 3 sections.
Semi-quantitative (SQ)-RT-PCR
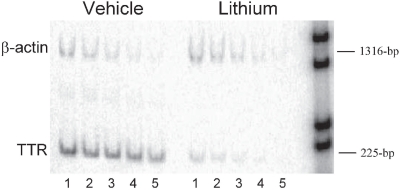
The expression of β-actin did not appear to change between control and lithium treated tissue whereas TTR expression appeared reduced at all dilutions (Figure 3). The normalized expression of TTR in tissue from vehicle treated rats was 799.3 and from lithium-treated tissue was 49.8. Relative expression of TTR following lithium treatment was therefore reduced by 94% compared with vehicle.
Figure 3.
Semi-quantitative-RT-PCR from lithium (n = 3) and vehicle-treated rats (n = 3). Expression levels were studied using oligonucleotides directed towards TTR: primers 5’- AAT ACG CAG AGG TGG TTT TC-3’ and 5’-GGC ATC TTC CCG AGT TG-3’ and to β-actin: primers 5’-TTG TAA CCA ACT GGG ACG ATA TGG-3’ and 5’-GAT CTT GAT CTT CAT GGT GCT AGG-3’. Shown is an example of the accumulation of product with 30 cycles of PCR for a serial dilution of template cDNA ranging from; lane 1 = 10 ng, 2 = 5 ng, 3 = 2.5 ng, 4 = 1.25 ng and 5 = 0.625 ng. Product sizes are indicated relative to 1 kb DNA markers (M).
Discussion
Twenty-eight days of lithium treatment significantly down regulated TTR mRNA expression in the rat. In-situ hybridization demonstrated an average 25% decrease in TTR mRNA expression in the choroid plexus from rats treated chronically with lithium when compared to vehicle treated animals. Confirmation that mRNA for house-keeping genes was not significantly affected by treatment demonstrated that the decrease in TTR mRNA expression was not due to a non-specific loss of tissue in the lithium treated brain sections. The effect of lithium was confirmed in dissected brain tissue containing both hippocampus and choroid plexus using SQ-RT-PCR with a 94% decrease in TTR mRNA in the pooled treated sample. While there is no clear explanation why SQ-RT-PCR detected a larger down-regulation of TTR mRNA expression, it is not unusual to observe discrepancies between absolute levels of mRNA detected using different experimental techniques (Riesewijk et al 2003). Importantly SQ-RT-PCR confirmed the same direction of altered TTR mRNA expression following 28 days of treatment with lithium chloride.
The present study did not investigate whether decreased TTR mRNA expression resulted in a reduced level of protein in the CSF. A previous study found that a dose of nicotine that produced an increase of approximately 60% in TTR mRNA expression measured using SQ-RT-PCR also significantly increased TTR protein levels in the CSF by ~12% (Li et al 2000).
It is unclear exactly what the consequences of a reduction in TTR levels might be for neurotransmission within the CNS. Neurochemical measures from the TTR-null mouse suggest that a lifetime absence produces an increase in the levels of norepinephrine in the limbic forebrain (Sousa et al 2004). A more recent study has also demonstrated increased levels of the amidated neuropeptide transmitters neuropeptide Y and substance P as a consequence of overexpression of peptidylglycine α-amidating mono-oxygenase (Nunes et al 2006) in TTR-null mice. TTR has been shown also to sequester the protein beta-amyloid (Aβ), the major component of the senile plaques found in Alzheimer brains, and prevent its aggregation in vitro (Schwarzman et al 1994). In addition, selective antibody blockade of TTR in a mutant mouse that over-expresses the amyloid precursor protein (APPSW) leads to increased Aβ deposition and cell death (Stein et al 2004). Previous studies have shown that ginkgo biloba (Watanabe et al 2001), omega 3 fatty acids (Puskas et al 2003), nicotine (Li et al 2000) or estradiol (Tang et al 2004) produce increased TTR mRNA expression and this was considered to be a beneficial effect. Although we have shown that lithium down-regulates TTR, it may also reduce Aβ levels by inhibiting its production both in vitro and in transgenic mice (Su et al 2004).
With regard to the role of lithium in affective disorder, the lithium-induced reduction in the levels of TTR mRNA may be consistent with observation that TTR-null mice behave in a manner similar to mice given antidepressants prior to the forced swim test (Sousa et al 2004). This may or may not be mediated through neuropeptide Y which is increased in both TTR-null mice (Nunes et al 2005) and following chronic lithium administration in rats (Zachrisson et al 1995). Central administration of NPY also demonstrates antidepressant activity when administered centrally (Redrobe et al 2002). On the other hand, TTR protein levels in the CSF of some depressed patients have been found to be reduced (Sullivan et al 1999), suggesting that there may be no simple relationship between TTR function and mood state. Although lithium is used clinically to augment antidepressant drug therapy (Fawcett 2003) its primary indication is as a mood stabilizer or anti-manic agent in bipolar disorder. In the absence of a useful animal model of bipolar disorder, further studies with additional mood stabilizing agents such as carbamazepine or sodium valproate may help to determine if a decrease in TTR is specific to lithium or whether it has significance with regard to the clinical efficacy of mood stabilizers in general.
Acknowledgments
This research was generously supported by Organon Laboratories Ltd. The authors would like to thank Mark Anderson at Organon for preparation of rat brains and Nik Harries for carrying out SQ-RT-PCR.
Footnotes
References
- Blay P, Nilsson C, Owman C, et al. Transthyretin expression in the rat brain: effect of thyroid functional state and role in thyroxine transport. Brain Res. 1993;632:114–20. doi: 10.1016/0006-8993(93)91145-i. [DOI] [PubMed] [Google Scholar]
- Dickson PW, Aldred AR, Marley PD, et al. Rat Choroid-Plexus Specializes in the Synthesis and the Secretion of Transthyretin (Prealbumin) - Regulation of Transthyretin Synthesis in Choroid-Plexus Is Independent from That in Liver. J Biol Chem. 1986;261:3475–8. [PubMed] [Google Scholar]
- Ewald H, Mors O, Koed K, et al. Susceptibility loci for bipolar affective disorder on chromosome 18? A review and a study of Danish families. Psychiatr Genet. 1997;7:1–12. doi: 10.1097/00041444-199700710-00001. [DOI] [PubMed] [Google Scholar]
- Fawcett JA. Lithium combinations in acute and maintenance treatment of unipolar and bipolar depression. J Clin Psychiatry. 2003;64(Suppl 5):32–7. [PubMed] [Google Scholar]
- Henry B, Crossman AR, Brotchie JM. Effect of repeated L-DOPA, bromocriptine, or lisuride administration on preproenkephalin-A and preproenkephalin-B mRNA levels in the striatum of the 6-hydroxydopamine-lesioned rat. Exp Neurol. 1999;155:204–20. doi: 10.1006/exnr.1998.6996. [DOI] [PubMed] [Google Scholar]
- Herbert J, Wilcox JN, Pham KT, et al. Transthyretin: a choroid plexus-specific transport protein in human brain. The 1986 S. Weir Mitchell award. Neurology. 1986;36:900–11. doi: 10.1212/wnl.36.7.900. [DOI] [PubMed] [Google Scholar]
- Ikonomov OC, Manji HK. Molecular mechanisms underlying mood stabilization in manic-depressive illness: the phenotype challenge. Am J Psychiatry. 1999;156:1506–14. doi: 10.1176/ajp.156.10.1506. [DOI] [PubMed] [Google Scholar]
- Li MD, Kane JK, Matta SG, et al. Nicotine enhances the biosynthesis and secretion of transthyretin from the choroid plexus in rats: implications for beta-amyloid formation. J Neurosci. 2000;20:1318–23. doi: 10.1523/JNEUROSCI.20-04-01318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes AF, Saraiva MJ, Sousa MM. Transthyretin knockouts are a new mouse model for increased neuropeptide Y. Faseb Journal. 2006:19. doi: 10.1096/fj.05-4106fje. [DOI] [PubMed] [Google Scholar]
- Palha JA, Fernandes R, de Escobar GM, et al. Transthyretin regulates thyroid hormone levels in the choroid plexus, but not in the brain parenchyma: study in a transthyretin-null mouse model. Endocrinology. 2000;141:3267–72. doi: 10.1210/endo.141.9.7659. [DOI] [PubMed] [Google Scholar]
- Puskas LG, Kitajka K, Nyakas C, et al. Short-term administration of omega 3 fatty acids from fish oil results in increased transthyretin transcription in old rat hippocampus. Proc Natl Acad Sci U S A. 2003;100:1580–5. doi: 10.1073/pnas.0337683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Samadani U, Porcella A, et al. Decreased expression of hepatocyte nuclear factor 3 alpha during the acute-phase response influences transthyretin gene transcription. Mol Cell Biol. 1995;15:1364–76. doi: 10.1128/mcb.15.3.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Fournier A, et al. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology. 2002;26:615–24. doi: 10.1016/S0893-133X(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Riesewijk A, Martin J, van Os R, et al. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003;9:253–64. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- Riisoen H. Reduced prealbumin (transthyretin) in CSF of severely demented patients with Alzheimer’s disease. Acta Neurol Scand. 1988;78:455–9. doi: 10.1111/j.1600-0404.1988.tb03687.x. [DOI] [PubMed] [Google Scholar]
- Schwarzman AL, Gregori L, Vitek MP, et al. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci U S A. 1994;91:8368–72. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Hatterer JA, Herbert J, et al. Low levels of transthyretin in the CSF of depressed patients. Am J Psychiatry. 1999;156:710–15. doi: 10.1176/ajp.156.5.710. [DOI] [PubMed] [Google Scholar]
- Sousa JC, Grandela C, Fernandez-Ruiz J, et al. Transthyretin is involved in depression-like behaviour and exploratory activity. J Neurochem. 2004;88:1052–8. doi: 10.1046/j.1471-4159.2003.02309.x. [DOI] [PubMed] [Google Scholar]
- Stauder AJ, Dickson PW, Aldred AR, et al. Synthesis of transthyretin (pre-albumin) mRNA in choroid plexus epithelial cells, localized by in situ hybridization in rat brain. J Histochem Cytochem. 1986;34:949–52. doi: 10.1177/34.7.3458812. [DOI] [PubMed] [Google Scholar]
- Stein TD, Anders NJ, DeCarli C, et al. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: support for the amyloid hypothesis. J Neurosci. 2004;24:7707–17. doi: 10.1523/JNEUROSCI.2211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Ryder J, Li B, et al. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-beta precursor protein processing. Biochemistry. 2004;43:6899–908. doi: 10.1021/bi035627j. [DOI] [PubMed] [Google Scholar]
- Tang YP, Haslam SZ, Conrad SE, et al. Estrogen increases brain expression of the mRNA encoding transthyretin, an amyloid beta scavenger protein. J Alzheimers Dis. 2004;6:413–420. doi: 10.3233/jad-2004-6409. [DOI] [PubMed] [Google Scholar]
- Watanabe CM, Wolffram S, Ader P, et al. The in vivo neuromodulatory effects of the herbal medicine ginkgo biloba. Proc Natl Acad Sci U S A. 2001;98:6577–80. doi: 10.1073/pnas.111126298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachrisson O, Mathe AA, Stenfors C, et al. Region-Specific Effects of Chronic Lithium Administration on Neuropeptide-y and Somatostatin Messenger-Rna Expression in the Rat-Brain. Neurosci Lett. 1995;194:89–92. doi: 10.1016/0304-3940(95)11735-f. [DOI] [PubMed] [Google Scholar]