Abstract
A growing body of literature suggests that human adipose-derived stromal cells (hASCs) possess developmental plasticity both in vitro and in vivo, and might represent a viable cell source for therapeutic angiogenesis and tissue engineering. We investigate their phenotypic similarity to perivascular cell types, ability to contribute to in vivo microvascular remodeling, and ability to modulate vascular stability. We evaluated hASC surface expression of vascular and stem/progenitor cell markers in vitro, as well as any effects of PDGF-BB and VEGF165 on in vitro hASC migration. To ascertain in vivo behavior of hASCs in an angiogenic environment, hASCs were isolated, expanded in culture, labeled with a fluorescent marker, and injected into adult nude rat mesenteries that were stimulated to undergo microvascular remodeling. 10, 30, and 60 days after injection, tissues from anesthetized animals were harvested and processed with immunohistochemical techniques to determine hASC quantity, positional fate in relation to microvessels, and expression of endothelial and perivascular cell markers. After 60 days, 29% of hASCs exhibited perivascular morphologies compared to 11% of injected human lung fibroblasts. hASCs exhibiting perivascular morphologies also expressed markers characteristic of vascular pericytes: smooth muscle α-actin (SMA) (10%) and NG2 (8%). In tissues treated with hASCs, vascular density was significantly increased over age-matched controls lacking hASCs. This study demonstrates that hASCs express pericyte lineage markers in vivo and in vitro, exhibit increased migration in response to PDGF-BB in vitro, exhibit perivascular morphology when injected in vivo, and contribute to increases in microvascular density during angiogenesis by migrating toward vessels.
Keywords: adipose-derived stromal cells, microcirculation, pericyte, angiogenesis
Much attention has been devoted to the plasticity of different adult cell types, termed “adult stem cells” or “progenitor cells”, in injury responses and as a therapeutic tool. Circulating [1,2], bone marrow-derived [3,4,5], and tissue-derived [6] progenitor cells have been implicated in the process of microvascular remodeling. An expanding body of literature suggests that cells within the stromal-vascular fraction (SVF) of human adipose tissue possess substantial developmental plasticity, both in vitro [7] and in vivo [8,9]. These stromal cells have alternatively been referred to as processed lipoaspirate cells (PLA), adipose-derived stem cells, and adipose-derived stromal cells. We refer to these cells as adherent adipose-derived stromal cells (ASCs) to distinguish them from stromal vascular fraction (SVF) cells, which have not been separated based on adherence to tissue culture plastic. The cell surface phenotype of adherent hASCs differs from freshly isolated SVF cells, undergoing changes in response to variables such as exposure to tissue culture plastic, duration in culture, and exposure to specific medias/supplements [8,9,10,11,12,13,14]. Previous studies suggest that stromal cells isolated from adipose tissue on the basis of adherence to tissue culture plastic have a remarkably consistent molecular and cell surface profile [8,15,16]. Interestingly, this profile is quite similar to that of stromal cells isolated from bone marrow (also referred to as mesenchymal stem cells (MSCs) [7,11,17,18,19]), yet an easily definable phenotype remains elusive.
Human ASCs are readily available as they can be harvested in large quantities using minimally-invasive techniques, and they can be expanded in vitro [20]. In addition, previous work has shown that hASCs can be genetically modified to secrete proangiogenic proteins [9], making this cell population an appealing and practical candidate for translation of autologous transplantation strategies into the clinical setting. These cells have been shown to differentiate into chondrogenic, myogenic, osteogenic, and adipogenic cells in the presence of lineage-specific induction factors in culture [20]. Moreover, adipose-derived stromal cells have been shown to differentiate into endothelial cells [8,10,14,21], form vascular-like sprouts in matrigel [8], enhance neovascularization in an ischemic hindlimb model [8,9,10], and secrete angiogenic and anti-apoptotic growth factors [10], suggesting a potential for this cell population in therapeutic vascularization and tissue engineering of vascularized constructs. It has been hypothesized that the pro-angiogenic activity of human adipose-derived stromal cells is a combined result of their ability to produce angiogenic growth factors and to differentiate into endothelial cells [8,9,10,14,21]. Additionally, several recent studies have shown in vitro evidence that hASCs can assume a pericyte role; however, data supporting functional benefit to the vasculature in vivo have not yet been produced [22,23,24], leaving the in vivo role of hASCs as perivascular cells in question.
Although most previous work has focused on the role of endothelial cell migration and proliferation during angiogenesis, a critical component of microvascular growth is the recruitment of perivascular support cells (such as pericytes and smooth muscle cells) to the abluminal surface of the microvessel wall. This step is important for vessel maintenance via prevention of microvascular regression [15], physical guidance of capillary sprouts [25], and regulation of capillary permeability [26]. Furthermore, it has been suggested that pericytes can differentiate into vascular smooth muscle cells in response to growth factor signals and function to transform a capillary into a contractile arteriole, thus participating in the process of arteriogenesis [27,28].
Since it has been suggested that pericytes contribute to microvessel growth [25] and maintenance [15], we tested the hypothesis that hASCs function as microvascular support cells by analyzing their perivascular investment in relation to changes in total vascular density. We show on a single cell level that hASCs are capable of expressing perivascular-cell markers in vitro and in vivo, responding to PDGF-BB with increased migration in vitro, exhibiting pericyte-like morphologies in vivo by migrating to the abluminal surface of microvessels and conforming to the curvature of the microvessel in a manner that is consistent with pericyte (and not endothelial) cell behavior, and increasing total microvascular length when injected into remodeling rat mesenteries compared to mesenteries receiving vehicle control (no cells) or hLFs. Therefore, we present pericyte-like behavior as a role for human adipose-derived stromal cells in the promotion of vascular stability and the enhancement of microvascular growth in vivo.
Materials and Methods
Isolation, Culture, and Labeling of hASCs
Subcutaneous adipose tissue was obtained from 14 female patients undergoing elective surgical procedures in the Department of Plastic Surgery, University of Virginia (age range: 30-56; mean = 42 years old) for in vivo experiments. The University of Virginia’s Human Investigation Committee approved tissue harvest protocols. Adipose tissue came either from intraoperative suction lipectomy (n=6) or from laboratory liposuction of panniculectomy specimens (n=8). Cells used in scratch test experiments were isolated by intraoperative suction lipectomy from a single 38 year old female donor.
Cells were isolated from adipose tissue using methods previously described [11,20]. Briefly, harvested tissue was washed several times and enzymatically dissociated [11,29]. Dissociated tissue was filtered to remove debris, and the resulting cell suspension was centrifuged. Pelleted stromal cells were recovered and washed several times. Contaminating erythrocytes were lysed with an osmotic buffer, and the stromal cells were plated onto tissue culture plastic. Cultures were washed with buffer 24-48 hours after plating to remove unattached cells, and then re-fed with fresh medium. Plating and expansion medium consisted of DMEM/F12 (Invitrogen) with 10% FBS (Invitrogen) and 1% antibiotic-antimycotic (Invitrogen). Cultures were maintained at 37°C with 5% CO2 and were fed three times per week.
Cells were grown to confluence after the initial plating (passage 0; P=0), typically within 10-14 days. Adherent cells were released with either 0.5% trypsin-EDTA or Accutase cell detachment medium (Innovative Cell Technologies) and replated at 2,000 cells/cm2 (P=1). Cell cultures were passaged every 7-8 days until analysis. All cells used for injection studies were between passage 2 and 4, corresponding to approximately 11 or fewer total population doublings.
One day prior to injection, cells were labeled with the fluorescent marker DiI (5uM) according to manufacturer’s instructions (Molecular Probes, OR). Cells were rinsed, trypsinized, counted, and resuspended for injection. The use of DiI as a label for identifying progenitor cells in vivo has been well documented [16,30,31,32]. Furthermore, plated hASCs were labeled with DiI and maintained in culture to confirm that DiI fluorescence did not diminish visibly over time or with cell division.
Immuno-phenotypic Characterization of hASCs
The vascular-related cell surface phenotype of hASCs was analyzed using flow cytometry. Freshly isolated (SVF) cells and cultured hASCs were evaluated for their expression of the following cell surface proteins over time in culture: CD31 (PECAM-1) (BD Bioscience, San Diego, CA), CD34 (BD Bioscience; Santa Cruz Biotechnology, Inc., CA; and Caltech Labs, CA), CD133 (Miltenyi Biotech, CA), CD144 (VE-Cadherin) (BD Bioscience), CD140b (PDGF-ß receptor) (BD Bioscience), and NG2 (Beckman Coulter, FL). Flow cytometry was performed on a Becton Dickinson FACS Calibur, as previously described [11]. A minimum of 10,000 events were counted for each analysis. HLA-ABC was used as a positive control for each flow trial. Isotype-matched (negative) controls were also performed in all cases.
In Vitro Scratch Test
Isolated ASCs were allowed to grow to confluence on culture-treated plastic (Corning) at P=3 (37°C, 5% CO2). Cells were cultured for 24 hours prior to experimentation in DMEM/F12 with 0% FBS and 1% antibiotic/antimycotic. A 1000 μl pipette tip was used to scratch each dish five times before cells were washed with DPBS.
The cells were then subjected to culture in DMEM/F12 containing no serum, 1% antibiotic/antimycotic and either 10 ng/ml recombinant human PDGF-BB (R&D Systems, Minneapolis, MN) or 20 ng/ml recombinant human VEGF165 (Biovision Inc., Mountain View, CA). In a separate experiment, scratch wounds were allowed to heal with either 10 ng/ml PDGF-BB, 500 ng/ml PDGF-BB antibody (Chemicon Int., Temecula, CA), 1 μg/ml PDGF receptor β antibody (Sigma Biosciences, St. Louis, MO), 10 ng/ml PDGF-BB plus 500 ng/ml PDGF-BB antibody, or 10 ng/ml PDGF-BB plus 1 μg/ml PDGF β-receptor antibody. Individual untreated controls containing no additives were run in parallel for each of these two experimental designs. All cells were used at passage 3.
Dishes were imaged at 0, 12, 20, and 30 hours after introduction of scratches. In all cases, scratch healing was determined by measuring the shortest distance between scratch edges in each field of view. At least 16 fields of view were analyzed for each condition at each time point comparing PDGF and VEGF effects on scratch closure, and at least 51 fields of view were analyzed for each condition at each time point comparing the abilities of PDGF antibody and PDGF β-receptor antibody to abrogate scratch closure in the presence of PDGF.
Animal Studies
Experiments were performed using sterile techniques according to the guidelines of the University of Virginia Animal Care and Use Committee. 72 male nude rats (NCI) weighing 250±20 grams were divided into six study groups: 1) hASC injection (1×106 cells), 2) hASC injection (1×106 cells) and 48-80 stimulation, 3) human lung fibroblast (hLF) injection (1×106 cells), 4) hLF injection (1×106 cells) and 48-80 stimulation, 5) vehicle control (sterile PBS), 6) vehicle control and 48-80 stimulation.
Stimulation of Microvascular Remodeling with Compound 48/80 and Cell Injection
Compound 48/80 (condensation product of N-methyl-p-methoxyphenylethylamine with formaldehyde; Sigma) is a pharmacological agent known to act specifically on mast cells by inducing degranulation. Injection of Compound 48/80 into the rat mesentery stimulates well-characterized microvascular growth and remodeling in the mesenteric vasculature [25,33], and this small animal model is a well-established assay for studying cellular and molecular mechanisms of angiogenesis [34,35]. Compound 48/80 was injected I.P. (1 ml/100 gram animal weight) in 0.9% sterile NaCl on the first 5 consecutive study days. 2 doses of each concentration (100, 200, 300, and 400 μg/ml) were administered per day separated by 8 hours on the first four days. On day 5, rats in these study groups received a single dose of 500 μg/ml. To rule out any direct effects of Compound 48/80 on hASC survival, proliferation, and differentiation, cultured hASCs (P=2) were exposed to either 100 or 5 μg/ml of Compound 48/80 in the media for six days.
On day 4 of 48-80 injections, 1×106 DiI labeled hASCs or hLFs (WI-38 cell line, No. CCL-75, ATCC) in 0.5 ml sterile PBS were injected I.P. using syringes with 25 3/8 G, 0.5 inch needles.
Harvesting of Mesenteric Tissue
Rats were anesthetized with intramuscular injections of ketamine (80 mg/kg b.w.), atropine (0.08 mg/kg b.w.), and xylazine (8 mg/kg b.w.). 6 mesenteric windows were harvested from each animal 10, 30, or 60 days after cell injection. Tissues were whole-mounted on gelatin-coated slides.
Immunohistochemistry
To determine if injected hASCs expressed markers consistent with a perivascular cell phenotype, tissues were immunostained for an array of markers known to be expressed by smooth muscle cells and pericytes: NG2, SMA, PDGF receptor β, and desmin [36,37]. Tissues were washed in phosphate buffered saline (PBS) + 0.1% saponin 3 times for 10 minutes and immunolabeled with lectin from Bandeiraea simplicifolia (BSI-lectin) FITC-conjugate (1:100, Sigma) or Alexa Fluor 647-conjugate (1:100, Molecular Probes), and/or antibody to smooth muscle α-actin (SMA) using purified FITC-conjugated clone 1A4 mouse monoclonal anti-SMA (1:500, Sigma), diluted in PBS buffer containing 0.1% saponin and 2% bovine albumin (Fisher Scientific) at pH 7.4 (incubation for 1 hour at room temperature). Tissues were also stained with perivascular cell markers [36], including antibodies to: NG2 (1:150, rabbit polyclonal, Chemicon), Desmin (1:100, mouse anti-human clone D33, DAKO, Denmark), and PDGF-βR (1:100, rabbit polyclonal, Santa Cruz Biotechnology). Cy2-conjugated secondary antibodies were applied for 1 hour at room temperature: 1 & 3) goat anti-rabbit IgG, and 2) goat anti-mouse IgG-fab fragment (1:100, Jackson Immunoresearch, PA). In the final wash cycle, Hoechst 33342 (1×10-6 mM) or TOTO-3 (1:1000; Molecular Probes) was added for visualization of nuclei.
Image Acquisition and Data Analysis
Mesenteric tissues were examined with a Nikon Eclipse TE2000-E microscope equipped with confocal accessories (Nikon D-Eclipse C1) using 20X Nikon water/oil immersion and 60X Nikon oil immersion objectives. Images were digitized and analyzed using Scion Image software version 4.0.2 (Scion Corporation). The number of DiI-positive cells per tissue area and total microvessel length were quantified. Nuclei were visualized with TOTO-3 and Hoechst 33342 to confirm the presence of DiI-labeled hASCs and hLFs.
While there is no single marker/protein that defines a pericyte phenotype, NG2, SMA, and desmin are considered to be supportive of, and consistent with, this lineage [36]. Pericytes are perhaps best defined by their histological and anatomical positions and shape, with the “sine qua non” being their close physical contact with microvascular endothelial cells.
Therefore, to compliment and expand upon our immunophenotypic findings, detected cells were examined for pericyte-like morphology, defined here as cells whose processes extend along vessels in a manner that conforms to the curvature of the vessel and whose cell bodies are no more than 5 micrometers from the abluminal surface of the endothelium. Morphometrists were blinded to the treatment group at the time of analysis. The cell behavior quantified using this method is distinct from smooth muscle cell morphology, which is characterized by wrapping of the smooth muscle cell around the abluminal endothelial surface in a direction perpendicular to the vessel axis and parallel to adjacent smooth muscle cells. Therefore, we term the cell morphology observed here “pericyte-like” as opposed to “perivascular-like,” which encompasses both pericyte and smooth muscle cell morphology.
Scratch test data was collected using a Nikon TE2000-E2 equipped with confocal accessories (Nikon D-Eclipse C1) using a 10X Nikon air objective and an Olympus Microfire digital camera. This data was analyzed using ImageJ ver. 1.37 software.
Statistical Analysis
Results are presented in the form of mean ± standard error. Comparisons for in vivo data were made using the statistical analysis tools provided by SigmaPlot 5.0 (Systat Software, Inc., Chicago, IL). Data were tested for normality and analyzed by one-way ANOVA followed by non-paired Tukey’s T-test. Significance was asserted at p<0.05. In vitro experiments were analyzed using SigmaStat 3.5 (Systat Software, Inc.) and are presented in the form of ± one standard deviation. This data was analyzed by unpaired t-test or Mann-Whitney Rank Sum test when normality tests failed.
Results
Immuno-Characterization of hASCs
The vascular-related cell surface profile of hSVF and hASCs were analyzed using flow cytometry and/or immunohistochemistry (SMA, Desmin). A very small percentage (2.5% or less) of early passage hASCs stain positively for markers of mature and/or activated endothelial cells (CD31, CD144), and these phenotypic markers become undetectable by passage 5 (Figure 1A). Under the described isolation and culture conditions, less than 1% of hASCs stain for the progenitor/stem cell marker CD133 (data not shown), whereas 9-97% of them stain positively for CD34, with specific expression levels depending on both the length in culture and the specific antibody utilized (Figure 1B). hASCs consistently stain for several markers consistent with a perivascular phenotype [36] including NG2 (Figure 1C, mean of P=1 through P=4 equals 15±6%, n=3), PDGF receptor β (CD140b; 76-97% by flow; mean = 90%), and smooth muscle actin (SMA; approximately 25% by immunocytochemistry—data not shown). Flow cytometry data showed that Compound 48/80 did not enhance NG2 expression (data not shown).
Figure 1. In Vitro Characterization of hASC Surface Marker Expression.

(A) Average expression (± standard deviation) of CD31 (white) and CD144 (black) is minimal and declines to 0%. (B) Detected expression of CD34 depends on the specific antibody used (8G12: white, 581: black, BI-3C5: gray). (C) Average expression (± standard deviation) of NG2 using flow cytometry.
hASCs Migrate Toward PDGF-BB In Vitro
When hASCs were allowed to migrate into an in vitro scratch wound assay, dishes supplemented with 10 ng/ml PDGF-BB showed significantly more wound closure than either dishes supplemented with 20 ng/ml VEGF165 or unsupplemented dishes at 12, 20, and 30 hours (Figure 2A). Dishes treated with VEGF exhibited more wound closure at each of these time points than unsupplemented dishes as well. In the negative control studies, hASC migration in the presence of PDGF-BB was significantly inhibited by both the addition of excess PDGF-BB antibody and excess soluble PDGF receptor β at all time points (Figure 2B). Unsupplemented control scratch wounds showed significantly less scratch closure at 30 hours than all conditions except PDGF receptor β reagent controls (soluble receptor β addition without PDGF-BB supplementation).
Figure 2. In Vitro Migration of hASCs in the Presence of PDGF-BB or VEGF165.

(A) Relative hASC migration (± standard deviation; * = p<0.001) into untreated scratch wounds or wounds treated with VEGF165 or PDGF-BB. (B) Inhibition of PDGF-BB-induced migration using antibodies to PDGF-BB and PDGF receptor β (± standard deviation; * = p<0.001; ‡ = p<0.005; † = p<0.01; # = p<0.05).
Total Numbers of hASCs and hLFs Retained in Mesentery is Dependent on Stimulus and Time
The total number of hASCs in the mesenteric tissue exhibited a biphasic response, decreasing from day 10 to 30 and increasing from day 30 to 60 (Table 1). In contrast, the total number of hLFs in mesenteric tissues remained relatively constant, yet was significantly diminished when averaged over all three analysis time points compared to hASCs. When hASCs or hLFs were injected into tissues that had not received Compound 48/80 treatment, they were initially (day 10) undetectable in number (Table 1, Day 10). Without Compound 48/80 treatment hASC numbers at the late time point (Day 60) were significantly diminished compared to what was measured in Compound 48/80-treated tissues at this time point (Table 1, Day 60, compare top two rows). This was not the case with hLFs at day 60, which showed statistically similar values regardless of Compound 48/80 treatment (Table 1, Day 60, compare bottom two rows).
Table 1.
Total number of injected cells per mm2 in 48/80-treated and un-treated tissues.
| Cell Type | Treatment Type | Day 10 | Day 30 | Day 60 |
|---|---|---|---|---|
| hASC | +48/80 | 202±58*,† | 9±5 | 63±5*,† |
| -48/80 | 0±0 | 6±6 | 4±4* | |
| hLF | +48/80 | 21±9 | 17±3 | 21±3 |
| -48/80 | 0±0 | 5±2 | 55±6 |
significantly different from corresponding hLF (human lung fibroblast) study group,
significantly different from hASCs (human adipose-derived stromal cells) without 48/80 treatment (-48/80), p≤0.05.
hASCs Exhibit Perivascular Morphology In Vivo
hASCs (Figure 3A-D) and hLFs (Figure 3E) are easily identified in the mesenteric tissue, which is a thin tissue (approximately 100 μm thick) that permits en face visualization of entire microvascular networks and single-cell resolution of microvessel and cell morphology. As early as 10 days following injection, hASCs are visible in inflamed mesenteric tissue and upwards of 20% exhibit pericyte-like morphology (Figure 4A), as defined above. hLFs at this time point also exhibit this morphology to a statistically similar extent. This cell behavior by hASCs and hLFs was observed to a much lesser degree after 30 days, but increased after 60 days in vivo (Figure 4A), and was independent of cell passage number (P=2 to P=4) at the time of injection (data not shown). After 60 days, a significantly higher number of hASCs than hLFs exhibited pericyte-like morphologies in tissues treated with Compound 48/80 (Figure 4A). Cell counts and flow cytometry confirmed that subjecting hASCs to Compound 48/80 in vitro did not directly promote hASC survival but, in fact, reduced cell proliferation and viability (data not shown).
Figure 3. Pericyte-like Localization and Surface Marker Expression of hASCs in Rat Mesentery.

Rat mesenteric tissues injected with human cells were harvested 60 days later and immunostained to visualize microvessels. A) Some DiI(+) hASCs express NG2 (yellow, arrows), some do not (red), and some exhibit pericyte-like morphologies (vessel wrapping) and are aligned with capillaries that express BSI-lectin (blue). B) Some hASCs expressing SMA (yellow) and exhibiting pericyte-like morphologies (arrows) along BSI-lectin-positive capillaries (blue). C-D) Examples of DiI-labeled SMA-expressing hASCs (yellow) in mesenteric tissue co-stained with SMA and BSI-lectin (both stains are green). E) Some hLFs (red) exhibit pericyte-like morphologies (arrows) along BSI-lectin-positive capillary (blue) and SMA-positive venules (green), although hLFs do not express SMA and total vessel density is reduced compared to hASC-injected tissue, as seen in B. F) No DiI(+) cells are present in control, un-injected tissues (green: BSI-lectin). Scale bar = 25 μm in A,B,E,F; scale bar = 20 μm in C,D.
Figure 4. Quantification of In Vivo Pericyte-like Localization and Surface Marker Expression in 48-80-stimulated tissues.

(A) Percentage of hASCs and hLFs that exhibit pericyte-like morphologies regardless of pericyte marker expression. (B) Percentage of hASCs and hLFs in the tissue that exhibit pericyte-like morphologies (but not pericyte markers). (C) Percentage of total hASCs in the tissue that express pericyte markers (but not morphology). (D) Percentage of total hASCs in the tissue that both exhibit pericyte-like morphologies and express pericyte markers. Error bars represent standard error; * = (p≤0.05).
hASCs Express Perivascular Cell Markers In Vivo
We quantified the relationship between perivascular marker expression and pericyte-like morphologies by injected human cells. Although we never observed hASCs or hLFs in an endothelial cell location or via co-labeling with BSI-lectin (an endothelial cell marker), hASCs express a number of perivascular cell (pericytes and smooth muscle cell) markers in vivo according to immunohistochemistry (Figure 4C,D). Of the sub-population of hASCs not expressing perivascular cell markers, significantly more hASCs than hLFs in the tissue exhibited pericyte-like morphologies (Figure 4B) at day 60. Of the subpopulation of hASCs not exhibiting a pericyte-like morphology, significantly more expressed NG2 and SMA compared to those that expressed desmin (Figure 4C) at day 60. More of the hASCs not associated with vessels expressed NG2 than PDGF receptor β, as well. A subpopulation that comprises approximately 8% of all hASCs in the tissue both exhibits pericyte-like cell morphologies and also expresses NG2. Likewise, a subpopulation encompassing ~10% of all hASCs both exhibit pericyte-like cell morphologies and also express SMA (Figure 4D). No hLFs were observed expressing NG2 or desmin at any timepoint. SMA expression in hLFs at day 60 was 2.48% of observed hLFs. No hLFs were observed expressing any pericyte markers while exhibiting a pericyte-like morphology.
Vascular Density of Microvessels Is Affected by hASCs at Early and Late Time Points
Since some hASCs exhibited pericyte-like morphologies in vivo, and native pericytes in the microvasculature have been shown to contribute to microvessel maintenance and prevent vascular regression, we investigated the functional effect of adipose-derived cells on microvascular length density in networks stimulated to undergo remodeling. Compound 48/80 stimulation alone (no cells), is known to evoke peak vascular remodeling responses between 14 and 20 days after delivery [33]. Accordingly, our data shows that even by day 10, Compound 48/80 increases vascular length density approximately 35% in the absence of cells compared to vascular length density in mesenteries receiving neither Compound 48/80 nor hASCs (Figure 5; compare 10 day white bars between 5A and 5B). 10 days after hASC injection, length density, or total vascular length, increased significantly in tissues treated with hASCs relative to tissues treated with hLFs (Figure 5A; compare black bar to gray bar). This observation is independent of Compound 48/80 treatment (Figure 5B). After 30 days, there was no significant difference in length density between treatment groups in 48-80 stimulated (Figure 5A) or un-stimulated tissues (Figure 5B). By day 60, the presence of hASCs in both Compound 48/80 stimulated (Figure 5A) and un-stimulated tissues (Figure 5B) evokes significant length density increases compared to that of hLF-treated and vehicle control-treated tissues. In stimulated tissues, this increase in length density coincides temporally with the elevated percentage of hASCs exhibiting a pericyte-like morphology compared to hLFs (Figure 4A). Furthermore, in tissues receiving hASCs, vascular length density at day 60 is elevated to levels comparable to those measured on day 10, regardless of Compound 48/80 treatment (Figure 5A, 5B).
Figure 5. Functional Impact of hASCs on Vascular Length Density In Vivo.

Vascular length density for vehicle control (no cells: white) vs. hASC (black) vs. hLF (gray) in (A) 48-80-treated tissues and (B) tissues not treated with 48-80. Error bars represent standard error; * = p≤0.05.
Correlation between hASCs Perivascular Cell Marker Expression, Morphology and Vascular Density
To determine if hASC pericyte-like morphology and marker expression were correlated with changes in vascular length density, the percentage of total hASCs exhibiting pericyte-like morphologies (Figure 6A) and the percentage of total hASCs exhibiting pericyte-like morphologies and expressing perivascular cell markers (Figure 6B) was plotted against vessel length density quartiles (for example, the 4th quartile represents data from the tissue specimens whose vascular length densities fall in the top 25th percentile) at day 60. hLFs did not exhibit these correlations with vascular length density (data not shown).
Figure 6. Distribution of hASCs With Respect to Length Density Quartile.
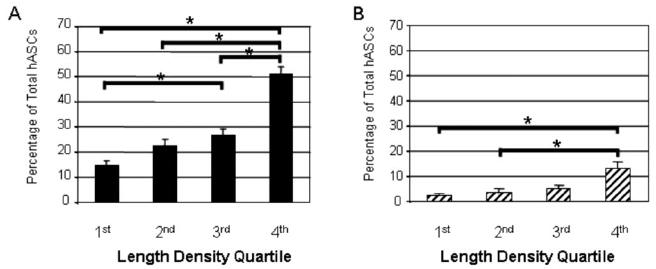
A) Percentage of total hASCs in the tissue that exhibit pericyte-like-morphologies at day 60, and B) percentage of total hASCs that exhibit pericyte-like morphologies and also express pericyte markers for each quartile of length density at day 60. Error bars represent standard error; * = significantly different (p≤0.05).
Discussion
hASCs Exhibit A Pericyte-like Phenotype both In Vitro and In Vivo
A characterization of hASC in vitro phenotype showed that 15% or more of all isolated hASCs express pericyte markers (NG2, PDGF receptor β, and/or SMA), while only a small number express endothelial markers (CD31 and CD144). Much of the observed endothelial marker expression is lost between passages 3 and 5; however, it is unclear if this decrease is due to cell death/selection and/or de-differentiation. In the light of the fact that hASCs were never observed co-staining with BSI-lectin in vivo, there was no evidence for cultured hASCs assuming endothelial cell morphology in this in vivo model. NG2 and SMA are known to be expressed by quiescent [37] and remodeling pericytes and smooth muscle cells [36], and the fact that these markers are expressed by hASCs both before and after injection (moreover by a subpopulation of hASCs that also exhibits pericyte-like morphology) suggests that a subfraction of isolated hASCs may be perivascular cells or have the potential to differentiate into perivascular cells. Pericyte marker expression of hASCs after in vivo injection is reinforced by the realization of pericyte-like morphology or abluminal “wrapping”. Increased in vitro migration in the presence of PDGF-BB offers a potential mechanism through which hASCs would be able to behave as pericytes in vivo. The increase in migration elicited by VEGF165 is significantly more modest, but is still significant with respect to controls. This can be a result of either the small population of isolated hASCs expressing endothelial surface markers or a population of pericytes within the hASC population exhibiting chemoattraction toward VEGF [36]. The ability to migrate in response to PDGF-BB coupled with in vivo expression of PDGF receptor β and morphological “wrapping” of blood vessels in vivo suggests that hASCs use this mechanism to localize to vessels and assume a pericyte-like phenotype in vivo.
The reason for the apparent increase in pericyte marker expression relative to endothelial marker expression may be as simple as the fact that the hASCs in this study were cultured before use. It is possible that culturing hASCs could select for pericyte-like cells over endothelial cells via their relative abilities to adhere to culture plastic. Alternatively, the genetic expression profiles of the hASCs may be altered by the artificial conditions of culture (adhesion mechanisms/forces, media ingredients, oxygenation, pH), and that the cells are truly differentiating into pericyte-like cells.
As reported both in these experiments and those performed by other groups [13,14,39], time in culture is a factor for expression levels of CD34, a molecule generally recognized as a marker of hematopoietic stem/progenitor cells, as well as microvascular endothelial cells, bone marrow stromal progenitors, and fibroblast-like dendritic cells from adult dermis and adipose tissue [40]. Approximately 1% of early passage hASCs were found to be CD133+, a protein expressed by hematopoietic stem cells and/or endothelial progenitor cells [41]. This is consistent with Miranville et al., who reported 1.5-5.3% of freshly isolated SVF cells to express the CD133 marker, with exact levels varying depending on the source depot of adipose tissue [10]. The low level of CD133 expression in culture and loss of CD34 expression over time in culture could mean that time in culture causes hASC lineage commitment instead of subselection through adhesion.
It has been shown that detection and quantification of CD34 on a given cell population can be highly variable depending on the class of antibody that is employed, as well as the particular antibody conjugate [42]. Our findings for hASCs are consistent with this work, demonstrating higher levels of CD34 expression/detection in early passage cells compared to later passage cells, and when using Class III antibodies (8G12 and 581) compared to Class I antibody (BI-3C5 [42]). The qualitative implication of these quantitative discrepancies is not clear at this time, however; the potential significance of distinct CD34-positive hASC subpopulations deserves further evaluation.
hASCs Participate in Microvascular Growth and Maintenance
The fact that neither hASCs nor hLFs are detectable at day 10 in tissues untreated with Compound 48/80 suggests that either Compound 48/80 or the pro-angiogenic, inflamed tissue environment created by this stimulus promotes hASC retention/survival in vivo early on. The former explanation can be ruled out by in vitro studies (data not shown), indicating that if Compound 48/80 has any direct effect on hASCs, it is to moderately reduce, not enhance, their proliferative capabilities. This suggests, instead, that it is the environment created by Compound 48/80 that initially enhances hASC presence in the mesentery (Table 1, Day 10).
The lack of a significant difference in length density between 48-80-stimulated and un-stimulated tissues at day 30 is to be expected based on the transient nature of the remodeling response to Compound 48/80, whereby newly sprouted vessels gradually regress after the peak remodeling response, causing length density to return to control levels after the inflammatory response has subsided. The subsequent increase in length density at day 60 in 48-80-stimulated tissues coincides temporally with the elevated percentage of hASCs exhibiting a pericyte-like phenotype compared to hLFs (Figure 4, 5), suggesting that the presence of hASCs with pericyte-like phenotypes and the increase in vascular density are causally related. This supports the possibility that the presence of hASCs in the tissue at late time points provides longer lasting support to vessels that would normally regress in vehicle control- or hLF-treated tissues. Figure 6 shows that stimulated tissues with the highest length densities also have the highest percentages of hASCs exhibiting pericyte-like morphology and expressing pericyte markers. This suggests that there is a significant, and possibly causative, correlation between vascular length density and pericyte-like behavior in the inflamed environment.
Although ASC counts (Table 1) suggest that the inflamed tissue environment created by Compound 48/80 stimulation is supportive of cell proliferation or cell survival/migration, the vascular density results suggest that, in tissues un-stimulated by Compound 48/80, ASCs are able to persist in the in vivo environment and elicit a potent vascular density response at later time points, even though relatively few ASCs are invested in the mesenteric tissue. It is possible that although ASCs are not invested in the mesenteric tissue (and, therefore, are undetected) in animals untreated by Compound 48/80, they may be affecting the angiogenic environment through a paracrine mechanism. Thus the biphasic nature of the length density measurements and ASC investment in rat mesenteries could be a superposition of tissue remodeling in response to inflammation from initial injections and the paracrine angiogenic secretions of injected ASCs over time.
These two proposed mechanisms for increasing vascular length density are not identical, so we may hypothesize that the inflamed environment caused by Compound 48/80 treatment provides a cue for ASCs to assume a pericyte-like phenotype and associate directly with vessels in the mesenteric tissue instead of maintaining a default paracrine support phenotype. In comparison, the less inflamed environment of peritoneal cavities untreated with Compound 48/80 may be experiencing increased length densities at late time points due primarily to the angiogenic environment (and activation of native pericytes) provided by secretions from ASCs that are performing paracrine instead of pericyte-like roles. If large enough in magnitude, paracrine secretions may account for increases in vascular length density without a significant presence of ASCs in the mesenteric tissue itself.
Conclusions
This study presents a description of the in vivo behavior of unsorted, adherent hASCs on the single cell level, and suggests that this behavior is indicative of a vascular support or maintenance role. We show that injected hASCs show increased migration in response to VEGF165 and PDGF-BB in vitro, express markers of perivascular cells both in vivo and in vitro, persist over time in vivo, exhibit pericyte-like morphologies around microvessels, and contribute to vascular growth and/or maintenance, as reflected by increases in vascular density compared to vehicle or cell control-treated tissues. This work suggests that hASCs might be an effective source of perivascular cells for therapeutic delivery or in the engineering of tissue equivalents, where perivascular cell support signals are likely be necessary for long term vessel maintenance [43].
Future work is needed to determine if these behaviors are consistent with a vascular growth or vascular maintenance role. However, it has been shown that these cells produce antiapoptotic growth factors as well as angiogenic growth factors, including GM-CSF, HGF, bFGF, VEGF, and TGF-β, suggesting that both roles are possible and consistent with those of native pericytes [9,19]. Whether injected hASCs are able to differentiate into perivascular cells, if and how this is mediated by the in vivo tissue environment, and the relative importance of isolated hASCs expressing a perivascular phenotype prior to injection remain to be determined through future studies. Future work should also aim to decouple the presence of ASCs from their angiogenic secretions either by injecting appropriate amounts of those secretions over the 60 day period or silencing key proangiogenic secretions prior to ASC injection.
Acknowledgments
This work was supported by the National Institutes of Health grant HL72141 (Katz) and by a charitable gift from Dr. and Mrs. Peyton Weary (Katz) and by the University of Virginia Department of Biomedical Engineering (Peirce). The authors would like to thank the faculty and staff of the Department of Plastic Surgery for facilitating the harvest of adipose tissue and Peter Stapor for assistance with image analysis.
Footnotes
Author contributions: P.J.A.: Conception and design, Administrative support, Collection and assembly of data, Data analysis and interpretation, Manuscript writing; H.S.: Collection and assembly of data; A.M.B.: Collection and assembly of data; A.C.T.: Collection and assembly of data; A.J.K.: Financial support, Provision of study material and patients; S.M.P.: Conception and design, Financial support, Administrative support, Provision of study material, Collection and assembly of data, Data analysis and interpretation, Final approval of manuscript.
References
- 1.Iba O, Matsubara H, Nozawa Y, et al. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106:2019–2025. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 2.Peichev M, Naiyer AJ, Zhu Z, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Hemostas, Thombosis, and Vasc Biol. 2000;95:952–958. [PubMed] [Google Scholar]
- 3.Asahara T, Haruchika M, Tomono T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Rajantie I, Ilmonen M, Alminaite A, et al. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeliet P, Luttun A. The role of the bone marrow-derived stem cells in (therapeutic) angiogenesis. Thrombos & Haemostas. 2001;86:289–297. [PubMed] [Google Scholar]
- 6.Majka SM, Jackson KA, Kienstra KA, et al. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 8.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells. Circulation. 2004;109:r23–r30. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 9.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:r52–r58. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 10.Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 11.Katz AJ, Tholpady A, Tholpady S, et al. Cell Surface and Transcriptional Characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 12.Boquest AC, Shahdadfar A, Frønsdal K, et al. Isolation and Transcription Profiling of Purified Uncultured Human Stromal Stem Cells: Alteration of Gene Expression after In Vitro Cell Culture. Mol Biol Cell. 2005;16:1131–1131. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerrose TE, De Ugarte DA, Hofling AA, et al. In Vivo Distribution of Human Adipose-Derived Mesenchymal Stem Cells in Novel Xenotransplantation Models. Stem Cells. 2007;25:220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of Human Adipose-Derived Cells: Temporal Changes in Stromal-Associated and Stem Cell–Associated Markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodeling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 16.Barresi V, Belluardo N, Sipione S, et al. Transplantation of prodrug-converting neural progenitor cells for brain tumor therapy. Cancer Gene Therapy. 2003;10:396–402. doi: 10.1038/sj.cgt.7700580. [DOI] [PubMed] [Google Scholar]
- 17.De Ugarte DA, Alfonso Z, Zuk PA, et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Letters. 2003;89:267–270. doi: 10.1016/s0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 18.Ibberson D, Tremain N, Gray A. What is in a name? Defining the molecular phenotype of marrow stromal cells and their relationship to other stem/progenitor cells. Cytotherapy. 2001;35:409–411. doi: 10.1080/146532401753277274. [DOI] [PubMed] [Google Scholar]
- 19.Kilroy GE, Foster SJ, Wu X, et al. Cytokine Profile of Human Adipose-Derived Stem Cells: Expression of Angiogenic, Hematopoietic, and Pro-Inflammatory Factors. J Cell Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 20.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering. 2001;7:211–227. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 21.Sengenes C, Miranville A, Maumus M, et al. Chemotaxis and Differentiation of Human Adipose Tissue CD34+/CD31- Progenitor Cells: Role of SDF-1 Released by Adipose Tissue Capillary Endothelial Cells. Stem Cells. 2007 Sep;25(9):2269–2276. doi: 10.1634/stemcells.2007-0180. [DOI] [PubMed] [Google Scholar]
- 22.Kang YJ, Jeon ES, Song HY, et al. Role of c-Jun N-terminal Kinase in the PDGF-Induced Proliferation and Migration of Human Adipose Tissue-Derived Mesenchymal Stem Cells. J Cell Biochem. 2005;95:1135–1145. doi: 10.1002/jcb.20499. [DOI] [PubMed] [Google Scholar]
- 23.Traktuev D, Merfeld-Clauss S, Li J, et al. A Population of Multipotent CD34-Positive Adipose Stromal Cells Share Pericyte and Mesenchymal Surface Markers, Reside in a Periendothelial Location, and Stabilize Endothelial Networks. Circ Res. 2008 Jan 4;102(1):77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 24.Zannettino ACW, Paton S, Arthur A, et al. Multipotent Human Adipose-Derived Stromal Stem Cells Exhibit a Perivascular Phenotype In Vitro and In Vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 25.Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell & Tissue Res. 1992;270:469–474. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- 26.Hellstrom M, Gerhardt H, Kalen M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirschi KK, Rohovsky SA, Beck LH, et al. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1998;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- 28.Peirce SM, Skalak TC. Microvascular Remodeling: A Complex Continuum Spanning Angiogenesis to Arteriogenesis. Microcirculation. 2003;10:99–111. doi: 10.1038/sj.mn.7800172. [DOI] [PubMed] [Google Scholar]
- 29.Katz AJ. Mesenchymal Cell Culture: Adipose Tissue. In: Atala A, Lanza RP, editors. Methods of Tissue Engineering. Academic Press; 2002. pp. 277–286. [Google Scholar]
- 30.Iwaguro H, Yamaguchi J, Kalka C, et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 31.Weber A, Pedrosa I, Kawamoto A, et al. Magnetic resonance mapping of transplanted endothelial progenitor cells for therapeutic neovascularization in ischemic heart disease. European J Cardio-Thoracic Surg. 2004;26:137–143. doi: 10.1016/j.ejcts.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131:177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Norrby K, Jakobsson A, Sorbo J. Mast-cell-mediated angiogenesis: a novel experimental model using the rat mesentery. Virchows Archiv B Cell Pathology. 1986;52:195–206. doi: 10.1007/BF02889963. [DOI] [PubMed] [Google Scholar]
- 34.Anderson CR, Ponce AM, Price RJ. Immunohistochemical identification of an extracellular matrix scaffold that microguides capillary sprouting in vivo. J Histochem & Cytochem. 2004;52:1063–1072. doi: 10.1369/jhc.4A6250.2004. [DOI] [PubMed] [Google Scholar]
- 35.Bocci G, Danesi R, Benelli U, et al. Inhibitory effect of suramin in rat models of angiogenesis in vitro and in vivo. Cancer Chemotherapy & Pharmacol. 1999;43:205–212. doi: 10.1007/s002800050885. [DOI] [PubMed] [Google Scholar]
- 36.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 37.Murfee WL, Skalak TC, Peirce SM. Differential Arterial/Venous Expression of NG2 Proteoglycan in Perivascular Cells Along Microvessels: Identifying a Venule-Specific Phenotype. Microcirculation. 2005;12:151–160. doi: 10.1080/10739680590904955. [DOI] [PubMed] [Google Scholar]
- 38.Hasumi Y, Klosowska-Wardega A, Furuhashi M, et al. Identification of a subset of pericytes that respond to combination therapy targeting PDGF and VEGF signaling. Int J Cancer. 2007;121:2606–2614. doi: 10.1002/ijc.22999. [DOI] [PubMed] [Google Scholar]
- 39.Oedayrajsingh-Varma MJ, Breuls RGM, Schouten TE, et al. Phenotypical and Functional Characterization of Freshly Isolated Adipose Tissue-Derived Stem Cells. Stem Cells Dev. 2007;16:91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 40.Lanza F, Healy L, Dutherland DR. Structural and functional features of the CD34 antigen: an update. J Biol Reg and Homeostatic Agents. 2001;15:1–13. [PubMed] [Google Scholar]
- 41.Hristov M, Erl W, Weber PC. Endothelial progenitor cells isolation and characterization. Trends in Cardiovas Med. 2003;13:201–206. doi: 10.1016/s1050-1738(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 42.Croockewit AJ, Raymakers RAP, Preijers FWMB, et al. The role of different CD34 epitopes in detection and positive selection of CD31+ bone marrow and peripheral blood stem cells. Scand J Immunol. 1998;47:82–90. doi: 10.1046/j.1365-3083.1998.00261.x. [DOI] [PubMed] [Google Scholar]
- 43.Bouhadir JH, Mooney DJ. Promoting angiogenesis in engineered tissues. J Drug Targeting. 2001;9:397–406. doi: 10.3109/10611860108998775. [DOI] [PubMed] [Google Scholar]


