Abstract
The nuclear factor of activated T cells (NFAT) transcription factors are regulated by calcium/calcineurin signals and play important roles in T cells, muscle, bone, and neural tissue. NFAT is expressed in the epidermis, and although recent data suggest that NFAT is involved in the skin’s responses to ultraviolet radiation (UVR), the wavelengths of radiation that activate NFAT and the biological function of UV-activated NFAT remain undefined. We demonstrate that NFAT transcriptional activity is preferentially induced by UVB wavelengths in keratinocytes. The derived action spectrum for NFAT activation indicates that NFAT transcriptional activity is inversely associated with wavelength. UVR also evoked NFAT2 nuclear translocation in a parallel wavelength-dependent fashion and both transcriptional activation and nuclear translocation were inhibited by the calcineurin inhibitor cyclosporin A. UVR also evoked NFAT2 nuclear translocation in three-dimensional skin equivalents. Evidence suggests that COX-2 contributes to UV-induced carcinogenesis. Inhibiting UV-induced NFAT activation in keratinocytes, reduced COX-2 protein induction, and increased UV-induced apoptosis. COX-2 luciferase reporters lacking functional NFAT binding sites were less responsive to UVR, highlighting that NFAT is required for UV-induced COX-2 induction. Taken together, these data suggest that the proinflammatory, antiapoptotic, and procarcinogenic functions of UV-activated COX-2 may be mediated, in part, by upstream NFAT signaling. Flockhart, R. J., Diffey, B. L., Farr, P. M., Lloyd, J., Reynolds, N. J. NFAT regulates induction of COX-2 and apoptosis of keratinocytes in response to ultraviolet radiation exposure.
Keywords: UVB, action spectrum, skin equivalents, cyclosporin A
The nuclear factor of activated t cells (NFAT) family of transcription factors was first identified in T cells and is established as playing a central role in transcription during immune responses (1). In immune cells, NFAT is involved in transcription of genes, including interleukin (IL) -2, -3, -4, -5, -8, and -13; granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor α (TNF-α), and IFN-γ (2). NFAT is also functional in muscle (3, 4), bone (5), skin (6,7,8), and neural tissue (9, 10), exerting diverse functions ranging from embryonic development of cardiac valves (4) to neuronal apoptosis (10). Five members of the NFAT family have been identified: NFAT1 (NFATp, NFATc2) (11), NFAT2 (NFAT c, NFATc1) (12), NFAT3 (NFATc4) (13), and NFAT4 (NFATx) (13, 14), which are all regulated by the calcium-dependent serine-, threonine- phosphatase calcineurin, the most recently discovered NFAT protein, NFAT5 (also named TonEBP) (15, 16), is not regulated by calcineurin (15).
NFAT is predominantly located in an inactive hyperphosphorylated state in the cytoplasm (17). Increased concentration of intracellular calcium ([Ca2+]i) activates calcineurin that dephosphorylates NFAT (17), evoking nuclear translocation where NFAT is able to drive gene transcription in cooperation with other transcription factors, including AP-1 (18). NFAT-dependent gene transcription halts when [Ca2+]i decreases and NFAT translocates back to the cytoplasm following rephosphorylation by NFAT kinases (19), including glycogen synthase kinase-3 (GSK-3) (20,21,22) and casein kinase-1 (CK-1) (23, 24).
Ultraviolet radiation (UVR) is a potent environmental carcinogen. The radiation emitted by the sun can be divided into three main regions: ultraviolet, visible, and infrared, collectively termed optical radiation (25). UVR comprises wavelengths from 100 to 400 nm, which are further divided into three main wavebands: UVA (400–315 nm), UVB (315–280 nm), and UVC (280–100 nm) (25). Effects of UVR on skin range from mild inflammation and erythema to hyperplasia, burns, photoageing, and cutaneous malignancies (26). DNA mutations resulting from chronic exposure to sunlight and UVB radiation, in particular, are regarded as a major risk factor for nonmelanoma skin cancer (27,28,29). DNA mutations caused by UVA likely arise indirectly via oxidative stress or by photosensitisation reactions through absorption of UVA by unidentified chromophores (28). Conversely, UVR is used therapeutically for treatment of skin disorders, including psoriasis and atopic eczema (30). In the case of psoriasis, successful clearing is wavelength dependent, with UVB radiation being most effective in unsensitized skin (31, 32). It is, therefore, important to consider wavelength-specific responses and employ biologically relevant wavelengths and doses when investigating biological responses to UVR. Investigating the molecular targets of UVR and the signal transduction pathways activated by UVR will increase understanding of both detrimental and therapeutic effects.
It has recently been shown that UVA increases DNA-binding of NFAT2 in human fibroblasts (33) and that UVB/UVC radiation activates NFAT in a mouse cell line and in mouse skin in vivo (34). Solar UVC radiation is absorbed by the atmospheric ozone layer and does not present an environmental hazard. To date, there has been no comparison of the ability of different, physiologically relevant wavelengths to activate NFAT and biological functions of UV-activated NFAT are poorly understood.
Cyclooxygenases, of which there are two isoforms, are enzymes involved in conversion of arachidonic acid to prostaglandins (PGs). COX-1 is expressed constitutively in most tissues and is a housekeeping enzyme. COX-2 is expressed at undetectable/low levels in normal, non-neoplastic tissue, but increased COX-2 expression has been reported during inflammation and in precancerous lesions and neoplasms (35,36,37,38). Increased COX-2 activity enhances migration, angiogenesis, and apoptotic resistance, which are well recognized traits of cancer progression (35, 36). UVR increases COX-2 expression in cultured human keratinocytes (39) and COX-2 protein levels become elevated in the epidermis of human skin following UVB irradiation and also in squamous cell carcinomas and actinic keratoses (39). Pharmacological inhibition of COX-2 has been shown to reduce epidermal PG content and tumor development in mice chronically irradiated with UVB (40). A recent study of heterozygous COX-2 knockout mice showed that COX-2 is required for the development of UV-induced skin tumors (41), and COX-2 knockout mice displayed increased UV-induced apoptosis in the epidermis (41). The COX-2 promoter contains binding sites for several transcription factors, including NFAT (42). The role of NFAT in UV-induced COX-2 induction has not been investigated, and the role played by NFAT in UV-driven apoptosis in skin is not understood.
We demonstrate that UVR increases NFAT transcriptional activity and evokes nuclear translocation of NFAT2 in a wavelength-dependent fashion in HaCaT keratinocytes. The action spectrum for NFAT activation was derived, which highlighted that NFAT transcriptional activation by UVR is inversely associated with wavelength. NFAT is required for both maximal UV-induced activation of the COX-2 promoter and induction of COX-2 protein. Inhibiting activation of NFAT using cyclosporin A (CsA) also increased apoptosis of keratinocytes following UV irradiation. Taken together, these data suggest that the proinflammatory and procarcinogenic role played by COX-2 in response to UVR may be mediated, in part, by upstream NFAT signaling.
MATERIALS AND METHODS
Cell culture and epidermal equivalents
Retroviral phoenix amphotropic (RPA) cells were a gift from G. Nolan (Stanford University, Palo Alto, CA, USA). HaCaT keratinocytes, a spontaneously immortalized keratinocyte cell line, were a gift from N. E. Fusenig (German Cancer Research Centre, Heidelberg, Germany). HaCaTs and RPA cells were cultured in Dulbecco’s modified Eagle medium (Lonza, Basel, Switzerland) supplemented with 10% fetal bovine serum, penicillin, and streptomycin in a humidified incubator at 37°C/5% CO2. Full thickness epidermal equivalents (FTEs) were purchased from Mattek (Ashland, MA, USA).
UV irradiation
Cells were irradiated in multiwell culture plates and prior to irradiation, culture medium was removed and replaced with PBS. After irradiation, the PBS was replaced with fresh medium. For experiments using CsA (Calbiochem, Gibbstown, NJ, USA), cells were incubated with 5 or 10 μM CsA for 16 h prior to irradiation. Lamps used were as follows (shorthand name and percentage of UVB emitted is shown in parentheses): Philips TL 20W/12 RS (TL-12, 53% UVB), Philips TL 20W/01 RS (TL-01, 79% UVB), Helarium R1.01 (Helarium, 16% UVB) and Helarium B1-12-40W (Arimed B, 4% UVB). Arimed B lamps provided the closest spectral match to natural sunlight in this study. The irradiance of all lamps was checked before each experiment using a calibrated photoradiometer. All UVR doses administered were biologically relevant in terms of erythemogenic capacity and ranged from 0.7–8.4 SED (standard erythemal dose). One SED is equivalent to an erythemal effective radiant exposure of 100 J/m2 normalized (according to the erythema action spectrum) at 297 nm. An exposure dose of 4 SED would be expected to induce moderate erythema on unacclimated white skin but minimal or no erythema on previously exposed skin (25).
Luciferase assays
NFAT transcriptional activity was measured using an NFAT luciferase reporter [p(NFAT)3-luc] (43) containing three repeats of the NFAT/AP-1-binding site from the murine IL-2 promoter (a gift from D. J. McKean, Mayo Foundation, Rochester, MN, USA). COX-2 luciferase reporters (gifts from M. A. Iniguez, Centro de Biologia Molecular, Madrid, Spain) containing either wild-type or mutated NFAT binding sites were used to assess the requirement of NFAT in COX-2 induction. The reporters have been described previously (42) but briefly, P-1900 contains the complete human COX-2 promoter, and the other reporters are derivatives of this, with binding site mutations as follows. The plasmid P-274 lacks NF-κB binding sites. The other COX-2 reporter plasmids are mutants derived from P-274 that lack either the distal NFAT binding site (P-dNFAT-mut), the proximal site (P-pNFAT-mut), or both sites (P-d/pNFAT-mut). In all luciferase experiments, transfection efficiency and cell viability were controlled for by cotransfecting a Renilla luciferase control vector (pRLTK; Promega, Southampton, UK), and reporter firefly luciferase values were normalized to the Renilla values. Luciferase assays were performed using the dual luciferase assay system (Promega). For UV experiments, cells were seeded in 24-well culture plates and transfected with 0.5 μg of firefly reporter plus 0.025 μg of Renilla control using Lipofectamine and Plus reagents (Invitrogen, Paisley, UK), according to the manufacturer’s instructions. The following day, cells were irradiated as described then lysed at the desired time point post-UV.
Action spectrum
The NFAT action spectrum was derived by a process of mathematical induction exploring three candidate models; a Gaussian function, a single exponential function of wavelength, and a function representing the sum of 2 exponentials. An optimization solution for each model was derived using the SOLVER facility in Excel (http://www.solver. com/), where it was found that a function representing the sum of 2 exponentials provided the closest match between the observed reciprocal dose for a given end point and that calculated using the model. The basis of the induction approach is to estimate an action spectrum that is described by fewer parameters than the number of sources used, calculate the weighted dose for an appropriate end point (increased NFAT transcriptional activity of 3-fold) and compare these calculated doses with those actually observed. The optimization process involves repeated adjustment of the various parameters until the closest agreement between the calculated and the observed values is achieved. The action spectrum for healing of psoriasis patients using psoralens plus UVA photochemotherapy (PUVA) was defined using this process (44) and mathematical induction (although a slightly different method) was also applied to derive a candidate action spectrum for skin cancer induction by UVR (27).
Retroviral transduction
Human NFAT2 cDNA was cloned from pEGFPc1-NFAT2 (45) [a gift from D. Cantrell, Imperial Cancer Research Fund (ICRF), London, UK] into the retroviral vector pLEGFPc1 (Clontech, Saint-Germain, France) using BspEI and HindIII restriction sites. The pLEGFP-NFAT2 and pLEGFP-empty (control) vector were transfected into the RPA packaging cell line and stable, viral-producing cell lines were obtained by selection using G418 (Invitrogen). Cells were then transferred to an incubator at 32°C for virus production. Target cells were seeded in multiwell culture plates, virus was added to each well plus 5 μg/ml polybrene (Sigma), and cells were transduced by centrifuging plates at 1500 rpm for 1.5 h using a Sanyo, Harrier 15/80 centrifuge (Sanyo Gallenkamp, Loughborough, UK).
GFP-NFAT2 imaging and immunostaining
For GFP-NFAT2 imaging experiments, cells were seeded on glass coverslips and retrovirally transduced with GFP-NFAT2 as described. Cells were then incubated for 48 h after transduction to allow for expression, and then cells were irradiated as described. Following irradiation, cells were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature (RT). Cells were then washed with PBS and mounted onto glass slides using Vectashield (Vector Labs, Peterborough, UK).
Immunostaining for endogenous NFAT2 in HaCaTs was performed using an antibody that binds to the N terminus of human NFAT2 (46) (a gift from N. Rice, Molecular Basis of Carcinogenesis Laboratory, Frederick, MD, USA). NFAT2 expression in skin equivalents was detected using an NFAT2 antibody, clone 7A6 (BD Biosciences, Oxford, UK). Cells were seeded, irradiated, and fixed, as detailed above. Following fixation, cells were permeabilized with 0.2% Triton-X (Sigma) in PBS, then blocked with 1% goat serum in PBS for 20 min, blocked with avidin D/biotin solutions (Vector Labs), then incubated at RT for 1 h with the NFAT2 antibody (1:100). Cells were then incubated with biotinylated anti-rabbit antibody (1:300; Vector Labs) for 1 h at RT, then incubated with streptavidin-Oregon Green 488 (Molecular Probes, Invitrogen) at 1:300 dilution for 30 min. Finally, cells were treated with 0.1μM TOTO-3 (Molecular Probes, Invitrogen), then mounted onto glass slides using Vectashield (Vector Labs). Cells were washed 3 times after each incubation. FTEs were frozen in liquid nitrogen, mounted using optimal cutting temperature (OCT) compound, and cut into 6-μm sections using a cryostat before attachment onto glass slides. Sections were fixed in 4% paraformaldehyde, and staining was performed as described above. All microscopy was performed using a Leica TCS SP II laser scanning confocal microscope (Leica Microsystems, Heidelberg, Germany). Imaging data were quantified using LCS Lite 2.61 software (Leica Microsystems) using regions of interest tools. The cytoplasmic and nuclear fluorescence values from each cell were quantified and the nuclear:cytoplasmic ratio for each cell was calculated.
Western blot analysis
Cell were lysed in buffer containing: 100 mM Tris-HCl (pH 7.4), sodium chloride (100 mM), sodium fluoride (25 mM), benzamidine (1 mM), EDTA (2 mM), sodium orthovanadate (0.1 mM), Triton-x100 (0.1%) plus a protease inhibitor cocktail (Sigma, P8340–1ML). Samples were probe sonicated for 2 × 5 s pulses at an amplitude of 10 μm. For electrophoresis, samples were combined with 4× sample buffer containing: 0.25M Tris-HCl (pH 8.0), 8% SDS, 40% glycerol, 10% β-mercaptoethanol, and bromphenol blue. Samples were then heated for 3 min at 90°C immediately prior to electrophoresis using 4–20% Novex Tris-glycine precast gels (Invitrogen). Samples were loaded (40 μg/well) and stacked at 100 V for ∼30 min followed by electrophoresis at 150 V for ∼1.5 h. Running buffer contained: 3.03 g/L Tris base, 14.42 g/L glycine and 1 g/L SDS, pH 8.5. Protein was then transferred to PVDF membrane (Amersham Biosciences, NY, USA) at 100 V for 1 h using buffer consisting of: 3.0 g/L Tris-base, 14.4 g/L glycine, 20% methanol and 0.1% SDS.
Membranes were blocked with 5% nonfat milk in TBS/T for 1 h at RT. COX-2 antibody (1:100) (Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc-19999) in TBS/T + 1% milk was incubated on the membrane for 1 h at RT. Horseradish peroxidase (HRP) -tagged secondary antibody (Upstate Biotechnology, Lake Placid, NY, USA) was applied at 1:2000 in TBS/T + 1% milk for 1 h at RT. Equal protein loading was confirmed by blotting for β-actin using a mouse anti-human β-actin antibody (Sigma) diluted at 1:10000 in TBS/T + 5% milk and incubated at RT for 45 min. The β-actin signal was detected using an anti-mouse HRP-tagged secondary antibody (Upstate Biotechnology) as described above. Signals were detected using ECL Plus reagent (Amersham), according to the manufacturer’s instructions. Membranes were imaged using a Fujifilm FLA-3000 fluorescent image analyzer.
Annexin V/7-amino-actinomycin D (7AAD) apoptosis assay
UV-induced apoptosis was studied by labeling cells with phycoerytherin-tagged (PE) Annexin V and the DNA dye 7AAD (both from BD Biosciences). Following irradiation, cells were trypsinized and washed with PBS. A master mix consisting of Annexin V binding buffer, Annexin V-PE, and 7AAD was made and 300 μl was added to each sample such that each sample was incubated with 300 μl buffer + 0.5 μl Annexin V-PE and 0.5 μl 7AAD. Samples were incubated in staining mix for 15 min prior to flow cytometry. Fifteen thousand cells per sample were analyzed using a FACScan flow cytometer (BD Biosciences) operating Cell Quest Pro software. Data were analyzed using Win MDI 2.8 (Scripps Institute, La Jolla, CA, USA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad, San Diego CA, USA).
RESULTS
UVR-induced transcriptional activation of NFAT is wavelength dependent
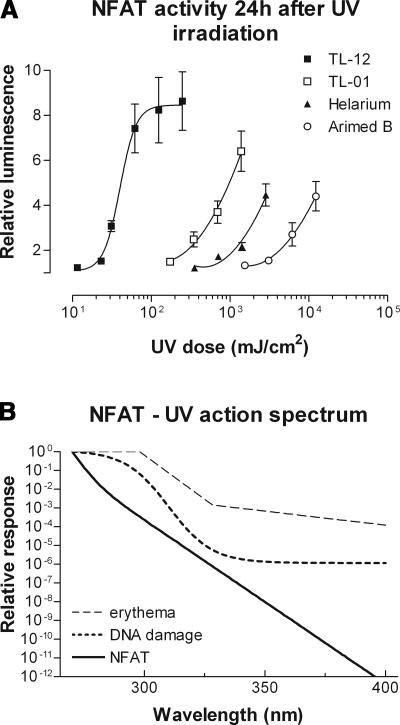
Transcriptional activity of NFAT was dose-dependently increased by all UV sources employed, and UVB-biased sources were better activators of NFAT compared to UVA-biased sources (Fig. 1A). The total UV dose required to increase NFAT activity 3-fold using Arimed B lamps (94% UVA) was 239 times greater than that required when using TL-12 lamps (53% UVB). From the data in Fig. 1A, the action spectrum for NFAT was derived, which illustrates that NFAT transcriptional activity is inversely associated with wavelength (Fig. 1B). The action spectrum for NFAT is distinct from the action spectra for erythema (47) and DNA damage (29).
Figure 1.
Ultraviolet radiation increases NFAT transcriptional activity: derivation of the NFAT action spectrum. A) NFAT luciferase activity was measured 24 h after UV irradiation with one of four different UV sources. All UV sources evoked a dose-dependent increase in NFAT transcriptional activity, with TL-12 lamps (53% UVB) being most effective. Values are means ± se from 3 independent experiments performed in triplicate. B) The action spectrum for NFAT was derived from the above luciferase data by a process of mathematical induction exploring three candidate models (see Materials and Methods). NFAT activity is inversely associated with wavelength. The action spectra for erythema (47) and general DNA damage (29) are shown for reference.
UVR-induced nuclear translocation of NFAT2 is wavelength dependent
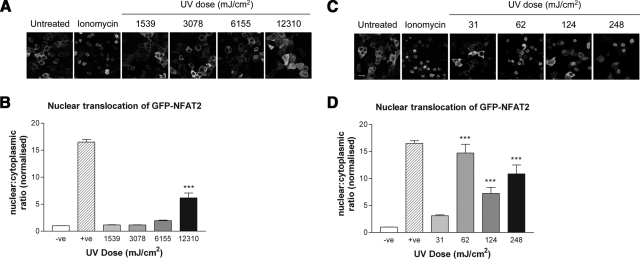
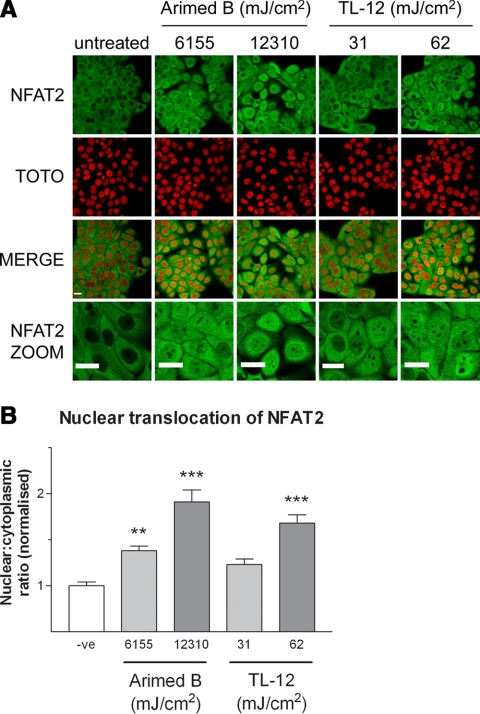
NFAT translocation in response to UVR was examined by immunostaining for endogenous NFAT and by imaging of GFP-tagged NFAT2 following irradiation with Arimed B and TL-12 lamps. Translocation of GFP-NFAT2 was observed with a UV-dose of ≥62 mJ/cm2 using TL-12 lamps (Fig. 2C, D), whereas translocation following Arimed B irradiation required a UV-dose of ≥12,310 mJ/cm2 (Fig. 2A, B). Figure 3 depicts a similar response when investigating the localization of endogenous NFAT2 by immunostaining. A significant increase in the nuclear:cytoplasmic ratio of NFAT2 was achieved by irradiating cells with ≥6155 mJ/cm2 using Arimed B lamps, whereas a significant increase was seen with TL-12 lamps at a UV dose of ≥62 mJ/cm2.
Figure 2.
UVR evokes wavelength-dependent nuclear translocation of GFP-NFAT2. Cells were retrovirally transduced with virus transmitting GFP-NFAT2 prior to irradiation. A, C) Images show nuclear translocation 24 h after irradiation. Arimed B irradiation (A) (≥12,310 mJ/cm2) and TL-12 irradiation (C) (≥62 mJ/cm2) evoke nuclear translocation of GFP-NFAT2. B, D) Quantification of nuclear translocation by calculating nuclear:cytoplasmic ratio of GFP-NFAT2 following Arimed B irradiation (B) and TL-12 irradiation (D). Values are means ± se from at least 12 fields of view (≥90 cells in total) obtained from 3 independent experiments. Negative controls (–ve) were mock irradiated, and positive controls (+ve) were treated with 1 μM ionomycin. ***P < 0.001 vs. negative control; one-way ANOVA. Scale bar = 50 μm.
Figure 3.
UVR evokes wavelength-dependent nuclear translocation of endogenous NFAT2. Cells were fixed 24 h after UV irradiation and immunostained for detection of endogenous NFAT2. A) Images represent the effect of UVR on endogenous NFAT2 localization 24 h after irradiation with Arimed B or TL-12 lamps. Digital zoom images are also included for clarity. B) Quantification of nuclear translocation by calculating nuclear:cytoplasmic ratio of NFAT2. Values are means ± se from 8 fields of view (≥161 cells in total) obtained from 3 independent experiments. Negative controls (–ve) were mock irradiated. **P < 0.01, ***P < 0.001 vs. negative control; one-way ANOVA. Scale bars = 50 μm.
CsA inhibits UV-induced activation of NFAT
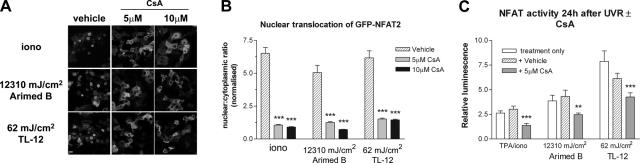
To assess the involvement of calcineurin and to validate an NFAT inhibitor for use in functional studies, cells were treated with the calcineurin inhibitor CsA prior to irradiation. Pretreating cells with 5 or 10 μM CsA prevented UV-induced nuclear translocation of GFP-NFAT2 (Fig. 4A, B). Pretreating cells with 5 μM CsA also reduced UV-induced NFAT transcriptional activation (Fig. 4C). Subsequently, CsA was used to assess the function of UV-activated NFAT.
Figure 4.
CsA inhibits UV-induced GFP-NFAT2 nuclear translocation and NFAT transcriptional activation. A) Cells retrovirally transduced with virus transmitting GFP-NFAT2 were treated with either vehicle or 5 or 10 μM CsA for 16 h prior to UV irradiation or ionomycin (1 μM) treatment. Images show nuclear translocation 24 h after irradiation or ionomycin treatment. B) Quantification of nuclear translocation by calculating nuclear:cytoplasmic ratio of GFP-NFAT2. Values are means ± se from 8 fields of view (≥90 cells in total) obtained from 3 independent experiments. C) Cells were treated with either vehicle or 5 μM CsA 16 h prior to UV irradiation or TPA/ionomycin (TPA/iono) treatment, and NFAT luciferase activity was measured 24 h later. Values are means ± se from 3 independent experiments performed in triplicate. **P < 0.01, ***P < 0.001 vs. vehicle control; one-way ANOVA.
NFAT is required for COX-2 induction by UVR
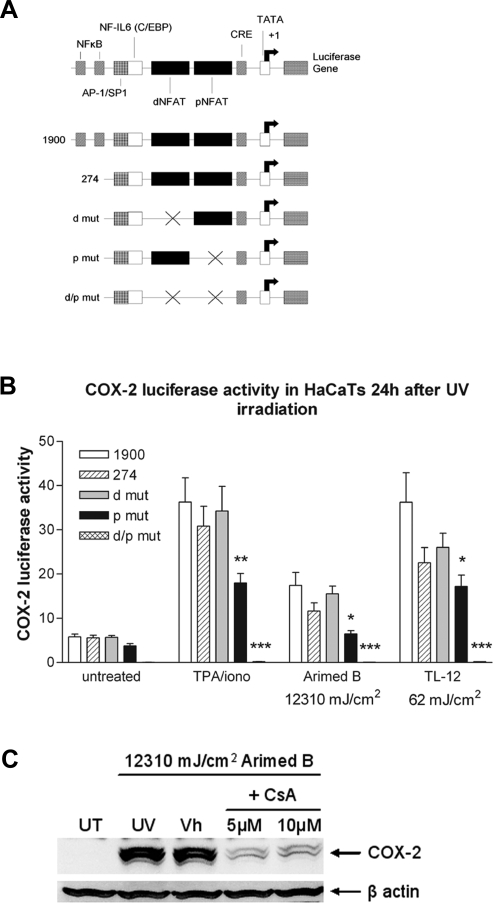
The COX-2 promoter contains two NFAT binding sites (Fig. 5A), and it is known that NFAT is essential for activation of the COX-2 promoter in T cells (42). We investigated involvement of NFAT in UV-induced COX-2 induction using COX-2 luciferase reporters that selectively lacked NFAT binding sites. COX-2 luciferase reporter assays indicate that the proximal and distal NFAT binding sites in the COX-2 promoter are required for maximal induction of the COX-2 promoter by UVR (Fig. 5B). Both Arimed B and TL-12 lamps induced activation of the reporter containing the complete COX-2 promoter (p1900). Interestingly, COX-2 promoter activity was induced more by TL-12 lamps than Arimed B lamps, and this wavelength-dependence parallels that of NFAT induction. UV-induced activity of the reporter lacking the distal NFAT binding site (dmut) did not differ compared to p274. UV-induced activity of the reporter lacking the proximal NFAT binding site (pmut), and the reporter lacking both distal and proximal sites (d/pmut) was significantly reduced compared to p274. This indicates that functional NFAT binding sites are required for maximal induction of the COX-2 promoter by UVR.
Figure 5.
NFAT is involved in UV-induced activation of the COX-2 promoter and UV-induced COX-2 protein production. A) Schematic diagram of the human COX-2 promoter and the promoters of COX-2 luciferase reporters used (adapted from ref. 42). B) COX-2 promoter activity 24 h after UV irradiation was measured by transfecting cells with luciferase reporter vectors that selectively lacked either one or both of the NFAT binding sites present in the COX-2 promoter. Positive controls (TPA/iono) were treated with TPA (50 nM) and ionomycin (1 μM). Values are means ± se from 3 independent experiments performed in quadruplicate. C) Western blot analysis of lysates prepared 24 h after Arimed B irradiation in the presence or absence of 5 or 10 μM CsA pretreatment. The two COX-2 bands migrate to 70 and 72 kDa, which are glycosylated variants of COX-2 protein that have been previously described (64, 65). *P < 0.05, ***P < 0.001 vs. control vector containing all NFAT binding sites (hatched bar); one-way ANOVA. UT, untreated; UV, UVR only; Vh, vehicle + UVR.
The involvement of NFAT in UV-induced COX-2 protein induction was assessed by treating cells with CsA prior to irradiation. Induction of COX-2 protein by Arimed B lamps was effectively blocked when cells were pretreated with 5 or 10 μM CsA (Fig. 5C). Taken together with the COX-2 promoter data (Fig. 5B), this illustrates that NFAT is involved in UV-induced COX-2 induction.
Inhibiting NFAT activation with CsA increases UV-induced apoptosis
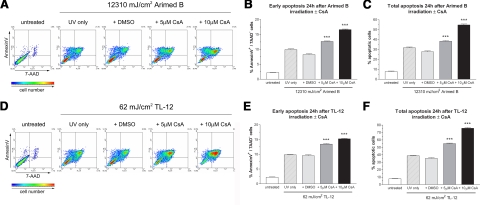
Apoptosis was quantified 24 h after irradiation in the presence or absence of doses of CsA shown to inhibit UV-induced activation of NFAT (Fig. 4). Irradiating cells with 12,310 mJ/cm2 using Arimed B lamps induced around 30% apoptosis that increased to ∼40 and ∼55% when cells were pretreated with 5 or 10 μM CsA, respectively (Fig. 6A, C). Irradiating cells with 62 mJ/cm2 using TL-12 lamps induced 40% apoptosis compared to 55 and 75% when cells were pretreated with 5 or 10 μM CsA, respectively (Fig. 6D, F). Early apoptotic populations were also similarly increased by CsA pretreatment (Fig. 6B, E; Arimed B and TL-12, respectively). CsA alone did not induce apoptosis (data not shown). These data show that inhibiting UV-induced activation of NFAT with CsA increases UV-induced apoptosis, suggesting an antiapoptotic role for UV-activated NFAT. Furthermore, doses of CsA (i.e., 1 μM) that were not sufficient to reduce UV-induced NFAT activation did not alter the amount of apoptosis induced by UVR (data not shown).
Figure 6.
Inhibiting NFAT with CsA increases apoptosis in response to UV irradiation. Cells were treated with 5 or 10 μM CsA prior to irradiation. Density plots show that CsA pretreatment dose dependently increased UV-induced apoptosis after both Arimed B (A) and TL-12 (D) irradiation. The percentage of Annexin V positive/7AAD negative cells (early apoptosis) following Arimed B (B) and TL-12 (E) irradiation and percentage of Annexin V positive/7AAD positive cells plus Annexin V positive/7AAD negative cells (total apoptosis) following Arimed B (C), and TL-12 (F) irradiation is summarized in bar charts that show the mean ± se percentage of positive cells from 3 independent, triplicate experiments. ***P < 0.001 vs. vehicle (DMSO) control; one-way ANOVA.
UVR induces NFAT2 nuclear translocation in a full-thickness epidermal equivalent model
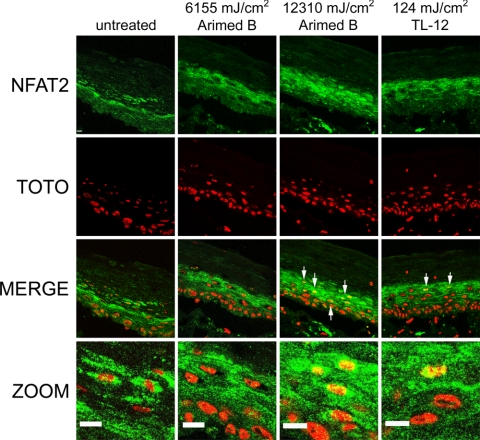
Full-thickness epidermal equivalents (FTEs) display in vivo-like morphological and growth characteristics and provide an excellent in vitro tool for studying biological responses in skin. NFAT2 was located in the cytoplasm of keratinocytes in untreated FTEs, and no cells stained positively for nuclear NFAT2 (Fig. 7). FTEs were irradiated with 6155 and 12,310 mJ/cm2 using Arimed B lamps and 124 mJ/cm2 using TL-12 lamps then fixed 24 h later. Irradiating cells with 6155 mJ/cm2 using Arimed B lamps did not induce NFAT2 nuclear translocation, whereas 12,310 mJ/cm2 Arimed B and 124 mJ/cm2 TL-12 irradiation evoked the appearance of cells staining positively for nuclear NFAT2 (Fig. 7).
Figure 7.
UVR evokes NFAT2 nuclear translocation in a skin equivalent model. Full-thickness epidermal equivalents were irradiated with 6155 and 12,310 mJ/cm2 using Arimed B lamps or 124 mJ/cm2 using TL-12 lamps. Specimens were fixed 24 h after irradiation, and immunostaining for detection of endogenous NFAT2 was performed. Arrows indicate positive nuclear staining of NFAT2. Scale bars = 50 μm.
DISCUSSION
Because wavelength is an integral factor that contributes to responses evoked by UVR, we focused particularly on the wavelength dependence of NFAT responses. UVR sources that exhibit a high ratio of UVB to total UVR, activated NFAT at much lower doses than lamps with a low ratio (and correspondingly high ratio of UVA to total UVR). The derived action spectrum for NFAT shows that NFAT activity is inversely associated with wavelength. Notably, the action spectrum for NFAT activation is distinct from the action spectra for erythema (47) and DNA damage (29). This implies that the chromophore for NFAT activation is not DNA and that it is also different from the (unknown) chromophore that is the principal trigger for erythema. It was shown prior to commencement of this study that UVB and UVC increased NFAT transcriptional activity in a mouse cell line and in mice in vivo (34). However, UVC is not physiologically important in the context of environmental solar exposure, and we are the first to compare the ability of physiologically relevant wavelengths to activate NFAT.
Since there are four calcineurin-regulated NFATs, we investigated the cellular localization of different NFAT proteins. GFP-NFAT2 translocates to the nucleus following irradiation with 62 mJ/cm2 TL-12 irradiation, whereas 12,310 mJ/cm2 is required when using Arimed B lamps. This highlights that nuclear translocation parallels the NFAT transcriptional data, and this observation was also similar for endogenous NFAT2. Interestingly, UVR did not evoke nuclear translocation of NFAT1, 3, or 4 (data not shown), and it appears that NFAT2 is the principal NFAT isoform activated by UVR. Isoform-specific activation of NFAT proteins has also been described elsewhere (48, 49), and this may be due to differences in the regulatory regions between NFAT proteins.
NFAT2 nuclear translocation in epidermal equivalents was also preferentially induced by UVB wavelengths. Irradiating epidermal equivalents with 6155 mJ/cm2 (3.5 SED) using Arimed B lamps did not induce an appearance of cells staining positively for nuclear NFAT2, whereas this was achieved by 124 mJ/cm2 (3.1 SED) TL-12 irradiation. Arimed B lamps (∼4% UVB) were able to induce NFAT2 nuclear translocation when a higher dose was applied (12,310 mJ/cm2, 7 SED) and notably, this effective dose was around 100 times higher than that required by TL-12 lamps (∼53% UVB), suggesting that UVB wavelengths are more potent activators of NFAT in epidermal equivalents. Furthermore, when irradiating cells with equally erythemogenic doses using Arimed B or TL-12 lamps (3.5 and 3.1 SED, respectively), the lamps that emitted a higher UVB to UVA ratio (TL-12 lamps) were much more effective at inducing NFAT2 activation. That TL-12 lamps were more effective activators of NFAT in epidermal equivalents than Arimed B lamps, is consistent with data showing that TL-12 lamps are more effective at inducing NFAT transcriptional activation and nuclear translocation in cultured keratinocytes in vitro.
Pretreating cells with CsA (at doses shown to inhibit UVR-induced NFAT activation) reduced the amount of COX-2 protein induced by UVR. This suggests an involvement of NFAT in UVR-driven induction of COX-2, and this was confirmed by COX-2 luciferase reporter experiments. The human COX-2 promoter contains binding sites for NF-κB, NF-IL6, CRE binding proteins, and NFAT (42). There are two NFAT binding sites in the COX-2 promoter denoted the distal- and proximal-NFAT binding sites that lie at regions −115 to −83 and −90 to −47, respectively. The reporter lacking the distal site did not respond differently compared to the control reporter containing both NFAT binding sites, whereas the reporter lacking the proximal site displayed significantly reduced activity compared to the control plasmid following UV radiation. The reporter lacking both the distal and proximal sites was not induced by UVR. This suggests that the proximal site may be more important for driving COX-2 in response to UVR and that the distal site may serve to support or enhance the activity of the proximal site. The distal NFAT site has been described as a “pure site,” which binds NFAT in the absence of other factors, whereas the proximal NFAT binding site lies adjacent to an AP-1 binding site (42). It is known that UVR induces AP-1 complex formation (26) and that NFAT and AP-1 often bind DNA in cooperation with each other (18). It is likely that UV-induced NFAT and AP-1 both bind the proximal NFAT binding site in the COX-2 promoter, thus accounting for the apparent increased primary importance of the proximal NFAT site. It was also observed that the reporter vector lacking the NF-κB elements (P-274) was induced to a lesser extent by UVR than the complete reporter (P-1900), suggesting involvement of NF-κB. In addition, we show that NFAT is involved in induction of COX-2 by UVR and it is likely that several elements, including NFAT, NF-κB, and CRE are required for maximal induction of COX-2 by UVR (50). Our data are further supported by a study that showed UVB reduced activity of GSK-3β and that overexpression of GSK-3β reduced COX-2 induction by UVB (51). GSK-3β is a protein kinase known to be important in regulating NFAT localization by inhibitory phosphorylation.
Comparing responses induced by Arimed B and TL-12 lamps also indicates that UVB wavelengths activate the COX-2 promoter more than UVA. This supports findings by Mahns et al. (52), who showed that induction of COX-2 in an artificial epidermis was largely UVB driven, and additionally, our data implicates NFAT in this response.
It is known that UVR induces apoptosis of keratinocytes, and the apoptotic response is likely to serve as a protective, anticarcinogenic response that prevents replication of cells harboring UV-induced DNA mutations. NFAT plays an antiapoptotic role during cardiomyocyte hypertrophy (53), in bronchial epithelial cells (54), and in granule neurons in the developing cerebellum (9). In our experiments, it was observed that doses of UVR that induced NFAT activation were also doses that induced appreciable levels of apoptosis. It was hypothesized that NFAT may be involved in mediating UV-induced apoptosis either positively or negatively. Pretreating HaCaT keratinocytes with doses of CsA shown to inhibit UV-induced NFAT activation increased the amount of UV-induced apoptosis, suggesting that UV-activated NFAT is antiapoptotic. These data support findings that show inhibiting NFAT with CsA resulted in decreased fibroblast viability following UV irradiation (33). Conversely, Yarosh et al. (55) suggest that NFAT plays a proapoptotic role in response to UVR by presenting data that show CsA reduces apoptosis of keratinocytes (55). However, this response is only seen when administering a highly cytotoxic dose of UVR (55). When irradiating cells with a lower dose of UVR, their data clearly show that CsA decreases viability (55). Our data support the similar responses found in fibroblasts (33).
In our experiments, CsA was used to inhibit UV-induced NFAT activation so that the biological function of UV-activated NFAT could be explored. It is not suggested that CsA per se could be used as an antineoplastic therapy for skin cancer. Prolonged treatment with CsA as an immunosuppressant in transplant medicine increases the chances of developing malignancy (56, 57). Increased cancer risk is largely attributed to reduced immunosurveillance that results from CsA-mediated inhibition of NFAT signaling in T cells (58). Although down-regulating NFAT in immune cells has procarcinogenic effects, up-regulated NFAT signaling in nonimmune cells can often be linked to a procarcinogenic outcome (59).
Increased NFAT transcriptional activity can contribute to both carcinoma invasion and migration in vitro (60), and data suggest that NFAT may promote tumor progression partly by up-regulating COX-2 (59). Increased COX-2 expression in colon carcinoma cells enhances cell invasion in vitro, and this was reduced by inhibiting NFAT (61). In addition, activating NFAT1 increased invasion of a breast cancer cell line, and this response was COX-2 dependent (62). Furthermore, NFAT2 has been shown to induce a transformed phenotype in fibroblasts (63). Our data suggest that NFAT signaling may be worthy of further study in the context of epidermal carcinogenesis in response to UVR.
CONCLUSIONS
We have demonstrated wavelength-dependent induction of NFAT by UVR, with UVB wavelengths being potent activators of NFAT. It is shown that inhibiting NFAT signaling causes increased apoptosis following irradiation, suggesting that UV-activated NFAT acts antiapoptotically in keratinocytes. NFAT is involved in induction of COX-2 by UVR and like NFAT; COX-2 is induced more effectively by UVB wavelengths. Taken together, our data suggest that the proinflammatory and procarcinogenic role played by COX-2 in response to UVR, may be mediated, in part, by upstream NFAT signaling.
Acknowledgments
This work was funded by The British Skin Foundation (R.J.F.) and Wellcome Trust Research Leave Fellowship (N.J.R.). The authors thank D. Cantrell (ICRF, London, UK) for NFAT2 cDNA, D. J. McKean (Mayo Foundation, Rochester, MN, USA) for the NFAT luciferase reporter vector, and M. A. Iniguez (Centro de Biologia Molecular, Madrid, Spain) for COX-2 luciferase reporter vectors.
References
- Shaw J P, Utz P J, Durand D B, Toole J J, Emmel E A, Crabtree G R. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Kiani A, Rao A, Aramburu J. Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity. 2000;12:359–372. doi: 10.1016/s1074-7613(00)80188-0. [DOI] [PubMed] [Google Scholar]
- Hill-Eubanks D C, Gomez M F, Stevenson A S, Nelson M T. NFAT regulation in smooth muscle. Trends Cardiovasc Med. 2003;13:56–62. doi: 10.1016/s1050-1738(02)00212-8. [DOI] [PubMed] [Google Scholar]
- Schulz R A, Yutzey K E. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol. 2004;266:1–16. doi: 10.1016/j.ydbio.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner E F, Mak T W, Serfling E, Takayanagi H. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Daraji W I, Grant K R, Ryan K, Saxton A, Reynolds N J. Localization of calcineurin/NFAT in human skin and psoriasis and inhibition of calcineurin/NFAT activation in human keratinocytes by cyclosporin A. J Invest Dermatol. 2002;118:779–788. doi: 10.1046/j.1523-1747.2002.01709.x. [DOI] [PubMed] [Google Scholar]
- Santini M P, Talora C, Seki T, Bolgan L, Dotto G P. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation. Proc Natl Acad Sci U S A. 2001;98:9575–9580. doi: 10.1073/pnas.161299698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, di Vignano A T, Sharov A A, Neilson J, Havrda M C, Roop D R, Botchkarev V A, Crabtree G R, Dotto G P. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell. 2005;8:665–676. doi: 10.1016/j.devcel.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Benedito A B, Lehtinen M, Massol R, Lopes U G, Kirchhausen T, Rao A, Bonni A. The transcription factor NFAT3 mediates neuronal survival. J Biol Chem. 2005;280:2818–2825. doi: 10.1074/jbc.M408741200. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy M T, Cluster A, Cai N S, Cadet J L. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey P G, Luo C, Kerppola T K, Jain J, Badalian T M, Ho A M, Burgeon E, Lane W S, Lambert J N, Curran T, Verdine G L, Rao A, Hogan P G. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993;262:750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- Northrop J P, Ho S N, Chen L, Thomas D J, Timmerman L A, Nolan G P, Admon A, Crabtree G R. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- Hoey T, Sun Y L, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Masuda E S, Naito Y, Tokumitsu H, Campbell D, Saito F, Hannum C, Arai K, Arai N. NFATx, a novel member of the nuclear factor of activated T cells family that is expressed predominantly in the thymus. Mol Cell Biol. 1995;15:2697–2706. doi: 10.1128/mcb.15.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez C, Aramburu J, Rakeman A S, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci U S A. 1999;96:7214–7219. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa H, Woo S K, Dahl S C, Handler J S, Kwon H M. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci U S A. 1999;96:2538–2542. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan P G, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- Horsley V, Pavlath G K. NFAT: ubiquitous regulator of cell differentiation and adaptation. J Cell Biol. 2002;156:771–774. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals C R, Sheridan C M, Turck C W, Gardner P, Crabtree G R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- Graef I A, Mermelstein P G, Stankunas K, Neilson J R, Deisseroth K, Tsien R W, Crabtree G R. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- Vyas D R, Spangenburg E E, Abraha T W, Childs T E, Booth F W. GSK-3beta negatively regulates skeletal myotube hypertrophy. Am J Physiol Cell Physiol. 2002;283:C545–C551. doi: 10.1152/ajpcell.00049.2002. [DOI] [PubMed] [Google Scholar]
- Zhu J, Shibasaki F, Price R, Guillemot J C, Yano T, Dotsch V, Wagner G, Ferrara P, McKeon F. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell. 1998;93:851–861. doi: 10.1016/s0092-8674(00)81445-2. [DOI] [PubMed] [Google Scholar]
- Okamura H, Garcia-Rodriguez C, Martinson H, Qin J, Virshup D M, Rao A. A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol Cell Biol. 2004;24:4184–4195. doi: 10.1128/MCB.24.10.4184-4195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffey B L. Sources and measurement of ultraviolet radiation. Methods. 2002;28:4–13. doi: 10.1016/s1046-2023(02)00204-9. [DOI] [PubMed] [Google Scholar]
- Heck D E, Gerecke D R, Vetrano A M, Laskin J D. Solar ultraviolet radiation as a trigger of cell signal transduction. Toxicol Appl Pharmacol. 2004;195:288–297. doi: 10.1016/j.taap.2003.09.028. [DOI] [PubMed] [Google Scholar]
- De Gruijl F R, Sterenborg H J, Forbes P D, Davies R E, Cole C, Kelfkens G, van Weelden H, Slaper H, van der Leun J C. Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice. Cancer Res. 1993;53:53–60. [PubMed] [Google Scholar]
- Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. UV-induced skin damage. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- Setlow R B. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc Natl Acad Sci U S A. 1974;71:3363–3366. doi: 10.1073/pnas.71.9.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T. Skin disorders that improve by exposure to sunlight. Clin Dermatol. 1998;16:59–65. doi: 10.1016/s0738-081x(97)00170-3. [DOI] [PubMed] [Google Scholar]
- Fischer T, Alsins J, Berne B. Ultraviolet-action spectrum and evaluation of ultraviolet lamps for psoriasis healing. Int J Dermatol. 1984;23:633–637. doi: 10.1111/j.1365-4362.1984.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Parrish J A, Jaenicke K F. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359–362. doi: 10.1111/1523-1747.ep12520022. [DOI] [PubMed] [Google Scholar]
- Maziere C, Morliere P, Louandre C, Conte M A, Gomilla C, Santus R, Antonicelli F, Hornebeck W, Maziere J C. Low UVA doses activate the transcription factor NFAT in human fibroblasts by a calcium-calcineurin pathway. Free Radic Biol Med. 2005;39:1629–1637. doi: 10.1016/j.freeradbiomed.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattjus P, Ma W Y, Rincon M, Chen N Y, Brown R E, Dong Z. Involvement of nuclear factor of activated T cells activation in UV response. Evidence from cell culture and transgenic mice. J Biol Chem. 2000;275:9143–9149. doi: 10.1074/jbc.275.13.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke C. Role of COX-2 inhibitors in cancer therapy. Cancer Invest. 2004;22:271–282. doi: 10.1081/cnv-120030216. [DOI] [PubMed] [Google Scholar]
- Meric J B, Rottey S, Olaussen K, Soria J C, Khayat D, Rixe O, Spano J P. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol. 2006;59:51–64. doi: 10.1016/j.critrevonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Onguru O, Casey M B, Kajita S, Nakamura N, Lloyd R V. Cyclooxygenase-2 and thromboxane synthase in non-endocrine and endocrine tumors: a review. Endocr Pathol. 2005;16:253–277. doi: 10.1385/ep:16:4:253. [DOI] [PubMed] [Google Scholar]
- Sinicrope F A, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev. 2004;23:63–75. doi: 10.1023/a:1025863029529. [DOI] [PubMed] [Google Scholar]
- Buckman S Y, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, Pentland A P. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19:723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- Fischer S M, Lo H H, Gordon G B, Seibert K, Kelloff G, Lubet R A, Conti C J. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 1999;25:231–240. [PubMed] [Google Scholar]
- Akunda J K, Chun K S, Sessoms A R, Lao H C, Fischer S M, Langenbach R. Cyclooxygenase-2 deficiency increases epidermal apoptosis and impairs recovery following acute UVB exposure. Mol Carcinog. 2007;46:354–362. doi: 10.1002/mc.20290. [DOI] [PubMed] [Google Scholar]
- Iniguez M A, Martinez-Martinez S, Punzon C, Redondo J M, Fresno M. An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J Biol Chem. 2000;275:23627–23635. doi: 10.1074/jbc.M001381200. [DOI] [PubMed] [Google Scholar]
- Hedin K E, Bell M P, Kalli K R, Huntoon C J, Sharp B M, McKean D J. Delta-opioid receptors expressed by Jurkat T cells enhance IL-2 secretion by increasing AP-1 complexes and activity of the NF-AT/AP-1-binding promoter element. J Immunol. 1997;159:5431–5440. [PubMed] [Google Scholar]
- Farr P M, Diffey B L, Higgins E M, Matthews J N. The action spectrum between 320 and 400 nm for clearance of psoriasis by psoralen photochemotherapy. Br J Dermatol. 1991;124:443–448. doi: 10.1111/j.1365-2133.1991.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Turner H, Gomez M, McKenzie E, Kirchem A, Lennard A, Cantrell D A. Rac-1 regulates nuclear factor of activated T cells (NFAT) C1 nuclear translocation in response to Fcepsilon receptor type 1 stimulation of mast cells. J Exp Med. 1998;188:527–537. doi: 10.1084/jem.188.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyakh L, Ghosh P, Rice N R. Expression of NFAT-family proteins in normal human T cells. Mol Cell Biol. 1997;17:2475–2484. doi: 10.1128/mcb.17.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay A F, Diffey B L. A reference action spectrum for ultraviolet induced erythema in human skin. CIE J. 1987;6:17–22. [Google Scholar]
- Abbott K L, Friday B B, Thaloor D, Murphy T J, Pavlath G K. Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol Biol Cell. 1998;9:2905–2916. doi: 10.1091/mbc.9.10.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh C, Carew J A, Kim J, Hogan P G, Rao A. T-cell receptor stimulation elicits an early phase of activation and a later phase of deactivation of the transcription factor NFAT1. Mol Cell Biol. 1996;16:3945–3954. doi: 10.1128/mcb.16.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Chen W, Gonzales M S, Finch J, Inoue H, Bowden G T. Role of cyclic AMP-responsive element in the UVB induction of cyclooxygenase-2 transcription in human keratinocytes. Oncogene. 2001;20:5164–5172. doi: 10.1038/sj.onc.1204667. [DOI] [PubMed] [Google Scholar]
- Tang Q, Gonzales M, Inoue H, Bowden G T. Roles of Akt and glycogen synthase kinase 3beta in the ultraviolet B induction of cyclooxygenase-2 transcription in human keratinocytes. Cancer Res. 2001;61:4329–4332. [PubMed] [Google Scholar]
- Mahns A, Wolber R, Stab F, Klotz L O, Sies H. Contribution of UVB and UVA to UV-dependent stimulation of cyclooxygenase-2 expression in artificial epidermis. Photochem Photobiol Sci. 2004;3:257–262. doi: 10.1039/b309067a. [DOI] [PubMed] [Google Scholar]
- Pu W T, Ma Q, Izumo S. NFAT transcription factors are critical survival factors that inhibit cardiomyocyte apoptosis during phenylephrine stimulation in vitro. Circ Res. 2003;92:725–731. doi: 10.1161/01.RES.0000069211.82346.46. [DOI] [PubMed] [Google Scholar]
- Ding J, Li J, Xue C, Wu K, Ouyang W, Zhang D, Yan Y, Huang C. Cyclooxygenase-2 induction by arsenite is through a nuclear factor of activated T-cell-dependent pathway and plays an antiapoptotic role in Beas-2B cells. J Biol Chem. 2006;281:24405–24413. doi: 10.1074/jbc.M600751200. [DOI] [PubMed] [Google Scholar]
- Yarosh D B, Pena A V, Nay S L, Canning M T, Brown D A. Calcineurin inhibitors decrease DNA repair and apoptosis in human keratinocytes following ultraviolet B irradiation. J Invest Dermatol. 2005;125:1020–1025. doi: 10.1111/j.0022-202X.2005.23858.x. [DOI] [PubMed] [Google Scholar]
- Penn I. Occurrence of cancers in immunosuppressed organ transplant recipients. Clin Transpl. 1998:147–158. [PubMed] [Google Scholar]
- Sheil A G. Cancer in immune-suppressed organ transplant recipients: aetiology and evolution. Transplant Proc. 1998;30:2055–2057. doi: 10.1016/s0041-1345(98)00539-9. [DOI] [PubMed] [Google Scholar]
- Botti C, Seregni E, Ferrari L, Martinetti A, Bombardieri E. Immunosuppressive factors: role in cancer development and progression. Int J Biol Markers. 1998;13:51–69. doi: 10.1177/172460089801300201. [DOI] [PubMed] [Google Scholar]
- Buchholz M, Ellenrieder V. An emerging role for Ca2+/calcineurin/NFAT signaling in cancerogenesis. Cell Cycle. 2007;6:16–19. doi: 10.4161/cc.6.1.3650. [DOI] [PubMed] [Google Scholar]
- Jauliac S, Lopez-Rodriguez C, Shaw L M, Brown L F, Rao A, Toker A. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002;4:540–544. doi: 10.1038/ncb816. [DOI] [PubMed] [Google Scholar]
- Corral R S, Iniguez M A, Duque J, Lopez-Perez R, Fresno M. Bombesin induces cyclooxygenase-2 expression through the activation of the nuclear factor of activated T cells and enhances cell migration in Caco-2 colon carcinoma cells. Oncogene. 2007;26:958–969. doi: 10.1038/sj.onc.1209856. [DOI] [PubMed] [Google Scholar]
- Yiu G K, Toker A. NFAT induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2. J Biol Chem. 2006;281:12210–12217. doi: 10.1074/jbc.M600184200. [DOI] [PubMed] [Google Scholar]
- Neal J W, Clipstone N A. A constitutively active NFATc1 mutant induces a transformed phenotype in 3T3–L1 fibroblasts. J Biol Chem. 2003;278:17246–17254. doi: 10.1074/jbc.M300528200. [DOI] [PubMed] [Google Scholar]
- Patel R N, Attur M G, Dave M N, Patel I V, Stuchin S A, Abramson S B, Amin A R. A novel mechanism of action of chemically modified tetracyclines: inhibition of COX-2-mediated prostaglandin E2 production. J Immunol. 1999;163:3459–3467. [PubMed] [Google Scholar]
- Sevigny M B, Li C F, Alas M, Hughes-Fulford M. Glycosylation regulates turnover of cyclooxygenase-2. FEBS Lett. 2006;580:6533–6536. doi: 10.1016/j.febslet.2006.10.073. [DOI] [PubMed] [Google Scholar]