Abstract
Background
Basic studies have suggested that cross-talk exists between the endothelin-A receptor (ET-AR) and tumor necrosis factor signaling pathway. This study tested the hypothesis that administration of an ET-AR antagonist at the separation from cardiopulmonary bypass would alter the tumor necrosis factor activation in the early post operative period.
Methods
Patients (n = 44) were randomized to receive bolus infusion of vehicle, 0.1, 0.5, 1, or 2 mg/kg of the ET-AR antagonist (sitaxsentan) at the separation from cardiopulmonary bypass (n=9, 9, 9, 9, and 8 respectively). Plasma levels of tumor necrosis factor-α and soluble tumor necrosis factor receptor 1 and 2 were measured.
Results
Compared to the vehicle group at 24 hours, plasma levels of tumor necrosis factor-α and tumor necrosis factor receptor 2 (indicative of receptor activation) were reduced in the 1 mg/kg ET-AR antagonist group (by ~13 pg/mL and ~0.5 ng/mL respectively; p<0.05). Plasma tumor necrosis factor receptor I levels also decreased (by ~1 ng/mL) following infusion of the higher doses of the ET-AR antagonist and remained lower (by ~3 ng/mL) at 24 hours post infusion (p<0.05). In addition, a dose effect was observed between the ET-AR antagonist and these indices of tumor necrosis factor activation (p<0.01).
Conclusions
This study demonstrated a mechanistic relationship between the ET-AR and tumor necrosis factor receptor activation in the post cardiac surgery period. Thus, in addition to the potential cardiovascular effects, a selective ET-AR antagonist can modify other biological processes relevant to the post cardiac surgery setting. (word count:245/250)
Keywords: Cardiopulmonary Bypass (CPB), Inflammation, Cytokines
INTRODUCTION
Coronary revascularization is one of the most common adult cardiac surgical procedures, which can often require cardiopulmonary bypass (CPB). While CPB provides for myocardial quiescence and a bloodless operative field, myocardial reperfusion and separation from CPB can be associated with transient cardiovascular compromise in the post operative setting. Coincident with the separation from CPB, is the release of bioactive molecules including endothelin (ET) and cytokines [1–9]. While mechanistic links remain to be established, ET release and cytokine activation have been associated with a protracted post operative course following CPB [6–9]. Past studies have demonstrated that ET has diverse biological effects on myocyte contractility and upon the vasculature [10–12]. The cardiovascular effects of ET are mediated through two unique receptor subtypes: ET-A receptor (ET-AR) and ET-B receptor (ET-BR). ET-AR mediates local and systemic vasoconstriction and has negative inotropic effects [10–12]. In contradistinction, ET-BR mediates the release of vasodilatory factors, such as nitric oxide, as well as facilitates ET clearance [13, 14]. Accordingly, a number of selective ET-AR antagonists have been developed and are currently under clinical investigation for pulmonary hypertension [15]. One such selective ET-AR antagonist, sitaxsentan [16], has been utilized by this laboratory in a dose ranging and safety study in patients undergoing coronary revascularization requiring CPB [17]. However, several unanswered questions remained from this initial dose ranging study which include the potential interactions that exist between ET-AR and the cytokine cascade.
Cytokines are a diverse group of molecules that form a part of the immune response and affect the cardiovascular system by regulating inflammation, myocardial function, and cell apoptosis [18–19]. Tumor necrosis factor-α (TNF) is a pro-inflammatory cytokine which has been reported to increase following CPB [3, 9]. TNF is a membrane bound molecule, which is cleaved by a protease and released, resulting in binding to the TNF receptors, TNFRI and TNFRII [20]. Subsequent to TNF binding, the extracellular TNF/TNFRI and TNF/TNFRII complexes are released into the interstitial space and can be detected in the systemic circulation [4, 21]. Thus measurements of relative changes in TNF/TNFRI and TNF/TNFRII within the systemic circulation provide an index of TNFR activation. Past in vitro and in vivo studies have demonstrated ET can induce the biosynthesis and release of TNF [22, 23]. Therefore, the hypothesis of this study is that administration of a selective ET-AR antagonist immediately following separation from CPB would alter the TNF receptor profile in a dose dependent manner.
METHODS
Overview
This study utilized plasma samples obtained from a previous dose ranging and safety study which a selective ET-AR antagonist was administered following CPB [17]. The patient demographics, hemodymanics, and operative statistics were fully described in this past study and accordingly are only briefly described below.
Patients
This study obtained full approval by the Human Subjects Review Committee of the Medical University of South Carolina (HR11122). Patients undergoing elective coronary artery bypass surgery requiring CPB provided informed consent to participate in the sub-study. Exclusion criteria included: emergent revascularization, stroke or thrombo-embolic event within 3 months preceding surgery, previous myocardial infarction, documented coagulopathy; hepatic dysfunction as defined by aspartate transaminase (AST) or alanine transaminase (ALT) >1.5 times the upper limit of normal, and chronic renal insufficiency as defined by a creatinine >2.5 mg/dL or requirement for dialysis. All patients were male and averaged 62 ± 1 years of age.
Operative Procedure
Standard induction and maintenance of anesthesia was accomplished with a combination of sufentanil, midazolam and isoflurane. All patients received the full Hammersmith dose of aprotinin prior to surgery. No patients were administered corticosteroids during surgery. Prior to CPB, systemic heparinization was accomplished with a heparin dose of 400 units/kg and was administered to maintain an activated clotting time of > 400 seconds. CPB was accomplished with a Sarns membrane oxygenator (Terumo/Sarns, Ann Arbor, MI) and a hemoconcentration pack with Smart Tubing circuits (Sorin Group, Arvada, CO). A cell saver was utilized during CPB (Haemonetics, Brainetree MA) and wound aspirate was returned following the separation from CPB. CPB was maintained at a cardiac index of 2.0 to 2.4 l/min/m2 and cardioplegia was accomplished with antrograde normothermic administration of a blood crystalloid solution as previously described. Cardiac arrest was maintained with routine intervals of administration of cardioplegic solution in a retrograde fashion. Patients were not actively cooled during CPB and were re-warmed to a rectal temperature of 36.5°C prior to CPB separation. At the termination of CPB, heparin was neutralized with protamine in a 1:1 ratio.
Endothelin-A Receptor Antagonist, Randomization, and Sampling Times
The selective ET-AR antagonist utilized in this study was sitaxsentan sodium (TBC11251Na) which reaches a steady-state level within 30 minutes following intravenous administration and has a half-life of approximately 6 hours [24]. This study was performed under FDA IND#52,527.
Patients (n=44) were assigned to treatment groups by an independent clinical research nurse the night prior to surgery by a predetermined randomized table. The treatment groups included administration of vehicle (saline bolus, n=9), 0.1 mg/kg (n=9), 0.5 mg/kg (n=9), 1.0 mg/kg (n=9), or 2.0 mg/kg (n=8) of the selective ET-AR antagonist. The ET-AR antagonist was infused at the separation of CPB to correspond with the peak release of ET from the myocardial interstitium and to avoid confounding effects of hemoconcentration during CPB [1]. The average duration of infusion was 7 ± 1 minutes. The following time points were used: Baseline (following placement of arterial and pulmonary catheters, but prior to the onset of CPB), immediately following cross clamp release, Time 0 (immediately at cessation of CPB and following vehicle/ET-AR antagonist infusion), 0.5, 6, and 24 hours post CPB. Blood samples were collected at the designated time points for the determination of plasma ET and cytokine levels.
Endothelin and Cytokine Assays
Plasma levels of ET were analyzed by a radioimmunoassay (RPA 545, Amersham, Piscataway, NJ) which has been previously validated by this laboratory [1, 7, 8]. Analysis of plasma TNF, TNFRI, and TNFRII levels was performed using multiplex suspension array technology [25]. To determine the potential effects of the ET-AR antagonist on other classical cytokines previously observed with CPB, the pro-inflammatory cytokine interleukin-6 and the anti-inflammatory cytokine interleukin-10 were also measured by a multiplex suspension array. Undiluted plasma was incubated for 2 hours at room temperature with analyte specific monoclonal antibodies conjugated to polystyrene beads in a 96 well filter plate. The wells were washed three times and incubated for 1 hour at room temperature with secondary biotinylated antisera (Invitrogen, Camarillo, CA). The wells were washed again prior to incubation for 30 minutes with streptavidin R-phycoerythin. Finally, the wells were washed and the analyte/bead complexes were re-suspended within the filter plate. The identification and quantification of the analyte/bead complexes were determined by flow cytometry with dual excitation lasers (Bio-Plex Suspension Array Workstation, Bio-Rad, Hercules, CA). Using this approach, the inter- and intra-assay variation was ≤ 10% and the level of detection was 0.1 pg/mL. Analyte concentrations were corrected for hemodilution.
Data Analysis
Categorical analysis of patient medications and transfusions were evaluated by Pearson’s chi-squared analysis to determine differences between treatment groups. The duration of hypothermia, CPB, and cross clamp time were compared between treatment groups by Bonferroni adjusted analysis of variance. Prior to and immediately following infusion of the ET-AR antagonist, plasma levels of ET and cytokines were evaluated by a two-way analysis of variance to determine the effects of time or ET-AR antagonist treatment. Potential correlations between plasma levels prior to vehicle/ET-AR antagonist infusion of ET and TNFRI, or TNFRII were analyzed by Spearman’s rank correlation. The absolute changes of plasma levels ET and cytokine levels were computed at 0.5, 6, and 24 hours following infusion of the ET-AR antagonist. If the analyte value was within 25% of the detection limits, the analyte value was assigned a numerical value of zero for the purposes of computing the absolute change. A one-sided t-test was used to determine if an analyte level significantly changed from the 0 hour/infusion value. The absolute changes of plasma ET and cytokines levels following the infusion of the ET-AR antagonist were evaluated by a two-way analysis of variance to determine the effects of time, ET-AR antagonist dose, or potential interactions. If any time or dose effects were observed after the initial two-way analysis of variance, the effect was further examined with the Wilcoxon signed rank test. All analysis was performed with the statistical package Intercooled Stata V 8.0 (College Station, TX). Values are reported as mean ± standard error of the mean (SEM) and statistical significance was determined at p<0.05.
RESULTS
Clinical Results
Forty-four patients were enrolled and successfully completed the study. Pre-operative medications were not different across the treatment groups for the following: non-steroidal anti-inflammatory drugs (i.e. aspirin: 73%, p = 0.20), nitrates (27%, p = 0.97), diuretics (i.e. hydrochlorothiazide: 18%, p = 0.94), renin angiotension aldosterone system antagonists (64%, p = 0.27), calcium channel antagonists (23%, p = 0.30), and beta adrenergic antagonists (73%, p = 0.28). The duration of hypothermia (below 35°C: 37 ± 4 minutes, p = 0.33), CPB (86 ± 4 minutes, p = 0.75), and cross clamp time (68 ± 3 minutes, p = 0.52) were not different across treatment groups. Patient medications during the intra-operative period also did not differ across treatment groups for positive inotropic agents (i.e. epinephrine and dopamine: 37%, p = 0.95), alpha adrenergic agonists (i.e. phenylephrine: 78%, p = 0.93), and nitrates (12%, p = 0.21). The frequency of patients receiving transfusions following the separation from CPB (defined as whole blood, packed red blood cells, fresh frozen plasma, and/or platelets, 55% p = 0.70) was not different across treatment groups.
Endothelin and Cytokine Plasma Levels
The plasma levels of ET and cytokines are shown in Table 1 and have been segmented with respect to treatment and time: baseline, cross clamp release, and immediately following the separation from CPB and the infusion of the ET-AR antagonist (0 hour). There was not a significant time effect with respect to plasma ET levels; however, a secondary treatment effect was observed with the infusion of the ET-AR antagonist. Specifically, immediately following the ET-AR antagonist infusion, an increase in plasma ET concentrations occurred. This effect is likely due to the displacement of ET from the ET-AR and is consistent with a ligand-receptor-antagonist interaction [26]. It has been previously reported that an acute infusion of an ET receptor antagonist can cause an increase in plasma ET [27]. Plasma interleukin-6 levels increased in a time dependent manner, but there was no significant treatment effect. There was no significant time or treatment effect with respect to interleukin-10 or TNF. Plasma TNFRI and TNFRII levels increased with respect to time, but not treatment. In order to examine whether an inter-relationship existed between plasma ET and TNFR levels, a correlation analysis was performed. This analysis was performed on the time points prior to the ET-AR antagonist infusion, in order to obviate the confounding effects of these later time points with respect to antagonist-receptor interactions. A significant correlation was observed between plasma ET and TNFRI levels (r = 0.25, p = 0.02), and a positive correlation, although not reaching statistical significance, was observed between plasma ET and TNFRII levels (r = 0.19, p = 0.08). In summary, while cytokine levels changed from baseline as a function of CPB, the changes were equivalent across the randomized groups. Thus, cytokine levels were equivalent and uniform for all treatment groups up to, and immediately following the separation of CPB and the infusion of the ET-AR antagonist.
Table 1.
Plasma concentrations of endothelin and cytokines at baseline, cross clamp release (CCR), and 0 hour.
| Time |
Two-way ANOVA |
||||||
|---|---|---|---|---|---|---|---|
| Baseline | CCR | 0 Hour: Infusion | F-value | P-value | |||
| Endothelin (fmol/mL) | |||||||
| Treatment | Vehicle | 2.8 ± 0.4 | 3.3 ± 0.4 | 2.9 ± 0.4 | Time | 1.02 | 0.36 |
| 0.1 mg/kg | 3.0 ± 0.5 | 3.9 ± 0.6 | 3.8 ± 1.1 | Treatment | 4.15 | 0.04 | |
| 0.5 mg/kg | 2.5 ± 0.4 | 3.7 ± 0.5 | 4.4 ± 1.0 | ||||
| 1 mg/kg | 4.1 ± 0.6 | 5.1 ± 0.9 | 4.1 ± 1.1 | ||||
| 2 mg/kg | 3.3 ± 0.7 | 4.9 ± 1.1 | 5.8 ± 1.4 | ||||
| Interleukin 6 (pg/mL) | |||||||
| Treatment | Vehicle | 8.0 ± 2.2 | 46.4 ± 26.1 | 88.1 ± 47.2 | Time | 9.80 | <0.01 |
| 0.1 mg/kg | 9.1 ± 2.3 | 39.2 ± 9.0 | 80.4 ± 20.6 | Treatment | 0.05 | 0.83 | |
| 0.5 mg/kg | 8.5 ± 2.1 | 49.6 ± 11.0 | 79.0 ± 16.9 | ||||
| 1 mg/kg | 10.3 ± 1.8 | 40.4 ± 15.6 | 95.2 ± 44.0 | ||||
| 2 mg/kg | 8.4 ± 2.4 | 39.3 ± 10.5 | 71.2 ± 17.9 | ||||
| Interleukin 10 (pg/mL) | |||||||
| Treatment | Vehicle | 3.0 ± 0.9 | 17.1 ± 8.2 | 18.0 ± 8.1 | Time | 2.04 | 0.13 |
| 0.1 mg/kg | 4.0 ± 1.8 | 30.7 ± 20.5 | 36.5 ± 24.5 | Treatment | 0.03 | 0.86 | |
| 0.5 mg/kg | 3.2 ± 0.7 | 13.2 ± 7.2 | 14.2 ± 6.4 | ||||
| 1 mg/kg | 5.2 ± 1.1 | 10.7 ± 4.3 | 26.4 ± 12.1 | ||||
| 2 mg/kg | 3.6 ± 0.8 | 7.0 ± 1.8 | 6.4 ± 1.3 | ||||
| Tumor Necrosis Factor (TNF:pg/mL) | |||||||
| Treatment | Vehicle | 4.8 ± 1.8 | 0.5 ± 0.1 | 0.7 ± 0.1 | Time | 0.03 | 0.97 |
| 0.1 mg/kg | 2.9 ± 0.8 | 1.9 ± 0.6 | 2.3 ± 0.7 | Treatment | 1.79 | 0.18 | |
| 0.5 mg/kg | 3.8 ± 1.0 | 1.2 ± 0.3 | 0.9 ± 0.2 | ||||
| 1 mg/kg | 7.3 ± 1.9 | 15.5 ± 8.3 | 27.2 ± 15.1 | ||||
| 2 mg/kg | 4.6 ± 1.9 | 5.6 ± 3.2 | 4.02 ± 2.1 | ||||
| TNF Receptor I (ng/mL) | |||||||
| Treatment | Vehicle | 2.73 ± 1.35 | 2.34 ± 0.30 | 5.00 ± 1.01 | Time | 13.62 | <0.01 |
| 0.1 mg/kg | 2.16 ± 0.71 | 2.67 ± 0.16 | 4.02 ± 0.23 | Treatment | 0.10 | 0.75 | |
| 0.5 mg/kg | 2.12 ± 0.60 | 3.18 ± 0.54 | 4.86 ± 0.55 | ||||
| 1 mg/kg | 2.65 ± 0.84 | 3.54 ± 0.69 | 7.14 ± 1.26 | ||||
| 2 mg/kg | 1.80 ± 0.28 | 2.59 ± 0.11 | 5.24 ± 0.71 | ||||
| TNF Receptor II (ng/mL) | |||||||
| Treatment | Vehicle | 0.53 ± 0.08 | 0.59 ± 0.05 | 1.03 ± 0.18 | Time | 11.22 | <0.01 |
| 0.1 mg/kg | 0.46 ± 0.09 | 0.62 ± 0.07 | 0.85 ± 0.05 | Treatment | 1.01 | 0.32 | |
| 0.5 mg/kg | 0.53 ± 0.05 | 1.02 ± 0.29 | 1.32 ± 0.22 | ||||
| 1 mg/kg | 0.60 ± 0.13 | 0.73 ± 0.13 | 1.35 ± 0.27 | ||||
| 2 mg/kg | 0.50 ± 0.06 | 0.72 ± 0.07 | 1.11 ± 0.15 | ||||
0 Hour: Separation from cardiopulmonary bypass and time of infusion. Treatment indicates patient randomized to vehicle or ET-AR Antagonist. ANOVA: analysis of variance. Vehicle: n = 9, 0.1 mg/kg: n=9, 0.5 mg/kg: n=9, 1 mg/kg: n=9, 2 mg/kg: n=8.
Endothelin and Cytokine Plasma Levels Following ET-AR Antagonist Infusion
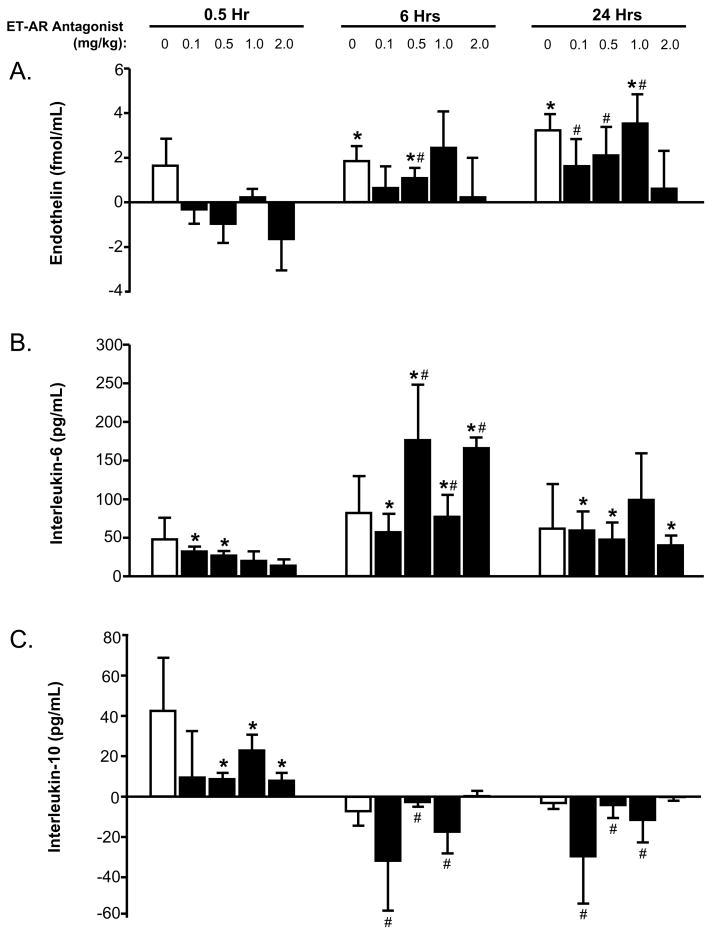
The absolute changes in plasma levels of ET and cytokines were computed at 0.5, 6, and 24 hours following the infusion of increasing doses of the ET-AR antagonist and vehicle (Figure 1 and 2). At 24 hours post infusion, plasma ET levels were increased, except in the high dose ET-AR antagonist group. Plasma interleukin-6 levels were increased at 6 hours post infusion in all of the ET-AR antagonist groups. In contrast, the plasma interleukin-10 levels were decreased following infusion of the higher doses of the ET-AR antagonist, and this effect persisted at longer time points. At 24 hours post infusion, plasma TNF levels were reduced in the higher dose of the ET-AR antagonist when compared to the vehicle group. Plasma TNFRI levels decreased immediately following infusion of the higher doses of the ET-AR antagonist, and this effect persisted at longer time points. At 24 hours post infusion, plasma TNFRII levels were reduced in the higher doses of the ET-AR antagonist when compared to the vehicle group.
Figure 1.
The absolute changes in plasma endothelin (panel A), interleukin-6 (panel B), and interleukin-10 (panel C) concentrations were computed at 0.5, 6, and 24 hours following infusion of increasing doses of the endothelin-A receptor (ET-AR) antagonist (0.1–2.0 mg/kg, black bars) or vehicle (0 mg/kg, white bars). At 24 hours post infusion, plasma endothelin levels were increased, except in the high dose ET-AR group. Plasma interleukin-6 levels were increased at 6 hours post infusion in all of the ET-AR antagonist groups. In contrast, interleukin-10 levels were reduced following infusion of the higher doses of the ET-AR antagonist, and this effect persisted at longer time points. (*p<0.05 vs. immediate post infusion time point, #p<0.05 vs. respective 0.5 hour time point)
Figure 2.
The absolute changes in plasma tumor necrosis factor-α (TNF: panel A), TNF Receptor I (panel B), and TNF Receptor II (panel C) concentrations were computed at 0.5, 6, and 24 hours following infusion of increasing doses of the endothelin-A receptor (ET-AR) antagonist (0.1–2.0 mg/kg, black bars) or vehicle (0 mg/kg, white bars). At 24 hours post infusion, plasma TNF levels were reduced in the higher dose of the ET-AR antagonist when compared to the vehicle group. Plasma TNF Receptor I levels decreased immediately following infusion of the higher doses of the ET-AR antagonist, and this effect persisted at longer time points. Similarly at 24 hours post infusion, plasma TNF Receptor II levels were reduced in the higher doses of the ET-AR antagonist when compared to the vehicle group. (*p<0.05 vs. immediate post infusion time point, #p<0.05 vs. respective vehicle group)
Following the infusion of the ET-AR antagonist, the effects of time and dose on plasma ET and cytokine levels were determined by a two-way analysis of variance (Table 2). In this analysis there were two main treatment effects: time following infusion of vehicle/ET-AR antagonist and dose (0–2.0 mg/kg). Plasma ET levels were significantly affected by both time and dose, but no interaction occurred. Plasma levels of interleukin-6 and interleukin-10 were affected with time, but not by dose. In contrast, the plasma levels of TNF, TNFRI, and TNFRII were not affected by time, but a significant dose effect was observed. There was no interaction between time and dose with respect to the indices of TNFR activation. In conclusion, the ET-AR antagonist dose significantly affected the TNF signaling pathway without altering the other cytokines.
Table 2.
The absolute changes of endothelin and cytokine levels from infusion of the endothelin-A receptor (ET-AR) antagonist (vehicle or sitaxsentan 0.1, 0.5, 1, and 2 mg/kg) was evaluated by a two-way analysis of variance to determine the effects of time, dose, and potential interactions.
| Analyte | Time |
ET-AR Antagonist Dose |
Interaction |
|||
|---|---|---|---|---|---|---|
| F-Value | P-Value | F-Value | P-Value | F-Value | P-Value | |
| Endothelin | 5.50 | <0.01 | 2.41 | 0.05 | 0.18 | 0.99 |
| Interleukin 6 | 7.24 | <0.01 | 0.40 | 0.81 | 1.30 | 0.25 |
| Interleukin 10 | 6.75 | <0.01 | 1.55 | 0.19 | 0.50 | 0.85 |
| Tumor Necrosis Factor-α (TNF) | 0.51 | 0.60 | 4.65 | <0.01 | 0.09 | 0.99 |
| TNF Receptor I | 2.16 | 0.12 | 3.88 | <0.01 | 0.49 | 0.86 |
| TNF Receptor II | 2.79 | 0.07 | 3.78 | <0.01 | 0.82 | 0.59 |
COMMENT
Separation from cardiopulmonary bypass (CPB) is often associated with the release of bioactive molecules which include endothelin (ET) and cytokines [1–9]. ET causes systemic and coronary vasoconstriction as well as negative inotropic effects primarily mediated through the ET-A receptor subtype (ET-AR) [10–13]. The pro-inflammatory cytokine, tumor necrosis factor-α (TNF), has been associated with negative inotropic effects and cell apoptosis [18–20]. Accordingly, the overall goal of this study was to examine the potential influence of ET-AR activation upon indices of TNF activation following coronary revascularization requiring CPB. Following the administration of a selective ET-AR antagonist at the separation from CPB, plasma levels of TNF and soluble TNF receptors (indicative of TNF activation) were significantly lower in the early post CPB period. The ET-AR antagonist had a significant dose effect on the indices of the TNF signaling pathway, which was not observed with the other cytokines released in the post CPB period. In addition to the established vasoconstrictive and negative inotropic effects of the ET-AR, this study provides clinical evidence for an additional biological role of modification of the TNF activation pathway.
ET antagonists have been utilized in cardiovascular diseases such as pulmonary hypertension and congestive heart failure [15–16]. Non-selective ET antagonist, i.e. equally inhibiting ET-AR and the ET-B receptor subtype (ET-BR), have demonstrated transient hemodynamic improvements with long term treatment being associated with liver toxicity and worsening of clinical status [28]. One potential explanation for the undesirable effects of non-selective ET antagonists is the inhibition of the beneficial vasodilatory and ET clearance mechanisms of the ET-BR. Accordingly, a number of selective ET-AR antagonists have been developed and are currently under clinical investigation for pulmonary hypertension [15]. This laboratory has previously shown that the selective ET-AR antagonist, sitaxsentan, reduced pulmonary vascular resistance following separation from CPB in an initial dose ranging and safety study [17]. However, several unanswered questions remain which include the potential interactions that exist between ET-AR and the TNF signaling pathway.
TNF is released in the post CPB period and has been associated with a protracted post operative course including longer intensive care unit stay, greater inotropic support, and multiple organ dysfunction [8, 9]. In this study, plasma TNF levels were lower with the infusion of the ET-AR antagonist in the post CPB period. TNF is synthesized and released from a variety of cell types which results in the binding and activation of the TNF receptors (TNFR). Following activation, the TNFR complexes are cleaved and released into the circulation. In this study, there was a reduction in the TNFR activation with the infusion of the ET-AR antagonist, indicated by reduced plasma levels of soluble TNFR. The reduction of TNF and TNFR activation with the ET-AR antagonist signifies attenuation of the inflammatory response which may be beneficial in the post CPB period. Based upon the findings of past reports and the present study, a prospective study designed to determine the relationship between ET-AR inhibition, post operative outcome, and cytokine profile is warranted.
The immune response in the post CPB period also includes the release of other cytokines such as, the pro-inflammatory interleukin-6 and the anti-inflammatory interleukin-10 [3, 4]. Interleukin-10 has been shown to have beneficial effects by suppressing production of TNF and interleukin-6 [29]. This study demonstrated that plasma interleukin-6 and interleukin-10 levels following infusion were significantly affected by time, but both cytokines were independent of effects by the ET-AR antagonist dose. In this study, the ET-AR antagonist selectively altered the TNF signaling pathway without significantly affecting a potentially beneficial cytokine, interleukin-10.
Previous pharmacological interventions for the attenuation of pro-inflammatory cytokines released in the post CPB period have included corticosteroids [30, 31]. The infusion of corticosteroids reduced circulating levels of TNF to a similar degree found in this study [31]. However, corticosteroids can be associated with non-specific/undesired effects including postoperative hyperglycemia, pulmonary dysfunction, and prolonged ventilatory time [30]. In contrast, the infusion of a selective ET-AR antagonist in a previous dose ranging and safety study demonstrated beneficial hemodynamic effects with no significant differences in adverse events in the perioperative period when compared to the vehicle [17]. Taken together, these results suggest that reducing ET-AR activation may possess a favorable profile with respect to reducing the deleterious effects of TNF.
Study Limitations and Summary
Past studies demonstrated circulating levels of ET and TNF can be associated with a protracted post operative course [5–9]. The present study design (duration and sample size) prevented the examination of potential relationships between the post operative outcome, plasma ET levels, and the cytokine profile. The duration of the present study extended to 24 hours post CPB; however, the effects of ET-AR inhibition on plasma levels of ET, cytokines, and TNF receptors throughout the remaining hospitalization remains unknown.
The present study focused upon the potential inter-relationship between ET-AR activation and cytokine release. However, multiple biological pathways are activated following separation from CPB and it is unlikely that the ET signaling pathway is mutually exclusive. For example, the inflammatory response includes complement activation in the early post CPB period [32]. The involvement of ET-AR activation in other biological signaling cascades relevant to cytokine release remains to be established.
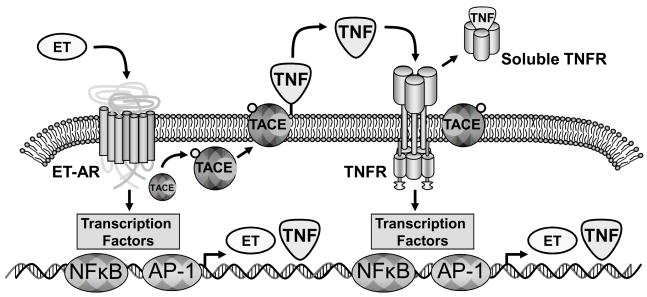
Past in vitro and in vivo experimental models have established that ET-AR activation can induce the synthesis and release of TNF [22, 23]. Binding of ET to the ET-AR can result in the induction of an intracellular cascade which culminates in both pre-transcriptional and transcriptional events (Figure 3) [22, 33]. Specifically, ET-AR can result in the formation of phosphorylation intermediates, which in turn could cause phosphorylation and mobilization of TNF convertase to the cell membrane [33, 34]. TNF convertase in turn would cause solubilization of membrane bound TNF and ultimately binding to the TNFR complex [20]. The binding of TNF to the TNFR will ultimately be proteolytically processed and yield soluble TNFR complexes [21]. Stimulation of both the ET-AR and TNFR will cause binding to transcription factor sites such as nuclear factor κ-B and activating protein-1, which can cause the formation of ET and TNF [20, 35–38]. As such, it is possible that ET-AR and TNFR signaling results in amplification and a positive feedback loop of the ET and TNF cascade. In the present study, the index of TNFR activation was attenuated in the ET-AR antagonist group in the post CPB period. The present study provides clinical evidence of an inter-relationship between the ET-AR and TNF release and activation in the post operative period following CPB.
Figure 3.
Schematic of the potential interaction of the endothelin-A receptor (ET-AR) and the tumor necrosis factor-α (TNF) pathway. Binding of endothelin (ET) to the ET-AR caused the induction of an intracellular cascade which culminates in both pre-transcriptional and transcriptional events. Specifically, ET-AR can result in the formation of phosphorylation intermediates, which in turn could cause phosphorylation and mobilization of TNF convertase (TACE) to the cell membrane. TACE in turn would cause solubilization of membrane bound TNF and ultimately binding to the TNF receptor complex (TNFR). The binding of TNF to the TNFR will ultimately be proteolytically processed and yield soluble TNFR complexes, which can be quantified in the plasma. Stimulation of both the ET-AR and TNFR will cause binding to transcription factor sites such as nuclear factor κ-B (NFκB) and activating protein 1 (AP-1), which can cause the formation of ET and TNF. Thus, stimulation of the ET-AR and TNFR thereby form an amplification loop. The present study demonstrated that selective ET-AR can directly modify TNFR activation.
Acknowledgments
This study was supported by an unrestricted grant from Encysive Pharmaceuticals, Houston, TX, NIH grant HL075488, HL056603, HL057952 and NIH Minority Supplement Research Grant.
Abbreviations
- CPB
Cardiopulmonary Bypass
- CCR
Cross Clamp Release
- ET
Endothelin
- ET-AR
Endothelin A Receptor
- ET-BR
Endothelin B Receptor
- TNF
Tumor Necrosis Factor-α
- TNFR
Tumor Necrosis Factor Receptor
Footnotes
Presented at the 54th Annual Meeting of the Southern Thoracic Surgical Association, November 9, 2007, Bonita Springs, FL.
References
- 1.Multani MM, Ikonomidis JS, Kim PY, et al. Dynamic and differential changes in myocardial and plasma endothelin in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;129:584–90. doi: 10.1016/j.jtcvs.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 2.St Rammos K, Koullias GJ, Hatzibougias JD, Argyrakis NP, Panagopoulos PG. Plasma endothelin-1 levels in adult patients undergoing coronary revascularization. Cardiovasc Surg. 1996;4:808–12. doi: 10.1016/s0967-2109(96)00036-1. [DOI] [PubMed] [Google Scholar]
- 3.Wan S, DeSmet JM, Barvais L, Goldstein M, Vincent JL, LeClerc JL. Myocardium is a major source of proinflammatory cytokines in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1996;112:806–11. doi: 10.1016/S0022-5223(96)70068-5. [DOI] [PubMed] [Google Scholar]
- 4.Wei M, Kuukasjärvi P, Laurikka J, et al. Inflammatory cytokines and soluble receptors after coronary artery bypass grafting. Cytokine. 2001;15:223–8. doi: 10.1006/cyto.2001.0920. [DOI] [PubMed] [Google Scholar]
- 5.Dorman BH, Bond BR, Clair MJ, et al. Temporal synthesis and release of endothelin within the systemic and myocardial circulation during and after cardiopulmonary bypass: Relation to postoperative recovery. J Cardiothorac Vasc Anesth. 2000;14:540–5. doi: 10.1053/jcan.2000.9451. [DOI] [PubMed] [Google Scholar]
- 6.Bond BR, Dorman BH, Clair MJ, et al. Endothelin-1 during and after cardiopulmonary bypass: Association to graft sensitivity and postoperative recovery. J Thorac Cardiovasc Surg. 2001;122:358–64. doi: 10.1067/mtc.2001.114936. [DOI] [PubMed] [Google Scholar]
- 7.Sander M, von Heymann C, von Dossow V, et al. Increased interleukin-6 after cardiac surgery predicts infection. Anesth Analg. 2006;102:1623–9. doi: 10.1213/01.ane.0000215998.21739.48. [DOI] [PubMed] [Google Scholar]
- 8.Bittar MN, Carey JA, Barnard JB, et al. Tumor necrosis factor alpha influences the inflammatory response after coronary surgery. Ann Thorac Surg. 2006;81:132–8. doi: 10.1016/j.athoracsur.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Khabar KSA, ElBarbary MA, Khouqeer F, Devol E, Al-Gain S, Al-Halees Z. Circulating endotoxin and cytokines after cardiopulmonary bypass: Differential correlation with duration of bypass and systemic inflammatory response/multiple organ dysfunction syndromes. Clin Immunol and Immunopathol. 1997;85:97–103. doi: 10.1006/clin.1997.4413. [DOI] [PubMed] [Google Scholar]
- 10.Joffs C, Walker CA, Hendrick JW, et al. Endothelin receptor subtype A blockade selectively reduces pulmonary pressure after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2001;122:365–70. doi: 10.1067/mtc.2001.114938. [DOI] [PubMed] [Google Scholar]
- 11.MacCarthy PA, Grocott-Mason R, Prendergast BD, Shah AM. Contrasting inotropic effects of endogenous endothelin in the normal and failing human heart: Studies with an intracoronary ET A receptor antagonist. Circulation. 2000;101:142–7. doi: 10.1161/01.cir.101.2.142. [DOI] [PubMed] [Google Scholar]
- 12.Dorman BH, New RB, Bond BR, et al. Myocyte endothelin exposure during cardioplegic arrest exacerbates contractile dysfunction after reperfusion. Anesth Analg. 2000;90:1080–5. doi: 10.1097/00000539-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Verhaar MC, Strachan FE, Newby DE, et al. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-b receptor blockade. Circulation. 1998;97:752–6. doi: 10.1161/01.cir.97.8.752. [DOI] [PubMed] [Google Scholar]
- 14.Bremnes T, Paasche JD, Mehlum A, Sandberg C, Bremnes B, Attramadal H. Regulation and intracellular trafficking pathways of the endothelin receptors. J Biol Chem. 2000;275:17596–604. doi: 10.1074/jbc.M000142200. [DOI] [PubMed] [Google Scholar]
- 15.Battistini B, Berthiaume N, Kelland NF, Webb DJ, Kohan DE. Profile of past and current clinical trials involving endothelin receptor antagonists: The novel “-sentan” class of drug. Exp Biol Med. 2006;231:653–95. [PubMed] [Google Scholar]
- 16.Barst RJ, Rich S, Widlitz A, Horn EM, McLaughlin V, McFarlin J. Clinical efficacy of sitaxsentan, an endothelin-A receptor antagonist, in patients with pulmonary arterial hypertension: Open-label pilot study. Chest. 2002;121:1860–8. doi: 10.1378/chest.121.6.1860. [DOI] [PubMed] [Google Scholar]
- 17.Ikonomidis JS, Hilton EJ, Payne K, et al. Selective endothelin-A receptor inhibition after cardiac surgery: A safety and feasibility study. Ann Thorac Surg. 2007;83:2153–61. doi: 10.1016/j.athoracsur.2007.02.087. [DOI] [PubMed] [Google Scholar]
- 18.Kapadia S, Lee J, Torre-Amione G, Birdsall HH, Ma TS, Mann DL. Tumor necrosis factor-α gene and protein expression in adult feline myocardium after endotoxin administration. J Clin Invest. 1995;96:1042–52. doi: 10.1172/JCI118090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krown KA, Page MT, Nguyen C, et al. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98:2854–65. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–95. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 21.Jansen J, van der Poll T, Levi M, et al. Inhibition of the release of soluble tumor necrosis factor receptors in experimental endotoxemia by anti-tumor necrosis factor-α antibody. J Clin Immunol. 1995;15:45–50. doi: 10.1007/BF01489489. [DOI] [PubMed] [Google Scholar]
- 22.Ruetten H, Thiemermann CH. Endothelin-1 stimulates the biosynthesis of tumour necrosis factor in macrophages: ET-Receptors, signal transduction and inhibition by dexamethasone. J Physiol Pharmacol. 1997;48:675–88. [PubMed] [Google Scholar]
- 23.Yang TL, Chen MF, Jiang JL, Xie QY, Li YP, Li YJ. The endothelin receptor antagonist decreases ischemia/reperfusion-induced tumor necrosis factor production in isolated rat hearts. Int J Cardiol. 2005;100:495–8. doi: 10.1016/j.ijcard.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 24.O’Callaghan DS, Gaine SP. Sitaxsentan: an endothelin-A receptor antagonist for the treatment of pulmonary arterial hypertension. Int J Clin Pract. 2006;60:475–81. doi: 10.1111/j.1368-5031.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 25.Gasz G, Lenard L, Racz B, et al. Effect of cardiopulmonary bypass on cytokine network and myocardial cytokine production. Clin Cardiol. 2006;29:311–5. doi: 10.1002/clc.4960290708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith CM, Reynard AM. Essentials of Pharmacology. 1. Philadelphia, PA: WB Saunders Co; 1995. p. 9. [Google Scholar]
- 27.Weber C, Schmitt R, Birnboeck H, et al. Pharmacokinetics and pharmacodynamics of the endothelin-receptor antagonist bosentan in healthy human subjects. Clin Pharmacol Ther. 1996;60:124–37. doi: 10.1016/S0009-9236(96)90127-7. [DOI] [PubMed] [Google Scholar]
- 28.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 29.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol. 1994;153:811–6. [PubMed] [Google Scholar]
- 30.Morariu AM, Loef BG, Aarts LP, et al. Dexamethasone: Benefit and prejudice for patients undergoing on-pump coronary artery bypass grafting: A study on myocardial, pulmonary, renal, intestinal, and hepatic injury. Chest. 2005;128:2677–87. doi: 10.1378/chest.128.4.2677. [DOI] [PubMed] [Google Scholar]
- 31.Liakopoulos OJ, Schmitto JD, Kazmaier S, et al. Cardiopulmonary and systemic effects of methylprednisolone in patients undergoing cardiac surgery. Ann Thorac Surg. 2007;84:110–9. doi: 10.1016/j.athoracsur.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Hoel TN, Videm V, Mollnes TE, et al. Off-pump cardiac surgery abolishes complement activation. Perfusion. 2007;22:251–6. doi: 10.1177/0267659107084142. [DOI] [PubMed] [Google Scholar]
- 33.Clerk A, Kemp TJ, Harrison JG, Mullen AJ, Barton PJR, Sugden PH. Up-regulation of c-jun mRNA in cardiac myocytes requires the extracellular signal-regulated kinase cascade, but c-Jun N-terminal kinases are required for efficient up-regulation of c-Jun protein. Biochem J. 2002;368:101–10. doi: 10.1042/BJ20021083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soond SM, Everson B, Riches DWH, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFα-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci. 2005;118:2371–80. doi: 10.1242/jcs.02357. [DOI] [PubMed] [Google Scholar]
- 35.Muller DN, Mervaala EMA, Schmidt F, et al. Effect of bosentan on NF-κB, inflammation, and tissue factor in angiotensin II-Induced End-Organ Damage. Hypertension. 2000;36:282–90. doi: 10.1161/01.hyp.36.2.282. [DOI] [PubMed] [Google Scholar]
- 36.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–7. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 37.Douthwaite JA, Lees DM, Corder R. A role for increased mRNA stability in the induction of endothelin-1 synthesis by lipopolysaccharide. Biochem Pharmacol. 2003;66:589–94. doi: 10.1016/s0006-2952(03)00336-8. [DOI] [PubMed] [Google Scholar]
- 38.Guha M, Bai W, Nadler JL, Natarajan R. Molecular mechanisms of tumor necrosis factor α gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and –independent pathways. J Biol Chem. 2000;275:17728–39. doi: 10.1074/jbc.275.23.17728. [DOI] [PubMed] [Google Scholar]