Abstract
In CD45 deficient animals there is a severe defect in thymocyte positive selection, resulting in an absence of mature T cells and the accumulation of thymocytes at the double positive stage of development. However, the signaling defect(s) responsible for the block in development of mature single positive T cells are not well characterized. Previous studies have found that early signal transduction events in CD45 deficient cell lines and thymocytes are markedly diminished following stimulation with anti-CD3. Nevertheless, there are also situations in which T cell activation and TCR signaling events can be induced in the absence of CD45. For example, CD45 independent TCR signaling can be recovered upon simultaneous antibody cross-linking of CD3 and CD4 compared to cross-linking of CD3 alone. These data suggest that CD45 may differentially regulate TCR signaling events depending on the nature of the signal and/or on the differentiation state of the cell. In the current study we have assessed the role of CD45 in regulating primary thymocyte activation following physiologic stimulation with peptide. Unlike CD3 mediated stimulation, peptide stimulation of CD45 deficient thymocytes induces diminished, but readily detectable TCR mediated signaling events such as phosphorylation of TCR-associated zeta, ZAP70, LAT, and Akt, and increased intracellular calcium concentration. In contrast, phosphorylation of ERK, which is essential for positive selection, is more severely affected in the absence of CD45. These data suggest that CD45 has a selective role in regulating different aspects of T cell activation.
Keywords: T cells, Kinases/Phosphatases, Cell Differentiation, Cell Activation, Thymus
Introduction
The CD45 protein tyrosine phosphatase plays a critical role in promoting T cell activation and development (1). Experiments in a variety of CD45 deficient T cell tumor lines, as well as data from CD45 deficient mice have demonstrated that early events in T cell receptor signal transduction pathways are severely impaired in the absence of CD45 (2–6). These observations are primarily attributed to the positive regulatory role of CD45 in promoting the activity of the src family kinase, Lck, which then helps to initiate TCR-mediated signal transduction pathways by inducing tyrosine phosphorylation of CD3/zeta ITAM residues and association and activation of the ZAP70 tyrosine kinase. CD45 promotes Lck activity by de-phosphorylating the negative regulatory carboxy-terminal tyrosine on Lck, maintaining Lck in an open active configuration (7–10). The importance of CD45-dependent regulation of Lck activity for T cell activation is further supported by experiments in which reconstitution of CD45 deficient cell lines and mice with constitutively active Lck is able to rescue the T cell activation and development deficit seen in the absence of CD45 (11–13).
Despite the recognized role of CD45 in promoting Lck activity and potentiating TCR signaling, CD45 also has been suggested to down-regulate T cell activation. In vitro experiments have indicated that CD45 has the potential to de-phosphorylate TCR-associated CD3ζ and ZAP70, and to de-phosphorylate the positive regulatory tyrosine phosphorylation site within the catalytic domain of Lck (14–16). It remains unclear how these apparently contradictory roles are regulated in vivo. One possibility is that CD45 plays a predominantly positive regulatory role prior to T cell activation by maintaining an active pool of Lck, but may also down regulate ongoing T cell receptor dependent signaling cascades due to redistribution of CD45 in different signaling complexes or membrane compartments after T cell activation (17–20). Thus, the precise role of CD45 in regulating the duration and strength of different TCR-dependent signal transduction pathways remains uncertain. In particular, there has been very little reported on the role of CD45 in regulating T cell activation and TCR signal transduction in primary cells in response to stimulation by antigen and antigen presenting cells.
There are several lines of evidence indicating that the dependence of CD45 for TCR signaling is not absolute, suggesting that CD45 may play a role in differentially regulating or tuning distinct parameters of T cell activation. As noted above, experiments in CD45 deficient tumor cell lines have shown defects in the response to CD3 antibody mediated cross-linking in the absence of CD45 that is recovered by CD45 genetic reconstitution. However, the defects in stimulation are variable in different cell lines, suggesting that some CD45-independent TCR-dependent signaling events can also occur. For example, in the CD45 deficient BW thymoma cell line, high doses of immobilized anti-CD3 induce similar levels of IL-2 production when compared to CD45 reconstituted cell lines (21).
In vivo analysis of T cell development in CD45 deficient mice also suggests that CD45-independent T cell activation can occur. CD45 deficient mice have a near complete block in thymic development at the double positive stage indicating a failure in positive selection (22–25). However the transition from double negative to double positive cells is less severely affected, suggesting that some signaling through the pre-TCR is able to occur in the absence of CD45. This phenotype contrasts with the phenotype of mice deficient in other molecules involved in TCR signaling, such as Lck/Fyn, LAT or SLP-76, where there is a defect in pre-TCR signaling resulting in a block in development at the double negative stage (26–30). In addition, previous studies of CD45 deficient mice crossed with various TCR transgenic strains have evaluated T cell activation and development by assessing positive and negative selection in the thymus. In all of these studies there has been a consistent block in positive selection regardless of the TCR transgenic strain (22–25). However, negative selection in male HY transgenic animals and also upon superantigen stimulation is reduced in efficiency but readily detectable in CD45 deficient animals, indicating that TCR signals sufficient to mediate negative selection can occur in the absence of CD45 (22, 24). One possible interpretation of this data is that signaling in response to strong TCR signals which normally induce negative selection can occur independently of CD45, while lower avidity signals required for positive selection are more CD45 dependent. Alternatively, specific signaling pathways critical for positive selection may be more dependent on CD45 than signaling events that mediate negative selection.
Further evidence of CD45 independent TCR signaling comes from data that, in contrast to CD3 cross-linking, simultaneous antibody cross-linking of CD3 and CD4 induces biochemical signaling events in CD45 deficient cells similar to that seen in wild type control cells (5, 31). Since physiologic stimulation with peptide typically involves co-receptor signaling, we hypothesized that CD45 independent signaling pathways may be more evident using this mode of stimulation.
In the current study we have assessed the role of CD45 in regulating TCR signaling and thymic development in the AND TCR transgenic mouse, bred onto a homozygous IEk background. Previous studies in this system have indicated that the AND TCR interaction with self peptides/IEk complex is relatively strong resulting in a significant amount of negative selection compared to selection on other MHC backgrounds (32, 33). In addition, selection of mature, Class II restricted, CD8− T cells can occur (although to a lesser extent than wild type mice) in CD4 deficient mice indicating that positive selecting signals are strong enough to occur in the absence of co-receptor signaling (33, 34). Despite the increase in strength of signal inherent in this system, we find that CD45 is absolutely required for positive selection of mature AND T cells. In these mice, the absence of CD45 yields a block in thymic development in the early double positive stage similar to previous studies of CD45 deficient mice (22–24). Notwithstanding the block in development, biochemical analysis of TCR signaling pathways following agonist peptide stimulation indicate that TCR-dependent signal transduction events as indicated by TCRζ, ZAP70 and LAT tyrosine phosphorylation, as well as calcium mobilization are reduced in magnitude, but can be induced in the absence of CD45. However, we find that activation of the ERK MAP kinase pathway, which is essential for positive selection, is much more severely impaired in CD45 deficient thymocytes in response to peptide stimulation. These data suggest that CD45 may differentially affect TCR mediated signal transduction events and provide a possible explanation for the block in thymocyte positive selection seen in CD45 deficient animals.
Materials and Methods
Mice
CD45 exon 9 null mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and have been backcrossed 7–9 times onto the B10.Br background and intercrossed with AND TCR transgenic mice (23, 32). Mice were bred and maintained at the George Washington University animal facility (Washington, DC). Animal procedures conform to institutional animal protocol guidelines.
Peptide
Moth cytochrome c peptide, pMCC, VFAGLKKANERADLIAYLKQATK (a.a. 81–103), was synthesized by the W.M. Keck Foundation Biotechnology Resource Laboratory (New Haven, CT) and purified by high pressure liquid chromatography prior to use.
Antibodies
Polyclonal rabbit anti-mouse TCRζ, and ZAP70 specific antibodies have been described previously (35). Anti-mouse CD4 (GK1.5), and CD8a (53–6.72), CD69, CD3ε, Vαll were purchased from BD Biosciences (Palo Alto, CA) in unlabeled or fluorescent conjugated forms. Anti-mouse LAT was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-specific antibodies to Lck, LAT, PLC-γ, Akt, ZAP-70, and ERK were purchased from Cell Signaling Technology (Beverly, MA). Pan-specific anti-phosphotyrosine antibody (4G10) was purchased from Upstate (Charlotteville, VA). Goat anti-rat Ig and HRP-conjugated Goat anti-rabbit Ig was purchased from Jackson Immunoresearch (West Grove, PA).
Preparation of Double Positive Thymocytes
Double Positive (DP) thymocytes were purified from total thymocytes from CD45+/− and CD45−/− AND TCR trangenic mice by flow cytometry cell sorting with an antibody against CD8α (53-6.72). Purity of the recovered CD4+CD8+TCRβlow DP thymocytes was >90% as determined by flow cytometric analysis. To insure that the sorting process and bound CD8a antibody were not impacting the basal status or activation of the thymocytes, we compared unlabeled presorted and sorted cells. This comparison demonstrated similar induction of tyrosine phosphorylation between presorted and sorted cells indicating that neither the sorting process nor the CD8α antibody had any observable impact on basal or post-activation phosphorylation status (data not shown).
Cell Stimulation
For antibody-mediated stimulation, thymocytes were pre-incubated with 5 μg/mL anti-CD3 (2C11) and/or 1 μg/mL anti-CD4 (GK1.5) for 10 minutes on ice. Bound antibodies were cross-linked with goat anti-rat Ig at 50 μg/mL and immediately incubated at 37 °C for the indicated amount of time. For stimulation with peptides, T cell depleted splenocytes were loaded with 50 μg/mL pMCC for 4 hours at 37 °C in HBSS supplemented with 5% FCS. The APCs were rapidly pelleted with thymocytes at 1:1 ratio and incubated at 37 °C for the indicated amount of time and then detergent soluble lysates were prepared (34).
Cell Lysis, Immunoprecipitation and Immunoblotting
Cell lysates containing equal number of cells were prepared by lysis in TNE buffer (20 mM Tris (pH 7.5), 150 mM NaCl, 5 mM EDTA,), supplemented with complete Mini protease inhibitors (Roche Applied Science, Indianapolis, IN), 1 mM Na3VO4, and either 1% Nonidet P-40 (NP-40) or 1% N-dodecyl-beta-D-maltoside (Maltoside) as indicated. Western blot analysis was done following SDS polyacrylamide electrophoresis and transfer onto nitrocellulose paper (BioRad, Hercules, CA). General and specific tyrosine phosphorylation was detected with indicated antibodies followed by goat anti-rabbit coupled horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA). All of the immunoblots were visualized with the ECL chemiluminescent detection system and images were taken on film. Where indicated, bands from immunoblots were quantified by densitometry (Molecular Dynamics, Sunnyvale, CA) and the relative degree of phosphorylation was corrected for total amounts of each specific protein.
Flow cytometric analysis of ERK activation
Following peptide stimulation for the indicated times, the thymocytes were immediately labeled with fluorescent conjugated antibodies to CD4 and CD8 on ice for 1 minute. Intracellular Erk phosphorylation was evaluated utilizing fluorescent conjugated anti-phospho-p44/42 MAPK (E10)(Cell Signaling Technology) following fixation and permeabilization utilizing the Fix & Perm Cell Permeabilization Kit (CalTag, Burlingame, CA).
Calcium mobilization
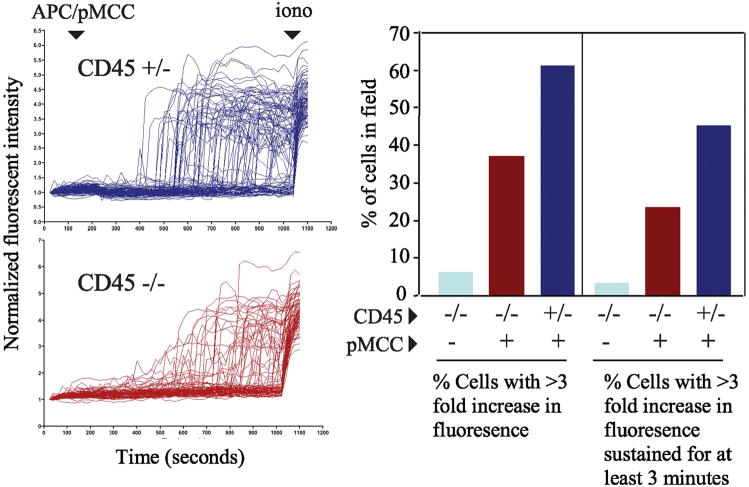
Purified CD4+CD8+ thymocytes cells were loaded with 5μM fluo-4/AM ester (Molecular Probes, Eugene, OR) and plated on a 96 well plate. Cell images were obtained every 20 seconds with a Nikon Eclipse TE200 (Nikon, Melville, NY) system and calcium influx was assessed with the Biorad 1024 laser confocal microscope (Biorad, Hercules, CA). After scanning was initiated 4 × 106 APCs that were previously pulsed with 20μM pMCC peptide were added to the thymocytes. The initial average fluorescence of each cell was normalized to one and the results were expressed as changes in normalized fluorescence over time. The percentage of responding cells was determined by dividing the number of cells in the field whose fluorescence increased 3-fold or was sustained 3-fold for more than 3 minutes by the number of cells in the field that did not. Ionomycin was added at a concentration of 666 ng/mL at the end of scanning as a positive control.
CD69 Expression Assay
Thymocytes from CD45+/− or CD45−/− AND TCR transgenic mice were stimulated in the presence of T cell depleted splenocytes with indicated concentration of peptide and/or PMA and/or ionomycin. After an overnight incubation, expression of CD4, CD8, Vα11 and CD69 were simultaneously evaluated via flow cytometry. All CD69 expression data presented is gated on CD4+CD8+Vα11+ population.
Results
Differential induction of tyrosine phosphorylation following stimulation with anti-CD3 or anti-CD3/CD4 in CD45 deficient thymocytes
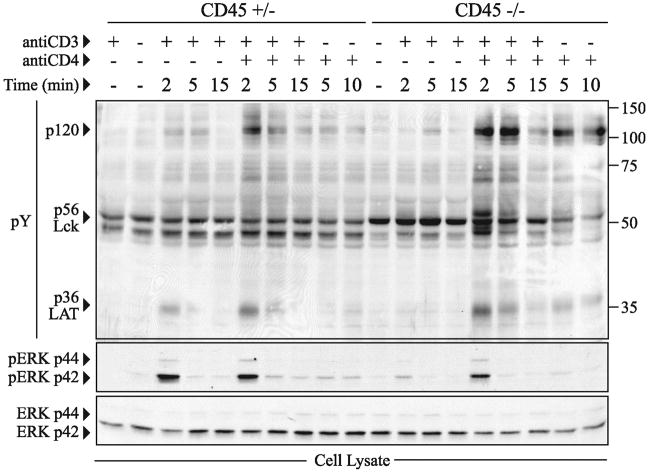
It is well established that there is a profound defect in TCR signal transduction events following stimulation of CD45 deficient cell lines and thymocytes with antibodies to CD3 (1). However, previous data using CD45 deficient tumor cell lines have suggested that the defect in signaling can be overcome by simultaneous cross-linking of both CD3 and CD4 (5, 31). This is presumably due to juxtaposition of CD3 and CD4, and to the ability of anti-CD4 antibodies to cross-link CD4-associated Lck molecules, promoting autophosphorylation of the positive regulatory tyrosine within the catalytic domain of Lck, facilitating Lck activation. In order to confirm these findings in primary cells, we isolated thymocytes from CD45+/− and CD45−/− mice and assessed protein tyrosine phosphorylation following anti-CD3 cross-linking alone or anti-CD3 and anti-CD4 cross-linking in combination (Figure 1). Similar to the previous studies, anti-CD3 cross-linking induces little if any tyrosine phosphorylation in CD45−/− thymocytes when compared to CD45+/− thymocytes (6). In contrast, there is no apparent defect in overall tyrosine phosphorylation or ERK activation in CD45 deficient cells upon simultaneous cross-linking with anti-CD3 and anti-CD4. Indeed, tyrosine phosphorylation of some proteins following anti-CD3 and anti-CD4 cross-linking or CD4 cross-linking alone is augmented in CD45 deficient thymocytes when compared to controls (see arrowheads at p36 and p120, Figure 1).
Figure 1.
Combined crosslinking of CD3 and CD4 induces tyrosine phosphorylation independently of CD45. (A) NP-40 soluble cell lysates of total thymocytes (2 × 106) from CD45+/− or CD45−/− mice expressing polyclonal TCR were analyzed for total tyrosine phosphorylation (pY) by western blot following stimulation with either CD3 and/or CD4. The pY blot was then stripped and probed for phosphorylated ERK (pERK) and repeated again for total ERK. The data shown are representative of two independent experiments.
Thymic development in CD45 deficient AND T cell receptor transgenic mice
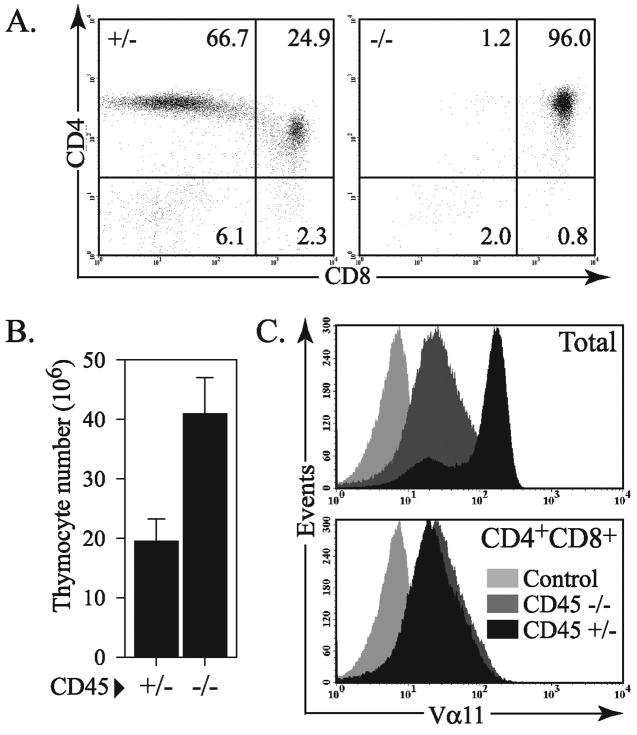
Since antibody engagement of the CD4 co-receptor promoted TCR-dependent signaling in CD45 deficient cells, we hypothesized that APC-mediated stimulation with agonist peptide/MHC Class II complexes that interact with the TCR and co-receptor, may similarly induce CD45-independent signaling. In order to evaluate antigen specific signaling, we bred the CD45 deficient mice with the AND T cell receptor transgenic mice that have specificity for a peptide from moth cytochrome c. As shown in Figure 2, the CD45 deficient AND transgenic mice have a near complete block in thymic development at the double positive stage similar to previous reports of other TCR transgenic strains (22–25). In wild type (or CD45+/−) AND transgenic mice there is a very strong selection bias evident for the development of single positive CD4 T cells.
Figure 2.
A severe positive selection defect in CD45−/− AND TCR transgenic mice. (A) Representative flow cytometry data assessing CD4 and CD8 expression of the total thymocyte population from CD45+/− or CD45−/− AND TCR transgenic mice. (B) Mean number of total thymocytes from CD45+/− or CD45−/− AND TCR transgenic mice (n=3). (C) Expression of the Vα11 chain of the AND TCR from total thymocytes and CD4+CD8+ double positive thymocytes. These data are representative of at least three independent experiments.
Previous reports describing thymocyte development in (non-TCR transgenic) CD45 deficient mice have described a decrease in total thymocyte number (22–24). This is in contrast to our results described in Figure 2 where AND TCR transgenic CD45 deficient mice typically have a modest increase in total thymocytes compared to wild type or CD45 heterozygous littermates (Figure 2B). This is a result of the high degree of negative selection that occurs in AND TCR transgenic mice on a homozygous IEk background, resulting in a relatively small thymus (32, 33). These findings emphasize the fact that there is a very strong requirement for CD45 for positive selection and the development of single positive mature T cells even when the positively selecting signal is relatively strong. In contrast, CD45 appears less important for pre-TCR signaling and development of double positive cells.
CD45-independent and dependent signal following stimulation with peptide pulsed antigen presenting cells
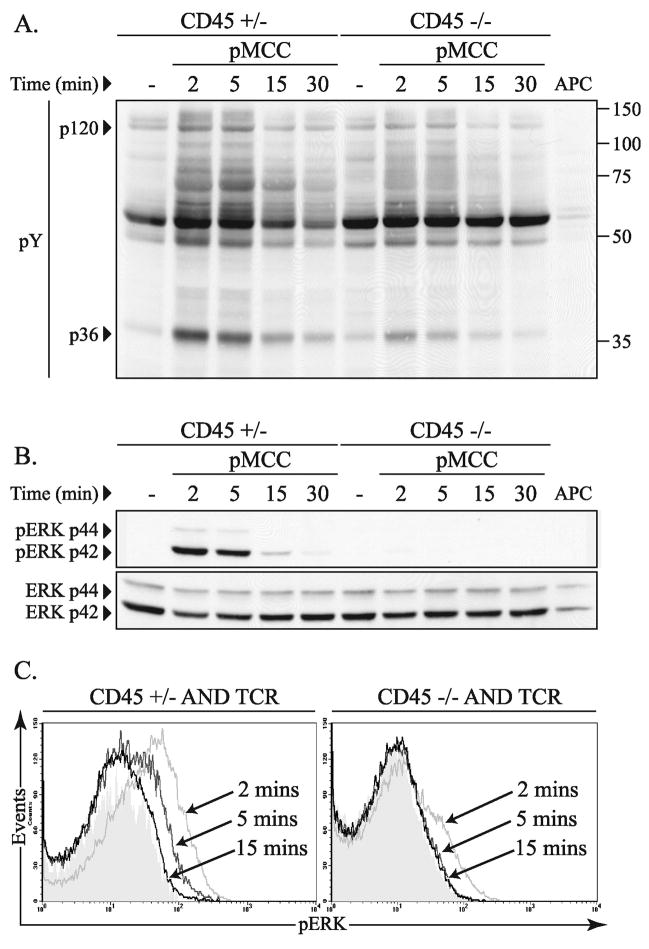
In order to assess CD45 dependent regulation of T cell receptor signal transduction pathways following peptide stimulation, total thymocytes from CD45+/− and CD45−/− AND TCR transgenic mice were isolated and stimulated with moth cytochrome C peptide (pMCC) pulsed T-depleted spleen cells as antigen presenting cells (Figure 3A). Although induction of total tyrosine phosphorylation was less robust, the overall pattern of tyrosine phosphorylation was similar in cell lysates from CD45−/− total thymocytes compared to CD45+/− thymocytes. This is in contrast to the near undetectable tyrosine phosphorylation response to stimulation with antibodies to CD3 (Figure 1).
Figure 3.
Peptide dependent thymocyte activation in the absence of CD45. (A) Total thymocytes (2 × 106) from CD45+/− or CD45−/− TCR transgenic mice were analyzed for induction of total tyrosine phosphorylation (pY) over time by western blot following stimulation with pMCC (50 μg/ml) pulsed T-depleted spleen cells as antigen presenting cells. (B) The pY blot shown in panel A was then stripped and probed for phosphorylated ERK (pERK) and repeated again for total ERK. The data shown are representative of three independent experiments. (C) Representative histograms of intracellular ERK phosphorylation in thymocytes from CD45+/− and CD45−/− mice following peptide stimulation for the indicated times. The cells were labeled with CD4 and CD8 in addition to antibodies to phosphorylated ERK. The data shown are gated on the double positive (CD4+, CD8+) cells and are representative of three independent experiments.
Although initiation of early tyrosine phosphorylation in response to agonist peptide signaling is evident in the absence of CD45, we went on to evaluate more down-stream TCR-dependent signal transduction events. Specifically, activation of the ras/ERK signaling cascade is known to be critical for positive selection and any defects in this pathway would correlate with the block in T cell development seen in the absence of CD45 (36). Indeed, when ERK activation was assessed following stimulation with agonist peptide (data is from the same blot shown in Figure 3A), there was a marked defect in ERK activation in the absence of CD45 compared to CD45+/− control thymocytes (Figure 3B). These data suggest that ERK activation is relatively more dependent on CD45 than induction of tyrosine phosphorylation in other signaling molecules.
Since the difference in ERK activation between CD45+/− and CD45−/− thymocytes may be magnified due to the large number of mature TCRhi, single positive T cells from thymocytes of control animals we also evaluated the induction of phosphorylated ERK by intracellular staining and flow cytometric analysis, gating specifically on the double positive cells. In agreement with the data in Figure 3B, ERK activation is severely compromised in the CD45 deficient thymocytes following stimulation with agonist peptide-pulsed antigen presenting cells (Figure 3C). Low levels of ERK phosphorylation within a subset of the CD45 deficient cells is detectable within 2 minutes after activation, however this is not sustained, and no evidence for ERK activation is seen at later time points. In comparison, CD45+/− DP cells exhibit substantially higher and more sustained ERK activation when compared to CD45−/− DP cells similar to the western blot data (Figures 3B, 4).
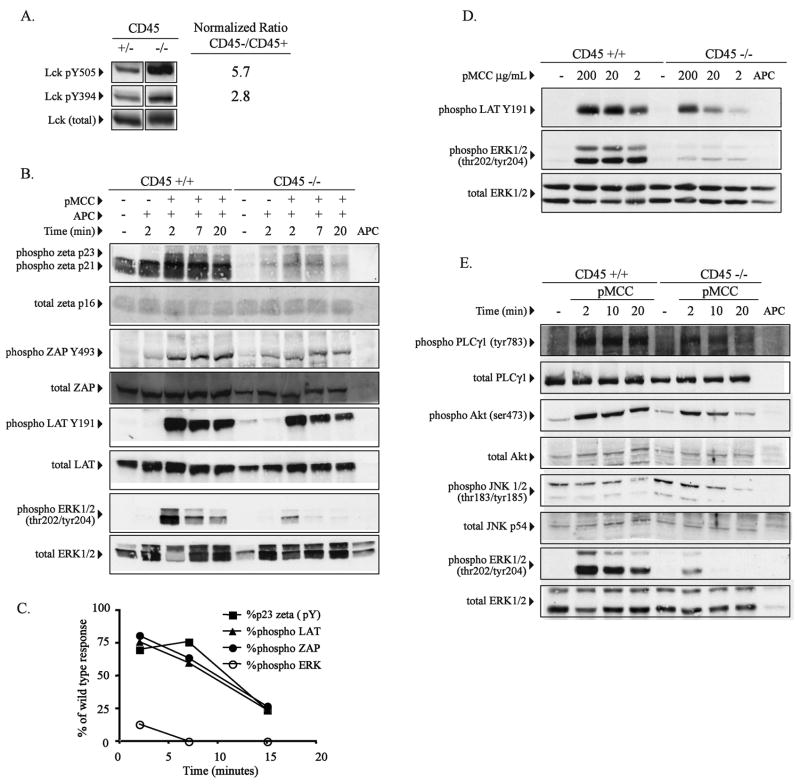
Figure 4.
ERK specific phosphorylation defect in double positive CD45 deficient thymocytes following stimulation with peptide. (A) Purified CD4+CD8+ double positive (DP) thymocytes from CD45+/+ or CD45−/− AND TCR transgenic mice were analyzed for Lck tyrosine phosphorylation at Y505 and Y394 using phosphospecific antibodies. Relative degree of phosphorylation at each site was quantified by densitometry after normalization for total Lck. The fold increase in tyrosine phosphorylation in CD45 deficient cells is indicated. (B) Purified DP thymocytes from CD45+/+ or CD45−/− AND TCR transgenic mice were stimulated with peptide pulsed APC (50 μg/ml, pMCC) as described in Figure 3 for the indicated time periods and analyzed for specific phosphosphorylation of TCR-zeta, ZAP70, LAT and ERK by western blot using the indicated phospho-specific antibodies. TCRzeta phosphorylation was detected using a pan-specfic phosphotyrosine antibody. The blots were then stripped and probed for total protein where indicated. (C) Densitometric analysis of the data in panel B is graphically represented following normalization for T cells using the total zeta western blot data. Data shown is the percent of the response of CD45 deficient cells compared to control CD45+ cells. (D) Purified DP thymocytes from wild type or CD45 deficient AND TCR transgenic mice were stimulated with APC’s pulsed with the indicated doses specific peptide for 5 minutes and phosphorylation of LAT and ERK1/2 was detected using the indicated phosphospecific antibodies. (E) As in panel B, purified DP thymocytes from wild type or CD45 deficient AND TCR transgenic mice were stimulated with peptide-pulsed APC for the indicated periods of time and activation of PLC-γ1, AKT, JNK1/2, and ERK1/2 was detected using the indicated phosphospecific antibodies. The data shown in each panel are representative of at least two independent experiments for each molecule. Data within each panel is derived from the same set of cell lysates.
Note that the defect in ERK activation in CD45 deficient cells contrasts with the data in Figure 1, where ERK activation was induced in CD45 deficient thymocytes following CD3/CD4 antibody cross-linking. Thus, physiologic stimulation with agonist peptide appears to overcome defects in membrane proximal TCR signal transduction events evident upon anti-CD3 stimulation, but does not fully restore T cell activation as seen upon anti-CD3 and CD4 cross-linking.
Selective regulation of ERK phosphorylation by CD45 in CD4+CD8+ double positive (DP) thymocytes following stimulation with peptide
Further analysis of indivdual components of the TCR signal transduction cascade also suggest that there is a specific defect in ERK activation in the absence of CD45, while other TCR-dependent signaling events are relatively CD45-independent following peptide stimulation. In order to examine CD45-dependent regulation of specific biochemical signaling events following peptide stimulation we performed a series of experiments using purified CD4+CD8+ double positive thymocytes from both CD45 deficient and control mice to alleviate the confounding single positive CD4 T cell population in the control CD45+ mice.
Since Lck is the best defined substrate for CD45, we initially assessed CD45 dependent changes in Lck tyrosine phosphorylation using phospho-specific antibodies to the negative regulatory carboxy-terminal tyrosine (Lck-Y505), as well as the regulatory tyrosine within the kinase domain associated with kinase activity (Lck-Y394). Consistent with previous reports, Lck is hyperphosphorylated at both sites in the absence of CD45, although phosphorylation at the negative regulatory site is more severely affected (Figure 4A) (16, 37).
In order to evaluate the functional activity of Lck in double positive thymocytes we stimulated the cells with agonist peptide and assessed tyrosine phosphorylation of TCR-associated zeta, ZAP-70, and LAT using a pan phosphotyrosine antibody to assess zeta chain phosphorylation and phosphospecific antibodies for ZAP70 Y493 and LATY191. Induction of TCRζ, ZAP70, and LAT phosphorylation are dependent on Lck activity and would be predicted to be severely impaired in CD45 deficient animals (38). As seen in Figure 4B, phosphorylation of TCRζ p23, ZAP70, and LAT were all induced following peptide stimulation in the absence of CD45. Although CD45 deficient cells exhibited modest decreases in sustained antigen-induced tyrosine phosphorylation compared to control thymocytes, activation was readily detectable.
In marked contrast, ERK phosphorylation was below detectable levels within 3 minutes after activation in the same experiment (Figure 4B). Densitometric analysis reveals that the role of CD45 in promoting TCR-zeta p23, ZAP-70, and LAT phosphorylation is most evident at later time points, while phosphorylation early after stimulation (2 minutes in this experiment), is only reduced by 20–30% compared to control CD45+ cells (Figure 4C). This is in contrast to the marked defect ERK activation at early time points and an even more pronounced defect (less than 1% of control) at later time points.
Importantly, in contrast to agonist peptide stimulation, basal levels of thymocyte TCRζ (p21) phosphorylation were markedly reduced in the absence of CD45 (Figure 4B, lane 1 vs lane 6). These data indicate that the low avidity signaling that occurs in vivo in response to TCR interaction with self-peptides is dependent on CD45 expression. However the current data indicate that agonist peptide signaling sufficient to induce saturated levels of CD3ζ chain phosphorylation (p23), ZAP70 and LAT phosphorylation can occur in the absence of CD45. The CD45-independent regulation of LAT phosphorylation compared to the CD45-dependent regulation of ERK phosphorylation is further shown upon stimulation with different doses of agonist peptide (Figure 4D). In this experiment, purified double positive thymocytes from CD45 deficient mice demonstrated a profound defect in ERK activation 5 minutes after stimulation with high doses of the agonist peptide, pMCC, while LAT phosphorylation was readily detectable. In contrast wild type control double positive thymocytes were induced to activate ERK following stimulation with low doses of the agonist peptide similarly to high doses of peptide.
In additional independent experiments we assessed other more “downstream” signal transduction events in addition to ERK in CD45 deficient thymocytes. Similarly to the data described above, PLCγ, AKT, JNK1/2 phosphorylation were all readily detectable in the absence of CD45, while ERK phosphorylation was markedly diminished in the same experiement (Figure 4D). These data suggest a specific role for CD45 in the regulation of ERK activation, and are consistent with a model in which ERK activity is regulated independently of other “downstream” signaling events such as AKT phosphorylation.
There does not appear to be a general defect in ERK activation in CD45 deficient thymocytes, since PMA stimulation of CD45 deficient cells or stimulation with anti-CD3/CD4 induces ERK activation similar to control mice (Figure 1, data not shown). In total, these data suggest that ERK is capable of being activated in CD45 deficient cells, and indicate that CD45 serves as a critical positive regulator of TCR-dependent ERK activation following physiologic peptide stimulation.
Diminished sustained calcium mobilzation in individual CD45- double positive thymocytes following peptide stimulation
In addition to ERK activation, TCR-induced increases in cytoplasmic calcium concentration is also required for efficient positive selection (39). In order to evaluate the role of CD45 in regulating calcium mobilization in individual double positive thymocytes, cells were monitored for changes in calcium concentration by video laser microscopy following stimulation with peptide-pulsed antigen presenting cells as previously described (40). As shown in Figure 5, both CD45+ and CD45 deficient thymocytes were induced to increase intracellular calcium concentration in a peptide-dependent manner. However, upon analysis of the CD45 deficient thymocytes, there were approximately 50% fewer responding cells, and those cells were also less likely to exhibit a sustain increase in intracellular calcium.
Figure 5.
Partial defect in sustained calcium mobilzation in individual CD45- double positive thymocytes following peptide stimulation. (A) Purified double positive thymocytes were loaded with 5μM fluo-4/AM ester and individual cell images were obtained every 20 seconds using the Biorad 1024 laser confocal microscope. After scanning was initiated 4 × 106 APCs (T-depleted splenocytes) that were previously pulsed with 50 μg/ml pMCC peptide were added to the thymocytes. The initial average fluorescence of each cell was normalized to one and the results were expressed as changes in normalized fluorescence over time. Ionomycin was added at the end of scanning as a positive control to ensure that changes in calcium were detectable in the imaged cell population. (B) The percentage of responding cells was determined by dividing the number of cells in the field whose fluorescence increased 3-fold or was sustained 3-fold for more than 3 minutes by the total number of cells in the field. For CD45+ cells n=93, for CD45 deficient cells n=89. The data are representative of 2 independent experiments.
Defect in CD69 induction in CD45 deficient cells
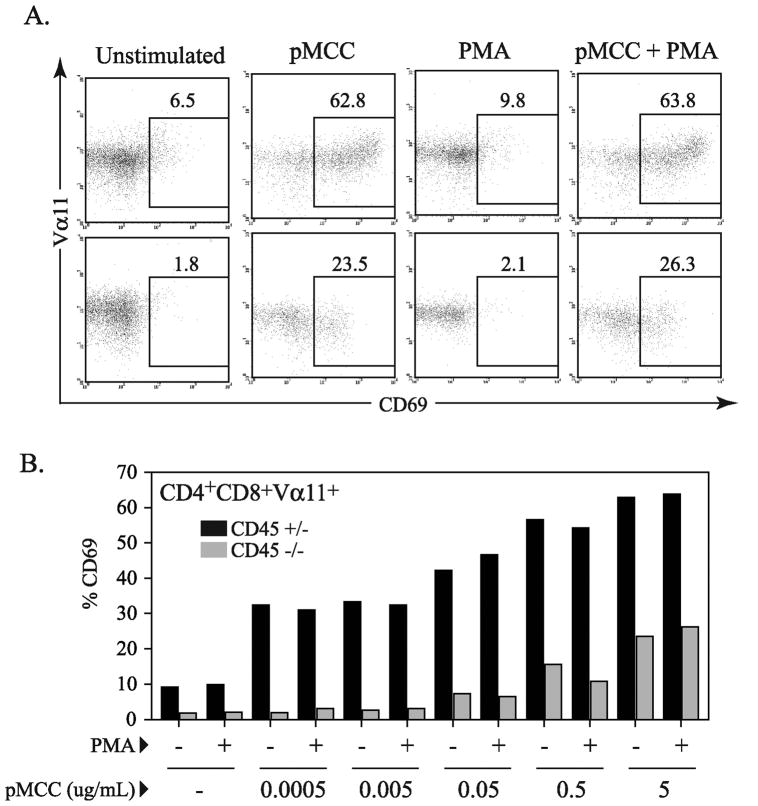
As suggested by the calcium mobilization data, CD45 may also affect other signaling events that are important for appropriate TCR-mediated induction of gene transcription and T cell development in addition to ERK activation. Since PMA was able to induce ERK activation in the CD45 deficient cells, we assessed whether PMA could complement the defect(s) in TCR signaling seen upon peptide stimulation and promote more downstream indicators of thymocyte activation. In these experiments, thymocytes were stimulated with agonist peptide alone or in combination with PMA, and induction of CD69 expression was assessed (Figure 6). A relatively low dose of PMA that was sufficient to induce ERK activation, but insufficient to induce CD69 expression alone was used in these experiments (Figures 6). This dose of PMA was able to complement the induction of CD69 expression in combination with exogenous ionomycyin, while higher doses of PMA induced CD69 expression in CD45 deficient cells in the absence of other signals (data not shown).
Figure 6.
Defect in CD69 induction in CD45 deficient thymocytes. (A) Representative dot plots displaying CD69 expression gated on CD4+CD8+ population after overnight stimulation with 5μg/mL pMCC and/or PMA at 0.1ng/mL. (B) Graph of %CD69 expression from flow cytometry data of thymocytes from CD45+/− or CD45−/− AND TCR transgenic mice after overnight stimulation in the presence of T cell depleted spleenocytes with varied concentrations of peptide (serial 10 fold dilutions with highest concentration at 5 μg/ml) and/or PMA at 0.1 ng/mL. All CD69 expression data presented is gated on CD4+CD8+Vα11+ population. This data is representative of three independent experiments.
Previous reports have indicated that ERK activation is critical for stimulating CD69 expression, and that induction of CD69 expression is associated with TCR signals that promote positive selection (41, 42). Thus, these experiments were based on the hypothesis that peptide stimulation alone would result in insufficient signaling to induce CD69 expression, while the addition of PMA (to activate ERK) to peptide stimulated cells would correct the defect in ERK activation and stimulate an increase in CD69 expression. Contrary to this hypothesis, we observed that across a broad range of peptide concentrations, neither peptide alone, nor peptide with PMA was able to induce CD69 expression in CD45−/− thymocytes similar to CD69 expression on CD45+/− thymocytes (Figure 6). Importantly, at the concentrations used in our studies, the combination of PMA and ionomycin but not either alone, was sufficient to induce similar levels of CD69 expression in both CD45 deficient and control cells.
Discussion
In this report we have evaluated the role of CD45 in regulating TCR receptor signal transduction events in primary thymocytes following physiologic stimulation with peptide ligand. While reduced, membrane proximal tyrosine phosphorylation events can be induced in double positive thymocytes in response to agonist peptide stimulation in the absence of CD45. This contrasts with the response to anti-CD3 stimulation in which induction of tyrosine phosphorylation is more dependent on CD45 activity. There are several distinctions between stimulation with peptide and anti-CD3 that may explain the differential dependence on CD45. For example, TCR recognition of MHC Class II/peptide complexes also involves ligation and cross-linking of CD4. Since antibody cross-linking of CD3 and CD4 together also stimulates CD45-independent T cell activation, it is likely that MHC Class II interaction with CD4 may similarly promote CD4-dependent signaling events (most likely via Lck activation) in the absence of CD45. In addition to involving co-receptors, APC dependent activation of T cells also involves a variety of other co-stimulatory and adhesive interactions that may also enhance or stabilize TCR signaling beyond that seen with anti-CD3 cross-linking.
The finding that TCRζ chain, LAT, and ZAP70 phosphorylation can be induced in CD45 deficient thymocytes following peptide stimulation suggests that Lck activity is not completely dependent on CD45. Many studies have shown that Lck activity is critical for TCRζ chain phosphorylation and ZAP70/TCR association and phosphorylation (38). CD45 is known to promote Lck activity by dephosphorylating the carboxy-terminal tyrosine and maintaining Lck in an open and active conformation (9, 43). This promotes Lck kinase activity and also facilitates the adapter role of Lck by freeing the SH2 domain. Thus, the predominantly held model is that CD45 activity is critical for generating Lck-dependent membrane proximal TCR signal transduction events. Previous studies in CD45 deficient cell lines using anti-CD3 stimulation have generally shown defects in early TCR signal transduction events and have supported this model (4, 6, 44, 45). However, our current data suggest that Lck activity is not entirely dependent on CD45. This is consistent with previous studies in CD45 deficient cells that have suggested a complex role for CD45 in regulating Lck kinase activity both positively and negatively (16, 46). This is attributed to the finding that CD45 also de-phosphorylates the positive regulatory tyrosine within the catalytic domain of Lck, resulting in little net change in Lck kinase activity (47). However, regardless of the role of CD45 in promoting Lck kinase activity, it is clear that CD45-dependent de-phosphorylation of the carboxy-terminal tyrosine is critical for promoting the adaptor function of the Lck SH2 domain that may facilitate TCR signal transduction independently of Lck enzymatic activity (10, 48, 49). Our data suggests that the requirement for CD45 in promoting both the enzymatic and adaptor functions of Lck is not absolute.
An explanation for these results may be that distinct pools of Lck exist within the T cell and are differentially regulated by CD45. In support of this model, we have recently found that CD45 predominantly regulates tyrosine phosphorylation of co-receptor associated Lck, while non-co-receptor associated Lck phosphorylation is regulated independently of CD45 (50). These data support a model in which CD45 is required to maintain activity of co-receptor associated Lck that in turn is critical for transmitting signals that activate ERK. In contrast, non-co-receptor associated Lck may be able to function in the absence of CD45 and (at least in response to a strong agonist peptide) promotes phosphorylation of CD3 ITAMs, ZAP70 and LAT. In addition to different pools of Lck, there may also be different pools of CD45 that differentially regulate T cell activation. This model is consistent with recent data from Miceli and colleagues indicating that distinct pools of CD45 have distinct roles in TCR signal transduction pathways (51). Similar to our findings, CD45 targeted to non-lipid raft domains was particularly critical for ERK activation while CD45 expression was not required to induce LAT phosphorylation. In contrast CD45 constitutively targeted to lipid-raft domains had an overall negative effect on both ERK and LAT phosphorylation.
One possible interpretation of our data is that there is a quantitative defect in signal strength in the absence of CD45. In this view, CD45 deficient thymocytes may receive partial signals sufficient to induce early tyrosine phosphorylation events, but which are insufficiently strong or sustained to promote more downstream signal transduction sufficient for ERK activation. In contrast to this interpretation is other data indicating that maximal ERK activation can be induced with low doses of agonist peptide or upon low avidity interactions with the TCR using partial agonist peptides (52). Indeed, the stringent requirement of CD45 for ERK activation, in the presence of LAT and ZAP70 phosphorylation is the opposite of the signaling phenotype seen following TCR stimulation with low-potency partial-agonist peptides. Low avidity partial-agonist peptides typically induce sustained ERK activation while failing to induce significant ZAP70 or LAT phosphorylation (53–55).
Overall these data support the concept ERK activation may be regulated independently of other parameters of TCR signaling and that CD45 has a qualitative role in regulating distinct signal transduction events. The precise molecular basis for this differential regulation ERK activation by CD45 remains unclear. Although it is interesting to speculate that it may be related to previous reports examining the role of Lck SH3 domain in regulating T cell activation and development. The signaling phenotype in CD45 deficient thymocytes is remarkably similar to cells that express a mutated Lck SH3 domain (W97ALck), where there is a similar selective defect in ERK activation following TCR stimulation, while ZAP70 and LAT phosphorylation are relatively unaffected (56, 57). In addition, thymocytes from “knock-in” mice with a mutated Lck SH3 domain have a relatively mild defect early in thymocyte development and progress through the pre-TCR checkpoint to double positive cells, but have a more severe defect in positive selection similar (though less pronounced) to CD45 deficient mice (57). In Lck SH3 domain mutant cell lines the defect in ERK activation has been attributed to a defect in Raf activation in the golgi, while initial Ras activation is not severely affected (58). Our current data suggest that CD45 may also be particularly important for this role of Lck. Furthermore our data suggest that co-receptor associated Lck which is regulated by CD45 may have a specialized role in facilitating ERK activation.
Since ERK activation is required for the development of single positive T cells, the defect in ERK activation correlates well with the block in positive selection seen in CD45 deficient mice. In addition, the ability to activate LAT independently of CD45 may also explain why pre-TCR signaling and transition from double negative to double positive cells is less affected in CD45 deficient thymocytes, while pre-TCR signaling is more severely compromised in mice deficient in Lck/Fyn or LAT (26–30). However, although there was a severe defect in ERK phoshorylation in the absence of CD45, pharmacologic activation of ERK with low doses of PMA in combination with peptide stimulation did not rescue the defect in CD69 expression present in the CD45 deficient thymocytes. This may be due to the non-physiologic nature of PMA signaling, but also may indicate that there are defects in signal transduction events in addition to ERK activation in the absence of CD45. This is consistent with earlier data indicating that constitutive activation of the ras/MAPK pathway is not sufficient to promote mature T cell development of CD4+CD8+ thymocytes (59).
In summary, our results indicate that the generation of different TCR signal transduction pathways in developing thymocytes is not equally dependent on CD45 activity. This is particularly evident upon stimulation with a strong agonist peptide, where early TCR signal transduction events can be induced in the absence of CD45 expression. These results are similar to recent reports evaluating immunoreceptor signal transduction in CD45 deficient B cells and NK cells (60–62). Upon BCR cross-linking or following ligation of activating NK cell receptors, early tyrosine phosphorlation events are induced in the absence of CD45, sufficient to promote selective aspects of both B and NK cell activation. In the presence of T cell help, CD45 deficient B cells undergo initial antigen dependent activation in vivo and germinal center formation, but this response is abbreviated and transient due to defects in B cell survival. Similarly, CD45 deficient NK cells can be induced to exhibit cytotoxic function but have severe defects in cytokine and chemokine production. Also, similarly to CD45 deficient thymocytes, both NK cells and B cells have severe defects in ERK activation. In total these data identify CD45 as a critical, but selective regulator of signal transduction events that impacts on immune cell development and effector function.
Acknowledgments
We are grateful to Matthew Raymond and Anastas Popratiloff for help with the calcium mobilization assay, and Dallen Herzog for animal care and overall excellent technical assistance.
Footnotes
This work was supported in part by grants from the Arthritis Foundation, American Cancer Society, and NIH AI42963 (D.L.). This manuscript was prepared in partial fulfillment of the requirements for a Ph.D. degree in the Institute for Biomedical Sciences, George Washington University (R.F.).
References
- 1.Hermiston ML, Xu Z, Weiss A. CD45: A critical regulator of signaling thresholds in immune cells. Ann Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 2.Pingel JT, Thomas ML. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell. 1989;58:1055–1065. doi: 10.1016/0092-8674(89)90504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koretzky GA, Picus J, Thomas ML, Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature. 1990;346:66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- 4.Volarevic S, Niklinska BB, Burns CM, Yamada H, June CH, Dumont FJ, Ashwell JD. The CD45 tyrosine phosphatase regulates phosphotyrosine homeostasis and its loss reveals a novel pattern of late T cell receptor-induced Ca2+ oscillations. J Exp Med. 1992;176:835–844. doi: 10.1084/jem.176.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiroo M, Goff L, Biffen M, Shivnan E, Alexander D. CD45 tyrosine phosphatase-activated p59fyn couples the T cell antigen receptor to pathways of diacylglycerol production, protein kinase C activation and calcium influx. EMBO J. 1992;11:4887–4897. doi: 10.1002/j.1460-2075.1992.tb05595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone JD, Conroy LA, Byth KF, Hederer RA, Howlett S, Takemoto Y, Holmes N, Alexander DR. Aberrant TCR-mediated signaling in CD45-null thymocytes involves dysfunctional regulation of lck, fyn, TCR-ζ, and ZAP-70. J Immunol. 1997;158:5773–5782. [PubMed] [Google Scholar]
- 7.Cahir McFarland ED, Hurley TR, Pingel JT, Sefton BM, Shaw A, Thomas ML. Correlation between Src family member regulation by the protein tyrosine phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proc Natl Acad Sci USA. 1993;90:1402–1406. doi: 10.1073/pnas.90.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustelin T, Altman A. Dephosphorylation and activation of the T cell tyrosine kinase p56lck by the leukocyte common antigen (CD45) Oncogene. 1990;5:809–813. [PubMed] [Google Scholar]
- 9.Ostergaard HL, Shackelford DA, Hurley TR, Johnson P, Hyman R, Sefton BM, Trowbridge IS. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci USA. 1989;86:8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sieh M, Bolen JB, Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of lck. EMBO J. 1993;12:315–321. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duplay P, Alcover A, Fargeas C, Sekaly RP, Branton PE. An activated epidermal growth factor receptor/lck chimera restores early T cell receptor-mediated calcium response in a CD45-deficient T cell line. J Biol Chem. 1996;271:17896–17902. doi: 10.1074/jbc.271.30.17896. [DOI] [PubMed] [Google Scholar]
- 12.Pingel S, Baker M, Turner M, Holmes N, Alexander DR. The CD45 tyrosine phosphatase regulates CD3-induced signal transduction and T celll development in recombinase-deficient mice: restoration of pre-TCR function by active p56(lck) Eur J Immunol. 1999;29:2376–2384. doi: 10.1002/(SICI)1521-4141(199908)29:08<2376::AID-IMMU2376>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Seavitt JR, White LS, Murphy KM, Loh DY, Perlmutter RM, Thomas ML. Expression of the p56(lck) Y505 mutation in CD45-deficient mice rescues thymocyte development. Mol Cell Biol. 1999;19:4200–4208. doi: 10.1128/mcb.19.6.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mustelin T, Williams S, Tailor P, Couture C, Zenner G, Burn P, Ashwell JD, Altman A. Regulation of the p70ZAP tyrosine protein kinase in T cells by the CD45 phosphotyrosine phosphatase. Eur J Immunol. 1995;25:942–946. doi: 10.1002/eji.1830250413. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa T, Itoh M, Krueger NX, Streuli M, Saito H. Specific interaction of the CD45 protein tyrosine phosphatase with tyrosine-phosphorylated CD3 ζ chain. Proc Natl Acad Sci USA. 1994;91:10928–10929. doi: 10.1073/pnas.91.23.10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Oro U, Sakaguchi K, Appella E, Ashwell JD. Mutational analysis of lck in CD45-negative T cells: dominant role of tyrosine 394 phosphorylation in kinase activity. Mol Cell Biol. 1996;16:4996–5003. doi: 10.1128/mcb.16.9.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitenberg D, Boutin Y, Lu D, Bottomly K. Biochemical association of CD45 with the T cell receptor complex:regulation by CD45 isoform and during T cell activation. Immunity. 1999;10:701–711. doi: 10.1016/s1074-7613(00)80069-2. [DOI] [PubMed] [Google Scholar]
- 18.Edmonds SD, Ostergaard HL. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J Immunol. 2002;169:5036–5042. doi: 10.4049/jimmunol.169.9.5036. [DOI] [PubMed] [Google Scholar]
- 19.Freiberg BA, Maslanik KHW, Delli J, Kappler J, Zaller D, Kupfer A. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 20.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novak T, Farber D, Leitenberg D, Cheol Hong S, Johnson P, Bottomly K. Isoforms of the transmembrane tyrosine phosphatase CD45 differentially affect T cell recognition. Immunity. 1994;1:109–119. doi: 10.1016/1074-7613(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 22.Kishihara K, Penninger J, Wallace VA, Kundig TM, Kawai K, Wakeham A, Timms E, Pfeffer K, Ohashi PS, Thomas MT, Furlonger C, Paige CJ, Mak TW. Normal B lymphocyte development but imparied T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 23.Byth KF, Conropy LA, Howlett S, Smith AJH, May J, Alexander DR, Holmes N. CD45-Null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development in the selection of CD4+CD8+ thymocytes and in B cell maturation. J Exp Med. 1996;183:1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mee PJ, Turner M, Basson MA, Costello PS, Zamoyska R, Tybulewicz VL. Greatly reduced efficiency of both positive and negative selection of thymocytes in CD45 tyrosine phosphatase-deficient mice. Eur J Immunol. 1999;29:2923–2933. doi: 10.1002/(SICI)1521-4141(199909)29:09<2923::AID-IMMU2923>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Kung C, Pingel JT, Heikinheimo M, Klemola T, Varkila K, Yoo LI, Vuopala K, Poyhonen M, Uhari M, Rogers M, Speck SH, Chatila T, Thomas ML. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat Med. 2000;6:343–345. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- 26.Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A, Davidson D, Mak TW. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 27.Groves T, Smiley P, Cooke MP, Forbush K, Perlmutter RM, Guidos CJ. Fyn can partially substitute for Lck in T lymphocyte development. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 28.van Oers NS, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. alpha beta T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, Long EO, Love PE, Samelson LE. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 30.Clements JL, Yang B, Ross-Barta SE, Eliason SL, Hrstka RF, Williamson RA, Koretzky GA. Requirement for the leukocyte-specific adaptor protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 31.Deans JP, Kanner SB, Torres RM, Ledbetter JA. Interaction of CD4:lck with the T cell receptor/CD3 complex induces early signaling events in the absence of CD45 tyrosine phophatase. Eur J Immunol. 1992;22:661–668. doi: 10.1002/eji.1830220308. [DOI] [PubMed] [Google Scholar]
- 32.Kaye J, Hsa ML, Sauron ME, Jameson SC, Gasciogne N, Hendrik SM. Selective development of CD4+ T cells in transgenic mice express a class II MHC restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 33.Matechak EO, Killeen N, Hedrick SM, Fowlkes BJ. MHC Class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity. 1996;4:337–347. doi: 10.1016/s1074-7613(00)80247-2. [DOI] [PubMed] [Google Scholar]
- 34.Leitenberg D, Boutin Y, Constant S, Bottomly K. CD4 regulation of T cell receptor signaling and T cell differentiaion following stimulation with peptides of different affinites for the T cell receptor. J Immunol. 1998;161:1194–1203. [PubMed] [Google Scholar]
- 35.Boutin Y, Leitenberg D, Tao X, Bottomly K. Distinct biochemical signals characterize agonist and altered peptide ligand-induced differentiation of naive CD4 T cells into Th1 and Th2 subsets. J Immunology. 1997;159:5802–5809. [PubMed] [Google Scholar]
- 36.Alberola-Ila J, Hernandez-Hoyos G. The Ras/MAPK cascade and the control of positive selection. Immunol Rev. 2003;191:79–96. doi: 10.1034/j.1600-065x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 37.McNeill L, Salmond RJ, Cooper JC, Carret CK, Cassady-Cain RL, Roche-Molina M, Tandon P, Holmes N, Alexander DR. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity. 2007;27:425–437. doi: 10.1016/j.immuni.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 39.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Ann Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 40.Leitenberg D, Constant S, Lu D, Smith BR, Bottomly K. CD4 and CD45 regulate qualitatively distinct patterns of calcium mobilization in individual CD4+ T cells. Eur J Immunol. 1995;25:2445–2451. doi: 10.1002/eji.1830250906. [DOI] [PubMed] [Google Scholar]
- 41.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of Th1 CD4+ T cells through IL-12 produced by listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 44.Koretzky GA, Kohmetscher MA, Kadleck T, Eiss AW. Restoration of T cell receptor-mediated signal transduction by transfection of CD45 into a CD45-deficient variant of the Jurkat T cell line. J Immunol. 1992;149:1138–1142. [PubMed] [Google Scholar]
- 45.Volarevic S, Niklinska BB, Burns CM, June CH, Weissman AM, Ashwell JD. Regulation of TCR signaling by CD45 lacking transmembrane and extracellular domains. Science. 1993;260:541–544. doi: 10.1126/science.8475386. [DOI] [PubMed] [Google Scholar]
- 46.Baker M, Gamble J, Tooze R, Higgins D, Yang FT, O’Brien PC, Coleman N, Pingel S, Turner M, Alexander DR. Develoment of T-leukemias in CD45 tyrosine phophatase-deficient mutant lck mice. EMBO J. 2000;19:4644–4654. doi: 10.1093/emboj/19.17.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Oro U, Ashwell JD. Cutting edge: The CD45 tyrosine phosphatase is an inhibitor of lck activity in thymocytes. J Immunol. 1999;162:1879–1883. [PubMed] [Google Scholar]
- 48.Collins TL, Burakoff SJ. Tyrosine kinase activity of CD4-associated p56lck may not be required for CD4-dependent T-cell activation. Proc Natl Acad Sci U S A. 1993;90:11885–11889. doi: 10.1073/pnas.90.24.11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H, Littman DR. A kinase-independent function of lck in potentiating antigen-specific T cell activation. Cell. 1993;74:633–644. doi: 10.1016/0092-8674(93)90511-n. [DOI] [PubMed] [Google Scholar]
- 50.Falahati R, Leitenberg D. Changes in the role of the CD45 protein tyrosine phosphatase in regulating Lck tyrosine phosphorylation during thymic development. J Immunol. 2007;178:2056–2064. doi: 10.4049/jimmunol.178.4.2056. [DOI] [PubMed] [Google Scholar]
- 51.Zhang M, Moran M, Round J, Low TA, Patel VP, Tomassian T, Hernandez JD, Miceli MC. CD45 signals outside of lipid rafts to promote ERK activation, synaptic raft clustering and IL-2 production. J Immunol. 2005;174:1479–1490. doi: 10.4049/jimmunol.174.3.1479. [DOI] [PubMed] [Google Scholar]
- 52.Leitenberg D, Falahati R, Lu D, Takeda A. CD45-associated protein regulates the response to low-potency T cell stimulation and promotes CD45 association with CD3/TCR and lck. Immunology. 2007 doi: 10.1111/j.1365-2567.2007.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chau LA, Madrenas J. Phospho-LAT-independent activation of ras-mitogen activated protein kinase pathway: a differential recruitment model of TCR partial agonist signaling. J Immunol. 1999;163:1853–1858. [PubMed] [Google Scholar]
- 54.Leitenberg D, Bottomly K. Regulation of naive T cell differentiation by varying the potency of TCR signal transduction. Sem Immunol. 1999;11:283–292. doi: 10.1006/smim.1999.0184. [DOI] [PubMed] [Google Scholar]
- 55.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 56.Denny MF, Kaufman HC, Chan AC, Straus DB. The lck SH3 domain is required for activation of the mitogen-activated protein kinase pathway but not the initiation of T cell antigen receptor signaling. J Biol Chem. 1999;274:5146–5152. doi: 10.1074/jbc.274.8.5146. [DOI] [PubMed] [Google Scholar]
- 57.Rudd ML, Tua-Smith A, Straus DB. Lck SH3 domain function is required for T-cell receptor signals regulating thymocyte development. Mol Cell Biol. 2006;26:7892–7900. doi: 10.1128/MCB.00968-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M, Ong SS, Rajwa B, Thieu VT, Geahlen RL, Harrison ML. The SH3 domain of Lck modulates T-cell receptor dependent activation of extracellular signal-regulated kinase through activation of Raf-1. Mol Cell Biol. 2008;28:630–641. doi: 10.1128/MCB.00150-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swat W, Shinkai Y, Cheng HL, Davidson L, Alt FW. Activated Ras signals differentiation and expansion of CD4+CD8+ thymocytes. Proc Natl Acad Sci USA. 1996;93:4683–4687. doi: 10.1073/pnas.93.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huntington ND, Xu Y, Nutt SL, Tarlinton DM. A requirement for CD45 distinguishes Ly49D-mediated cytokine and chemokine production from killing in primary natural killer cells. J Exp Med. 2005;201:1421–1433. doi: 10.1084/jem.20042294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huntington ND, Xu Y, Puthalakath H, Light A, Willis SN, Strasser A, Tarlinton DM. CD45 links the B cell receptor with cell survival and is required for the persistance of germinal centers. Nat Immunol. 2006;7:190–198. doi: 10.1038/ni1292. [DOI] [PubMed] [Google Scholar]
- 62.Hesslein DG, Takaki R, Hermiston ML, Weiss A, Lanier LL. Dysregulation of signaling pathways in CD45-deficient NK cells leads to differentially regulated cytotoxicity and cytokine production. Proc Natl Acad Sci USA. 2006;103:7012–7017. doi: 10.1073/pnas.0601851103. [DOI] [PMC free article] [PubMed] [Google Scholar]