Abstract
Pn-AMP1, Pharbitis nil antimicrobial peptide 1, is a small cysteine-rich peptide implicated in host-plant defense. We show here that Pn-AMP1 causes depolarization of the actin cytoskeleton in Saccharomyces cerevisiae and Candida albicans. Pn-AMP1 induces rapid depolarization of actin cables and patches within 15 min. Increased osmolarity or temperature induces transient actin depolarization and results in increased sensitivity to Pn-AMP1, while cells conditioned to these stresses show less sensitivity. Mutations in components of a cell wall integrity pathway (Wsc1p, Rom2p, Bck1p and Mpk1p), which regulate actin repolarization, result in increased sensitivity to Pn-AMP1. A genetic screen reveals that mutations in components of the α-1,6-mannosyltransferase complex (Mnn10p, Mnn11p and Och1p), which regulate mannosylation of cell wall proteins, confer resistance to Pn-AMP1. FITC-conjugated Pn-AMP1 localizes to the outer surface of the cell with no significant staining observed in spheroplasts. Taken together, these results indicate that cell wall proteins are determinants of resistance to Pn-AMP1, and the ability of a plant defense protein to induce actin depolarization is important for its antifungal activity.
Keywords: Actin cytoskeleton, Cell wall integrity pathway, Hevein-like peptide, Plant antifungal protein, Yeast
Introduction
Plants produce a variety of cysteine/glycine-rich small (2–9 kDa) antimicrobial proteins, which are believed to play a defensive role in plants due to their potent in vitro activity and in planta resistance (reviewed in García-Olmedo et al. 1998, Selitrennikoff 2001). Many studies on these proteins have shown that the cytotoxicity of thionins is caused by an electrostatic interaction with negatively charged membrane phospholipids, followed by either pore formation or interaction with a certain lipid domain (Florack and Stiekema 1994). Plant defensins induce membrane permeabilization in fungi, resulting in Ca2+ uptake, K+ efflux, alkalinization of the medium and membrane potential changes. Sphingolipids in the plasma membrane appear to act as putative binding sites for Dahlia merckii defensin (DmAMP1) in Saccharomyces cerevisiae (Thevissen et al. 2000, Thomma et al. 2002). The mode of action of hevein and hevein-like peptides has been thought to be binding cell wall chitin in growing hyphae and consequently inhibiting cell wall biosynthesis. However, the different degrees of antifungal activity among the hevein-type peptides and their molecular actions remain largely unknown.
The actin cytoskeleton in S. cerevisiae has been implicated in polarized growth, secretion, endocytosis, bipolar bud site selection and organelle trafficking (Adams and Pringle 1984, Novick and Botstein 1985, Drubin et al. 1993, Kubler and Riezman 1993, Yang et al. 1997). The actin cytoskeleton has two major filamentous components, cortical patches and cytoplasmic cables. Actin patches are foci of actin and actin-binding proteins at the plasma membrane, whereas cables are bundles of actin filaments that are oriented along the mother-bud axis, toward sites of polarized growth (Adams and Pringle 1984, Mulholland et al. 1994). The yeast actin cytoskeleton responds to environmental stimuli including temperature shifts, osmotic shock, mating pheromone and certain agents such as latrunculin toxin via signaling pathways (Kamada et al. 1995, Delley and Hall 1999, Davenport et al. 1995, Read et al. 1992, Ayscough et al. 1997).
Recent studies suggest that heat-induced cell wall stress might be sensed by the plasma membrane protein Wsc1p and its signal transmitted to the small G-protein Rho1p via a guanine nucleotide exchange factor Rom2p (Gray et al. 1997). Rho1p induces the activation of a yeast homolog of mammalian protein kinase C (Pkc1p). Pkc1p activates the mitogen-activated protein (MAP) kinase cascade consisting of MAP-KKK Bck1p (Lee and Levin 1992), a pair of MAPKKs Mkk1p and Mkk2p (Irie et al. 1993) and a MAP kinase Mpk1p (Mazzoni et al. 1993), which controls actin polarization and transcription of cell wall biosynthesis genes. Mutations in this pathway result in a mild lysis defect, growth arrest as small-budded cells either at high temperature or at all temperatures and actin depolarization (Novick and Botstein 1985, Schmidt and Hall 1998, Delley and Hall 1999). The phenotype of these mutants is attributable to a deficiency in cell wall growth, organization of cortical actin, or both. These results suggest that the Pkc1p-Mpk1p pathway and its putative upstream regulators play a regulatory role in the maintenance of cell wall integrity during polarized cell growth as well as in actin reorganization in response to environmental stimuli (Davenport et al. 1995, Kamada et al. 1995, Helliwell et al. 1998).
The yeast cell wall is an essential and dynamic structure, composed of mannoproteins, fibrous β-1,3-glucan, branched β-1,6-glucan and a minor chitin component, which mainly contributes to the insolubility of the fibers (Klis 1994, Lipke and Ovalle 1998). The β-1,3-glucan-chitin complex is the major constituent of the inner wall, and β-1,6-glucan links the components of the inner and outer walls. On the outer surface of the wall are mannoproteins, which are extensively O- and N-glycosylated. The mannoproteins are densely packed and determine the surface properties of hydrophobicity, electrical charge, immunogenicity, flocculence, sexual agglutinability and porosity. Digestion of cell walls in the absence of an osmotic protector leads to cell lysis due to high internal turgor pressure. Thus, substances that interfere with cell wall synthesis are potential antifungal agents, and yeast cell wall components have critical roles in signal transduction and controlling the effectiveness of antifungal agents by perception of environmental stimuli (Yun et al. 1997, Ibeas et al. 2000).
Previously, we demonstrated that hevein-type Pharbitis nil anti-microbial peptides (Pn-AMPs) exhibit growth inhibitory activity toward several phytopathogens and S. cerevisiae (Koo et al. 1998). Also, overexpression of Pn-AMP cDNA confers enhanced disease resistance in transgenic tobacco and tomato challenged with Phytophthora parasitica and Phytophthora capsici, respectively (Koo et al. 2002, Lee et al. 2003). Pn-AMP1 is somewhat unique among plant defensins in that it is equally effective against traditional plant pathogens and laboratory yeast strains. Thus, we utilize yeast as a model to investigate the molecular mechanism of Pn-AMP1. Here, we present the results that a plant antifungal peptide induces growth arrest through depolarization of the actin cytoskeleton, while the yeast cell wall integrity pathway suppresses the action of Pn-AMP.
Results
Differential sensitivity to Pn-AMP1 among yeast strains
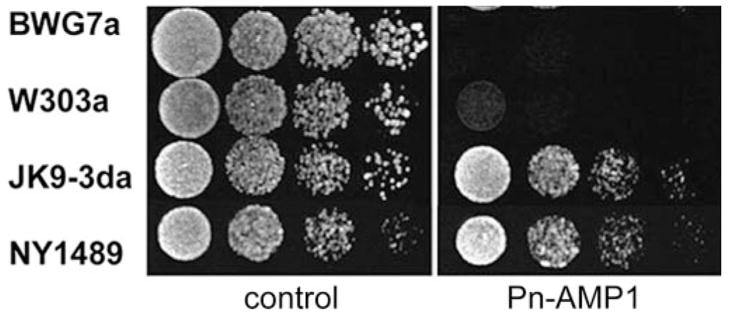
Pn-AMP1, isolated from seeds of morning glory (P. nil), is a 4.3 kDa cysteine/glycine-rich antimicrobial peptide homologous to hevein, which exhibits potent antifungal activity against both chitin-containing and chitin-lacking fungi in the cell wall (Koo et al. 1998). To examine whether laboratory S. cerevisiae strains have an equal sensitivity to Pn-AMP1, four different strains were grown on YPD plates containing Pn-AMP1. Significantly different degrees of sensitivity to Pn-AMP1 were shown among the strains (Fig. 1). Pn-AMP1 concentrations required for 50% growth inhibition (IC50) ranged from 2.3 to 26.7 μM (data not shown). BWG7a was the strain most sensitive to Pn-AMP1, and NY1489 was 10-fold less sensitive. This range of IC50s is similar to the range of values, 0.7–6 μM, that previously inhibited growth of various phytofungi (Koo et al. 1998).
Fig. 1.
Differential sensitivity to Pn-AMP1 among yeast strains. of 0.01, and Exponentially growing cells were diluted to an OD600 nm serial 5-fold dilutions were spotted on a YPD plate supplemented with 7.5 μM Pn-AMP1. The cells were allowed to grow for 2 d at 28°C.
Pn-AMP1 induces cell-cycle-independent growth arrest
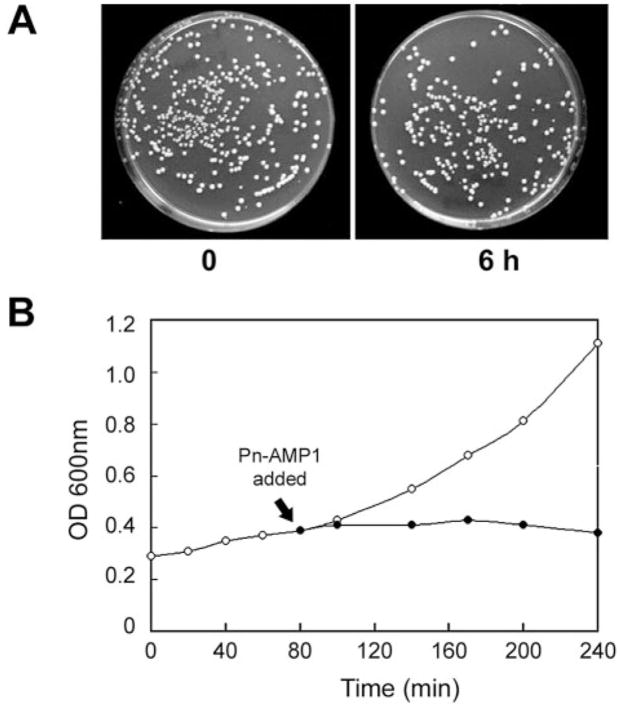
To investigate whether Pn-AMP1 induces cell death, the viability of S. cerevisiae BWG7a following treatment with 7.5 μM Pn-AMP1 was assessed by counting the number of treated cells that could form colonies on YPD plates. After 6 h of Pn-AMP1 incubation, the cells were washed with water and plated. The viability of cells following incubation with Pn-AMP1 did not drop significantly over the 6-h time course, with >70% of cells able to form colonies within 2 d (Fig. 2A). Under the same conditions except for washing with YPD medium instead of water, >80% of cells showed viability (data not shown), indicating that Pn-AMP1 induces reversible cell growth arrest instead of cell death. This growth arrest was fairly rapid; when cell growth was assessed by measuring turbidity (OD600 nm), growth arrest was observed at the earliest times measured, within 15–20 min of Pn-AMP1 addition (Fig. 2B).
Fig. 2.
Pn-AMP1 induces cell growth arrest. (A) Effect of Pn-AMP1 on viability. BWG7a cells were incubated with or without 7.5 μM Pn-AMP1 for 6 h at 28°C. The cells were washed with sterile water and plated on YPD for 2 d at 28°C. (B) The effect of Pn-AMP1 on growth rate was assessed by addition of 7.5 μM Pn-AMP1 to a growing cell culture at the indicated time. Growth of treated (filled circles) or untreated cells (open circles) was determined by OD measurements at 600 nm.
This finding led us to ask whether Pn-AMP1 arrests growth at a certain stage of the cell cycle. Asynchronously growing BWG7a cells were treated with 7.5 μM Pn-AMP1 for 1–16 h, and cells were examined under the light microscope. The treated cells did not arrest with a uniform morphology, and the percentage of cells in various growth stages was similar to that before addition of Pn-AMP1, indicating that Pn-AMP1-induced cell arrest was not due to a block in a certain stage of the cell cycle (data not shown).
Pn-AMP1 induces different morphogenic alterations according to mode of growth
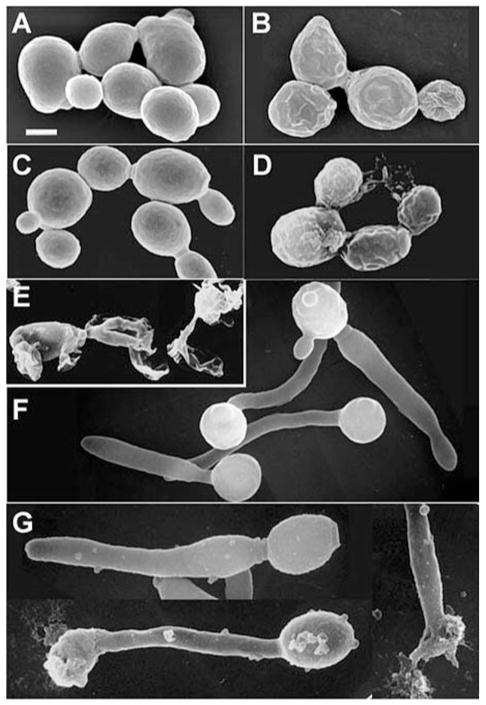
To get an understanding of Pn-AMP1’s mode of action, we looked for morphological changes by scanning electron microscopy (SEM). BWG7a cells were treated with 7.5 μM Pn-AMP1 for 30 min in YPD medium, fixed and processed for SEM. Cells exposed to Pn-AMP1 showed wrinkling of the surface, especially in daughters, compared with the smooth surface of untreated cells (Fig. 3A, B). However, we have demonstrated previously that Pn-AMP1 induces hyphal bursts in the phytopathogens Botritis cinerea and P. parasitica (Koo et al. 1998), raising the concern that Pn-AMP1’s mode of action is different for S. cerevisiae than for phytofungi. To address this difference, we investigated morphological changes using the dimorphic yeast Candida albicans, which can grow alternately between a yeast phase and a hyphal phase, depending on environmental conditions. When the yeast form of C. albicans was treated with an inhibitory concentration (25 μM) of Pn-AMP1, all cells showed surface wrinkling similar to that of S. cerevisiae. Approximately 40% of cells also contained aggregates on the surface caused by leakage of cytoplasmic materials (Fig. 3D). However, exposure of filamentous C. albicans to Pn-AMP1 resulted in swelling along the hyphae and bursts in the hyphal tip (Fig. 2G) as observed previously in the phytopathogens. Since the morphological effects of Pn-AMP1 on C. albicans depend on its phase of growth, it is likely that Pn-AMP1 has only one mode of action but that cells with different forms of growth respond somewhat differently. Pn-AMP1’s primary mode of action is not likely to be cell lysis since C. albicans cells treated with an inhibitory concentration (15 μM) of tobacco osmotin, from another class of antifungal proteins that specifically induces cell lysis in fungi and yeast (Woloshuk et al. 1991, Yun et al. 1997), rapidly lysed without swelling (Fig. 3E).
Fig. 3.
Effect of Pn-AMP1 on cell surface morphology. S. cerevisiae BWG7a (A, B) or C. albicans (C–G) cells were grown on poly-L-lysine-coated coverslips in the absence (A, C, F) or presence (B, D, G) of Pn-AMP1 or (E) tobacco osmotin for 30 min in YPD medium. Hyphal growth of C. albicans was induced by incubating at 37°C for 2 h (F, G). The cells were fixed and processed as described in Materials and Methods. Scale bar is 2 μm.
Pn-AMP1 induces depolarization of the actin cytoskeleton
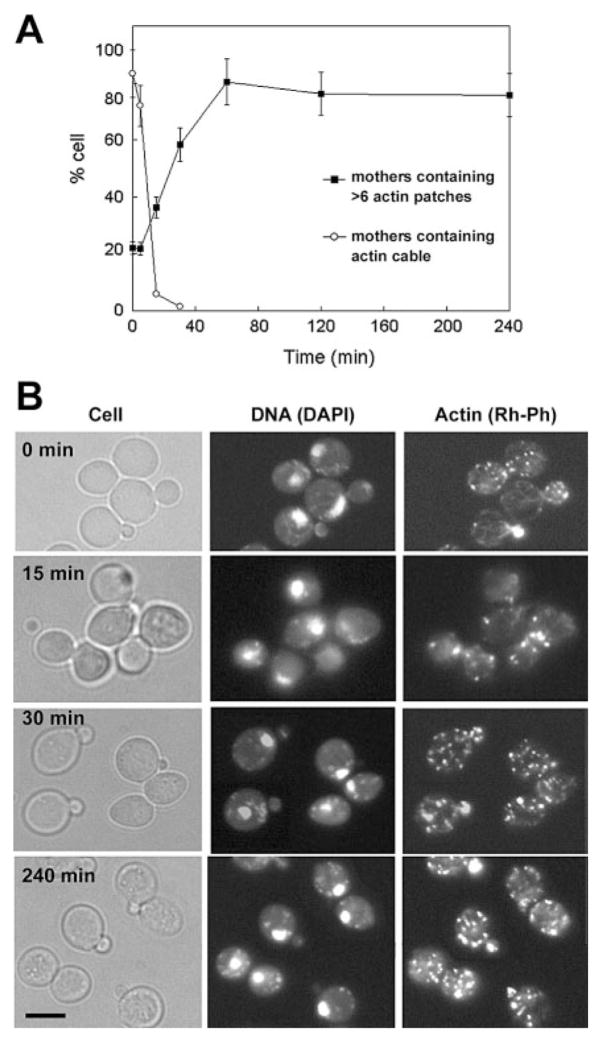
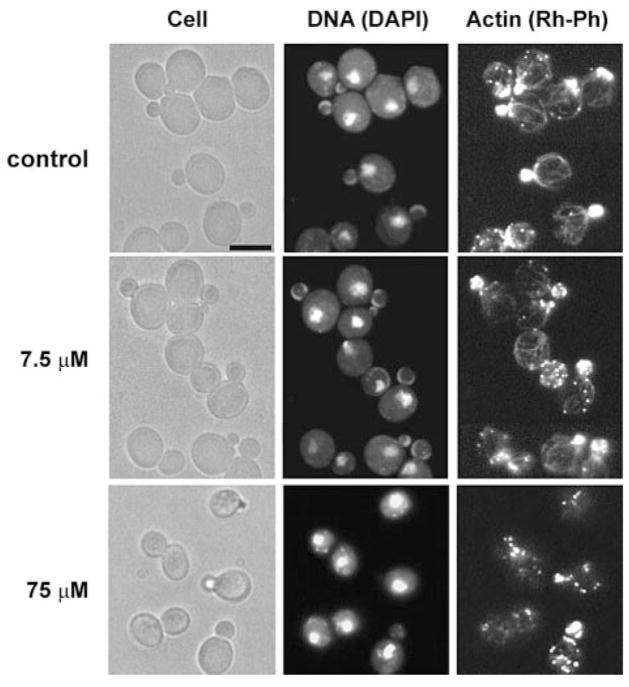
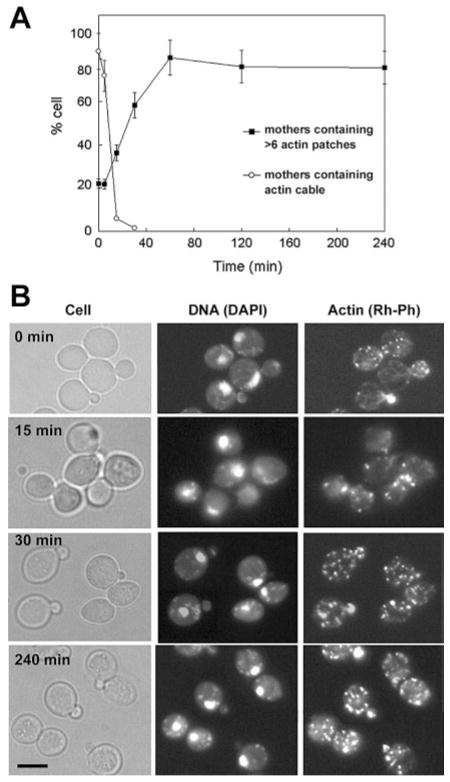
Growth arrest and morphological changes can be caused by disruptions of the actin cytoskeleton. Thus, we examined whether Pn-AMP1 alters the yeast actin cytoskeleton. After treatment with 7.5 μM Pn-AMP1, BWG7a cells were fixed and stained with an F-actin-staining dye, rhodamine-labeled phalloidin (Rh-Ph) and a DNA-staining dye, 4′,6-diamidino-2-phenylindole (DAPI). In untreated cells, actin cables were polarized along the mother-bud axis, and actin patches were concentrated in the bud (Fig. 4). In contrast, in Pn-AMP1-treated cells, actin cables disappeared rapidly, with only 8% of cells having any observable cables after 15 min. No cables were seen after 30 min. Cells also displayed an increase in randomly distributed cortical actin patches in all phases of the cell cycle. The percentage of cells containing more than six actin patches in the mother of small- and medium-budded cells was determined, as a measure of depolarization. Cells with depolarized actin patches began to increase within 15 min, and nearly all cells contained depolarized actin patches within 1 h. The actin depolarization persisted over a 4-h time-course. DAPI staining showed normal nuclear segregation patterns in all the cells examined. To investigate whether growth arrest is correlated with actin depolarization, we observed the actin cytoskeleton of NY1489 cells after Pn-AMP1 treatment for 1 h at growth-inhibitory (75 μM) or subinhibitory (7.5 μM) concentrations. While the majority of NY1489 cells (>80%) showed normal actin cables and polarized actin patches at a subinhibitory concentrations, almost no actin cables and highly depolarized actin patches were observed in the cells treated with an inhibitory concentration (Fig. 5), indicating that actin depolarization is a specific phenotype correlating with growth arrest.
Fig. 4.
Pn-AMP1 induces rapid depolarization of the actin cytoskeleton. BWG7a cells were incubated with 7.5 μM Pn-AMP1 for the indicated times, fixed with formaldehyde and then stained with Rh-Ph and DAPI to visualize the actin cytoskeleton and nuclei, respectively. (A) The percentages of small-budded cells exhibiting loss of actin cables or depolarization of actin patches were determined as described in Materials and Methods. n-values for each point were 130–270 cells. Error bars are the SEP. (B) Representative images of cells. Cells were observed by Nomarski microscopy (left), by fluorescence microscopy of DAPI staining to detect nuclei (center) and with Rh-Ph to detect F-actin (right). Scale bar is 5 μm.
Fig. 5.
Pn-AMP1 depolarizes the actin cytoskeleton at concentrations that inhibit growth. NY1479 cells were incubated with 7.5 or 75 μM Pn-AMP1 for 1 h, fixed with formaldehyde and then stained with Rh-Ph and DAPI to visualize the actin cytoskeleton and nuclei, respectively. Cells were observed by Nomarski microscopy (left), by fluorescence microscopy of DAPI staining to detect nuclei (center) and with Rh-Ph to detect F-actin (right). Scale bar is 5 μm.
To investigate whether Pn-AMP1-induced actin depolarization is unique to a budding yeast mode of growth, we observed the actin cytoskeleton in C. albicans after treatment with 25 μM Pn-AMP1 for 15 min. Both yeast and filamentous forms of C. albicans cells (>95%) stained with Rh-Ph showed highly depolarized actin patches along hyphae and hyphal tips (Fig. 6H) under conditions that induced cell swelling and bursts in the SEM analysis. This indicates that actin depolarization is induced by Pn-AMP1, regardless of a cell’s form of growth.
Fig. 6.
Pn-AMP1 induces rapid depolarization of the actin cytoskeleton in C. albicans. Cells were incubated in the absence (A, B, E, F) or presence (C, D, G, H) of 25 μM Pn-AMP1 for 15 min, fixed with formaldehyde and stained with Rh-Ph. Cells were observed by Nomarski microscopy (A, C, E, G) and by fluorescence microscopy (B, D, F, H) to detect F-actin. Hyphal growth of C. albicans was induced by incubating at 37°C for 2 h (E–H). Scale bars (A–D, E–H) are 5 μm.
Hyperosmotic and heat stress result in increased sensitivity to Pn-AMP1 correlated with actin depolarization
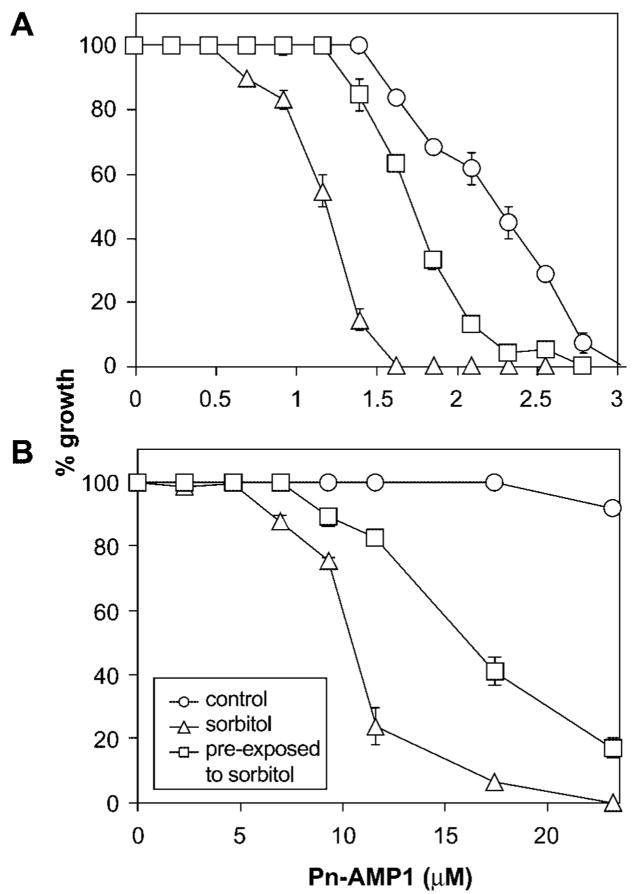
Osmotic stabilizers, such as 1 M sorbitol, are known to suppress cell wall lysis in several mutants, including components of the cell wall integrity pathway (Lee and Levin 1992, Irie et al. 1993). Osmotic support also partially suppresses the toxicity of a pore-forming killer toxin (Komiyama et al. 1996). If actin depolarization and growth arrest by Pn-AMP1 are the consequence of cell wall damage or defects, osmotic support should suppress the action of Pn-AMP1. To test this hypothesis, cell growth was measured after treatment with various concentrations of Pn-AMP1 in the presence or absence of sorbitol. Unexpectedly, exposure to sorbitol resulted in increased sensitivity to Pn-AMP1 in both BWG7a and NY1489 cells (Fig. 7). The yeast actin cytoskeleton is known to be transiently depolarized in response to sorbitol (Davenport et al. 1995). As observed in previous studies, exposure to 1 M sorbitol induced actin depolarization by 40–60 min in both BWG7a and NY1489 cells, but actin polarization was normal after 3–4 h in almost all the cells (data not shown). Thus, the Pn-AMP1 sensitivity of cells pre-exposed to sorbitol for 4 h were compared with that of cells without pre-exposure to determine whether the increased sensitivity seen in sorbitol is a consequence of actin depolarization. Indeed, pre-exposed cells, which had recovered actin polarization, did show less sensitivity to Pn-AMP1 than cells not pre-treated to the same osmotic stress (Fig. 7).
Fig. 7.
Hyperosmotic stress enhances Pn-AMP1 sensitivity. Exponentially growing BWG7a (A) or NY1489 (B) cells were pre-incubated in YPD medium with or without 1 M sorbitol for 4 h and then diluted to OD600 nm 0.01 in YPD medium containing 1 M sorbitol and the indicated concentration of Pn-AMP1. After incubation for 16–20 h at 28°C, growth was determined by measuring OD600 nm. As a control, cells were treated with Pn-AMP1 in YPD medium without pre-treatment with sorbitol (see Materials and Methods for detail). Growth inhibition was determined as described above. Error bars are the SEM (n = 3, P < 0.01 for most concentrations with differential growth inhibition).
Like osmotic stress, temperature stress is also known to induce transient depolarization of the actin cytoskeleton and inhibit cell growth in budding yeast (Kamada et al. 1995, Delley and Hall 1999). A temperature shift from 28°C to 37°C induced actin depolarization at ~50 min in >90% of cells, and repolarization occurred within 4 h in almost all cells (data not shown). Overnight cultures at 28°C were freshly regrown for 4 h either at the same temperature or at 37°C. The sensitivity of the same number of both cells to Pn-AMP1 was determined based on growth at 37°C. The temperature shift from 26°C to 37°C resulted in significantly increased sensitivity to Pn-AMP1, and preconditioning cells at 37°C reduced the increase in sensitivity to Pn-AMP1 in both BWG7a and NY1489 (Fig. 8). Taken together, the observations of differential sensitivity in pre-treated versus control cells to given stresses indicates that the main action of Pn-AMP1 is actin depolarization rather than cell wall defects or osmotic sensitivity.
Fig. 8.
Heat stress enhances Pn-AMP1 sensitivity. Exponentially growing BWG7a (A) and NY1489 (B) cells were grown at 28°C or 37°C for 4 h, diluted to an OD600 nm of 0.01, and treated with the indicated concentration of Pn-AMP1 for 16–20 h at 37°C. As a control, cells were kept at 28°C during the assay (see Materials and Methods for detail). Error bars are the SEM (n = 3, P < 0.01 for most concentrations with differential growth inhibition).
WSC1-mediated cell wall integrity pathway suppresses the action of Pn-AMP1
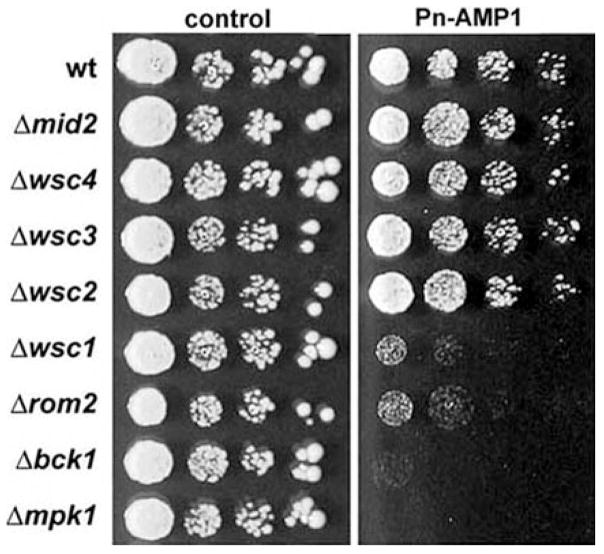
Components of the Pkc1-Mpk1 pathway as well as its upstream regulating factors play a key regulatory role in maintenance of the integrity of the cytoskeleton and the cell wall in S. cerevisiae, including responses to osmotic shock and heat shock (Davenport et al. 1995, Kamada et al. 1995, Gray et al. 1997, Delley and Hall 1999). Null mutations in the Pkc1-Mpk1 pathway result in cell lysis. This phenotype is thought to be a consequence of a defect in polarization of the actin cytoskeleton, resulting from decreased ability to transport and target secretory vesicles necessary for biosynthesis of the cell wall (Cid et al. 1995). To examine whether mutations of this pathway result in increased sensitivity to Pn-AMP1, we determined the sensitivity of several mutants to Pn-AMP1. Since null mutations of Rho1 or Pkc1 resulted in lethality or a severe cell lysis defect, we tested wsc1, wsc2, wsc3, wsc4, mid2, rom2, bck1 and mpk1 null mutants compared with their wild-type parent, S. cerevisiae JK9-3da. The wsc1 mutant, lacking one of the putative cell surface sensors for cell integrity (Gray et al. 1997, Delley and Hall 1999), displayed increased sensitivity to Pn-AMP1 (Fig. 9). Cells lacking Rom2p also showed increased sensitivity to Pn-AMP1. Mutations in the components downstream of Pkc1p, Bck1p and Mpk1p resulted in higher sensitivity than mutations in the upstream components Wsc1p and Rom2p. This result agrees with the recent report that Bck1p and Mpk1p are necessary for repolarization of actin, whereas Pkc1p regulates both depolarization and repolarization of the actin cytoskeleton through an unidentified effector branch (Delley and Hall 1999).
Fig. 9.
Mutations in the Wsc1-Mpk1 pathway result in increased Pn-AMP1 sensitivity. Cultures of exponentially growing wild-type JK9-3da or various mutants of the cell wall integrity pathway were diluted to OD600 nm 0.1, and serial 5-fold dilutions were spotted on a YPD plate containing Pn-AMP1. The cells were allowed to grow for 2 d at 28°C.
To investigate the effect of Pn-AMP1 on the actin cytoskeleton of these mutants, we counted the number of cells with depolarized actin patches in medium- or small-budded cells after staining with Rh-Ph. When cells were treated with an inhibitory concentration of Pn-AMP1, a similar degree of actin depolarization was observed in all mutant strains and wild type (> 90%). This result suggests that Pn-AMP1 induces actin depolarization independently from the Pkc-Mpk1 pathway and that the increased sensitivity of Pkc1-Mpk1 pathway mutants is due to the loss of ability to repair actin organization, which normally antagonizes Pn-AMP1-induced actin depolarization.
Putative cell wall glycoproteins determine Pn-AMP1 sensitivity
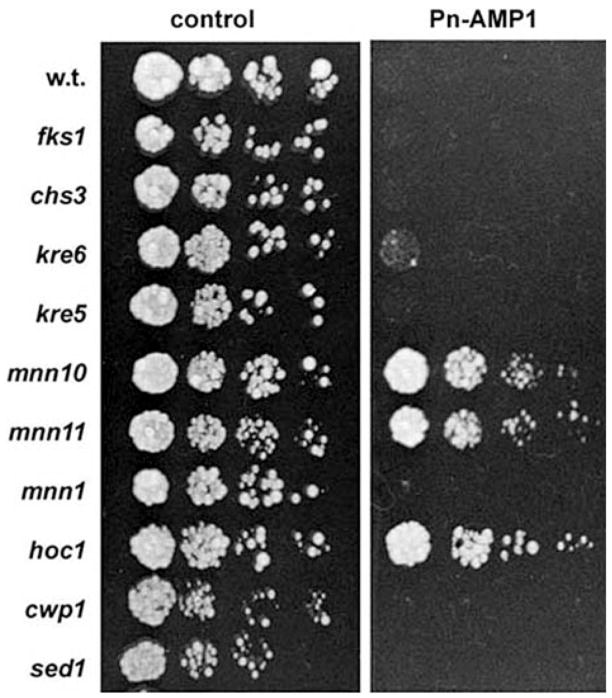
In order to identifying the target molecules for Pn-AMP1 we utilized a genetic approach, screening mutant BWG7a cells constructed by transformation of a genomic library carrying random Tn3:Leu2 gene insertions (Burns et al. 1994). Cells showing Pn-AMP1 resistance (ARE) were isolated by growth on YPD plates containing 7.5 μM Pn-AMP1. After selecting individual clones, the Tn3:Leu2 inserted loci were determined by DNA sequencing. Analysis of the tagged sequences revealed that most mutations were placed within the coding regions of the MNN10, MNN11, HOC1, KEX2 and SIR3 genes. Of these, Mnn10p, Mnn11p and Hoc1p are known components of the Golgi α-1,6-mannosyltransferase complex, which regulates the mannosylation of cell wall proteins (Jungmann and Munro 1998). To confirm these results, we generated mnn10, mnn11 and hoc1 null mutants and examined their sensitivity to Pn-AMP1 (Fig. 10). As seen for the ARE mutants, the three null mutants showed a 3-fold increased resistance in IC50 values relative to that of wild-type BWG7a cells. In addition, mutations in MNN9 and ANP1, which encode other components of the Golgi α-1,6-mannosyltransferase complex, conferred a similar level of increased resistance in the W303a genetic background (data not shown). However, disruption of MNN1, an α-1,3 mannosyltransferase (Yip et al. 1994) or genes that encode two abundant cell wall mannoproteins, CWP1 and SED1 (Van der Vaart et al. 1997), did not change sensitivity to Pn-AMP1.
Fig. 10.
Mutations in genes encoding α-1,6-mannosyltransferase result in increased Pn-AMP1 sensitivity. Aliquots (2.5 μl) from exponentially growing cultures of wild-type BWG7a and mannosylation or cell wall mutants, including fks1, chs3, kre6, kre5, mnn10, mnn11, mnn1, hoc1, cwp1 and sed1, were diluted to OD600 nm 0.1, and serial 5-fold dilutions were spotted on YPD plates with or without 15 μM Pn-AMP1. The cells were allowed to grow for 2 d at 28°C.
Pn-AMP1 has a chitin-binding activity (Lee et al. 2003) as observed in other small hevein-like peptides, which has led to the hypothesis that chitin is the biological target of Pn-AMP1. However, no mutants defective for chitin synthesis were isolated in our genetic screen. To address the question of whether chitin or other cell wall components are involved in the action of Pn-AMP1, we examined Pn-AMP1 sensitivity in cell wall mutants. The yeast cell wall is composed of β-1,3-glucan, β-1,6-glucan, chitin and mannan. The major catalytic enzymes that regulate β-1,3-glucan and chitin biosynthesis are encoded by the FKS1 (Inoue et al. 1995) and CHS3 genes (Shaw et al. 1991), respectively. The major catalytic enzyme for β-1,6-glucan has not yet been identified, so we examined kre5 and kre6 mutants because these genes have been implicated in β-1,6-glucan biosynthesis (Meaden et al. 1990, Roemer and Bussey 1991). When all these mutant cells were grown on YPD plates containing Pn-AMP1, none of them showed a detectable alteration of their sensitivity to Pn-AMP1. Only a slightly increased resistance was observed in the kre6 mutant (Fig. 10).
To examine whether target molecules for Pn-AMP1 reside on the cell wall or the plasma membrane or both, the binding of Pn-AMP1 to yeast cells and spheroplasted cells was further analyzed using FITC-conjugated Pn-AMP1. FITC-conjugated Pn-AMP1 showed the same level of biological activity as native peptide (data not shown). After incubating with 7.5 μM FITC-Pn-AMP1 for 30 min, cells were washed and the subcellular localization of Pn-AMP1 was examined by confocal fluorescence microscopy. A much higher level of FITC-Pn-AMP1 binding was observed over the cell surface in intact cells, whereas no detectable level of binding was found in spheroplasts (Fig. 11), indicating that the target molecules for Pn-AMP1 reside on the cell wall. This result was confirmed by washing the FITC-Pn-AMP1-treated cells or spheroplasts and quantifying the bound FITC with a fluorometer (data not shown). Taken together, these results indicate that specific mannan(s) are required for the activity of Pn-AMP1 in the cell wall.
Fig. 11.
Localization of Pn-AMP1 to the cell wall. BWG7a cells (A, B) or spheroplasts (C, D) were incubated with 7.5 μM FITC-Pn-AMP1 for 30 min in YPD or YPD/1 M sorbitol medium, respectively. After washing, fluorescence was observed with a confocal microscope (B, D), and whole cell images were observed by Nomaraski microscopy (A, C). Scale bar is 5 μm. (E) Growth inhibition assay. The activity of Pn-AMP1 and FITC-Pn-AMP1 was compared using BWG7a in liquid cultures. Values are the average of three independent experiments and are normalized to the OD600 nm of control cultures without Pn-AMP1 or FITC-Pn-AMP.
Discussion
We found that Pn-AMP1 causes actin depolarization correlated with growth arrest in S. cerevisiae and C. albicans. While most Pn-AMP1-treated cells showed disruption of actin cables and depolarization of cortical patches within 15 min at concentrations that inhibited cell growth, no significant changes in actin polarity were observed at a subinhibitory concentration (Fig. 4, 5). This may be a unique mechanism of Pn-AMP1 since the tobacco pathogenesis-related protein osmotin causes rapid cell lysis (Fig. 3E) without changing numbers and patterns of actin patches, compared with untreated cells (data not shown). Our findings provide the genetic and cell biological basis for a number of important open issues concerning the mode of action of a plant defensin.
Pn-AMP1 induces various morphogenic alterations, such as cell bursts or wrinkling, in yeast (Fig. 3). These observations led to the question of whether Pn-AMP1’s main action is mediated by depolarization of actin or by osmotic sensitivity due to a cell wall defect. Several lines of evidences indicate that the main action of Pn-AMP1 is actin depolarization and cell bursts or wrinkling may be a downstream phenotype of actin depolarization, depending on mode of growth and species of fungi. First, cells carrying mutations in actin and some actin regulatory proteins are known to be osmotically sensitive (Munn et al. 1995, Moreau et al. 1996). Secondly, cells grown in osmotically stabilized media, such as 1 M sorbitol, are generally more resistant to osmotic lysis, but these cells were more sensitive to Pn-AMP1 (Fig. 7). Interestingly, >50% cells treated with 1 M sorbitol for 30 min showed the depolarization of actin cytoskeleton and cell wrinkling phenotype that was observed in Pn-AMP1-treated cells (data not shown), accounting for the additive or synergic effect of hyperosmotic stress. Moreover, cells adapted to this stress, which repolarize their actin cytoskeleton after these periods of stress, slightly recovered their resistance to Pn-AMP1 compared with non-adapted cells, strongly supporting our hypothesis. Thirdly, while Pn-AMP1 induced cell leakage preferentially in the filamentous form rather than the yeast forms, actin depolarization was observed in almost all cells, regardless of mode of growth (Fig. 3, 6). Fourthly, although mutants showing cell-wall-defective phenotypes, such as mpk1Δ, fks1Δ and mnn9Δ, are also known to be sensitive to osmotic stress (Torres et al. 1991, Yip et al. 1994, Garrett-Engele et al. 1995), these mutants were sensitive (mpk1Δ), indifferent (fks1Δ) or resistant (mnn9Δ) to Pn-AMP1, compared with wild-type cells (Fig. 9, 10).
The cell wall integrity pathway regulates depolarization and repolarization of the actin cytoskeleton and cell growth in response to extracellular stresses, such as heat shock and hypotonic shock (Davenport et al. 1995, Kamada et al. 1995, Schmidt and Hall 1998). Pn-AMP1 seems to induce the cell wall integrity pathway, but this induction is actually antagonistic to Pn-AMP1-induced growth arrest; loss of this pathway leads to Pn-AMP1 hypersensitivity (Fig. 9). Recent studies proposed that the upstream components of this pathway including Wsc1p, the Rho1p exchange factor Rom2p, Rho1p and Pkc1p are necessary both for actin depolarization and for repolarization. Depolarization is specifically dependent on a yet-to-be identified Pkc1 effector branch (Gray et al. 1997, Helliwell et al. 1998, Delley and Hall 1999), while repolarization is specifically dependent on the Pkc1 downstream branch that includes Bck1p and Mpk1p (Delley and Hall 1999). In addition, Mpk1p itself is greatly activated in response to the actin perturbation drug, latrunculin-B (Harrison et al. 2001). Since mutants downstream of Pkc1 (bck1Δ and mpk1Δ) were more sensitive to Pn-AMP1 than those upstream (wsc1Δ and rom2Δ), it is likely that the actin repolarization branch is antagonizing Pn-AMP1 activity (Fig. 9, 12). These observations suggest that yeast cell wall integrity pathway may play a key role not only in cell maintenance upon environmental stress but also in defense itself from host barriers under highly specialized plant-fungal interactions.
Fig. 12.
Proposed model of Pn-AMP1’s effects on the regulation of the actin cytoskeleton and its antagonism by the Wsc1-Mpk1 pathway (see Discussion for further details).
To identify target molecules for Pn-AMP1, we screened transposon-mediated yeast mutants for resistance to Pn-AMP1. Mutations in the genes encoding Mnn10p, Mnn11p, Hoc1p and Kex2p were isolated (Fig. 10). Although Pn-AMP1 has chitin-binding activity, growth arrest is probably mediated through cell wall mannoprotein(s) rather than chitin for several reasons. First, when glycoproteins pass through the secretory pathway, N-linked glycans undergo modifications; Mnn10p, Mnn11p and Hoc1p are subunits of a Golgi mannosyltransferase complex that elongates the mannan backbone of cell wall mannoproteins. Mutations in this complex result in a short backbone of mannan chains with 10–15 residues (Jungmann and Munro 1998). Kex2p protease is a Ca2+-dependent serine protease involved in endoproteolytic processing in the Golgi. Thus, cells carrying mutations in this gene may be defective in modification or processing of target molecules for Pn-AMP1 and subsequently exhibit resistance to the peptide. Secondly, the yeast cell wall is composed of chitin, β-1,3-glucan, β-1,6-glucan and mannoproteins. Mutations in genes encoding chitin synthase (chs3) or β-1,3-glucan synthase (fks1) did not confer resistance (Fig. 10). The enzyme for β-1,6-glucan synthesis is not known, but mutations that confer resistance to β-1,6-glucan-binding yeast killer toxin K1 are known and named KREs (Meaden et al. 1990, Roemer and Bussey 1991). However, the kre5 mutant also showed no change in resistance, and kre6 exhibited only slightly increased resistance, suggesting that glucans are not involved as Pn-AMP1 targets. Thirdly, we demonstrated previously that Pn-AMP1 had a similar growth-inhibiting activity against both chitin-containing phytofungi and non-chitin-containing oomycete pathogens both in vitro and in vivo (Koo et al. 1998, Koo et al. 2002, Lee et al. 2003).
Our findings constitute the first report that a plant antifungal protein causes growth arrest through actin depolarization in yeast. Although much remains to be determined about how Pn-AMP1 affects the actin cytoskeleton and which molecules are involved in the signals, our findings implicate cell wall mannoproteins and stress response pathways as being involved (Fig. 12). Thus, further study on Pn-AMP1 will provide greater understanding of the molecular mechanisms for antifungal peptides as well as for regulation of the actin cytoskeleton in response to environmental stimuli through cell wall proteins.
Materials and Methods
Strains
S. cerevisiae strains used in this study are listed in Table 1. A clinical isolate of C. albicans SC5314 (Gillum et al. 1984) was kindly provided by Dr. Gerald Fink, MIT, Cambridge, MA, U.S.A. Standard procedures for yeast media preparation and genetic experiments were followed (Rothstein 1985, Sherman 1991). The deletion mutants were isogenic derivatives of strains BWG7a and JK9-3da and were created by PCR-mediated gene disruption. The primers used for gene disruption are listed in Supplementary Table 1. Gene disruption was confirmed by Southern blotting and PCR.
Table 1.
List of yeast strains
| Strain | Genotype | Source |
|---|---|---|
| BWG7a | MATa ade1-100 leu2-3,112 ura3-52 his4-519 | Ray Bressan |
| W303a | MATa, can1-100, ade2-1, his3-11,15, leu2-3, 12, trp1-1, ura3-1 | Cesar Roncero |
| JK9-3da | MATa leu2-3,112 ura3-52 trp1 his4 rme1 HMLa | Delley and Hall (1999) |
| NY1489 | MATa ura3-52 trp1 his 3–200 | TerBush et al. (1996) |
| are1 | BWG7a mnn10::lacZ-mTn3 | This study |
| are2 | BWG7a mnn11::lacZ-mTn3 | This study |
| are3 | BWG7a hoc1::lacZ-mTn3 | This study |
| are4 | BWG7a kex2::lacZ-mTn3 | This study |
| PMC1 | BWG7a mnn10:: URA3 | This study |
| PMC2 | BWG7a mnn11:: URA3 | This study |
| PMC3 | BWG7a hoc1:: URA3 | This study |
| PMC4 | BWG7a kex2:: URA3 | This study |
| PMC5 | BWG7a chs3:: URA3 | This study |
| PMC6 | BWG7a fks1:: URA3 | This study |
| PMC7 | BWG7a kre5:: URA3 | This study |
| PMC8 | BWG7a kre6:: URA3 | This study |
| PMC9 | BWG7a mnn1:: URA3 | This study |
| PMC10 | BWG7a cwp1:: URA3 | This study |
| PMC11 | BWG7a sed1:: URA3 | This study |
| PMC12 | JK9-3da wsc4:: URA3 | This study |
| PMC13 | JK9-3da bck1:: URA3 | This study |
| PMC14 | JK9-3da mpk1:: URA3 | This study |
| PA39–1b | JK9-3da wsc1::kanMX4 | Delley and Hall (1999) |
| PA88–1a | JK9-3 da wsc2::kanMX4 | Delley and Hall (1999) |
| PA62–2d | JK9-3 da wsc3::kanMX4 | Delley and Hall (1999) |
| PE3-2a | JK9-3 da mid2::kanMX4 | Delley and Hall (1999) |
| AS138–1b | JK9-3 da rom2::URA3 | Delley and Hall (1999) |
Measurement of growth inhibition activity
Pn-AMP1 was purified from seeds of P. nil as described previously (Koo et al. 1998). Tobacco osmotin was kindly provided by Dr. Ray Bressan, Purdue University, Lafayette, IN, U.S.A. Antifungal activity of protein samples was assayed by microspectrophotometry of liquid cultures grown in 96-well microtiter plates as described (Thevissen et al. 2000). Briefly, 50 μl of the protein sample was mixed with 50 μl of yeast cells (final OD600 nm = 0.01) in YPD medium. The microplates were incubated at 28°C without shaking, and absorbance at 600 nm was recorded after 16–20 h of incubation. The IC50 was determined from dose-response curves. For spot assays, aliquots (2–3 μl) from an exponentially growing culture were adjusted to OD600 nm 0.01, and serial 5-fold dilutions were spotted on YPD plates containing various concentrations of Pn-AMP1. For viability assays of yeast cells, exponentially growing BWG7a cells were diluted to OD600 nm 0.01 in YPD medium containing 7.5 μM Pn-AMP1. After incubation for 0 and 6 h at 28°C, the cells were centrifuged at 1,000×g for 5 min, resuspended in water and plated on YPD for 2 d at 28°C. To measure Pn-AMP1 sensitivity in hyphal-growing C. albicans, cells were incubated at 37°C for 2 h in YPD medium on poly-L-lysine-coated slides and then treated with various concentrations of Pn-AMP1.
Effect of heat or hyperosmotic stress on Pn-AMP1 sensitivity
To analyze the effect of hyperosmotic stress on Pn-AMP1 sensitivity, cells (OD600 nm of 0.02) were mixed with an equal volume of YPD/2 M sorbitol medium in a 96-well microplate. In some controls, cells were first grown in YPD/1 M sorbitol medium for 4 h, then diluted to OD600 nm 0.01 with YPD/1 M sorbitol medium containing various concentrations of Pn-AMP1. To analyze the effect of heat stress on Pn-AMP1 sensitivity, cells (OD600 nm 0.01) grown at 28°C were treated with various concentrations of Pn-AMP1 and incubated either at the same temperature or at 37°C. To examine the effect of pre-treatment to heat stress, cells were grown at 37°C for 4 h, diluted to OD600 nm 0.01 with YPD pre-warmed to 37°C and treated with Pn-AMP1 at 37°C for 16–20 h in a microplate. The OD600 nm values were normalized to control cultures grown without Pn-AMP1 and given as percentages. Values are the mean of three experiments.
Localization of FITC-Pn-AMP1
FITC conjugation of Pn-AMP1 was performed according to the manufacturer’s protocols (FITC labeling kit, CalBiochem). Exponentially growing cells (OD600 nm 0.1 2 × 105) were incubated in 1 ml YPD medium containing 7.5 μM FITC-Pn-AMP1. After incubation for 30 min, cells were washed twice with YPD medium and resuspended in 100 μl YPD medium. Spheroplasts (2×105) were prepared using lyticase (Sigma) for cell wall digestion as described (Yun et al. 1997) and resuspended in 1 ml YPD 1 M sorbitol medium containing 7.5 μM FITC-Pn-AMP1 30 min. After washing twice with YPD/sorbitol medium, the spheroplasts were mounted on glass slides and viewed with a laser scanning confocal microscope (Carl Zeiss LSM410). Excitation was from a 488 nm argon laser, and the emission filter was a long-pass 515 nm filter.
Isolation of Pn-AMP1 resistance mutants
BWG7a cells were mutagenized by transformation with a transposon LacZ genomic library (gift of Dr. Michael Snyder, Yale University, New Haven, CT, U.S.A.) as described (Burns et al. 1994). About 10,000 transformants were selected on SC-Leu plates and pooled. Approximately 50,000 cells were plated on YPD with 7.5–10 μM Pn-AMP1. Twenty-three mutants were isolated by resistant phenotype. After rescue of insertion sites using the pRSQ-URA3 plasmid, we determined the chromosomal sequences flanking the insertion element as described by Burns et al. (1994). Searches for sequence similarities were carried out with the BLAST search protocol as implemented on the National Center for Biotechnology Information Server (NLM, NIH, Bethesda, MD, U.S.A.).
SEM analysis
SEM analysis was performed as described previously (Koo et al. 1998). Briefly, exponentially growing cells (20 μl of OD600 nm = 0.1) were attached to poly-L-lysine-coated glass coverslips for 2 h. Hyphal growth of C. albicans was induced at 37°C for 2 h on the cover glass. The medium was replaced with YPD medium containing Pn-AMP1 or tobacco osmotin. After incubation for 30 min or 1 h, the cells were washed, fixed and dehydrated with an alcohol series from 30% to 100% in a 26-well microplate. After critical point drying with CO2, samples on glass slides were mounted on carbon filters, and coated with gold in a sputtercoater. The samples were viewed with a Joel840 Scanning Electron Microscope at 20 kV.
Analysis of the actin cytoskeleton
Changes in filamentous actin in response to Pn-AMP1 treatment were analyzed by Rh-Ph staining. Asynchronous populations (OD600 nm 0.01) of wild-type and mutant cells were treated with various concentrations of Pn-AMP1 in YPD medium. Cells were fixed for 15 min in 3.7% formaldehyde by direct addition of a commercial 37% stock to the medium. Rh-Ph staining was performed as described previously (Karpova et al. 1998).
To assess depolarization of the actin cytoskeleton, budding cells with buds less than one-third the size of the mother cell were examined. Mothers having more than six cortical actin patches were scored as depolarized. Without Pn-AMP1 treatment, ~6% of the cells in each group showed depolarized patches. The number of cells counted was 60–270 cells for each time point. The standard error of percentage (SEP) was calculated as described (Spiegel 1975).
Supplementary Material
Acknowledgments
We thank Michael Hall (Department of Biochemistry, University of Basel, Switzerland), Cesar Roncero (Instituto de Microbiologia Bioquimica, Universidad de Salamanca, Spain), David Levin (Department of Biochemistry, Johns Hopkins University, MD, U.S.A.), Ray Bressan (Purdue University, U.S.A.) and Michael Snyder (Department of Molecular, Cellular and Developmental Biology, Yale University, CT, U.S.A.) for yeast strains, DNA constructs for gene disruption, and the mTn::LacZ DNA library. This work was supported by grants from Plant Diversity Research Center of 21st Century Frontier Research (PF0330401-00; MOST), Environmental Biotechnolgy Core Research Center (R15-2003-012-01002-0;KOSEF/MOST), Biogreen 21 program and NIH (GM47337). D.B. was supported by scholarship from the Brain Korea 21 program, Ministry of Education, Korea.
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- IC50
concentrations required for 50% growth inhibition
- MAP
mitogen-activated protein
- Pn-AMP
Pharbitis nil antimicrobial peptide
- Rh-Ph
rhodamine-labeled phalloidin
- SEM
scanning electron microscopy
Footnotes
Supplementary material mentioned in the article is available to online subscribers at the journal website www.pcp.oupjournals.org.
References
- Adams AE, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns N, Grimwade B, Ross-Macdonald PB, Choi EY, Finberg K, Roeder GS, Snyder M. Large-scale analysis of gene expression, protein localization and gene disruption in Saccharomyces cerevisiae. Genes Dev. 1994;8:1087–1105. doi: 10.1101/gad.8.9.1087. [DOI] [PubMed] [Google Scholar]
- Cid VJ, Duran A, del Rey F, Snyder MP, Nombela C, Sanchez M. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol Rev. 1995;59:345–386. doi: 10.1128/mr.59.3.345-386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport KR, Sohaskey M, Kamada Y, Levin DE, Gustin MC. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J Biol Chem. 1995;270:30157–30161. doi: 10.1074/jbc.270.50.30157. [DOI] [PubMed] [Google Scholar]
- Delley PA, Hall MN. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J Cell Biol. 1999;147:163–174. doi: 10.1083/jcb.147.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Jones HD, Wertman KF. Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol Biol Cell. 1993;4:1277–1294. doi: 10.1091/mbc.4.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florack DE, Stiekema WJ. Thionins: properties, possible biological roles and mechanisms of action. Plant Mol Biol. 1994;26:25–37. doi: 10.1007/BF00039517. [DOI] [PubMed] [Google Scholar]
- García-Olmedo F, Molina A, Alamillo JM, Rodriguez-Palenzuela P. Plant defense peptides. Biopolymers. 1998;47:479–491. doi: 10.1002/(SICI)1097-0282(1998)47:6<479::AID-BIP6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Garrett-Engele P, Moilanen B, Cyert MS. Calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar H(+)-ATPase. Mol Cell Biol. 1995;15:4103–4114. doi: 10.1128/mcb.15.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Gray JV, Ogas JP, Kamada Y, Stone M, Levin DE, Herskowitz I. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 1997;16:4924–4937. doi: 10.1093/emboj/16.16.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JC, Bardes ES, Ohya Y, Lew DJ. A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat Cell Biol. 2001;3:417–420. doi: 10.1038/35070104. [DOI] [PubMed] [Google Scholar]
- Helliwell SB, Schmidt A, Ohya Y, Hall MN. The Rho1 effector Pkc1, but not Bni1, mediates signaling from Tor2 to the actin cytoskeleton. Curr Biol. 1998;8:1211–1214. doi: 10.1016/s0960-9822(07)00511-8. [DOI] [PubMed] [Google Scholar]
- Ibeas JI, Lee H, Damsz B, Prasad DT, Pardo JM, Hasegawa PM, Bressan RA, Narasimhan ML. Fungal cell wall phosphomannans facilitate the toxic activity of a plant PR-5 protein. Plant J. 2000;23:375–383. doi: 10.1046/j.1365-313x.2000.00792.x. [DOI] [PubMed] [Google Scholar]
- Inoue SB, Takewaki N, Takasuka T, Mio T, Adachi M, Fujii Y, Miyamoto C, Arisawa M, Furuichi Y, Watanabe T. Characterization and gene cloning of 1, 3-β-D-glucan synthase from Saccharomyces cerevisiae. Eur J Biochem. 1995;231:845–854. doi: 10.1111/j.1432-1033.1995.tb20770.x. [DOI] [PubMed] [Google Scholar]
- Irie K, Takase M, Lee KS, Levin DE, Araki H, Matsumoto K, Oshima Y. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol Cell Biol. 1993;13:3076–3083. doi: 10.1128/mcb.13.5.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann J, Munro S. Multi-protein complexes in the cis Golgi of Saccharomyces cerevisiae with α-1, 6-mannosyltransferase activity. EMBO J. 1998;17:423–434. doi: 10.1093/emboj/17.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Jung US, Piotrowski J, Levin DE. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Karpova TS, Moltz SL, Riles LE, Guldener U, Hegemann JH, Veronneau S, Bussy H, Cooper JA. Depolarization of the actin cytoskeleton is a specific phenotype in Saccharomyces cerevisiae. J Cell Sci. 1998;111:2689–2696. doi: 10.1242/jcs.111.17.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis FM. Cell wall assembly in yeast. Yeast. 1994;10:851–869. doi: 10.1002/yea.320100702. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Ohta T, Urakami H, Shiratori Y, Takasuka T, Satoh M, Watanabe T, Furuichi Y. Pore formation on proliferating yeast Saccharomyces cerevisiae cell buds by HM-1 killer toxin. J Biochem (Tokyo) 1996;119:731–736. doi: 10.1093/oxfordjournals.jbchem.a021303. [DOI] [PubMed] [Google Scholar]
- Koo JC, Chun HJ, Park HC, Kim MC, Koo YD, Koo SC, Ok HM, Park SJ, Lee SH, Yun DJ, Lim CO, Bahk JD, Lee SY, Cho MJ. Over-expression of a seed specific hevein-like antimicrobial peptide from Pharbitis nil enhances resistance to a fungal pathogen in transgenic tobacco plants. Plant Mol Biol. 2002;50:441–452. doi: 10.1023/a:1019864222515. [DOI] [PubMed] [Google Scholar]
- Koo JC, Lee SY, Chun HJ, Cheong YH, Choi JS, Kawabata S, Miyagi M, Tsunasawa S, Ha KS, Bae DW, Han CD, Lee BL, Cho MJ. Two hevein homologs isolated from the seed of Pharbitis nil L. exhibit potent antifungal activity. Biochim Biophys Acta. 1998;1382:80–90. doi: 10.1016/s0167-4838(97)00148-9. [DOI] [PubMed] [Google Scholar]
- Kubler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 1993;12:2855–2862. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Levin DE. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol Cell Biol. 1992;12:172–182. doi: 10.1128/mcb.12.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee OS, Lee B, Park N, Koo JC, Kim HY, Prasad DT, Karigar C, Chun HJ, Jeong BR, Kim DH, Nam J, Yun JG, Kwak SS, Cho MJ, Yun DJ. Pn-AMPs, the hevein-like proteins from Pharbitis nil confers disease resistance against phytopathogenic fungi in tomato, Lycopersicum esculentum. Phytochemistry. 2003;62:1073–1079. doi: 10.1016/s0031-9422(02)00668-4. [DOI] [PubMed] [Google Scholar]
- Lipke PN, Ovalle R. Cell wall architecture in yeast: new structure and new challenges. J Bacteriol. 1998;180:3735–3740. doi: 10.1128/jb.180.15.3735-3740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni C, Zarov P, Rambourg A, Mann C. The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J Cell Biol. 1993;123:1821–1833. doi: 10.1083/jcb.123.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaden P, Hill K, Wagner J, Slipetz D, Sommer SS, Bussey H. The yeast KRE5 gene encodes a probable endoplasmic reticulum protein required for (1-6)-β-D-glucan synthesis and normal cell growth. Mol Cell Biol. 1990;10:3013–3019. doi: 10.1128/mcb.10.6.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau V, Madania A, Martin R, Winson B. The Saccharomyces cerevisiae actin-related protein Arp2 is involved in the actin cytoskeleton. J Cell Biol. 1996;134:117–132. doi: 10.1083/jcb.134.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A, Stevenson B, Geli M, Riezman H. end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol Biol Cell. 1995;6:1721–1742. doi: 10.1091/mbc.6.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Read EB, Okamura HH, Drubin DG. Actin- and tubulin-dependent functions during Saccharomyces cerevisiae mating projection formation. Mol Biol Cell. 1992;3:429–444. doi: 10.1091/mbc.3.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer T, Bussey H. Yeast β-glucan synthesis: KRE6 encodes a predicted type II membrane protein required for glucan synthesis in vivo and for glucan synthase activity in vitro. Proc Natl Acad Sci USA. 1991;88:11295–11299. doi: 10.1073/pnas.88.24.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. Cloning in yeast. In: Glover DM, editor. DNA Cloning. IRL Press; Oxford: 1985. pp. 45–66. [Google Scholar]
- Schmidt A, Hall MN. Signaling to the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:305–338. doi: 10.1146/annurev.cellbio.14.1.305. [DOI] [PubMed] [Google Scholar]
- Selitrennikoff CP. Antifungal proteins. Appl Environ Microbiol. 2001;67:2883–2894. doi: 10.1128/AEM.67.7.2883-2894.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JA, Mol PC, Bowers B, Silverman SJ, Valdivieso MH, Duran A, Cabib E. The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1991;114:111–123. doi: 10.1083/jcb.114.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:12–17. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Spiegel MR. Shaum’s Outline of Theory and Problems of Probability and Statistics. McGraw-Hill Book Company; New York: 1975. [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Thevissen K, Cammue BP, Lemaire K, Winderickx J, Dickson RC, Lester RL, Ferket KK, Van Even F, Parret AH, Broekaert WF. A gene encoding a sphingolipid biosynthesis enzyme determines the sensitivity of Saccharomyces cerevisiae to an antifungal plant defensin from dahlia (Dahlia merckii) Proc Natl Acad Sci USA. 2000;97:9531–9536. doi: 10.1073/pnas.160077797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Cammue BP, Thevissen K. Plant defensins. Planta. 2002;216:193–202. doi: 10.1007/s00425-002-0902-6. [DOI] [PubMed] [Google Scholar]
- Torres L, Martin H, Garcia-Saez MI, Arroyo J, Molina M, Sanchez M, Nombela C. A protein kinase gene complements the lytic phenotype of Saccharomyces cerevisiae lyt2 mutants. Mol Microbiol. 1991;5:2845–2854. doi: 10.1111/j.1365-2958.1991.tb01993.x. [DOI] [PubMed] [Google Scholar]
- Van der Vaart JM, te Biesebeke R, Chapman JW, Toschka HY, Klis FM, Verrips CT. Comparison of cell wall proteins of Saccharomyces cerevisiae as anchors for cell surface expression of heterologous proteins. Appl Environ Microbiol. 1997;63:615–620. doi: 10.1128/aem.63.2.615-620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshuk CP, Meulenhoff JS, Sela-Buurlage M, van den Elzen PJ, Cornelissen BJ. Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell. 1991;3:619–628. doi: 10.1105/tpc.3.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Ayscough KR, Drubin DG. A role for the actin cytoskeleton of Saccharomyces cerevisiae in bipolar bud-site selection. J Cell Biol. 1997;136:111–123. doi: 10.1083/jcb.136.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip CL, Welch SK, Klebl F, Gilbert T, Seidel P, Grant FJ, O’Hara PJ, MacKay VL. Cloning and analysis of the Saccharomyces cerevisiae MNN9 and MNN1 genes required for complex glycosylation of secreted proteins. Proc Natl Acad Sci USA. 1994;91:2723–2727. doi: 10.1073/pnas.91.7.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun DJ, Zhao Y, Pardo JM, Narasimhan ML, Damsz B, Lee H, Abad LR, D’Urzo MP, Hasegawa PM, Bressan RA. Stress proteins on the yeast cell surface determine resistance to osmotin, a plant anti-fungal protein. Proc Natl Acad Sci USA. 1997;94:7082–7087. doi: 10.1073/pnas.94.13.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.