Abstract
Anosognosia for motor impairment has been linked to lesions of the right hemisphere. However, left hemisphere damaged patients have often been excluded from investigation because of their associated language deficits. In this study we assessed anosognosia for motor disorders in a group of left hemisphere damaged patients using 2 tools that assess the presence of unawareness—a structured interview that is a common method of assessment of anosognosia in clinical settings, and a new tool, the Visual-Analogue Test for Anosognosia for Motor Impairment (VATAm; Della Sala, Cocchini, Beschin, & Cameron, in press). The structured interview relies heavily on language and enquires about general motor ability whereas the VATAm is less dependent on language abilities and enquires about specific motor tasks. Results suggest that the frequency of anosognosia in left brain damaged patients may have been underestimated due to methodological reasons, and that anosognosia for motor impairment can also be associated with lesions of the left hemisphere.
Keywords: anosognosia, unawareness, motor deficit, left hemisphere, aphasia
Anosognosia for motor impairment is considered to be a rare occurrence following left hemisphere damage (Adair, Schwartz, & Barrett, 2003; Cutting, 1978; Nathanson, Bergman, & Gordon, 1952; Stone, Halligan, & Greenwood, 1993; see Vuilleumier, 2004, for a review). Its frequency in the acute phase has been estimated as less than 4% (Baier & Karnath, 2005). In a study using injection of barbiturate (Wada test), Gilmore, Heilman, Schmidt, Fennell, and Quisling (1992) found that none of the eight participants showed unawareness following suppression of left hemisphere activity, reinforcing the idea that the left hemisphere plays little role in awareness for motor disorders. As a result, unawareness for right motor impairment has received very little attention. This has strong implications for theories of anosognosia, which have to account for this hemispheric asymmetry. For example, the motivational approach (Weinstein, 1991; Weinstein & Kahn, 1955) suggests that anosognosia for motor impairment reflects a psychological reaction to an unbearable reality. However, this account has been dismissed, mainly on the basis that it fails to explain why right, as opposed to left, hemiplegia rarely triggers the psychological mechanism of denial (e.g., Bisiach & Geminiani, 1991; Heilman, 2007). Therefore, the few attempts to reinterpret this psychodynamic approach of anosognosia have assumed deficits of specific right-sided monitoring (Venneri & Shanks, 2003) or emotion-regulation systems (Turnbull & Solms, 2007a, 2007b).
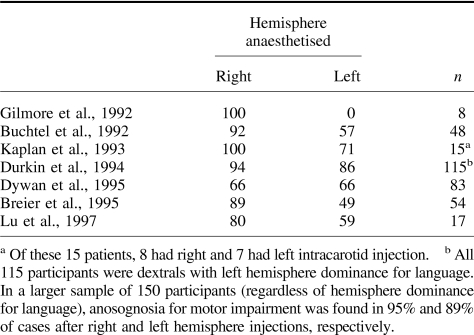
Studies using the Wada test that were carried out with a large group of volunteers (e.g., Dywan, McGlone, & Fox, 1995) showed a different pattern of results (see Table 1). Despite a trend toward a higher frequency of anosognosia following suppression of right hemisphere activity, presence of unawareness following suppression of left hemisphere activity ranged from 49% (Breier et al., 1995) to 86% (Durkin, Meador, Nichols, Lee, & Loring, 1994).
Table 1. Percentage of Volunteers Who Show Evidence of Unawareness Following Suppression of Activity of Left and Right Hemispheres (Wada Test).
Why, then, is unawareness among left brain damaged patients so rarely reported? A possible reason is a methodological bias. The typical methods used to assess anosognosia for motor impairment are structured interviews (Bisiach, Vallar, Perani, Papagno, & Berti, 1986; Cutting, 1978; Nathanson et al., 1952; Stone et al., 1993; see also Jehkonen, Laihosalo, & Kettunen, 2006, Tables 1 and 4), sometimes in conjunction with verbal estimates of motor ability (Berti, Làdavas, & Della Corte, 1996; Marcel, Tegnér, & Nimmo-Smith, 2004). These types of assessment rely heavily on the patient's language skills, resulting in the exclusion of left hemisphere damaged patients who may present with severe language deficits. The rate of exclusion of aphasics has been relatively high, ranging from 38% in Nathanson et al.'s (1952) study, to 48% in Stone et al.'s (1993) study and to 60% in Cutting's (1978) study. Unfortunately, no information is available about the exclusion rate of left hemisphere damaged patients in other studies (Baier & Karnath, 2005; Berti et al., 1996; Marcel et al., 2004). Data related to exclusion rates are particularly interesting considering that “there is no way of knowing whether the patients with global aphasia denied their deficits or not” (Nathanson et al., 1952, p. 383), and “that right hemiplegics at risk for developing anosognosia were the very patients in whom aphasia precluded its determination” (Cutting, 1978, p. 548). Hence, as recently suggested by Jehkonen et al. (2006) in their meta-analysis on anosognosia, there is a need to develop methods of assessment that rely less on verbal forms of communication.
In addition, some methods appear to be more sensitive in detecting lack of awareness. Frequency of anosognosia for motor impairment has been found much higher when, rather than enquiring about a general condition, patients were asked to estimate their ability in performing specific (bimanual) tasks (Marcel et al., 2004). However, the rating method also relies on patients' language ability.
It is therefore possible that by using a highly sensitive method to assess unawareness, and by finding an appropriate means of including aphasic patients, it should be possible to more effectively assess the presence of anosognosia for right motor disorders.
In this paper, we evaluated the presence of unawareness in a group of left hemisphere damaged patients using two different methods. The first is a classic structured interview that is often used both for research purposes and in the clinical setting (Berti et al., 1996). It enquires about general conditions and requires a relatively high degree of language skills. The other is a newly devised Visual-Analogue Test for Anosognosia for Motor Impairment (VATAm; Della Sala, Cocchini, Beschin, & Cameron, in press; see also http://homepages.gold.ac.uk/gcocchini/; to view and download the test). The VATAm assesses patients' awareness of their ability to perform specific motor tasks. It includes nonverbal stimuli to facilitate comprehension, a rating scale to encourage nonverbal responses, and control items to establish the reliability of patients' responses.
This study is not aimed at providing epidemiological data, instead it aims to investigate whether anosognosia for motor disorders following left brain damage could have been underestimated in the past.
Method
Participants
Forty-two left hemisphere damaged (LHD) patients were considered in the study according to the following inclusion criteria: (a) younger than 90 years of age; (b) no known psychiatric problems prior to brain damage; (c) evidence of recent vascular unilateral lesion on CT scan; (d) presence of motor impairment as detected by the Standard Neurological Examination for upper and lower limbs (Bisiach et al., 1986). Scores for each limb ranged from 0 (normal motor performance) to 3 (complete plegia). Scores of 1 and 2 were given for mild and moderate motor impairment, respectively. Poor performance on this test due to apraxia, tremor, or ataxia was not considered as evidence of motor impairment.
Nine patients (i.e., 21%) were excluded from the study because their severe language difficulties precluded comprehension of the test. Therefore 33 patients (17 women and 16 men) entered the study. Their mean age was 69.7 (SD = 11.8; range = 42 to 87) with an average formal education of 9.1 year (SD = 4.6; range = 2 to 19). The average motor impairment score was 2.2 (SD = 0.9; range = 0 to 3) and 2.1 (SD = 0.8; range = 1 to 3) for upper and lower limb, respectively. A subgroup of 14 patients showed complete plegia (i.e., score = 3) either in both limbs (9 patients) or for upper limb only (5 patients—whose lower limb score = 2). On average, patients were recruited for this study 73.8 days (SD = 46.0; range = 7 to 210) after brain damage. Seven patients were recruited during the acute phase (i.e., between 7 and 30 days postonset), 7 patients were recruited during the subacute phase (i.e., 31 to 60 days), and 19 patients were recruited during the chronic phase (i.e., 61 to 210 days).
For each patient, at least one caregiver was asked to participate in the study and provided information about the patient's motor disorders. All participants gave informed consent prior to their participation in the study. Examiners were informed that a new diagnostic tool to assess anosognosia was being piloted, but they were blind to the actual experimental hypotheses.
Anosognosia Assessment
Structured Interview
Patients were asked about their current condition, and about their motor abilities for upper limb. Questions were taken from Berti et al. (1996). In the first set of questions patients were asked: “Where are we? Why are you in hospital? How is your right arm? Can you move it?” If the patient answered “no” to the last question, then she/he was asked “Why can't you move your right arm?” If the patient denied the motor impairment, she/he was asked to “Please, touch my hand with your right hand.” The patient was then asked “Have you done it?” If the patient answered “no,” then she/he was asked “Why haven't you done it?” If the patient answered “I did” (i.e., she/he can move it), then she/he was asked “Are you sure? It is very strange because I have not seen your hand touching my hand.” The total score indicating unawareness for upper limb motor impairment ranged from 0 to 2. A score of 0 was given if the patient acknowledged his or her motor impairment (aware); a score of 1 was given if the patient did not acknowledge his or her motor impairment but recognized that she/he had not reached the examiner's hand (mild anosognosia); a score of 2 was given if the patient denied motor impairment even after she/he failed to reach the examiner's hand (severe anosognosia).
Patients were also asked about the motor impairment of their affected lower limb. First they were asked, “How is your right leg? Can you move it?,” and then “Can you walk without any difficulty?” Unawareness for lower limb motor impairment was indicated by the final score (range 0 to 2). A score of 0 was given if the patient either spontaneously reported his or her motor impairment when first asked about the reasons for being in the hospital (see above), or acknowledged the motor impairment when specifically questioned (aware); a score of 1 was given if the patient answered “fine” to the first question, but acknowledged the impossibility of walking (mild anosognosia); a score of 2 was given when the patient claimed to be able to walk (severe anosognosia).
Examiners were instructed to repeat the questions if necessary. The entire interview was administered despite possible associated communication difficulties. Patients' responses were considered unreliable when responses could not be clearly classified due to communication difficulties or when no reply could be obtained. These interviews were excluded from the study.
VATAm
Patients were asked to rate their motor abilities on simple tasks (e.g., climbing stairs) using a questionnaire that comprised drawings to illustrate each question. Della Sala et al. (in press) reported the norms for this test and observed that, in accordance with the literature (e.g., Cutting, 1978; Stone et al., 1993), 42% of right hemisphere damaged (RHD) patients showed some form of anosognosia for their motor impairment. This demonstrates that the VATAm is no more likely to elicit false positives than other available anosognosia assessments, including structured interviews.
The VATAm was piloted with people with aphasia, who helped to refine the drawings in terms of content and clarity. Often, minimal verbal stimuli consisting of the action alone, rather than a whole sentence, were presented to simplify and minimize the language structure. The examiner emphasized that the questions were about “you” (the patient) and about “now” (not related to premorbid condition) using gesture and pointing if necessary to make this clear. The practice item allowed further explanation, and repetition if required.
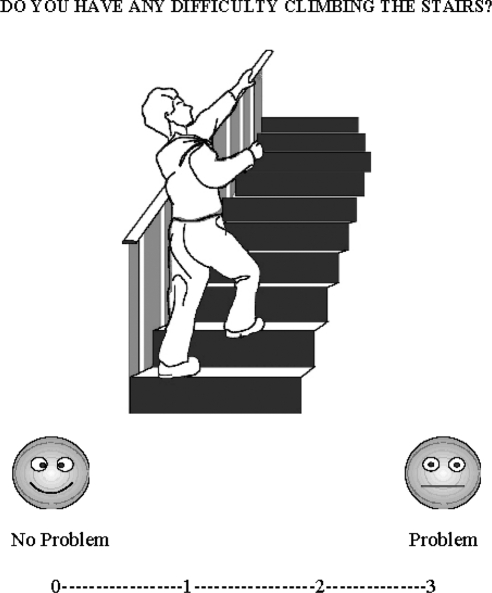
The test comprised 12 questions about the patient's ability to perform tasks that require the use of both hands (8 questions) or both feet (4 questions; “bilateral tasks”; e.g., walking; see the Appendix). The patients were asked to rate their ability using a 4-point visual-analogue scale. A rating of 0 indicated (no problem in carrying out the task) and a rating of 3 indicated (major difficulty or impossibility in carrying out the task). Ratings between 0 and 3 were displayed along a continuum. At the extremities of the continuum, there were written labels “no problem” and “problem” together with a smiling or nonsmiling face (see Figure 1). The format of the visual-analogue scale (i.e., 0 on the left and 3 on the right) was used for the entire test to avoid confusion across the trials. Patients rated their performance using a verbal response (e.g., rating “3” or “Problem”) or nonverbal response (i.e., pointing).
Figure 1. Example of a VATAm question for bipedal motor task.
Four additional “check questions,” which elicit obvious answers at either end of the continuum (fairly easy like “Do you have any difficulty drinking from a glass?”; or more difficult like “Do you have any difficulty juggling five balls in the air?”) were used to assess comprehension of the questions, reliability of responses, and to monitor any perseveration (see the Appendix). Ratings for the check questions were not included in the total scoring of anosognosia. However, participants who did not provide the expected answer (i.e., a rating of 0 or 1 for “easy” check questions and a rating of 3 or 2 for “difficult” check questions, respectively) to all check questions were excluded from further analyses. The written questions, drawings, and rating scale were placed on the ipsilesional side to avoid biases due to possible neglect and to facilitate motor responses with the ipsilesional limb. The examiner always ensured that the patients attended to the visual stimuli by pointing to them while asking each question.1
For each patient, one or two caregivers (with a personal or a professional relationship to the patient) were asked to rate the patient's motor capabilities using the same scale.
VATAm score
The total rating of the VATAm (i.e., patient's self-rating and caregiver's rating of the patient) ranged from 0 to 36. Each patient's self-evaluation was then subtracted from that of their caregiver. Where more than one caregiver rated a patient's motor skills, the patient's final self-evaluation was compared with the mean of the caregivers' ratings. A previous study reporting norms for the VATAm test (based on the discrepancy between 36 pairs of caregivers rating the motor performance of the same patients; Della Sala et al., in press) suggested that a patient/caregiver discrepancy equal to or higher than 6.3 should be considered as an indicator of unawareness. A discrepancy between 6.3 and 12.0 (i.e., average discrepancy for each question was no more than 1 rating point) was deemed as evidence of “mild anosognosia”; a value between 12.1 and 24.0 (i.e., average discrepancy for each question between 1 and 2 rating points) indicated “moderate anosognosia” and a value between than 24.1 and 36 (i.e., average discrepancy for each question between 2 and 3 rating points) indicated “severe anosognosia.”
As in the structured interview, examiners were instructed to repeat questions if necessary. Patients' responses were considered unreliable when no response was given or when responses could not be clearly classified due to communication difficulties. These questionnaires were excluded from the study.
General Cognitive Assessment
Anosognosia for left hemplegia has occasionally been associated with personal neglect (Bisiach et al., 1986) so patients were asked to perform the One Item Test (Bisiach et al., 1986). In this test, patients are asked to reach their contralesional hand using their ipsilesional one. Scores ranged from 0 (i.e., the patient promptly reach for the target) to 1 (i.e., the target is reached with hesitation and search), to 2 (i.e., the search is interrupted before the target is reached), to 3 (i.e., no movement toward the target is performed).
Anosognosia has also been associated with general impairment in abstract thinking (Levine, Calvanio, & Rin, 1991), therefore patients were asked to perform the vertical version of the Raven's Colored Matrices (Gainotti, D'Erme, Villa, & Caltagirone, 1986). The scores range from 36 (i.e., all responses correct) to 0 (i.e., all responses incorrect). Individual scores are adjusted by age and education. A final score below 18 indicates an impairment of abstract reasoning abilities (Basso, Capitani, & Laiacona, 1987).
Results
Structured Interview
In addition to the initial 9 patients who could not be assessed (see above), a further 13 patients could not be tested using the structured interview due to communication difficulties. Therefore, a total of 22 out of 42 patients (52.4%) could not be assessed using the structured interview. Presence and severity of anosognosia was evaluated for the remaining 20 patients. Of these, 2 patients (10%) showed anosognosia. They were assessed 50 and 70 days, respectively after brain damage. One showed severe anosognosia (i.e., score = 2) for the upper limb and mild anosognosia (i.e., score = 1) for the lower limb, whereas the other patient showed mild anosognosia for both limbs. Both patients were part of the subgroup of 14 patients (representing the 14.3% of the subgroup) with complete paresis of at least one limb.
VATAm
In addition to the 9 patients who were initially excluded because they were not able to be assessed, 3 further patients were excluded because their data were not reliable (i.e., they responded incorrectly to at least one check question). Therefore, 12 of the 42 patients (28.6%) could not be assessed using the VATAm, and the responses from 30 patients were considered in the final analyses. Twelve of these (i.e., 40%) showed evidence of unawareness. Two of them showed mild anosognosia, 7 moderate anosognosia, and 3 severe anosognosia. Motor impairment for patients showing mild, moderate, and severe anosognosia was 1.5 (SD = .5, range 1 to 2), 2.3 (SD = .7, range = 1 to 3), and 3 (SD = 0), respectively for upper limb; and 1.5 (SD = .5, range = 1 to 2), 1.9 (SD = .8, range = 1 to 3), and 2.7 (SD = .5, range = 2 to 3), respectively for lower limb. The average time postonset of these 12 patients was 104 days (SD = 50.37, range = 50 to 120). Two patients (1 with severe and 1 with moderate anosognosia) were in the subacute phase, and the other 10 (2 with severe, 6 with moderate, and 2 with mild anosognosia) were in the chronic phase.
Six of the 14 patients with complete paresis of at least one limb (i.e., 43%) showed evidence of moderate (3 patients) or severe (3 patients) anosognosia.
Comparison Between the Structured Interview and the VATAm
Only 10% of LHD patients showed anosognosia using the structured interview assessment, but up to 40% showed evidence of anosognosia using the VATAm. This difference is significant, χ2(1, N = 50) = 5.36, p < .05.
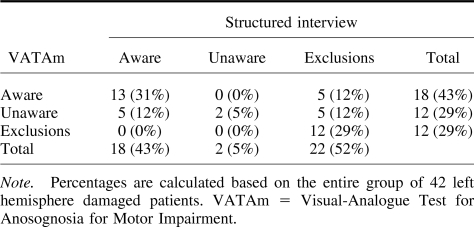
Table 2 shows the percentages of aware, unaware, and excluded patients according to the different methods of assessment. One-third of the patients (31%) were classed as aware of their motor disorders using both tests. Two patients (5%) were diagnosed as anosognosic according to both tests (severe using the VATAm; mild using the structured interview). Crucially there was evidence of anosognosia in 5 patients (12%) using the VATm alone, and in 1 of these the level was severe. On the contrary, no patients were classed as anosognosic using the structured interview alone. Finally, of the 22 patients who were excluded from the structured interview, 5 (23%) showed evidence of anosognosia (moderate in 4 patients and mild in 1) using the VATAm, but none of the patients who were excluded from the VATAm could be assessed using the structured interview.
Table 2. Number of Patients Who Were Excluded From the Study or Classified as Aware/Unaware Using the VATAm and the Structured Interview.
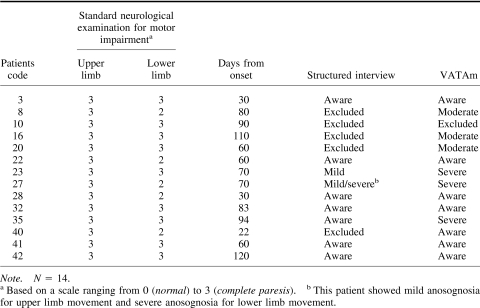
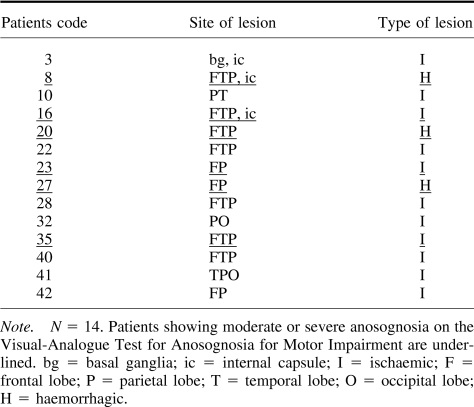
Of the 14 patients with complete paresis, 5 could not be assessed using the structured interview mainly due to their language deficits, whereas 4 of them could be assessed using the VATAm. In addition, only 2 of the 14 patients were considered to have anosognosia when the structured interview was used (see Table 3), whereas a further 4 were identified using the VATAm. These 4 patients were either excluded (3 patients) or considered aware (1 patient) when the structured interview was used.
Table 3. Structured Interview and VATAm Evaluation of Awareness in Patients With Complete Paresis.
VATAm Test–Retest Reliability
Thirty caregivers and 21 patients were retested (between 24 hr and 3 days later). Separated Pearson correlation analyses were carried out for caregivers and patients. Both groups showed very high correlation coefficients, that is, r = .961 (p < .001) and r = .932 (p < .001), respectively.
Anosognosia and Brain Lesion
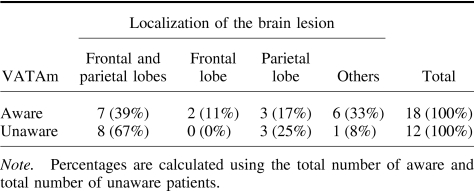
Neuroradiological scanning showed that 22 patients had ischemic lesions, 5 had hemorrhagic lesions, and 3 had both ischemic and hemorrhagic lesions. A recent literature review by Pia, Neppi-Modona, Ricci, and Berti (2004) reported an increased incidence of anosognosia when the lesion involved both parietal and frontal lobes. Our study shows that 67% of the patients who presented with anosognosia had lesions involving either the frontal or parietal lobe or both, whereas only 39% of the patients who were fully aware of their motor impairment showed the same lesion pattern (see Table 4). However, the association between lesions of the frontal and parietal lobes and the presence of anosognosia was not significant, χ2(1, N = 30) = 1.3, ns.). Subcortical lesions (involving basal ganglia, internal capsule, or the thalamus) were evident in 5 patients, and 2 of them showed anosognosia. Karnath, Baier, and Nagele (2005) claimed that the insular cortex may play a crucial role in awareness; however the only patient (case n. 2) with a lesion of this area alone did not show anosognosia. Vallar and Ronchi (2006) suggested that this type of discrepancy might be due to the inclusion of patients with mild forms of motor impairment. We have therefore considered the 14 patients with complete paresis separately (see Table 5). In accordance with Pia et al.'s conclusions, all 6 anosognosic (moderate and severe) patients within this subgroup had lesions in the fronto-parietal region, however, the association between these sites of lesion and the presence of anosognosia was not significant, χ2(1, N = 14) = 2.22, p = .136, ns.
Table 4. Number of Aware and Unaware Patients With Brain Lesions in Specific Areas.
Table 5. Site and Type of Brain Lesions of Patients With Complete Paresis.
Anosognosia and Neglect
One patient who showed anosognosia using the VATAm and 3 other patients who were fully aware of their motor impairments were not tested for personal neglect. Eight anosognosic patients and 12 patients who were aware of their motor impairment did not show any signs of personal neglect (score = 0). Two in each group obtained a score of 1, one anosognosic patient obtained a score of 2, and only one patient who was aware of his motor impairment showed evidence of personal neglect (i.e., score = 3). These data confirm that anosognosia and personal neglect double dissociate (e.g., Bisiach et al., 1986; Cutting, 1978; ), and suggest that anosognosia could not be accounted for solely by concomitant personal neglect.
Anosognosia and Nonverbal Abstract Thinking
One patient did not complete the Colored Matrices. The average score for anosognosic patients was 21.17 out of 36 (SD = 6.99; range = 7 to 32), whereas the patients who were aware of their motor impairment achieved an average score of 21.18 (SD = 6.99; range = 8 to 30). A t test analysis confirmed no significant difference, t(1) = .004, p = .997, ns. Four patients obtained a pathological score, 1 of whom had anosognosia.
Discussion
Previous studies suggested that anosognosia following LHD is uncommon (Baier & Karnath, 2005; Cutting, 1978; Marcel et al., 2004; Nathanson et al., 1952; Stone et al., 1993), but the relatively high rate of exclusion of LHD patients in previous studies and the method of assessment may have led to the underestimation of the frequency of unawareness for motor impairment among LHD patients.
In accordance with the literature, a relatively high percentage of LHD patients (52.4%) were excluded from the current study when anosognosia was assessed using the structured interview. In contrast, the exclusion rate decreased considerably (28.6%) when the VATAm was used. Because the examiners were not aware of the specific experimental question of the study, possible examiner bias cannot fully account for the relatively high number of patients who were excluded using the structured interview. The VATAm enabled nearly half of the aphasic patients who were excluded using the structured interview to be reliably assessed. The reasons for the relatively high number of aphasics who were able to complete the VATAm may be partly due to the nonverbal support provided by the drawings, which facilitated comprehension, but also to the response method, which allowed nonverbal responses (i.e., pointing).
Anosognosia was found in a significantly higher percentage (40%) of LHD patients when the VATAm was used in comparison with the structured interview. In addition, when patients with complete paresis were considered separately, the VATAm allowed us to identify three times the number of patients with severe and moderate degrees of anosognosia than the structured interview. More interesting, 23% of patients, who could not be tested with the structured interview, showed moderate or mild signs of anosognosia using the VATAm.
This result may reflect a higher rate of false positives (i.e., patients incorrectly considered as unaware); or it may represent a higher level of sensitivity of the VATAm in comparison with the structured interview. There is some evidence to support the hypothesis of increased sensitivity. First, in Della Sala et al.'s study (in press) 41% of RHD patients showed some degree of anosognosia when assessed using the VATAm. This falls within the expected range of unawareness found in several other studies using different methods of assessment (for a recent review, see Orfei et al., 2007), and suggests that the VATAm does not overestimate unawareness in the RBD group. Second, a diagnosis of anosognosia using the VATAm was not just based on the arbitrary examiner/caregiver's judgment of lack of awareness. A clear cut-off point has been identified to determine when the rating discrepancy between caregiver and patient should be considered pathological (norms in Della Sala et al., in press), and does not simply reflect different individuals' opinions. Third, the cut-off point and the scoring system of the VATAm appear to be more rigorous than those of the structured interview. In fact, minimal discrepancies (i.e., below the cut-off) between the patient's and the caregiver's ratings are acceptable and not interpreted as pathological. Only two patients showed a mild form of anosognosia using the VATAm; therefore, even if a very conservative criterion is adopted (see Baier & Karnath, 2005, for a discussion on possible biases in assessing the presence of mild forms of anosognosia), over 33% of the LHD patients in the present study showed moderate or severe anosognosia.
If aphasia is considered as the major reason for the different sensitivity levels of the two tests, this could be addressed more directly by devising, if possible, a nonverbal version of the structured interview. From the data that we collected, it seems that the different sensitivity levels of the two methods used to assess anosognosia, cannot be entirely explained by the nonverbal support provided by the VATAm. Indeed some severely anosognosic patients (as indicated by the VATAm) could be assessed using the structured interview. A possible reason for the higher sensitivity of the VATAm might be due to the fact that the structured interview enquires about general conditions (e.g., How is your arm?”), whereas the VATAm focuses on specific motor activities. This is supported by Marcel et al.'s (2004) findings that showed that the frequency of anosognosia for motor impairment following RHD was higher when patients were asked to estimate their ability to perform specific (bimanual and bipedal) tasks similar to those in the VATAm rather than when they were asked about their general condition. This could be particularly important for subacute and chronic patients (most of our LHD group) who are inevitably exposed over time to numerous comments about their condition. Patients may respond correctly to general questions about their deficits, yet may still be unaware of their condition when asked to evaluate their motor abilities in less common situations (see, e.g., Patient 35 reported in Table 3 for an illustration). Therefore, the higher sensitivity of the VATAm in comparison with that of the structured interview may be due to both decreased reliance on verbal communication and to the specific methods used to investigate abilities/deficits.
The current data suggest that anosognosia for motor deficits following LHD is a phenomenon that occurs more frequently than has been previously reported. These findings are in accordance with those studies that showed an association between inactivation of the left hemisphere and anosognosia (e.g., Dywan et al., 1995), and with recent neuroimaging studies that suggest that the left hemisphere is definitively involved in tasks requiring self-evaluation (Goldberg, Harel, & Malach, 2006; Lieberman, Jarcho, & Satpute, 2004; see also Morin, 2007). In addition, our study mainly comprised patients in the subacute and chronic phases, and our findings suggest that anosognosia in less acute phases is not a rare occurrence. This is in accordance with a substantial body of literature that has described patients with anosognosia in subacute and chronic phases (see Cocchini, Beschin, & Della Sala, 2002, Table 1).
A wider issue should be considered. There is clear evidence that damage to the right hemisphere leads to some degree of unawareness, and several explanations, not necessarily mutually exclusive, have been proposed. Some authors have suggested that dysfunction of right brain areas may be responsible for monitoring the veracity of mental contents (Venneri & Shanks, 2003). Turnbull and Solms (2007a; see also Turnbull, Evans, & Owen, 2005) suggested that, in accordance with the motivational approach, right-sided lesions may damage a right-sided emotion-regulation system, therefore these patients may deny their deficits because they have overwhelming difficulty in tolerating aversive emotional states, such as paresis.
However, damage to the left hemisphere has also been considered to be responsible for lack of awareness of language deficits (see Vuilleumier, 2000, for a review). Some authors have suggested that unawareness of aphasia following LHD may result from attentional or monitoring difficulties in comparing the actual output with the intended output (e.g., Maher, Rothi, & Heilman, 1994; Marshall, Robson, Pring, & Chiat, 1998; Shuren, Hammond, Maher, Rothi, & Heilman, 1995). In addition, if data from the present study were replicated, then the major criticisms (e.g., Heilman, 2007) raised against some theoretical approaches of anosognosia, such as the motivational theories, would not stand up and such theoretical interpretations could be reconsidered.
We did not identify a clear association between anosognosia and site of lesion, or between anosognosia and specific cognitive deficits. The left hemisphere does appear to be involved in the process of awareness of deficits, even though it may play a different role to that of the right hemisphere. This is in line with the growing opinion among researchers that anosognosia may be a multifaceted phenomenon (Marcel et al., 2004), and that several factors may underlie deficits of awareness (e.g., Cocchini et al., 2002; Davies, Davies, & Coltheart, 2005; Marcel et al., 2004; Orfei et al., 2007; Vuilleumier, 2004). Should this be the case, then different types and aspects of anosognosia will be identified, and diverse theoretical approaches could coexist in accounting for different aspects of unawareness. It would therefore not be surprising if, in accordance with our anatomical findings, the outcome of meta-analysis studies does not support a definitive pattern of brain lesions that are associated with anosognosia (e.g., Pia et al., 2004).
Acknowledgments
This research was supported by the Stroke Association (Grant TSA 06/02) and partially by the Wellcome Trust (Grant 078580). We thank Prof. Bono, Dr. Zaro, and Dr. Hamilton for their support in recruiting the patients in the hospitals in Italy (Reparto di Neurologia-Ospedale di Varese; Reparto di Riabilitazione-Ospedale di Gallarate) and Scotland (Woodend Hospital in Aberdeen).
VATAm Questions
Example: Do/would you have difficulty driving?
Do you have difficulty clapping your hands?
Do you have difficulty walking?
Do you have any difficulty washing your hands?
Check question: Do you have any difficulty jumping over a lorry?
Do you have any difficulty washing the dishes?
Check question: Do you have any difficulty drinking from a glass?
Do you have any difficulty putting on a pair of gloves?
Do you have any difficulty jumping?
Do you have any difficulty opening a jam jar?
Check question: Do you have any difficulty waving?
Do you have any difficulty climbing the stairs?
Do you have any difficulty opening a bottle?
Do you have any difficulty dealing a pack of cards?
Do you have any difficulty tying a knot?
Do you have any difficulty riding a bicycle?
Check question: Do you have any difficulty juggling five balls in the air?
Expected ratings for check questions 4 and 16 are 2 or 3; expected ratings for check questions 6 and 10 are 0 or 1.
Footnotes
As part of a routine psychometric assessment, 4 patients showed clear evidence of contralesional extrapersonal neglect on the Behavioural Inattention Test (BIT; Wilson, Cockburn, & Halligan, 1987). Three further patients showed mild ipsilesional neglect in some subtests of the BIT; in these cases the VATAm stimuli were displayed on the contralesional side.
References
- Adair J. C., Schwartz R. L., & Barrett A. M. (2003). Anosognosia. In Heilman K. M. & Valenstein E. (Eds.), Clinical Neuropsychology (pp. 185–214). Oxford, England: Oxford University Press. [Google Scholar]
- Baier B., & Karnath H. O. (2005). Incidence and diagnosis of anosognosia for hemiparesis revised. Journal of Neurology, Neurosurgery, and Psychiatry, 76, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso A., Capitani E., & Laiacona M. (1987). Raven's Coloured Progressive Matrices: Normative values on 305 adult normal controls. Functional Neurology, 2, 189–194. [PubMed] [Google Scholar]
- Berti A., Làdavas E., & Della Corte M. (1996). Anosognosia for hemiplegia, neglect dyslexia and drawing neglect: Clinical findings and theoretical considerations. Journal of International Neuropsychological Society, 2, 426–440. [DOI] [PubMed] [Google Scholar]
- Bisiach E., & Geminiani G. (1991). Anosognosia related to hemiplegia and hemianopia. In Prigatano G. P. & Schacter D. L. (Eds.), Awareness of deficit after brain injury (pp. 17–39). Oxford, England: Oxford University Press. [Google Scholar]
- Bisiach E., Vallar G., Perani D., Papagno C., & Berti A. (1986). Unawareness of disease following lesions of the right hemisphere: Anosognosia for hemiplegia and anosognosia for hemianopia. Neuropsychologia, 24, 471–482. [DOI] [PubMed] [Google Scholar]
- Breier J. L., Adair J. C., Gold M., Fennell E. B., Gilmore R. L., & Heilman K. M. (1995). Dissociation of anosognosia for hemiplegia and aphasia during left-hemisphere anesthesia. Neurology, 45, 65–67. [DOI] [PubMed] [Google Scholar]
- Buchtel H., Henry T., & Abou-Khalil B. (1992). Memory for neurological deficits during the intracarotid amytal procedure: A hemispheric difference. Journal of Clinical Experimental Neuropsychology, 14, 96–97. [Google Scholar]
- Cocchini G., Beschin N., & Della Sala S. (2002). Chronic anosognosia: A case report and theoretical account. Neuropsychologia, 40, 2030–2038. [DOI] [PubMed] [Google Scholar]
- Cutting J. (1978). Study of anosognosia. Journal of Neurology, Neurosurgery, and Psychiatry, 41, 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M., Davies A. A., & Coltheart M. (2005). Anosognosia and the two-factor theory of delusions. Mind & Language, 20, 209–236. [Google Scholar]
- Della Sala S., Cocchini G., Beschin N., & Cameron A. (in press). VATAm: Visual-analogue test for anosognosia for motor impairment: A new test to assess awareness for motor impairment. The Clinical Neuropsychologist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin M. W., Meador K. J., Nichols M. E., Lee G. P., & Loring D. W. (1994). Anosognosia and the intracarotid amobarbital procedure (Wada Test). Neurology, 44, 978–979. [DOI] [PubMed] [Google Scholar]
- Dywan C., McGlone J., & Fox A. (1995). Do intracarotid barbiturate injections offer a way to investigate hemispheric models of anosognosia? Journal of Clinical and Experimental Neuropsychology, 17, 431–438. [DOI] [PubMed] [Google Scholar]
- Gainotti G., D'Erme P., Villa G., & Caltagirone C. (1986). Focal brain and intelligence: A study with a new version of Raven's Coloured Matrices. Journal of Clinical and Experimantal Neuropsychology, 8, 37–50. [DOI] [PubMed] [Google Scholar]
- Gilmore R. L., Heilman K. M., Schmidt R. P., Fennell E. M., & Quisling R. (1992). Anosognosia during Wada testing. Neurology, 42, 925–927. [DOI] [PubMed] [Google Scholar]
- Goldberg I. I., Harel M., & Malach R. (2006). When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron, 50, 329–339. [DOI] [PubMed] [Google Scholar]
- Heilman K. M. (2007). Freud and neuropsychology: Comments related to anosognosia. Cortex, 43, 1091–1092. [DOI] [PubMed] [Google Scholar]
- Jehkonen M., Laihosalo M., & Kettunen J. (2006). Anosognosia after stroke: Assessment, occurrence, subtypes and impact on functional outcome reviewed. Acta Neurologica Scandinavica, 114, 293–306. [DOI] [PubMed] [Google Scholar]
- Kaplan R. F., Meadows M. E., Cohen R. A., Bromfield E. B., & Ehrenberg B. L. (1993). Awareness of deficit after the sodium amobarbital (Wada) test. Journal of Clinical Experimental Neuropsychology, 15, 383. [Google Scholar]
- Karnath H. O. Baier B., & Nagele T. (2005). Awareness of the functioning of one's own limbs mediated by the insular cortex? Journal of Neuroscience, 25, 7134–7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine D. N., Calvanio R., & Rin W. E. (1991). The pathogenesis of anosognosia for hemiplegia. Neurology, 41, 1770–1781. [DOI] [PubMed] [Google Scholar]
- Lieberman M. D., Jarcho J. M., & Satpute A. B. (2004). Evidence–based and intuition-based self-knowledge: An fMRI study. Journal of Personality and Social Psychology, 87, 421–435. [DOI] [PubMed] [Google Scholar]
- Lu L. H., Barrett A. M., Schwartz R. L., Cibula J. E., Gilmore R. L., Uthman B. M., et al. (1997). Anosognosia and confabulation during the Wada test. Neurology, 49, 1316–1322. [DOI] [PubMed] [Google Scholar]
- Maher L., Rothi L., & Heilman K. (1994). Lack of error awareness in an aphasic patient with relatively preserved auditory comprehension. Brain and Language, 46, 402–418. [DOI] [PubMed] [Google Scholar]
- Marcel A., Tegnér R., & Nimmo-Smith I. (2004). Anosognosia for plegia: Specificity, extension, partiality and disunity of bodily unawareness. Cortex, 40, 19–40. [DOI] [PubMed] [Google Scholar]
- Marshall J., Robson J., Pring T., & Chiat S. (1998). Why does monitoring fail in jargon aphasia? Comprehension, judgment, and therapy evidence. Brain and Language, 63, 79–107. [DOI] [PubMed] [Google Scholar]
- Morin A. (2007). Self-awareness and the left hemisphere: The dark side of selectively reviewing the literature. Cortex, 43, 1068–1073. [DOI] [PubMed] [Google Scholar]
- Nathanson M., Bergman P. S., & Gordon C. G. (1952). Denial of illness: Its occurrence in one hundred consecutive cases of hemiplegia. Archives of Neurology and Psychiatry, 68, 380–387. [PubMed] [Google Scholar]
- Orfei M. D., Robinson R. G., Prigatano G. P., Starkstein S., Rüsch N., Bria P., et al. (2007). Anosognosia for hemiplegia after stroke is a multifaceted phenomenon: A systematic review of the literature. Brain, 130, 3075–3090. [DOI] [PubMed] [Google Scholar]
- Pia L., Neppi-Modona M., Ricci R., & Berti A. (2004). Anatomy of anosognosia for hemiplegia: A meta-analysis. Cortex, 40, 367–378. [DOI] [PubMed] [Google Scholar]
- Shuren J. E., Hammond C. S., Maher L. M., Rothi L. J. G., & Heilman K. M. (1995). Attention and anosognosia: The case of a jargon aphasic patient with unawareness of language deficit. Neurology, 45, 376–378. [DOI] [PubMed] [Google Scholar]
- Stone S. P., Halligan P. W., & Greenwood R. J. (1993). The incidence of neglect phenomena and related disorders in patients with an acute right or left hemisphere stroke. Age and Ageing, 22, 46–52. [DOI] [PubMed] [Google Scholar]
- Turnbull O. H., Evans C. E. Y., & Owen V. (2005). Negative emotions and anosognosia. Cortex, 41, 67–74. [DOI] [PubMed] [Google Scholar]
- Turnbull O. H., & Solms M. (2007a). Awareness, desire, and false beliefs: Freud in the light of modern neuropsychology. Cortex, 43, 1083–1090. [DOI] [PubMed] [Google Scholar]
- Turnbull O. H., & Solms M. (2007b). Big issues, little issues… and non issues. Cortex, 43, 1031–1124. [Google Scholar]
- Vallar G., & Ronchi R. (2006). Anosognosia for motor and sensory deficits after unilateral brain damage: A review. Restorative Neurology and Neuroscience, 24, 247–257. [PubMed] [Google Scholar]
- Venneri A., & Shanks M. F. (2003). Belief and awareness: Reflections on a case of persistent anosognosia. Neuropsychologia, 42, 230–238. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. (2000). Anosognosia. In Bogousslausky J. & Cummings J. L. (Eds.), Behavior and mood disorders in focal brain lesions (pp. 465–519). Cambridge, England: Cambridge University Press. [Google Scholar]
- Vuilleumier P. (2004). Anosognosia: The neurology of beliefs and uncertainties. Cortex, 40, 9–17. [DOI] [PubMed] [Google Scholar]
- Weinstein E. A. (1991). Anosognosia and denial of illness. In Prigatano G. P. & Schacter D. L. (Eds.), Awareness of deficit after brain injury (pp. 240–257). Oxford, England: Oxford University Press. [Google Scholar]
- Weinstein E. A., & Kahn R. L. (1955). Denial of illness: Symbolic and physiological aspects. Springfield, IL: Thomas. [Google Scholar]
- Wilson B. A., Cockburn J., & Halligan P. (1987). Behavioural Inattention Test. Flempton, England: Thames Valley Test Company. [Google Scholar]