Abstract
Flaviviruses are a group of positive-stranded RNA viruses that cause a spectrum of severe illnesses globally in more than 50 million individuals each year. While effective vaccines exist for three members of this group (yellow fever, Japanese encephalitis, and tick-borne encephalitis viruses), safe and effective vaccines for several other flaviviruses of clinical importance, including West Nile and dengue viruses, remain in development. An effective humoral immune response is critical for protection against flaviviruses and an essential goal of vaccine development. The effectiveness of virus-specific antibodies in vivo reflects their capacity to inhibit virus entry and spread through several mechanisms, including the direct neutralisation of virus infection. Recent advances in our understanding of the structural biology of flaviviruses, coupled with the use of small-animal models of flavivirus infection, have promoted significant advances in our appreciation of the factors that govern antibody recognition and inhibition of flaviviruses in vitro and in vivo. In this review, we discuss the properties that define the potency of neutralising antibodies and the molecular mechanisms by which they inhibit virus infection. How recent advances in this area have the potential to improve the development of safe and effective vaccines and immunotherapeutics is also addressed.
Flaviviruses are a group of positive-stranded RNA viruses with a global impact on public health as a result of their widespread distribution and their ability to cause significant morbidity and mortality in humans. More than 75 different flaviviruses have been identified, roughly half of which are capable of causing disease in humans. The majority of flaviviruses are transmitted to humans through the bite of a mosquito or tick. Flavivirus infections result in clinical manifestations that range from febrile illnesses to encephalitis and haemorrhagic disease. Several members of this group, such as the four serotypes of dengue virus (DENV), and West Nile virus (WNV), are considered emerging or re-emerging pathogens because in the past decade the incidence of human disease has increased at an alarming rate (Ref. 1). Other flaviviruses of significant clinical importance include yellow fever virus (YFV), tick-borne encephalitis virus (TBEV), and Japanese encephalitis virus (JEV). The development of vaccines for several flaviviruses is being actively pursued with the goal of eliciting protective levels of neutralising antibody.
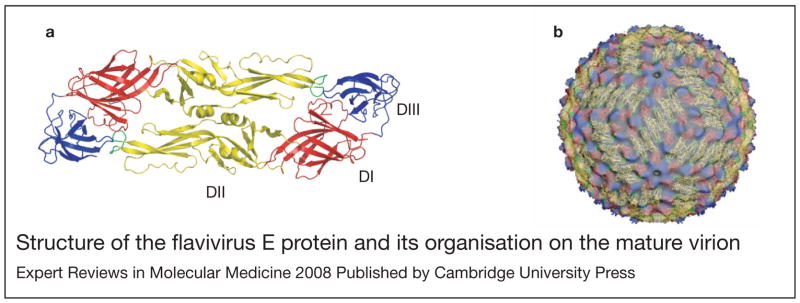
Flaviviruses are small (roughly 50 nm diameter) spherical virions composed of a single copy of an ~11 kb genomic RNA of positive polarity, the capsid protein (C), a lipid envelope derived from the endoplasmic reticulum, and two envelope glycoproteins: envelope (E) and pre-membrane/membrane (prM/M). The atomic structure of the E protein reveals an organisation of three distinct domains separated by short flexible ‘hinges’ (Refs 2, 3, 4, 5). Domain III (DIII) (Fig. 1a; shown in blue) is an immunoglobulin-like domain that is thought to mediate interactions between the virus and structures on the host cell involved in virus attachment (Refs 3, 6). Domain II (DII) (Fig. 1a; shown in yellow) is an elongated finger-like domain that contains the highly conserved hydrophobic fusion loop that interacts with the membranes of the target cell during fusion (Refs 7, 8, 9). Domain I (DI) (Fig. 1a; shown in red) is a β-barrel structure that connects DII and DIII via flexible hinges that participate in the conformational changes that drive the fusion process (Refs 2, 4). An amphipathic stretch of residues referred to as the stem anchor connects the E protein ectodomain to two transmembrane domains that anchor the E protein within the viral membrane. The stem anchor is thought to lie flat against the viral membrane of mature flaviviruses (Ref. 10), be intimately involved in the large rearrangements occurring within and between E proteins during the fusion process, and play a role in interactions with prM (Refs 11, 12). The structure of prM is presently unknown.
Figure 1. Structure of the flavivirus E protein and its organisation on the mature virion.
(a) Ribbon diagram of a dengue virus E protein dimer with domains II, I and III shown as yellow, red and blue ribbons, respectively. The fusion loop at the tip of DII is shown in green. (b) Cryoelectron reconstruction of the dengue virus mature virion illustrating the arrangement of E proteins on the virion with pseudo-icosahedral symmetry (for a detailed explanation of the icosahedral symmetry patterns of viruses see the weblink for the virus particle explorer: http://viperdb.scripps.edu/) (Ref. 21). Image kindly provided by Drs Richard Kuhn and Michael Rossmann, Purdue University, IN, USA.
Flaviviruses assemble at the endoplasmic reticulum and bud into the lumen as immature virions (Ref. 13). Cryoelectron microscopic reconstructions of WNV and DENV immature virions reveal an icosahedral arrangement of 60 trimeric spikes, each composed of prM–E heterodimers in which the prM protein is positioned at the tip of each E protein of the trimer (Refs 14, 15). In this position, prM may prevent low-pH-induced conformational changes that would inactivate the virus particle during egress through mildly acidic compartments of the secretory pathway (Refs 16, 17). During transit through the trans-Golgi network, prM is cleaved by a cellular furin-like protease. This required cleavage step promotes a rearrangement of E protein on the surface of the virion from a heterodimer (prM–E) into an antiparallel homodimer (E–E) and the formation of a mature virus particle (reviewed in Ref. 18). Cleavage results in the formation of a small virion-associated M peptide and the release of the N-terminal ‘pr’ portion of the protein (Ref. 19). By contrast to the spikes present on the immature precursor, mature flavivirus virions are relatively smooth and composed of 90 antiparallel dimers arranged with T = 3 pseudo-icosahedral symmetry (Refs 18, 20, 21) (Fig. 1b).
Following clathrin-mediated internalisation of a flavivirus into the cell, fusion is orchestrated by a series of conformational changes within and between E proteins arrayed on the surface of the virion in response to exposure to the mildly acidic environment of the endosome (Refs 22, 23, 24). These involve the dissociation of the E protein homodimers present on the mature virion (a reversible step) and the formation of E protein trimers (an irreversible step) (Refs 25, 26, 27). This process involves rotation between the three domains of the E protein and results in the projection of DII away from the surface of the virion while positioning the fusion loop to interact with the target cell membrane. Following insertion of the fusion loop into the target cell membrane, viral and cellular membranes are brought into close apposition as the E protein folds back upon itself with the stem anchor region fitting into grooves on the exterior of the trimer (Refs 8, 9). While structurally distinct, these rearrangements represent a functionally analogous process to the well-characterised fusion process of class I fusion glycoproteins such as influenza haemagglutinin and human immunodeficiency virus (HIV)-1 gp120 (reviewed in Refs 28, 29). Efforts are under way to develop small-molecule inhibitors of virus entry orchestrated by the class II fusion proteins of alpha- and flaviviruses (Refs 30, 31, 32), based on the demonstrated success of fusion and entry inhibitors that target the HIV-1 envelope proteins (Ref. 33).
Humoral immunity against flavivirus infection
Humoral immunity is an essential aspect of immune-mediated protection from flavivirus infection (Refs 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45). The primary target for neutralising antibodies is the E protein, although antibodies specific for prM and nonstructural (NS) proteins have been observed (Refs 46, 47, 48, 49, 50, 51, 52, 53, 54). Antibodies specific for NS proteins (proteins not incorporated into virions) do not directly neutralise virus infectivity, but may protect via other effector mechanisms of antibodies as discussed below. More than 12 distinct epitopes have been identified on the surface of the E protein that elicit antibodies characterised by varying degrees of neutralisation potency in vitro and efficacy in vivo (Refs 36, 55, 56, 57, 58, 59, 60, 61). The antigenic domains of E proteins were initially characterised by mapping and competition experiments with monoclonal antibodies (mAbs); three antigenic domains (C, A and B) were identified, which were later correlated with the structural domains DI, DII and DIII on the E protein (Fig. 1) (Ref. 61). Many of the most potent neutralising antibodies characterised to date recognise the upper lateral surface of DIII that protrudes off the surface of the virion (DIII-lr) (Refs 36, 55, 62). While humans produce antibodies of this specificity in response to natural infection, recent studies indicate that the human humoral immune response to flavivirus infection is narrower than anticipated, with antibody specificity focused on determinants around the fusion loop at the tip of DII. B-cell repertoire analysis of three WNV-infected humans revealed that only 8% of WNV-specific B-cell clones produced antibodies specific to DIII, whereas almost half produced antibody that bound determinants in DII, particularly the fusion loop (Ref. 63). Functional studies of the polyclonal response of WNV-infected mice, horses and humans indicate that the neutralisation activity of sera is not dependent upon antibodies directed against the DIII-lr epitope (Refs 64, 65).
Factors that determine the neutralisation potency of antibodies
Flavivirus neutralisation is a ‘multiple’ hit phenomenon requiring engagement by more than a single antibody (reviewed in Refs 66, 67). Neutralisation occurs when the number of antibodies bound to an individual virion exceeds a required threshold (Ref. 68). In this regard, two biochemical factors play a significant role in determining when an antibody exceeds the stoichiometric requirements for neutralisation: antibody affinity and the accessibility of epitopes on the virus particle.
The strength of binding between antibody and viral antigen (affinity) determines the fraction of epitopes on the virus particle occupied by antibody at any given concentration (referred to as antibody occupancy) and is a primary determinant of neutralisation potency (Ref. 69). Thus, it is no surprise that differences in neutralisation potency between antibodies often can be accounted for by differences in the strength of antibody–antigen interactions. Integrating data from measurements of the avidity of antibody–virion interactions and the concentration of antibody required to inactivate 50% of the virus allows an estimate of antibody occupancy when the virus is neutralised. For the most potent neutralising antibodies against flaviviruses, neutralisation appears to occur at a relatively low occupancy (Refs 68, 70). However, some high-affinity antibodies exhibit rather limited neutralisation potency and inhibit infection only at very high concentrations relative to their affinity for viral antigens. In fact, for some antibodies, even complete occupancy of epitopes on the virion is not sufficient to exceed the threshold for neutralisation (Ref. 68).
The pseudo-icosahedral arrangement of E proteins on the virion displays the E protein in three distinct chemical environments defined by proximity to the two-, three- or fivefold axes of symmetry (Refs 20, 21). From the perspective of the antibody, epitopes in each of these environments may be differentially accessible for antibody binding because of steric constraints imposed by adjacent E proteins on the virus particle. As a result, the number of sites available for binding may differ among structurally distinct epitopes on the virion. Accessibility is a significant factor that modulates antibody potency and shapes the occupancy requirements for neutralisation. Antibodies that bind highly exposed determinants may exceed the stoichiometric threshold for neutralisation by binding the virion at relatively low occupancy (Ref. 68). By contrast, epitopes that are predicted to be poorly exposed may require nearly complete occupancy to achieve threshold requirements for neutralisation (Refs 68, 71) (Fig. 2).
Figure 2. Relationship between epitope accessibility and the occupancy requirements for neutralisation.
The accessibility of epitopes recognised by two different antibodies on the mature West Nile virion is illustrated using molecular modelling: residues that form each determinant are illustrated as solid spheres. E proteins are coloured according to their proximity to the twofold, threefold or fivefold symmetry axes (blue, green and yellow, respectively). The number of accessible binding sites for each antibody is indicated on the left, whereas the ‘threshold’ for neutralisation is indicated as a red line [modelled in this instance as 30 monoclonal antibodies (mAbs) based on studies using the mAb E16] (Ref. 68). To exceed the threshold requirements for neutralisation, only a fraction of highly accessible determinants must be simultaneously occupied by antibody (a low occupancy requirement). By contrast, a significantly greater percentage of poorly accessible epitopes must be bound to achieve the same number of antibodies docked on the average virion (a high occupancy requirement). Not all epitopes appear to exist on the average virion at levels that exceed this threshold.
The stoichiometry of antibody binding that defines the neutralisation threshold for flaviviruses has been estimated for only one antibody and epitope (Ref. 68). The WNV-specific mAb E16, which binds an epitope on the lateral surface of DIII, potently neutralises virus infection in vitro (Ref. 36) and is protective in vivo even when administered several days after virus infection (Refs 72, 73). Structural studies of E16 in complex with DIII on the intact virion demonstrate that this antibody cannot bind E proteins around the fivefold axis of symmetry. Thus, this potently neutralising antibody can recognise only 120 of the 180 E proteins on WNV (Refs 74, 75). As this antibody neutralises at an occupancy of roughly 25%, it is predicted that approximately 30 mAbs are required for neutralisation (Ref. 68). By comparison, molecular modelling studies suggest that many of the other epitopes recognised by neutralising antibodies are less accessible on the mature virion (Refs 56, 68, 71). How antibodies bind ‘cryptic’ epitopes yet neutralise virus infectivity is difficult to explain using existing static models of virion structure and is an area of active investigation.
Mechanisms of neutralisation
At present, the cell biology of the flavivirus entry pathway is not well understood. The first step in the process likely involves the interaction of virions on the surface of target cells with one or more cellular factors. Presumably, these interactions trigger access for the virus to the endocytic pathway via clathrin- and Rab5-mediated endocytosis and transport processes (Refs 76, 77, 78, 79) where fusion occurs in a pH-dependent fashion. Cellular factors that play a required role as a flavivirus ‘receptor’ have not yet been rigorously defined despite considerable effort. While many candidate receptors have been proposed (reviewed in Ref. 80), due in part to the extremely broad tropism in vitro of many flaviviruses, it remains difficult to distinguish molecules that play an essential role in the virus entry pathway from those that promote more efficient and durable attachment of virions to the cell surface. Furthermore, the requirements and characteristics of a cellular receptor for flaviviruses have not been formally established as several lines of evidence suggest exposure to acidic pH is the sole requirement for the conformational changes in the E protein that drive membrane fusion (Refs 23, 81, 82).
Blocking virus attachment
Antibodies have the potential to neutralise the infectivity of flaviviruses by interfering with several steps of the virus entry pathway including attachment, internalisation and fusion. Antibody-mediated neutralisation of several viruses has been reported to occur by blocking the attachment of viruses to the cell (reviewed in Ref. 69). Perhaps the most well-characterised example are antibodies that block the binding of the HIV-1 envelope protein to the CD4 receptor or CCR5 coreceptor on T cells (reviewed in Ref. 83). While the cellular factors involved remain unclear, antibodies may block flavivirus infection by inhibiting the interactions between virions and the cell surface during the attachment step. mAbs specific for DIII have been shown in some studies to block infection at this stage (Ref. 84). Several lines of indirect evidence suggest DIII plays an important role in virus attachment, including: (1) DIII protrudes the farthest from the surface of the virion; (2) many of the mutations that impact tropism or virulence map to DIII (Refs 85, 86, 87, 88); and (3) soluble forms of DIII can block infection (Ref. 6). Thus, blockade of the binding step is an attractive model for the neutralising mechanism of some DIII-specific mAbs.
The calcium-dependent (C-type) lectin DC-SIGN (CD209) is a well-characterised ‘attachment factor’ for flaviviruses. CD209 plays an important role in the infection of immature monocyte-derived dendritic cells by DENV in vitro (Refs 89, 90), and polymorphisms in the CD209 promoter are associated with protection against dengue fever (Ref. 91). Furthermore, transfection of a variety of cells lines with plasmids encoding either CD209 or the related molecule CD209L increases their capacity to support DENV infection (Refs 89, 90, 92). However, CD209-augmented infection does not require internalisation of the attachment factor, suggesting other processes and molecules at the cell surface are involved (Ref. 92). Like DENV, infection of cells in vitro by WNV can be enhanced by interactions with CD209L and to a lesser extent CD209, but the role (if any) of these lectins during WNV infection in vivo has not been examined (Refs 93, 94). As the spatial arrangement of the carbohydrate-recognition domains at the end of CD209 and CD209L tetramers is an important aspect of efficient ligand binding, it is conceivable that antibodies docked on virions may inhibit flavivirus infection by disrupting required multivalent binding of the sugars arrayed on the E protein (Refs 95, 96). Thus, DIII-independent inhibition of virion attachment may also be a mechanism of neutralisation and may contribute to cell-type-dependent differences in neutralisation potency observed for some mAbs.
Inhibiting viral membrane fusion
mAbs also have the potential to neutralise infectivity at steps downstream of binding, perhaps by inhibiting the conformational changes in the E protein associated with membrane fusion. Pioneering electron microscopy studies by Gollins and Porterfield suggest that West Nile virions complexed with neutralising quantities of antibody can be internalised by target cells, suggesting a post-attachment mechanism of neutralisation (Ref. 97). Neutralisation by the WNV DIII-specific mAb E16 also occurs at a post-attachment step of the viral entry pathway (Ref. 74). Structural and cryoelectron reconstruction analysis suggests docking of this antibody to the virion imposes steric constraints on the low-pH-mediated rearrangements of the E proteins that drive fusion (Refs 75, 98). Of significant interest, the ability of mAbs to directly block flavivirus fusion has been recently demonstrated using TBEV. Using a cell-free system in which labelled flavivirus virions are induced to fuse with synthetic lipid membranes, mAbs to some but not all epitopes on the TBEV E protein directly blocked fusion (Ref. 99). Using similar approaches, the WNV DIII-specific mAb E16 has been shown to also directly and completely block membrane fusion (B. Thompson, J. Smit, M. Diamond and D. Fremont, unpublished).
While specific mechanisms of neutralisation can readily be demonstrated using individual in vitro approaches, neutralisation by a single antibody may occur via multiple mechanisms that operate simultaneously, depending on how many antibodies bind to the virion. For example, the WNV-specific mAb E16 neutralises infection at a relatively low occupancy of epitopes on the virion. Several lines of evidence suggest this inhibition occurs primarily by blocking the conformational changes in E protein required for fusion. However, when individual virions are coated with saturating quantities of E16, blockade of attachment may also occur (Ref. 74). Furthermore, antibodies that neutralise infection only at full occupancy may block fusion in some experimental contexts, but may not neutralise infection in the endosome via this mechanism where the concentration of antibody falls below that required for saturation and inhibitory function.
Viral clearance via Fc-dependent effector functions of antibodies
Antibodies may also inhibit flavivirus infection by activating Fc-dependent effector functions including complement activation. Studies using animal models of flavivirus infection highlight the importance of an intact complement system for humoral immunity (Refs 100, 101). Virus opsonisation with the classical pathway complement components C1q, C4b and C3b may promote the formation of C5b–C9 membrane attack components that result in direct lysis of the virion. The efficiency of this process for flaviviruses may be limited by the small surface area of viral membrane exposed in the context of the mature virion. Complement may also augment the neutralisation potency of antibodies directly by modulating the occupancy requirements for neutralisation: increasing antibody avidity or increasing the steric effects of bound antibody may more efficiently result in a blockade of virus attachment or fusion (Refs 66, 102, 103). Finally, neutralisation potency may also reflect complement-independent Fcγ-receptor-dependent clearance pathways. The differential capacity of antibodies of IgG subclasses to interact with complement and/or Fcγ receptors identifies a layer of complexity beyond epitope specificity for determining the potency of neutralising antibodies (Refs 104, 105).
Antibody-dependent enhancement of infection
Antibody-dependent enhancement of infection (ADE) describes the dramatic increase in infection of cells bearing Fcγ receptors or complement receptors in the presence of subneutralising concentrations of antibody or immune sera. In this context, antibodies are believed to play a role in exacerbating disease following DENV infection (Ref. 106). Clinical manifestations of DENV range from a self-limiting acute, febrile illness (dengue fever) to a potentially fatal syndrome characterised by plasma leakage and shock (dengue haemorrhagic fever; DHF) (Ref. 107). Four related serotypes of DENV circulate in nature, each capable of causing the full spectrum of DENV-related disease. Prospective clinical studies clearly demonstrate that sequential infection with two DENV serotypes is associated with a more severe disease course (Ref. 108). The most direct link between ADE and the clinical outcome of DENV infection comes from investigations of the unusually large number of DHF cases following primary infection observed in children of DENV-immune mothers during the first year of life (Ref. 109). At birth, DENV-specific passively acquired antibodies are present at a relatively high concentration and exhibit neutralising activity in vitro. However, as the infant ages, maternally acquired antibody wanes to levels that no longer neutralise virus, and allows for enhancement of infection in vitro (Ref. 110). The waning antibody titres of infants to levels that support ADE in vitro parallels the risk of DHF following primary DENV infection during the first year of life. In a broader context, antibodies elicited by primary infection with one serotype of DENV may bind related viruses introduced during secondary infection with reduced avidity, resulting in engagement of the virion with a stoichiometry that does not permit virus neutralisation yet can support ADE.
The phenomenon of ADE has been established using several viral systems in vitro (Refs 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130). Furthermore, in some circumstances, passive transfer of antibody has been shown to result in significant increases in viral load in animal models of DENV and WNV infection (Refs 131, 132, 133), as well as a more rapid progression to disease in heterologous challenge experiments performed with the related JEV and Murray Valley encephalitis viruses (Refs 114, 134). Gollins and Porterfield suggest that enhancement of flavivirus infection can be explained in part by more-efficient virus binding and internalisation in the presence of antibody (Refs 78, 135). These studies suggest that enhancement is an opsonic phenomenon in which antibodies increase the efficiency of virus attachment to the cell surface. However, other mechanisms are possible, including increasing the efficiency of post-attachment steps in the replication cycle following Fcγ-receptor-mediated signalling, delivery of antibody-bound virions to more favourable locations in the endocytic compartment, direct alterations in the fusion process, and the antibody-dependent release of autocrine or paracrine factors (cytokines, interferons, chemokines and nitric oxide) that modulate virus replication (Refs 136, 137, 138, 139, 140).
Clinical implications/applications
The most potent inhibitory mAbs against flaviviruses neutralise infection by engagement of a relatively small fraction of the available epitopes on the average virion (Refs 68, 70). As a practical consequence, neutralisation at low occupancy requires lower concentrations of antibody and can occur even with lower-affinity antibodies. By contrast, neutralisation by antibodies that recognise epitopes on poorly accessible structures requires engagement of a larger fraction of epitopes on the virion to reach the threshold required for neutralisation (Fig. 2). Thus, neutralisation is achieved only at relatively high concentrations of antibody. Of interest, some epitopes on the virion are accessible at a frequency very close to the threshold for neutralisation and thus may not elicit antibodies with significant neutralisation potential even at full occupancy.
Integrating new information that defines in biochemical terms the potency of neutralising antibodies into the design of next-generation flavivirus immunogens and antibody therapeutics may increase efficacy and reduce the potential for ADE. As the phenomena of neutralisation and enhancement appear related simply by the stoichiometry of antibody binding to individual virions, most epitopes have the capacity to elicit antibody capable of promoting ADE (Ref. 68). However, antibodies specific for determinants that are poorly accessible are not only less potent due to the large occupancy requirements for neutralisation, but also more likely to promote enhancement on Fcγ-receptor-bearing cells over a wide range of concentrations. Of note, many of the epitopes raised by natural infection exhibit these properties using in vitro tests, and are poorly protective in vivo (Refs 56, 63). Thus an important goal for vaccine development against flaviviruses in general, and the four serotypes of DENV in particular, will be to redirect the humoral immune response away from poorly accessible structures to target more accessible determinants that elicit highly potent neutralising antibodies.
In addition to their role as effector molecules in response to vaccination, antibodies may be effective therapeutics as suggested by clinical improvement in WNV-infected patients treated with immune γ-globulin (Refs 141, 142). Antibodies that neutralise infection at low occupancy (i.e. low plasma concentrations) have greater therapeutic potential and a decreased risk for ADE in vivo. In light of its potency in vitro and in vivo (Ref. 36), including the ability to protect rodent models of WNV infection even when administered several days post-infection (Refs 72, 73), human clinical trials with the humanised version of the WNV-specific mAb E16 are planned for the treatment of severe WNV disease.
Research in progress and outstanding questions
Based on recent studies, a composite picture has emerged as to the location of epitopes that are recognised by the most strongly neutralising antibodies. Although preliminary experiments suggest the most potently inhibitory mAbs against WNV block the pH-dependent fusion step, these results need to be confirmed with a larger panel of antibodies, including those that recognise related flaviviruses. The ongoing identification of attachment and entry receptors for flaviviruses will undoubtedly impact our understanding of antibody neutralisation. Cell-specific differences in receptor usage may affect the mechanism and potency of antibody inhibition. Moreover, further study is warranted to explain why crossreactive mAbs that recognise the fusion loop in DII, a cryptic epitope on the mature virion, differentially neutralise flaviviruses. The current static model of the arrangement of E proteins on the mature virion might require revision.
A fundamental understanding of the mechanisms of antibody-mediated neutralisation may have significant implications for the generation of novel antibody-based therapeutics, epitope-targeted vaccines, or peptide inhibitors of WNV infection. WNV and other flaviviruses may be well suited to ‘reverse vaccinology’ – the identification and targeting of specific structural protein epitopes that elicit protective antibodies. In this strategy, epitopes that are poorly protective would be eliminated or masked in favour of epitopes that elicit strongly protective antibodies. This could be achieved through selective epitope mutation or deletion, epitope masking with N-linked carbohydrates, subunit (i.e. DIII alone) vaccines, or through generation of novel variants that display desired epitopes. Vaccines that elicit potently neutralising antibodies that block fusion could be safer and more effective against a range of flavivirus infections.
Acknowledgments
Acknowledgements and funding
The authors thank members of their laboratories, and also Daved Fremont, Richard Kuhn and John Roehrig, for helpful discussions. The authors also thank the reviewers of the manuscript for their comments and suggestions. The work was supported by the Pediatric Dengue Vaccine Initiative (M.S.D. and T.C.P.), NIH [AI061373 (M.S.D.) and U54 AI057160 (Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research)], and the Intramural Research Program of NIAID at NIH.
References
- 1.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, et al. Conformational changes of the flavivirus E glycoprotein. Structure. 2004;12:1607–1618. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rey FA, et al. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 4.Modis Y, et al. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A. 2003;100:6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modis Y, et al. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol. 2005;79:1223–1231. doi: 10.1128/JVI.79.2.1223-1231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu JJ, et al. Inhibition of West Nile virus entry by using a recombinant domain III from the envelope glycoprotein. J Gen Virol. 2005;86:405–412. doi: 10.1099/vir.0.80411-0. [DOI] [PubMed] [Google Scholar]
- 7.Allison SL, et al. Mutational evidence for an internal fusion peptide in flavivirus envelope protein E. J Virol. 2001;75:4268–4275. doi: 10.1128/JVI.75.9.4268-4275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modis Y, et al. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 9.Bressanelli S, et al. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004;23:728–738. doi: 10.1038/sj.emboj.7600064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, et al. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat Struct Biol. 2003;10:907–912. doi: 10.1038/nsb990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allison SL, et al. Mapping of functional elements in the stem-anchor region of tick-borne encephalitis virus envelope protein E. J Virol. 1999;73:5605–5612. doi: 10.1128/jvi.73.7.5605-5612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, He R, Anderson R. PrM- and cell-binding domains of the dengue virus E protein. J Virol. 1999;73:2547–2551. doi: 10.1128/jvi.73.3.2547-2551.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie JM, Westaway EG. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J Virol. 2001;75:10787–10799. doi: 10.1128/JVI.75.22.10787-10799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Structure of immature West Nile virus. J Virol. 2007;81:6141–6145. doi: 10.1128/JVI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Structures of immature flavivirus particles. EMBO J. 2003;22:2604–2613. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinz FX, et al. Structural changes and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology. 1994;198:109–117. doi: 10.1006/viro.1994.1013. [DOI] [PubMed] [Google Scholar]
- 17.Guirakhoo F, et al. Fusion activity of flaviviruses: comparison of mature and immature (prM-containing) tick-borne encephalitis virions. J Gen Virol. 1991;72:1323–1329. doi: 10.1099/0022-1317-72-6-1323. [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 19.Stadler K, et al. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukhopadhyay S, et al. Structure of West Nile virus. Science. 2003;302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn RJ, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura T, Gollins SW, Porterfield JS. The effect of pH on the early interaction of West Nile virus with P388D1 cells. J Gen Virol. 1986;67:2423–2433. doi: 10.1099/0022-1317-67-11-2423. [DOI] [PubMed] [Google Scholar]
- 23.Gollins SW, Porterfield JS. pH-dependent fusion between the flavivirus West Nile and liposomal model membranes. J Gen Virol. 1986;67:157–166. doi: 10.1099/0022-1317-67-1-157. [DOI] [PubMed] [Google Scholar]
- 24.Holzmann H, et al. Tick-borne encephalitis virus envelope protein E-specific monoclonal antibodies for the study of low pH-induced conformational changes and immature virions. Arch Virol. 1995;140:213–221. doi: 10.1007/BF01309857. [DOI] [PubMed] [Google Scholar]
- 25.Stiasny K, et al. Structural requirements for low-pH-induced rearrangements in the envelope glycoprotein of tick-borne encephalitis virus. J Virol. 1996;70:8142–8147. doi: 10.1128/jvi.70.11.8142-8147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiasny K, et al. Role of metastability and acidic pH in membrane fusion by tick-borne encephalitis virus. J Virol. 2001;75:7392–7398. doi: 10.1128/JVI.75.16.7392-7398.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allison SL, et al. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J Virol. 1995;69:695–700. doi: 10.1128/jvi.69.2.695-700.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 29.Colman PM, Lawrence MC. The structural biology of type I viral membrane fusion. Nat Rev Mol Cell Biol. 2003;4:309–319. doi: 10.1038/nrm1076. [DOI] [PubMed] [Google Scholar]
- 30.Liao M, Kielian M. Domain III from class II fusion proteins functions as a dominant-negative inhibitor of virus membrane fusion. J Cell Biol. 2005;171:111–120. doi: 10.1083/jcb.200507075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai F, et al. Antiviral peptides targeting the west nile virus envelope protein. J Virol. 2007;81:2047–2055. doi: 10.1128/JVI.01840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hrobowski YM, Garry RF, Michael SF. Peptide inhibitors of dengue virus and West Nile virus infectivity. Virol J. 2005;2:49. doi: 10.1186/1743-422X-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves JD, Piefer AJ. Emerging drug targets for antiretroviral therapy. Drugs. 2005;65:1747–1766. doi: 10.2165/00003495-200565130-00002. [DOI] [PubMed] [Google Scholar]
- 34.Diamond MS, et al. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003;77:2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamond MS, et al. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003;16:259–278. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- 36.Oliphant T, et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tesh RB, et al. Immunization with heterologous flaviviruses protective against fatal West Nile encephalitis. Emerg Infect Dis. 2002;8:245–251. doi: 10.3201/eid0803.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, et al. Immunization of mice against West Nile virus with recombinant envelope protein. J Immunol. 2001;167:5273–5277. doi: 10.4049/jimmunol.167.9.5273. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Nathan D, et al. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating west nile virus infection in mice. J Infect Dis. 2003;188:5–12. doi: 10.1086/376870. [DOI] [PubMed] [Google Scholar]
- 40.Camenga DL, Nathanson N, Cole GA. Cyclophosphamide-potentiated West Nile viral encephalitis: relative influence of cellular and humoral factors. J Infect Dis. 1974;130:634–641. doi: 10.1093/infdis/130.6.634. [DOI] [PubMed] [Google Scholar]
- 41.Roehrig JT, et al. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann N Y Acad Sci. 2001;951:286–297. doi: 10.1111/j.1749-6632.2001.tb02704.x. [DOI] [PubMed] [Google Scholar]
- 42.Mathews JH, Roehrig JT. Elucidation of the topography and determination of the protective epitopes on the E glycoprotein of Saint Louis encephalitis virus by passive transfer with monoclonal antibodies. J Immunol. 1984;132:1533–1537. [PubMed] [Google Scholar]
- 43.Kreil TR, et al. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clin Exp Immunol. 1997;110:358–361. doi: 10.1046/j.1365-2249.1997.4311446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kreil TR, et al. Neutralizing antibodies protect against lethal flavivirus challenge but allow for the development of active humoral immunity to a nonstructural virus protein. J Virol. 1998;72:3076–3081. doi: 10.1128/jvi.72.4.3076-3081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beasley DW, et al. Protection against Japanese encephalitis virus strains representing four genotypes by passive transfer of sera raised against ChimeriVax-JE experimental vaccine. Vaccine. 2004;22:3722–3726. doi: 10.1016/j.vaccine.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 46.Vazquez S, et al. Immune response to synthetic peptides of dengue prM protein. Vaccine. 2002;20:1823–1830. doi: 10.1016/s0264-410x(01)00515-1. [DOI] [PubMed] [Google Scholar]
- 47.Pincus S, et al. Recombinant vaccinia virus producing the prM and E proteins of yellow fever virus protects mice from lethal yellow fever encephalitis. Virology. 1992;187:290–297. doi: 10.1016/0042-6822(92)90317-i. [DOI] [PubMed] [Google Scholar]
- 48.Falconar AK. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch Virol. 1999;144:2313–2330. doi: 10.1007/s007050050646. [DOI] [PubMed] [Google Scholar]
- 49.Colombage G, et al. DNA-based and alphavirus-vectored immunisation with prM and E proteins elicits long-lived and protective immunity against the flavivirus, Murray Valley encephalitis virus. Virology. 1998;250:151–163. doi: 10.1006/viro.1998.9357. [DOI] [PubMed] [Google Scholar]
- 50.Chung KM, et al. Antibody recognition of cell surface-associated NS1 triggers Fc-gamma receptor-mediated phagocytosis and clearance of West Nile Virus-infected cells. J Virol. 2007;81:9551–9555. doi: 10.1128/JVI.00879-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung KM, et al. Antibodies against West Nile Virus nonstructural protein NS1 prevent lethal infection through Fc gamma receptor-dependent and -independent mechanisms. J Virol. 2006;80:1340–1351. doi: 10.1128/JVI.80.3.1340-1351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shu PY, et al. Dengue NS1-specific antibody responses: isotype distribution and serotyping in patients with Dengue fever and Dengue hemorrhagic fever. J Med Virol. 2000;62:224–232. doi: 10.1002/1096-9071(200010)62:2<224::aid-jmv14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 53.Churdboonchart V, et al. Antibodies against dengue viral proteins in primary and secondary dengue hemorrhagic fever. Am J Trop Med Hyg. 1991;44:481–493. doi: 10.4269/ajtmh.1991.44.481. [DOI] [PubMed] [Google Scholar]
- 54.Gibson CA, Schlesinger JJ, Barrett AD. Prospects for a virus non-structural protein as a subunit vaccine. Vaccine. 1988;6:7–9. doi: 10.1016/0264-410x(88)90004-7. [DOI] [PubMed] [Google Scholar]
- 55.Beasley DW, Barrett AD. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J Virol. 2002;76:13097–13100. doi: 10.1128/JVI.76.24.13097-13100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oliphant T, et al. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol. 2006;80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crill WD, Chang GJ. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J Virol. 2004;78:13975–13986. doi: 10.1128/JVI.78.24.13975-13986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roehrig JT, Bolin RA, Kelly RG. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology. 1998;246:317–328. doi: 10.1006/viro.1998.9200. [DOI] [PubMed] [Google Scholar]
- 59.Roehrig JT, Mathews JH, Trent DW. Identification of epitopes on the E glycoprotein of Saint Louis encephalitis virus using monoclonal antibodies. Virology. 1983;128:118–126. doi: 10.1016/0042-6822(83)90323-9. [DOI] [PubMed] [Google Scholar]
- 60.Heinz FX, et al. A topological and functional model of epitopes on the structural glycoprotein of tick-borne encephalitis virus defined by monoclonal antibodies. Virology. 1983;126:525–537. doi: 10.1016/s0042-6822(83)80010-5. [DOI] [PubMed] [Google Scholar]
- 61.Mandl CW, et al. Antigenic structure of the flavivirus envelope protein E at the molecular level, using tick-borne encephalitis virus as a model. J Virol. 1989;63:564–571. doi: 10.1128/jvi.63.2.564-571.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sukupolvi-Petty S, et al. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol. 2007;81:12816–12826. doi: 10.1128/JVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Throsby M, et al. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile Virus. J Virol. 2006;80:6982–6992. doi: 10.1128/JVI.00551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oliphant T, et al. Induction of epitope-specific neutralizing antibodies against West Nile virus. J Virol. 2007;81:11828–11839. doi: 10.1128/JVI.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanchez MD, et al. The neutralizing antibody response against West Nile virus in naturally infected horses. Virology. 2007;359:336–348. doi: 10.1016/j.virol.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 66.Della-Porta AJ, Westaway EG. A multi-hit model for the neutralization of animal viruses. J Gen Virol. 1978;38:1–19. doi: 10.1099/0022-1317-38-1-1. [DOI] [PubMed] [Google Scholar]
- 67.Burton DR, Saphire EO, Parren PW. A model for neutralization of viruses based on antibody coating of the virion surface. Curr Top Microbiol Immunol. 2001;260:109–143. doi: 10.1007/978-3-662-05783-4_7. [DOI] [PubMed] [Google Scholar]
- 68.Pierson TC, et al. Stoichiometric requirements for antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe. 2007;1:135–146. doi: 10.1016/j.chom.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klasse PJ, Sattentau QJ. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol. 2002;83:2091–2108. doi: 10.1099/0022-1317-83-9-2091. [DOI] [PubMed] [Google Scholar]
- 70.Gromowski GD, Barrett AD. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology. 2007;366:349–360. doi: 10.1016/j.virol.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 71.Stiasny K, et al. Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. J Virol. 2006;80:9557–9568. doi: 10.1128/JVI.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morrey JD, et al. Humanized monoclonal antibody against West Nile virus envelope protein administered after neuronal infection protects against lethal encephalitis in hamsters. J Infect Dis. 2006;194:1300–1308. doi: 10.1086/508293. [DOI] [PubMed] [Google Scholar]
- 73.Morrey JD, et al. Defining limits of treatment with humanized neutralizing monoclonal antibody for West Nile virus neurological infection in a hamster model. Antimicrob Agents Chemother. 2007;51:2396–2402. doi: 10.1128/AAC.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nybakken GE, et al. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature. 2005;437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaufmann B, et al. West Nile virus in complex with the Fab fragment of a neutralizing monoclonal antibody. Proc Natl Acad Sci U S A. 2006;103:12400–12404. doi: 10.1073/pnas.0603488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krishnan MN, et al. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J Virol. 2007;81:4881–4885. doi: 10.1128/JVI.02210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Schaar HM, et al. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J Virol. 2007;81:12019–12028. doi: 10.1128/JVI.00300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gollins SW, Porterfield JS. Flavivirus infection enhancement in macrophages: an electron microscopic study of viral cellular entry. J Gen Virol. 1985;66:1969–1982. doi: 10.1099/0022-1317-66-9-1969. [DOI] [PubMed] [Google Scholar]
- 79.Chu JJ, Ng ML. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J Virol. 2004;78:10543–10555. doi: 10.1128/JVI.78.19.10543-10555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson R. Manipulation of cell surface macromolecules by flaviviruses. Adv Virus Res. 2003;59:229–274. doi: 10.1016/S0065-3527(03)59007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stiasny K, Koessl C, Heinz FX. Involvement of lipids in different steps of the flavivirus fusion mechanism. J Virol. 2003;77:7856–7862. doi: 10.1128/JVI.77.14.7856-7862.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stiasny K, et al. Membrane interactions of the tick-borne encephalitis virus fusion protein E at low pH. J Virol. 2002;76:3784–3790. doi: 10.1128/JVI.76.8.3784-3790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pantophlet R, Burton DR. GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol. 2006;24:739–769. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- 84.He RT, et al. Antibodies that block virus attachment to Vero cells are a major component of the human neutralizing antibody response against dengue virus type 2. J Med Virol. 1995;45:451–461. doi: 10.1002/jmv.1890450417. [DOI] [PubMed] [Google Scholar]
- 85.Jennings AD, et al. Analysis of a yellow fever virus isolated from a fatal case of vaccine-associated human encephalitis. J Infect Dis. 1994;169:512–518. doi: 10.1093/infdis/169.3.512. [DOI] [PubMed] [Google Scholar]
- 86.Mandl CW, et al. Attenuation of tick-borne encephalitis virus by structure-based site-specific mutagenesis of a putative flavivirus receptor binding site. J Virol. 2000;74:9601–9609. doi: 10.1128/jvi.74.20.9601-9609.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holzmann H, et al. A single amino acid substitution in envelope protein E of tick-borne encephalitis virus leads to attenuation in the mouse model. J Virol. 1990;64:5156–5159. doi: 10.1128/jvi.64.10.5156-5159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang WR, et al. Single amino acid codon changes detected in louping ill virus antibody-resistant mutants with reduced neurovirulence. J Gen Virol. 1993;74:931–935. doi: 10.1099/0022-1317-74-5-931. [DOI] [PubMed] [Google Scholar]
- 89.Tassaneetrithep B, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Navarro-Sanchez E, et al. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4:723–728. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sakuntabhai A, et al. Avariant in the CD209 promoter is associated with severity of dengue disease. Nat Genet. 2005;37:507–513. doi: 10.1038/ng1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lozach PY, et al. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J Biol Chem. 2005;280:23698–23708. doi: 10.1074/jbc.M504337200. [DOI] [PubMed] [Google Scholar]
- 93.Davis CW, et al. The location of asparagine-linked glycans on West Nile virions controls their interactions with CD209 (dendritic cell-specific ICAM-3 grabbing nonintegrin) J Biol Chem. 2006;281:37183–37194. doi: 10.1074/jbc.M605429200. [DOI] [PubMed] [Google Scholar]
- 94.Davis CW, et al. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pokidysheva E, et al. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell. 2006;124:485–493. doi: 10.1016/j.cell.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 96.Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 97.Gollins SW, Porterfield JS. A new mechanism for the neutralization of enveloped viruses by antiviral antibody. Nature. 1986;321:244–246. doi: 10.1038/321244a0. [DOI] [PubMed] [Google Scholar]
- 98.Nybakken GE, et al. Crystal structure of the west nile virus envelope glycoprotein. J Virol. 2006;80:11467–11474. doi: 10.1128/JVI.01125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stiasny K, et al. Probing the flavivirus membrane fusion mechanism by using monoclonal antibodies. J Virol. 2007;81:11526–11531. doi: 10.1128/JVI.01041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehlhop E, et al. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J Virol. 2005;79:7466–7477. doi: 10.1128/JVI.79.12.7466-7477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mehlhop E, Diamond MS. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J Exp Med. 2006;203:1371–1381. doi: 10.1084/jem.20052388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyer K, et al. Complement-mediated enhancement of antibody function for neutralization of pseudotype virus containing hepatitis C virus E2 chimeric glycoprotein. J Virol. 2002;76:2150–2158. doi: 10.1128/jvi.76.5.2150-2158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feng JQ, Mozdzanowska K, Gerhard W. Complement component C1q enhances the biological activity of influenza virus hemagglutinin-specific antibodies depending on their fine antigen specificity and heavy-chain isotype. J Virol. 2002;76:1369–1378. doi: 10.1128/JVI.76.3.1369-1378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schlesinger JJ, Chapman S. Neutralizing F(ab′)2 fragments of protective monoclonal antibodies to yellow fever virus (YF) envelope protein fail to protect mice against lethal YF encephalitis. J Gen Virol. 1995;76:217–220. doi: 10.1099/0022-1317-76-1-217. [DOI] [PubMed] [Google Scholar]
- 105.Schlesinger JJ, Foltzer M, Chapman S. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology. 1993;192:132–141. doi: 10.1006/viro.1993.1015. [DOI] [PubMed] [Google Scholar]
- 106.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 107.Rothman AL. Immunology and immunopathogenesis of dengue disease. Adv Virus Res. 2003;60:397–419. doi: 10.1016/s0065-3527(03)60010-2. [DOI] [PubMed] [Google Scholar]
- 108.Vaughn DW, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 109.Halstead SB, Nimmannitya S, Cohen SN. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med. 1970;42:311–328. [PMC free article] [PubMed] [Google Scholar]
- 110.Kliks SC, et al. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 111.Klimstra WB, et al. Targeting Sindbis virus-based vectors to Fc receptor-positive cell types. Virology. 2005;338:9–21. doi: 10.1016/j.virol.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 112.Iankov ID, et al. Immunoglobulin g antibody-mediated enhancement of measles virus infection can bypass the protective antiviral immune response. J Virol. 2006;80:8530–8540. doi: 10.1128/JVI.00593-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang KJ, et al. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J Immunol. 2006;176:2825–2832. doi: 10.4049/jimmunol.176.5.2825. [DOI] [PubMed] [Google Scholar]
- 114.Wallace MJ, et al. Antibody-dependent enhancement of Murray Valley encephalitis virus virulence in mice. J Gen Virol. 2003;84:1723–1728. doi: 10.1099/vir.0.18980-0. [DOI] [PubMed] [Google Scholar]
- 115.Takada A, et al. Antibody-dependent enhancement of Ebola virus infection. J Virol. 2003;77:7539–7544. doi: 10.1128/JVI.77.13.7539-7544.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Girn J, Kavoosi M, Chantler J. Enhancement of coxsackievirus B3 infection by antibody to a different coxsackievirus strain. J Gen Virol. 2002;83:351–358. doi: 10.1099/0022-1317-83-2-351. [DOI] [PubMed] [Google Scholar]
- 117.Littaua R, Kurane I, Ennis FA. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J Immunol. 1990;144:3183–3186. [PubMed] [Google Scholar]
- 118.Takeda A, Tuazon CU, Ennis FA. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science. 1988;242:580–583. doi: 10.1126/science.2972065. [DOI] [PubMed] [Google Scholar]
- 119.Tamura M, Webster RG, Ennis FA. Antibodies to HA and NA augment uptake of influenza Aviruses into cells via Fc receptor entry. Virology. 1991;182:211–219. doi: 10.1016/0042-6822(91)90664-w. [DOI] [PubMed] [Google Scholar]
- 120.Morens DM, Halstead SB. Measurement of antibody-dependent infection enhancement of four dengue virus serotypes by monoclonal and polyclonal antibodies. J Gen Virol. 1990;71:2909–2914. doi: 10.1099/0022-1317-71-12-2909. [DOI] [PubMed] [Google Scholar]
- 121.Morens DM, Halstead SB, Marchette NJ. Profiles of antibody-dependent enhancement of dengue virus type 2 infection. Microb Pathog. 1987;3:231–237. doi: 10.1016/0882-4010(87)90056-8. [DOI] [PubMed] [Google Scholar]
- 122.Gimenez HB, et al. Neutralizing and enhancing activities of human respiratory syncytial virus-specific antibodies. Clin Diagn Lab Immunol. 1996;3:280–286. doi: 10.1128/cdli.3.3.280-286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gimenez HB, Keir HM, Cash P. Invitro enhancement of respiratory syncytial virus infection of U937 cells by human sera. J Gen Virol. 1989;70:89–96. doi: 10.1099/0022-1317-70-1-89. [DOI] [PubMed] [Google Scholar]
- 124.Osiowy C, Horne D, Anderson R. Antibody-dependent enhancement of respiratory syncytial virus infection by sera from young infants. Clin Diagn Lab Immunol. 1994;1:670–677. doi: 10.1128/cdli.1.6.670-677.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mady BJ, et al. Antibody-dependent enhancement of dengue virus infection mediated by bispecific antibodies against cell surface molecules other than Fc gamma receptors. J Immunol. 1991;147:3139–3144. [PubMed] [Google Scholar]
- 126.Gould EA, Buckley A. Antibody-dependent enhancement of yellow fever and Japanese encephalitis virus neurovirulence. J Gen Virol. 1989;70:1605–1608. doi: 10.1099/0022-1317-70-6-1605. [DOI] [PubMed] [Google Scholar]
- 127.Barrett AD, Gould EA. Antibody-mediated early death in vivo after infection with yellow fever virus. J Gen Virol. 1986;67:2539–2542. doi: 10.1099/0022-1317-67-11-2539. [DOI] [PubMed] [Google Scholar]
- 128.Schlesinger JJ, Brandriss MW. Antibody-mediated infection of macrophages and macrophage-like cell lines with 17D-yellow fever virus. J Med Virol. 1981;8:103–117. doi: 10.1002/jmv.1890080204. [DOI] [PubMed] [Google Scholar]
- 129.Robinson WE, Jr, Montefiori DC, Mitchell WM. Antibody-dependent enhancement of human immunodeficiency virus type 1 infection. Lancet. 1988;1:790–794. doi: 10.1016/s0140-6736(88)91657-1. [DOI] [PubMed] [Google Scholar]
- 130.Halstead SB, O’Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goncalvez AP, et al. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci U S A. 2007;104:9422–9427. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Halstead SB. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979;140:527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- 133.Mehlhop E, et al. Complement protein C1q inhibits antibody-dependent enhancement of flavivirus infection in an IgG subclass-specific manner. Cell Host Microbe. 2007;2:417–426. doi: 10.1016/j.chom.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Broom AK, et al. Immunisation with gamma globulin to murray valley encephalitis virus and with an inactivated Japanese encephalitis virus vaccine as prophylaxis against australian encephalitis: evaluation in a mouse model. J Med Virol. 2000;61:259–265. doi: 10.1002/(sici)1096-9071(200006)61:2<259::aid-jmv13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 135.Gollins SW, Porterfield JS. Flavivirus infection enhancement in macrophages: radioactive and biological studies on the effect of antibody on viral fate. J Gen Virol. 1984;65:1261–1272. doi: 10.1099/0022-1317-65-8-1261. [DOI] [PubMed] [Google Scholar]
- 136.Chareonsirisuthigul T, Kalayanarooj S, Ubol S. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. J Gen Virol. 2007;88:365–375. doi: 10.1099/vir.0.82537-0. [DOI] [PubMed] [Google Scholar]
- 137.Suhrbier A, La Linn M. Suppression of antiviral responses by antibody-dependent enhancement of macrophage infection. Trends Immunol. 2003;24:165–168. doi: 10.1016/s1471-4906(03)00065-6. [DOI] [PubMed] [Google Scholar]
- 138.Hober D, et al. Antibody-dependent enhancement of coxsackievirus B4 infectivity of human peripheral blood mononuclear cells results in increased interferon-alpha synthesis. J Infect Dis. 2001;184:1098–1108. doi: 10.1086/323801. [DOI] [PubMed] [Google Scholar]
- 139.Lidbury BA, Mahalingam S. Specific ablation of antiviral gene expression in macrophages by antibody-dependent enhancement of Ross River virus infection. J Virol. 2000;74:8376–8381. doi: 10.1128/jvi.74.18.8376-8381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mahalingam S, Lidbury BA. Antibody-dependent enhancement of infection: bacteria do it too. Trends Immunol. 2003;24:465–467. doi: 10.1016/s1471-4906(03)00210-2. [DOI] [PubMed] [Google Scholar]
- 141.Hamdan A, et al. Possible benefit of intravenous immunoglobulin therapy in a lung transplant recipient with West Nile virus encephalitis. Transpl Infect Dis. 2002;4:160–162. doi: 10.1034/j.1399-3062.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 142.Shimoni Z, et al. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerg Infect Dis. 2001;7:759. doi: 10.3201/eid0704.010432. [DOI] [PMC free article] [PubMed] [Google Scholar]