Abstract
The adaptor and signaling proteins TRAF2, TRAF3 and cIAP1 and cIAP2 were suggested to inhibit alternative nuclear factor kappa B (NF-κB) signaling in resting cells by targeting NF-κB inducing kinase (NIK) to ubiquitin-dependent degradation, thus preventing processing of the NF-κB2 precursor protein p100 to release p52. However, the respective functions of TRAF2 and TRAF3 in NIK degradation and activation of alternative NF-κB signaling has remained elusive. We now show that CD40 or BAFF receptor activation resulted in TRAF3 degradation in a cIAP1-cIAP2- and TRAF2- dependent way due to enhanced cIAP1, cIAP2 TRAF3-directed ubiquitin ligase activity. Receptor-induced activation of cIAP1 and cIAP2 correlated with their K63-linked ubiquitination by TRAF2. Degradation of TRAF3 prevented association of NIK with the cIAP1-cIAP2-TRAF2 ubiquitin ligase complex, which resulted in NIK stabilization and NF-κB2-p100 processing. Constitutive activation of this pathway causes perinatal lethality and lymphoid defects.
Introduction
The Mammalian Rel-NF-κB family of transcription factors play important roles in innate and adaptive immune responses, inflammation, cell survival and cancer1, 2. In resting cells, inhibitory IκB molecules bind NF-κB dimers via ankyrin repeats and retain them in the cytoplasm1. The IκB kinase (IKK) complex, comprised of the catalytic subunits IKKα and IKKβ and the regulatory subunit IKKγ (NEMO), controls NF-κB activation by phosphorylating IκBs and targeting them to lysine (K) 48-linked polyubiquitination by the ubiquitin ligase (E3) SCFIκB, thereby inducing IκB proteasomal degradation3. This pathway, known as the classical or canonical NF-κB signaling pathway, mostly affects p50-RelA and p50-c-Rel heterodimers1. However, the Nfκb2 gene product p100, which contains IκB-like ankyrin repeats in its C-terminal region, is regulated through the alternative NF-κB signaling pathway4. In this pathway, p100, which is mainly associated with RelB in non-stimulated cells1, is phosphorylated by IKKα at its C-terminal region4, leading to K48-linked polyubiquitination also by the SCFIκB complex5. In this case, however, K48-linked polyubiquitination results in limited proteolysis of the C-terminal ankyrin repeats and release of the N-terminal p52 protein subunit that then forms heterodimers with RelB (p52-RelB)6. This alternative pathway is activated by lymphorganogenic cytokines, such as lymphotoxin (LT) α-β heterotrimers and B-cell survival and maturation factors, such as CD40 ligand (CD40L) and BAFF7–10. An important component of this pathway is the protein kinase NIK (encoded by Map3k14), which activates IKKα4, 6, 8
LTα-β, CD40L and BAFF belong to the tumor necrosis factor (TNF) superfamily, whose members signal via trimeric receptors (TNF receptors or TNFRs) and signaling proteins called TNFR associated factors (TRAFs)11, 12, that are key intermediates in both the classical and alternative NF-κB signaling pathways12, 13. The TRAFs share a C-terminal TRAF domain that mediates oligomerization as well as receptor binding and a variable number of Zinc (Zn) fingers within their N-terminal portion11, 14. TRAF2-6 also contain an N-terminal RING finger that is critical for E3 activity, which catalyzes formation of non-degradative K63-linked polyubiquitin chains13, a function demonstrated most clearly for TRAF215 and TRAF616. In the context of TNF receptor 1 (TNFR1)-activated classical NF-κB signaling, TRAF2 and TRAF5 act redundantly17, but the exact targets for their E3 activity remain contentious12. TRAF2 and TRAF6 are both involved in CD40-induced classical NF-κB activation11, 18, and TRAF6 is a critical mediator of NF-κB signaling downstream of Toll-like and interleukin 1 (IL-1) receptors (TLR-IL-1R)19. Although, some of these receptors, e.g, TLR4, engage TRAF3 to activate interferon (IFN) signaling, TRAF3 does not directly contribute to classical NF-κB signaling20. TRAF2, 5 and 6 also activate NF-κB signaling upon overexpression11, 21, 22, an activity not exhibited by TRAF320. Recently, TRAF3 was found to act as a negative regulator of TRAF2- and TRAF6-dependent MAP kinase (MAPK) activation23. These results strongly suggest that TRAF2 and TRAF3 have distinct signaling functions and activities.
Curiously, while TRAF2 ablation in B-cells attenuates classical NF-κB signaling, it constitutively activates the alternative pathway24. A similar phenotype is seen upon TRAF3 ablation, although TRAF3 does not directly impact classical NF-κB signaling25. Based on constitutive NF-κB2-p100 processing and presence of nuclear p52-RelB in TRAF2- or TRAF3- deficient cells, both molecules were described as “inhibitors” of alternative NF-κB signaling26. Activation of the alternative pathway was suggested to depend on receptor-induced TRAF3 degradation and stabilization of NIK27. However, ablation of TRAF2 also results in NIK accumulation28. Since TRAF2 is an E3 ligase and TRAF3 is similar in structure to TRAF2, it was assumed that both TRAFs target NIK to ubiquitin-dependent proteasomal degradation in non-stimulated cells26–28. However, the finding of activated alternative NF-κB signaling upon ablation of either TRAF2 or TRAF3 suggests that each of these molecules has a unique function that is not provided by the remaining family member.
The critical role of TRAF3 in NIK protein turnover is underscored by identification of TRAF3 mutations in human multiple myeloma (MM) that elevate NIK expression and activate p100 processing29, 30. Deregulated alternative NF-κB signaling also accounts for the postnatal lethality associated with TRAF3 ablation25. We noted that Traf2−/− mice, which also die perinatally31, resemble Traf3−/− mice32 and therefore we began to investigate the relative functions of these TRAFs in alternative NF-κB signaling. In particular, we became interested in the relationships between TRAF2 and TRAF3 and two other RING-containing components of the alternative pathway, cIAP1 and cIAP2, which belong to the IAP (inhibitors of apoptosis) family, characterized by presence of one or more BIR domains28–30, 33, 34. Many IAPs have anti-apoptotic functions, but their mechanisms of action are diverse33. The anti-apoptotic function of cIAP1 and cIAP2 is inhibited upon release of mitochondrial protein Diablo (Smac), which may serve both as an activator and a substrate of the E3 activity of these cIAPs35. Accordingly, Diablo mimics that bind to the same sites on cIAP1 and cIAP2 as does Diablo induce cIAP1 and cIAP2 self-ubiquitination and proteasomal degradation and stabilization of NIK 28, 34, 36, leading to the conclusion that cIAP1 and cIAP2 are required for NIK turnover28, 34. This possibility is supported by the finding that in MM, in which the linked genes encoding cIAP1 (BIRC2) and cIAP2 (BIRC3) are deleted, cells exhibit NIK accumulation and constitutive p100 processing29, 30. It was also observed that cIAP1 is degraded upon stimulation of melanoma cells with TWEAK, a TNF family member that activates alternative NF-κB signaling, leading to the suggestion that the first step in this pathway is receptor-induced cIAP degradation34. However, the exact roles of TRAF2, TRAF3 and cIAP1 and cIAP2 (‘cIAP1-cIAP2’) in CD40- or BAFF-R-induced alternative NF-κB signaling remained unclear.
We now show that Traf2−/− mice die postnatally and exhibit lymphoid defects due to constitutive NIK signaling, just like Traf3−/− mice. Yet, despite their nearly identical phenotypes, TRAF2 and TRAF3 have distinct signaling functions. TRAF2 is required for cytokine-induced TRAF3 degradation that is dependent on cIAP1-cIAP2, which act as receptor-activated TRAF3-directed ubiquitin ligases. Receptor-induced activation of cIAP1-cIAP2 depends on TRAF2, which acts as a K63-specific E3 for the cIAPs. TRAF3 itself is an adaptor that links a ubiquitin ligase complex consisting of TRAF2 and cIAP1- cIAP2 to NIK. We suggest that the first step in alternative NF-κB signaling triggered by CD40 or BAFF-R is TRAF2-dependent cIAP1-cIAP2 activation leading to TRAF3 degradation and disruption of the cIAP1-cIAP2-TRAF2-TRAF3-NIK complex.
Results
Deletion of NIK prevents postnatal lethality of Traf2−/− mice
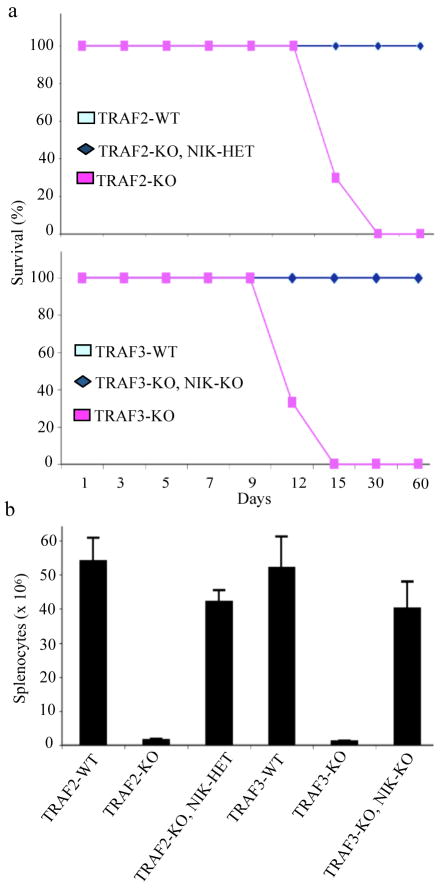
The postnatal phenotypes of Traf2−/− and Traf3−/− mice are similar31, 32. Since TRAF3 deficiency was proposed to cause postnatal lethality dependent on constitutive alternative NF-κB signaling that is rescuable by deletion of the Nfkb2 25, we examined whether TRAF2 ablation acts similarly and generated mice deficient in both TRAF2 and NIK. For comparison, we generated Traf3−/− mice also deficient in NIK (Traf3−/−Map3k14−/−). Deletion of only one Map3k14 allele prevented postnatal lethality and the runted stature of Traf2−/− mice, similar to the obligatory (we did not recover viable Traf3−/−Map3k14−/− mice in more than 50 crosses) deletion of both Map3k14 alleles in Traf3−/− mice (Fig. 1a and Supplementary Fig. 1a online). Splenic atrophy and reduced splenocyte numbers also were rescued by reducing the NIK dosage (Fig. 1b and Supplementary Fig. 1b online). Serum cortisol concentrations indicative of a stress response were elevated in Traf2−/− and Traf3−/− mice and deletion of NIK returned them to normal (Supplementary Fig. 1c online). Several inflammatory chemokine mRNAs were also elevated in Traf2−/− mouse embryonic fibroblasts (MEFs) but not to the same extent as in Traf3−/− MEFs (Supplementary Fig. 1d online). This suggests that the inflammatory response associated with TRAF2 deficiency is not as severe as the one associated with the TRAF3 deficiency, providing a plausible explanation for the dose-dependent effects of NIK in the two mutants.
Fig. 1. Deletion of NIK prevents postnatal lethality and splenic atrophy in Traf2−/− and Traf3−/− mice.
(a) Survival curves of mice of the indicated genotypes. (b) Spleen cellularity in mice of the indicated genotypes at P15. Results are averages ± s.d (n = 4). WT, wild-type; Traf2-KO, Traf2−/−; Traf3-KO, Traf3−/−; NIK-KO, Map3k14−/−; NIK-HET, Map3k14+/−.
Lymphoid abnormalities in TRAF2- and TRAF3-deficient mice
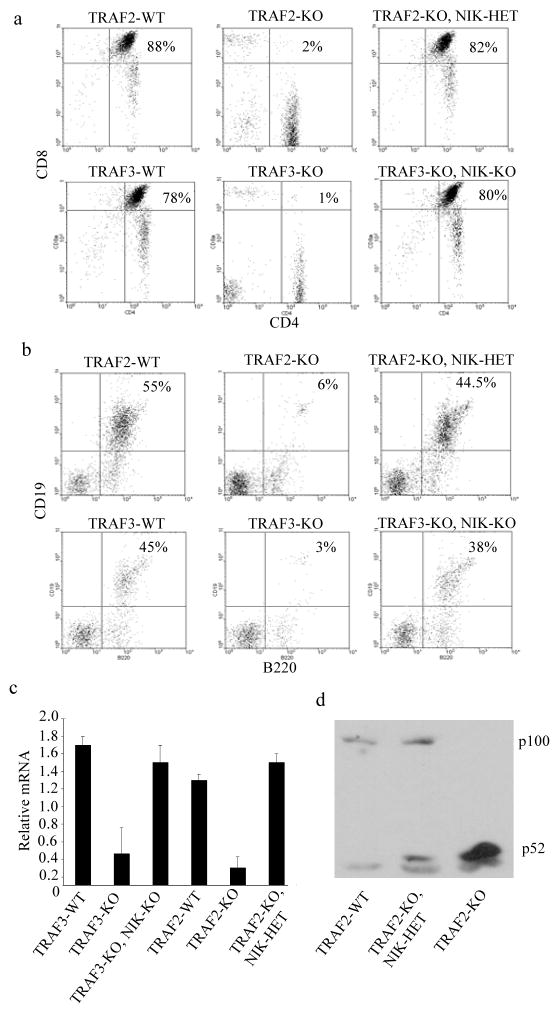
TRAF2−/− and TRAF3−/− mice have highly reduced numbers of CD4+CD8+ double-positive (DP) thymocytes 31, 32. Although the mechanism underlying this defect is not clear, it could be related to elevated circulating cortisol in these mice and the known ability of glucocorticoids to induce thymocyte apoptosis37, 38. Deletion of NIK restored normal numbers of DP thymocytes in both Traf2−/− and Traf3−/− mice (Fig. 2a).
Fig. 2. Reversal of lymphoid abnormalities in Traf2−/− and Traf3−/− mice by deletion of NIK.
(a) Flow cytometry of thymocytes from the indicated strains stained with anti-CD8 and anti-CD4. Numbers indicate percentages of double positive thymocytes. (b) Flow cytometry of BM cells isolated from the indicated strains stained with anti-CD19 and anti-B220. Numbers indicate percentages of CD19+B220+ Pre-B cells. (c) B220+ cells from the indicated mice were purified using magnetic beads. Total RNA was extracted and Pax5 mRNA was analyzed by quantitative RT-PCR. (d) Immunoblot for NF-κB2 p100 and p52 in splenic B-cells purified from the indicated mice. Representative figures from atleast 3 independent experiments were shown. WT, wild-type; Traf2-KO, Traf2−/−; Traf3-KO, Traf3−/−; NIK-KO, Map3k14−/−; NIK-HET, Map3k14+/−.
Traf2−/− and Traf3−/− mice harbor elevated numbers of marginal zone (MZ) B-cells and splenic B-cells lacking TRAF2 or TRAF3 survive longer than WT cells24–26, 39, which should result in B-cell accumulation and splenomegaly. However, Traf2−/− and Traf3−/− mice suffer from splenic atrophy and exhibit highly reduced splenocyte numbers31, 32. In both Traf2−/− and Traf3−/− mice BM B-cell development was defective due to impairment in the transition from the Pro-B to the Pre-B stage, characterized by CD19 expression (Fig. 2b) resulting in fewer BM B220+ cells. Expression of the mRNA for transcription factor Pax5, which regulates this transition40, was reduced in Traf2−/− and Traf3−/− B-cells but was restored upon NIK deletion (Fig. 2c), which also rescued normal B-cell development (Fig. 2b). These defects are also consistent with the published effects of glucocorticoids on B cell progenitors41.
Deletion of NIK prevented constitutive p100 processing in Traf2−/− B-cells (Fig. 2d), as previously shown for Traf3−/− B-cells25. Consistent with the effect on p100 processing, deletion of NIK in either Traf2−/− or Traf3−/− mice reduced MZ B-cell numbers and decreased splenic B-cell survival to WT levels (Supplementary Fig. 2a, b online). Collectively, these results indicate that the absence of TRAF2 causes very similar lymphoid defects as those associated with TRAF3 ablation. Some of these defects are likely to be the direct consequence of constitutive NIK-dependent signaling whereas others are probably caused by elevated serum cortisol.
TRAF2-deficiency stabilizes NIK, promotes p100 processing
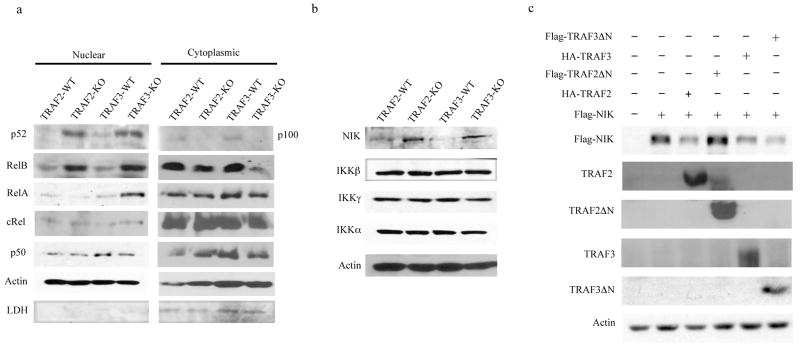
TRAF2 negatively regulates p100 processing in B-cells24. However, it was not clear whether TRAF2, like TRAF327, also induced NIK proteasomal degradation. To investigate this point we analyzed wild-type, Traf2−/− and Traf3−/− mouse embryonic fibroblasts (MEFs). Both Traf2−/− and Traf3−/− MEFs exhibited elevated NIK expression, constitutive p100 processing and nuclear p52 and RelB (Fig. 3a, b). NIK mRNA amounts were not considerably affected by ablation of either TRAF2 or TRAF3 (Supplementary Fig. 3a online), suggesting that the observed changes in NIK expression are post-transcriptional. Notably, Traf3−/− MEFs contained higher amounts of nuclear RelA than Traf2−/− cells (Fig. 3a), a finding that is consistent with the higher expression of NF-κB-dependent inflammatory chemokines in these cells (Supplementary Fig. 1d online). As shown for TRAF327, TRAF2 overexpression in HEK293T-cells induced proteasome-dependent NIK degradation (Fig. 3c and Supplementary Fig 3b-d online). This activity was dependent on the N-terminal portion of TRAF2 which contains its RING finger; by contrast, deletion of the N-terminal RING finger of TRAF3 did not compromise its ability to induce NIK degradation, which is in contrast to a previous report that the RING finger of TRAF3 is essential for inhibition of alternative NF-κB signaling42 (Fig. 3c). Overexpression of either cIAP1 or cIAP2 also led to NIK degradation (Supplementary Fig. 3c online).
Fig. 3. TRAF2 and TRAF3 are involved in NIK turnover and NF-κB2 p100 processing.
(a) Immunoblot of nuclear and cytoplasmic extracts from MEFs of the indicated genotypes analyzed for expression of the NF-κB proteins indicated. Lactate dehydrogenase (LDH) was evaluated to rule out cytoplasmic contamination of nuclear extracts. (b) Immunoblot of total lysates from MEFs of the indicated genotypes examined for NIK and IKK subunit expression. (c) Immunoblot of total lysates from HEK293T cells transfected with Flag-NIK, HA-TRAF2 (TF2), Flag-TRAF2ΔN, HA-TRAF3 and Flag-TRAF3ΔN expression vectors probed with anti-HA or anti-FLAG. WT, wild-type; Traf2-KO, Traf2−/−; Traf3-KO, Traf3−/−. Representative figures from 3–5 independent experiments were shown.
TRAF3 recruits TRAF2 and cIAP2 to NIK
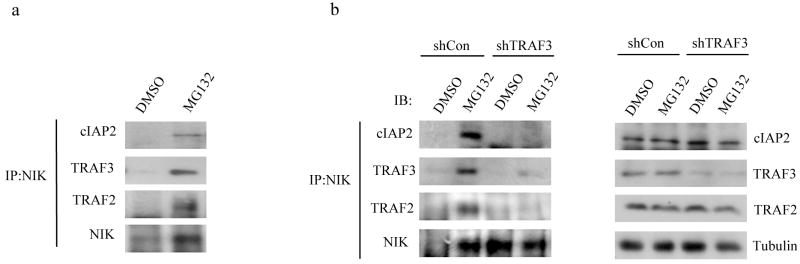
TRAF2, TRAF3 and cIAP1-cIAP2 are all involved in rapid NIK turnover in non-stimulated B-cells and their inactivation or deletion results in NIK accumulation and p100 processing24, 25, 28, 29, 34. Analyses of TRAF3 mutants in MM29, 30, suggested that TRAF3 binds to NIK and TRAF2, which is associated with cIAP1-cIAP243. We examined the interaction of cIAP2, TRAF2 and TRAF3 with NIK in primary B-cells. Since endogenous NIK is of very low abundance, due to its rapid turnover in non-stimulated cells, we incubated B-cells with the proteasome inhibitor MG132 to prevent NIK degradation. Immunoprecipitation confirmed that NIK associates with cIAP2, TRAF2 and TRAF3 (Fig. 4a). Depletion of TRAF3 by shRNA-mediated knockdown in BJAB lymphoma cells prevented association of both TRAF2 and cIAP2 with NIK (Fig. 4b).
Fig. 4. TRAF3 links TRAF2 and cIAP2 to NIK.
(a) Immunoprecipitation and immunoblot of splenic B-cell lysates after the cells were incubated with vehicle control (DMSO) or MG132 for 6 hrs to stabilize endogenous NIK. Association of cIAP2, TRAF2 and TRAF3 with NIK was analyzed. (b) Immunoprecipitation and immunoblot of BJAB human B cell lymphoma lysates after the cells were infected with control or TRAF3-specific shRNA lentiviruses. The cells were incubated with DMSO or MG132 for 6 hrs and NIK was immunoprecipitated from total cell lysates and co-precipitation of the indicated proteins was examined. Representative figures from 3–5 independent experiments were shown.
NIK stabilization is preceded by cIAP1-cIAP2- and TRAF2- dependent TRAF3 degradation
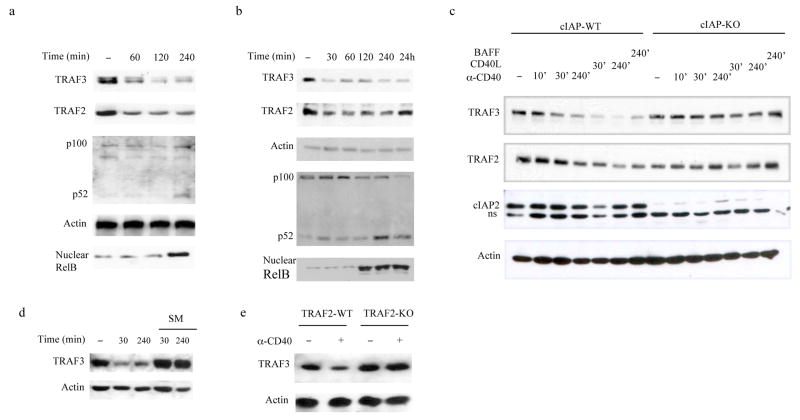
Rapid NIK turnover in resting cells seems to be the major mechanism that prevents premature activation of alternative NF-κB signaling. However, how engagement of receptors that control B-cell survival and development, e.g. CD40 and BAFF-R, activates this pathway is not clear. It was suggested that receptor-induced TRAF3 degradation is a critical regulatory step leading to NIK stabilization and p100 processing26, 27. However, a more recent report suggested that the first step is receptor-induced cIAP1-cIAP2 degradation34. We confirmed that CD40 or BAFF-R engagement result in rapid, proteasome dependent (Supplementary Fig. 4a online), TRAF3 degradation that preceded p100 processing (Fig. 5a, b). TRAF2 also decayed upon receptor activation but not as extensively as TRAF3. Moreover, TRAF3 decay preceded TRAF2 decay (Supplementary Fig. 4b online). As expected, CD40 engagement also led to NIK stabilization that paralleled receptor-induced p100 processing (Supplementary Fig. 4c online). CD40- or BAFF-R engagement also resulted in extensive TRAF3 and modest TRAF2 decay, only in cIAP1 and cIAP2- positive MM cells (Fig. 5c). No significant cIAP2 decay was seen and neither TRAF2 nor TRAF3 were degraded in MM cells lacking both cIAPs (Fig. 5c). Similar results were seen upon treatment of primary B-cells with a Smac mimic, SM, which induces rapid cIAP1 and cIAP2 degradation36 (Fig. 5d). CD40-induced TRAF3 degradation was also dependent on TRAF2, whose absence in B-cells and especially MEFs increased TRAF3 abundance (Fig. 5e and Supplementary Fig. 5a online). TRAF3 deficiency, however, had no impact on TRAF2 amounts (Supplementary Fig. 5b online).
Fig. 5. cIAP1-cIAP2 and TRAF2 are required for receptor-induced TRAF3 degradation which precedes NIK stabilization.
(a,b) Immunoblot of lysates from splenic B-cells purified and incubated with anti-CD40 antibody (a) or recombinant BAFF (b) for the indicated times. Total lysates as well as nuclear extracts were prepared and the indicated protein amounts were analyzed. (c) Immunoblot of lysates from wild-type and cIAP1 and cIAP2-double deficient (cIAP-KO) MM cells incubated with anti-CD40, CD40L or BAFF for the indicated time. Total lysates were prepared and expression of the indicated proteins was analyzed. ns, nonspecific band. (d,e) Immunoblot of lysates from splenic B-cells pre-incubated with or without Smac mimic (SM) and stimulated with anti-CD40 for the indicated time (d) or from wild-type and TRAF2-deficient B-cells stimulated or not with anti-CD40 for 4 hrs (e) analyzed for TRAF3 amounts. Representative figures from 3–5 independent experiments were shown.
Activation of cIAP2 E3 ligase activity towards TRAF3
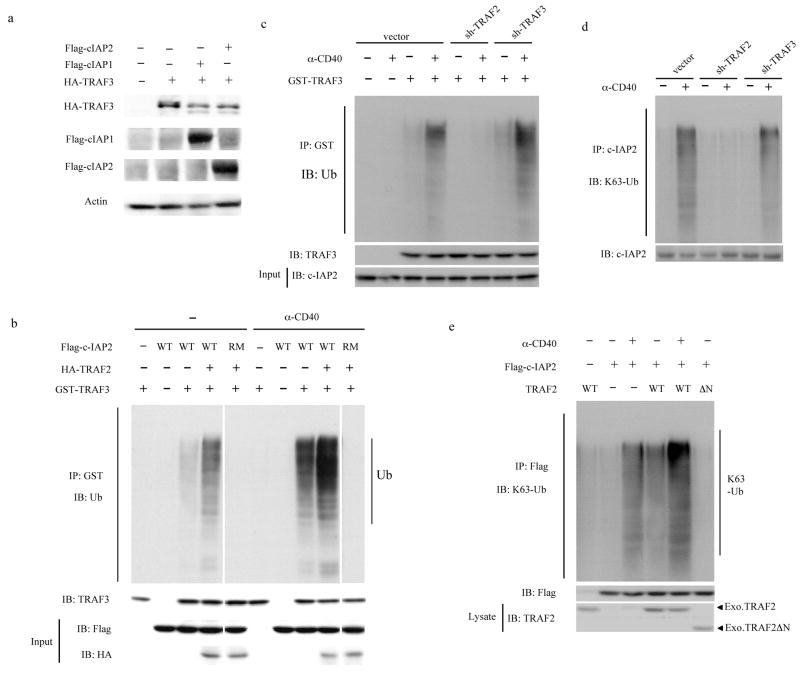
To better understand the mechanism of receptor induced TRAF3 degradation we used HEK293T cells stably expressing CD40 (HEK293T-CD40). Co-expression of TRAF3 with either cIAP1 or cIAP2 in these cells resulted in TRAF3 degradation in a proteasome dependent manner (Fig. 6a and Supplementary Fig. 6 online). We next examined whether CD40 engagement enhances the E3 activity of cIAP2 and whether cIAP2 can directly ubiquitinate TRAF3. Flag-tagged wild-type or RING finger mutated (RM) cIAP2 were transiently expressed in HEK293T-CD40 cells. HA-tagged TRAF2 was also transiently expressed in a separate culture of HEK293T-CD40 cells. After 48 hrs the cells were stimulated or not with anti-CD40 antibody and the cIAP2 and TRAF2 proteins were immunoprecipitated 2 hrs later. When incubated with recombinant GST-TRAF3 in an in vitro ubiquitination system44 that contained ubiquitin, ATP, E1 and a mixture of E2s, wild-type cIAP2 but not cIAP2(RM) led to TRAF3 polyubiquitination that was much more extensive if cIAP2 was isolated from CD40-stimulated cells (Fig. 6b). The TRAF3 polyubiquitinating activity of cIAP2 was further enhanced by the presence of TRAF2 in the reaction mix. However, when TRAF2 was incubated in this reaction together with cIAP2 (RM), no TRAF3 ubiquitination was observed (Fig. 6b) indicating that TRAF3 is not a direct substrate for the E3 ligase activity of TRAF2. Endogenous TRAF3 from CD40-stimulated B-cells was polyubiquitinated and this modification was inhibited by pretreatment of the CD40-stimulated cells with Smac mimic (Supplementary Fig. 7 online). The abundance of polyubiquitinated TRAF3 was augmented by treating the cells with a proteasome inhibitor, suggesting that the polyubiquitin chains were K48-linked. Accordingly, the polyubiquitinated form of TRAF3 found in CD40-stimulated cells did not react with an antibody that specifically recognizes K63-linked ubiquitin chains (Supplementary Fig. 7 and Ref. 23).
Fig. 6. CD40 ligation activates cIAP1-cIAP2 via TRAF2 to induce TRAF3 ubiquitination.
(a) Immunoblot of lysates from CD40-expressing HEK293T cells transfected with Flag-cIAP1, Flag-cIAP2, and HA-TRAF3 expression vectors as indicated. After 30 hrs lysates were prepared and analyzed for the proteins indicated. (b-e) Immunoblot of the products of in vitro or in vivo (d) ubiquitination reactions. (b) CD40-expressing 293T cells were transfected with Flag-cIAP2 (wild-type) or RING finger-mutated cIAP2 (RM). Non-transfected and cIAP2-transfected cells were incubated with or without anti-CD40 for 2 hrs, lysates were prepared, cIAP2 was immunoprecipitated and eluted from the immunocomplexes with a 3x-Flag peptide. Independently, HEK293T-CD40 cells were transfected with HA-tagged TRAF2 followed by incubation with or without anti-CD40 and TRAF2 was immunoprecipitated with anti-HA. GST-TRAF3 and cIAP2 were incubated in the presence or absence of immunoprecipitated TRAF2 in an in vitro ubiquitination reaction containing ubiquitin, ATP, E1 and a mixture of E2 ubiquitin conjugating enzymes. GST-TRAF3 was immunoprecipitated, and analyzed by anti-ubiquitin and anti-TRAF3 antibodies. cIAP2 and TRAF2 amounts were examined by immunoblotting with anti-Flag and anti-HA, respectively. (c) Wild-type, TRAF2- and TRAF3-depleted A20 cells were stimulated or not with anti-CD40 for 1 hr and the endogenous cIAP1 and cIAP2 were immunoprecipitated and incubated with GST-TRAF3 in the in vitro ubiquitination system described above. GST-TRAF3 was immunoprecipitated and its ubiquitination was examined as above. (d) wild-type, TRAF2- and TRAF3-depleted A20 cells were activated as above and cIAP1 and cIAP2 were immunoprecipitated and their ubiquitination was analyzed by immunoblotting with a K63-specific anti-ubiquitibin antibody (HWA4C4). (e) HEK293T-CD40 cells were transfected with Flag-cIAP2, TRAF2 and TRAF2ΔN expression vectors and after 24 hrs were stimulated or not with anti-CD40 for 1 hr. cIAP2 was immunoprecipitated using anti-Flag antibody and its ubiquintination was analyzed with a K63-specific anti-ubiquitin antibody (HWA4C4).
Endogenous cIAP1 and cIAP2 immunoprecipitated from CD40-stimulated wild-type or TRAF3-deficient, but not from TRAF2-deficient, A20 B cells also exhibited enhanced E3 activity towards TRAF3 relative to cIAP1 and cIAP2 from unstimulated cells (Fig. 6c). These results indicate that the E3 activity of cIAP1-cIAP2 towards TRAF3 is strongly stimulated upon CD40 engagement in a TRAF2-dependent manner. Since TRAF2 is also a ubiquitin ligase13 and its RING finger is required for induction of NIK degradation, we examined whether TRAF2 is an E3 for cIAP2. Indeed, endogenous cIAP2 was subjected to K63-linked polyubiquitination in CD40-stimulated wild-type and TRAF3-deficient, but not in TRAF2-deficient, A20 cells (Fig. 6d). In transfected HEK293T cells that express CD40, engagement of CD40 induced the K63-linked polyubiquitination of cIAP2 and this modification was strongly enhanced by co-expression of TRAF2, which also induced the K63-linked polyubiquitination of cIAP2 in non-stimulated cells, an effect not seen upon expression of a RING finger deleted TRAF2 (Fig. 6e). These results strongly suggest that TRAF2 is a K63-specific E3 ligase for cIAP2, whereas cIAP2 is K48-specific E3 ligase for TRAF3 (see model in Supplementary Fig. 8 online and discussion).
Discussion
The results described above indicate that although TRAF2 and TRAF3 are structurally similar RING finger proteins, whose ablation results in constitutive activation of alternative NF-κB signaling, the biochemical roles they play in this pathway are distinct. While TRAF3 acts as an adaptor that links an E3 ligase complex containing TRAF2 and cIAP1-cIAP2 to NIK in non-stimulated cells, TRAF2 is required for receptor-induced activation of cIAP1-cIAP2 that act as K48-specific ubiquitin ligases that induce TRAF3 degradation, which we propose is the first irreversible step committing the cell to activation of alternative NF-κB signaling. Receptor-induced TRAF3 degradation was observed previously and suggested to be responsible to NIK stabilization27, 45 and for MAPK activation23. However, the mechanism of receptor (CD40 or BAFF-R)-induced TRAF3 degradation remained elusive and it was even suggested that alternative NF-κB signaling is triggered by degradation of cIAP1-cIAP2, rather than TRAF3 degradation34. However, in numerous experiments conducted with both normal B-cells and B-cell derived tumors, we reproducibly detected rapid and nearly complete receptor-induced decay of TRAF3, partial TRAF2 degradation and hardly any cIAP1-cIAP2 degradation. We previously found that CD40 engagement results in rapid recruitment of a TRAF2-cIAP1-cIAP2 complex and TRAF3 to the receptor and that cIAP1-cIAP2 are required for receptor-induced TRAF3 degradation23. However, it was not examined in these studies whether receptor engagement activates cIAP1-cIAP2 ubiquitin ligase activity. The new results shown above demonstrate that CD40 occupancy stimulates the E3 activity of cIAP2 towards TRAF3, resulting in degradative K48-linked polyubiquitinaton of TRAF3. CD40-induced cIAP1-cIAP2 activation correlates with their K63-linked polyubiquitination and both effects are dependent on TRAF2. Thus, in addition to being an adaptor that recruits cIAP1-IAP2 and TRAF3 to the receptor, TRAF2 is also an E3 that activates cIAP1-cIAP2 most likely via their K63-linked ubiquitination. TRAF2, however, does not ubiquitinate TRAF3. These results provide the first demonstration of a ubiquitination cascade in which TRAF2 ubiquitinates and activates cIAP1-cIAP2, which then proceed to ubiquitinate TRAF3 (and to some extent TRAF2) and induce its degradation (Supplementary Fig. 8 online).
Unlike TRAF2, whose RING finger is required for cIAP1-cIAP2 activation and for NIK degradation, the RING finger of TRAF3 is dispensable for NIK degradation, supporting the conclusion that TRAF3 functions as an adaptor in this process and not as an E3. Although TRAF3, TRAF2 and cIAP1-cIAP2 are all engaged in degradative NIK polyubiquitination in non-stimulated cells, we suggest that receptor occupancy results in recruitment of a pool of the complexes formed by these molecules to the receptor, where TRAF2 enhances cIAP1-cIAP2 E3 activity by ubiquitination and targets it towards TRAF3 (Supplementary Fig. 8 online). Once receptor associated TRAF3 is degraded, NIK can no longer associate with the remaining cIAP1-cIAP2-TRAF2 complex.
It was shown that the absence of either TRAF2 or TRAF3 enhances the survival of B-cells and their accumulation in the MZ and we have confirmed these results. However, we also found that ablation of either TRAF2 or TRAF3 resulted in a markedly defective development of BM B-cells with impaired transition from CD19− to CD19+ stage. In addition, Traf2−/− and Traf3−/− mice exhibit thymic and splenic atrophy. Given the elevated concentration of circulating cortisol in these mice and the known lymphocydal activity of glucocorticoids37, 38, we presume that some of these defects are due to cortisol-induced killing of BM B-cell progenitors37, 41 and thymocytes as well as atrophy of lymphoid organs. However, the increased survival and accumulation of MZ B-cells are most likely direct consequences of constitutive alternative NF-κB signaling in these mice, as this pathway drives B-cell survival and accumulation in the MZ46. In support of this interpretation, a conditional deletion of TRAF3 using a CD19-driven Cre transgene, which does not result in perinatal mortality and stress, only led to increased B-cell survival and accumulation of autoreactive MZ B-cells39, a phenotype also produced by transgenic overproduction of BAFF, which results in prolonged activation of the alternative pathway46.
The perinatal lethality and stress-induced elevation in blood cortisol associated with TRAF3 ablation are due to uncontrolled activation of the alternative NF-κB pathway as they are reversed by Nfkb2 ablation25. Our results indicate that the perinatal lethality caused by ablation of TRAF2 is also due to uncontrolled activation of this pathway. However, it should be noted that loss of TRAF3 results in a more profound elevation of NF-κB target genes, some of which are likely to account for perinatal lethality and stress, than the absence of TRAF2. Correspondingly, Traf3−/− cells contain more RelA in their nuclei than Traf2−/− cells. It is plausible that activation of RelA, a hallmark of classical NF-κB signaling, is partially due to autocrine production of TNF or a similar cytokine that signals to the classical IKK complex via TRAF2.
Collectively, our results indicate that TRAF2 and TRAF3 play essential and non-redundant roles in the alternative NF-κB pathway. As an adaptor, TRAF3 recruits a TRAF2-cIAP1-cIAP2 E3 complex to NIK leading to its rapid turnover in resting cells. Upon CD40 or BAFF-R activation, TRAF2 ubiquitinates and activates cIAP1-cIAP2 to induce degradative K48-linked polyubiquitination of TRAF3. Degradation of TRAF3 prevents targeting of newly synthesized NIK by the TRAF2-cIAP1-cIAP2 ubiquitin ligase complex, allowing NIK accumulation and activation through autophosphorylation (Supplementary Fig. 8). This requirement for ongoing NIK synthesis explains the long delay in activation of alternative NF-κB signaling and its dependence on new protein synthesis.
Methods
Mice
Traf2+/− mice were obtained from Tak W. Mak (University of Toronto); Traf3+/− mice were from Hitoshi Kikutani (Osaka University, Japan) and Map3k14+/− (NIK-deficient) mice generated by Robert Schrieber (Washington University, St Louis) were obtained from Amgen Inc, and were intercrossed to generate the different strains used in the experiments described above. Mice were housed under conventional barrier protection according to UCSD and NIH guidelines, and mouse protocols were approved by the UCSD Institutional Animal Care Committee.
Splenic B-cell isolation and culture
Splenic single cell suspensions were prepared as described47. CD43− B-cells were isolated after removal of CD43+ cells with magnetic beads (MACS; Miltenyi) according to manufacturer’s protocol. B-cells or B-cell lines were stimulated with anti-CD40 (5 μg/ml; clone 3/23; Pharmingen), CD40L (500 ng/ml; PeproTech) or BAFF (200 ng/ml; PeproTech).
Flow Cytometry
Single cell thymus and spleen suspensions47, were incubated with the indicated antibodies and analyzed on a FACScalibur flow cytometer using CellQuest software (Beckton Dickinson, San Jose, CA). FITC-, PE- or biotin-labeled antibodies to: CD4, CD8, CD19, CD21, CD23, and CD45/B220 were from Pharmingen. Biotinylated antibodies were detected by Streptavidin-PE (PharMingen).
Immunochemical procedures
MEFs, B-cells and B-cell lines were lysed in RIPA buffer (10mM Tris-HCl pH7.4, 150mM NaCl, 1:NP40, 1% deoxycholate (w/v), 1mM PMSF) to prepare total lysates. Nuclear and cytoplasmic extracts were prepared as described47. Immunoprecipitations were performed by incubating lysates with the indicated antibodies overnight at 4°C. Samples were precleared by incubation with Sepharose-6B beads (Sigma-Aldrich) for 1 hr at 4°C, and immunoprecipitated with protein-G or protein-A-Sepharose (Amersham) for 1 hr at 4°C, after which the beads were washed extensively and the proteins were eluted. The samples were separated by SDS-PAGE and analyzed by immunoblotting with antibodies to: NIK (Cell Signaling and Sc-7211), IKKα (Imagenex), IKKβ (Upstate), IKKγ (Pharmingen), TRAF2 (Sc-876), TRAF3 (Sc-949), RelA (Sc-372), RelB (Sc-226), c-Rel (Sc-70), NF-κB1 (Sc-7178), LDH (Sc-27238), NF-κB2 (Upstate), cIAP1 (Sc-7943), Ub (Sc-8017) and cIAP2 (R&D). Preparation of the antibody (HWA4C4) specific for K63-linked polyubiquitin will be described elsewhere (H.W., A.M., S.A. Brown, J.R. Zhou, K. Forbes, P.H.T., H. Haecker, M.K. and D.A.A.V., in preparation). Blots were developed with ECL reagent (Amersham Pharmacia Biotech).
Short hairpin RNA (shRNA), lentiviruses and transduction
A 5′AGAGTCAGGTTCCGATGAT3′ oligonucleotide directed against h-TRAF3 was cloned into pLSLPw lentiviral vector, kindly provided by Peter M. Chumakov, Cleveland Clinic48 and lentivirus was prepared and packaged as described49. Cells were transduced with lentivirus in the presence of 5 mg/ml polybrene (Sigma). After 24 hrs, the virus-containing medium was replaced with selection medium containing 5 mg/ml puromycin (EMD). After cell growth was stable, the cells were used in the described experiments.
In vitro ubiquitination assay
In vitro ubiquitination was performed as described44. HEK-293T cells stably expressing CD40 were transfected with pcDNA-HA-TRAF2, pcDNA-Flag-cIAP2 WT and RM (kindly provided by Zeev Ronai and John Reed, respectively, Burnham Institute). After simulation with anti-CD40 the cells were lysed and immunoprecipitated with anti-Flag or anti-HA antibodies. Flag-cIAP2 was eluted from the protein-G beads using 3x-Flag peptide (Sigma) and incubated with GST-TRAF3 produced in E. coli in the absence or presence of HA-TRAF2 in a reaction mixture including ubiquitin, E1 and a mixture of E2 ubiquitin conjugating enzymes: UbcH2, UbcH3, Ubc5a, Ubc5b, Ubc5c, UbcH6, UbcH7, and UbcH10. Reactions were carried out at 30 °C for 30 min in 50 mM HEPES pH7.8 containing 4 mM ATP and 10 mM MgCl2. GST-TRAF3 was immunoprecipitated, gel-separated, and analyzed by immunoblotting with anti-ubiquitin and anti-TRAF3 antibodies. In other experiments we used cIAP1 and cIAP2 isolated from non-stimulated and stimulated A20 B cells. All subsequent incubations and procedures were as described above.
Supplementary Material
Acknowledgments
We thank Drs. Tak Mak and Dr. Hitoshi Kikutani and Amgen Inc for the Traf2− and Traf3− and Map3k14− mouse strains used in this study. We gratefully acknowledge Dr. Zeev Ronai and Dr. John Reed for providing us with some of the expression vectors used in this study and Santa Cruz Biotechnology for the donated antibodies. Research was supported by NIH grants to MK. AM and P.H.T were supported in part by The Kanzawa Medical Research Foundation and the American Lung Association, respectively. DAAV and HW were supported by the NIH, a Cancer Center Core grant and the American Lebanese Syrian Associated Charities. MK is an American Cancer Society Professor.
References
- 1.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 (Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Neriah Y. Regulatory functions of ubiquitination in the immune system. Nat Immunol. 2002;3:20–26. doi: 10.1038/ni0102-20. [DOI] [PubMed] [Google Scholar]
- 4.Senftleben U, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 5.Fong A, Sun SC. Genetic evidence for the essential role of beta-transducin repeat-containing protein in the inducible processing of NF-kappa B2/p100. J Biol Chem. 2002;277:22111–22114. doi: 10.1074/jbc.C200151200. [DOI] [PubMed] [Google Scholar]
- 6.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 7.Derudder E, et al. RelB/p50 dimers are differentially regulated by tumor necrosis factor-alpha and lymphotoxin-beta receptor activation: critical roles for p100. J Biol Chem. 2003;278:23278–23284. doi: 10.1074/jbc.M300106200. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz ZB, Weih DS, Sivakumar V, Weih F. RelB is required for Peyer’s patch development: differential regulation of p52-RelB by lymphotoxin and TNF. Embo J. 2003;22:121–130. doi: 10.1093/emboj/cdg004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coope HJ, et al. CD40 regulates the processing of NF-kappaB2 p100 to p52. Embo J. 2002;21:5375–5385. doi: 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 11.Bishop GA. The multifaceted roles of TRAFs in the regulation of B-cell function. Nat Rev Immunol. 2004;4:775–786. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 12.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;re13 doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 13.Chen ZJ, Bhoj V, Seth RB. Ubiquitin, TAK1 and IKK: is there a connection? Cell Death Differ. 2006;13:687–692. doi: 10.1038/sj.cdd.4401869. [DOI] [PubMed] [Google Scholar]
- 14.Chung JY, Park YC, Ye H, Wu H. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci. 2002;115:679–688. doi: 10.1242/jcs.115.4.679. [DOI] [PubMed] [Google Scholar]
- 15.Shi CS, Kehrl JH. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2) J Biol Chem. 2003;278:15429–15434. doi: 10.1074/jbc.M211796200. [DOI] [PubMed] [Google Scholar]
- 16.Deng L, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 17.Tada K, et al. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death. J Biol Chem. 2001;276:36530–36534. doi: 10.1074/jbc.M104837200. [DOI] [PubMed] [Google Scholar]
- 18.Ishida T, et al. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem. 1996;271:28745–28748. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 19.Lomaga MA, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hacker H, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 21.Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 22.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 23.Matsuzawa A, et al. Essential Cytoplasmic Translocation of a Cytokine Receptor-Assembled Signaling Complex. Science. 2008 doi: 10.1126/science.1157340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grech AP, et al. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004;21:629–642. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 25.He JQ, et al. Rescue of TRAF3-null mice by p100 NF-kappa B deficiency. J Exp Med. 2006;203:2413–2418. doi: 10.1084/jem.20061166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 28.Vince JE, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Keats JJ, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annunziata CM, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh WC, et al. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Cheng G, Baltimore D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 1996;5:407–415. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- 33.Vaux DL, Silke J. IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol. 2005;6:287–297. doi: 10.1038/nrm1621. [DOI] [PubMed] [Google Scholar]
- 34.Varfolomeev E, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Hu S, Yang X. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J Biol Chem. 2003;278:10055–10060. doi: 10.1074/jbc.M207197200. [DOI] [PubMed] [Google Scholar]
- 36.Li L, et al. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 37.Cohen JJ. Glucocorticoid-induced apoptosis in the thymus. Semin Immunol. 1992;4:363–369. [PubMed] [Google Scholar]
- 38.Dinarello CA, Mier JW. Lymphokines. N Engl J Med. 1987;317:940–945. doi: 10.1056/NEJM198710083171506. [DOI] [PubMed] [Google Scholar]
- 39.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nutt SL, Urbanek P, Rolink A, Busslinger M. Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 1997;11:476–491. doi: 10.1101/gad.11.4.476. [DOI] [PubMed] [Google Scholar]
- 41.Igarashi H, et al. Early lymphoid progenitors in mouse and man are highly sensitive to glucocorticoids. Int Immunol. 2005;17:501–511. doi: 10.1093/intimm/dxh230. [DOI] [PubMed] [Google Scholar]
- 42.He JQ, Saha SK, Kang JR, Zarnegar B, Cheng G. Specificity of TRAF3 in its negative regulation of the noncanonical NF-kappa B pathway. J Biol Chem. 2007;282:3688–3694. doi: 10.1074/jbc.M610271200. [DOI] [PubMed] [Google Scholar]
- 43.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 44.Gallagher E, et al. Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation and antibody production. Nat Immunol. 2007;8:57–63. doi: 10.1038/ni1421. [DOI] [PubMed] [Google Scholar]
- 45.Brown KD, Hostager BS, Bishop GA. Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein-Barr virus oncoprotein latent membrane protein 1 (LMP1) J Exp Med. 2001;193:943–954. doi: 10.1084/jem.193.8.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enzler T, et al. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25:403–415. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. J Exp Med. 2003;197:1613–1621. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 49.Gurova KV, Hill JE, Razorenova OV, Chumakov PM, Gudkov AV. p53 pathway in renal cell carcinoma is repressed by a dominant mechanism. Cancer Res. 2004;64:1951–1958. doi: 10.1158/0008-5472.can-03-1541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.